Abstract
Objective
Human fibrinogen concentrate (HFC) is approved by the Food and Drug Administration for use at 70 mg/kg to treat congenital afibrinogenemia. We sought to determine whether this dose of HFC increases fibrinogen levels in the setting of high-risk bleeding associated with aortic reconstruction and deep hypothermic circulatory arrest (DHCA).
Methods
This was a prospective, pilot, off-label study in which 22 patients undergoing elective proximal aortic reconstruction with DHCA were administered 70 mg/kg HFC upon separation from cardiopulmonary bypass (CPB). Fibrinogen levels were measured at baseline, just before, and 10 minutes after HFC administration, on skin closure, and the day after surgery. The primary study outcome was the difference in fibrinogen level immediately after separation from CPB, when HFC was administered, and the fibrinogen level 10 minutes following HFC administration. Additionally, postoperative thromboembolic events were assessed as a safety analysis.
Results
The mean baseline fibrinogen level was 317±49 mg/dL and fell to 235±39 mg/dL just before separation from CPB. After HFC administration, the fibrinogen level rose to 331±41 mg/dL (p<0.001) and averaged 372±45 mg/dL the next day. No postoperative thromboembolic complications occurred.
Conclusions
Administration of 70 mg/kg HFC upon separation from CPB raises the fibrinogen levels by approximately 100 mg/dL without an apparent increase in thrombotic complications during proximal aortic reconstruction with DHCA. Further prospective study in a larger cohort of patients will be needed to definitively determine the safety and evaluate the efficacy of HFC as a hemostatic adjunct during these procedures.
Keywords: bleeding, blood transfusion, blood coagulation, anesthesia, aortic reconstruction, deep hypothermia, circulatory arrest, fibrinogen, coagulopathy
Introduction
Surgery of the aortic arch with deep hypothermic circulatory arrest (DHCA) is often associated with coagulopathic bleeding as a result of coagulation factor consumption during prolonged periods of cardiopulmonary bypass (CPB) and hypothermia-related platelet dysfunction.1 Fibrinogen consumption is particularly exaggerated during these procedures, and the ability to replete fibrinogen represents an important element for the correction of the coagulopathy that develops in these procedures.1 Fibrinogen is traditionally replaced by transfusion with plasma or cryoprecipitate.2, 3 However, in addition to the increased morbidity and mortality risk that results from large volume blood product transfusion,3–5 the use of plasma or cryoprecipitate for fibrinogen replacement has several specific disadvantages.6 First, the amount of fibrinogen given with plasma or cryoprecipitate transfusion is unknown, prohibiting the ability to replete fibrinogen in a targeted manner. Second, cryoprecipitate and plasma delivery requires product thawing, which can result in critical delays in the setting of acute operative bleeding. Third, there is no virus inactivation or elimination process for cryoprecipitate, imparting the risk of virus transmission upon transfusion. Lastly, plasma and cryoprecipitate contain alloantigens, which can result in anaphylaxis or less severe hypersensitivity reactions.6
For these reasons, human fibrinogen concentrate (HFC) (CSL Behring, Marburg, Germany; RiaSTAP® in USA, Haemocomplettan® P in Europe) represents an attractive alternative for the correction of acquired fibrinogen deficiency compared to plasma or cryoprecipitate. HFC is a highly purified, lyophilized, virus-inactivated fibrinogen powder manufactured from human plasma. Current evidence suggests that fibrinogen concentrates are well-tolerated and can quickly restore hemostasis in patients with fibrinogen deficiencies.7, 8 Several European reports on the use of HFC for trauma-related massive hemorrhage and acquired perioperative fibrinogen deficiency, including in the setting of cardiothoracic surgery, have also been published previously.9–16 However, in the United States, HFC is reserved for replacement therapy in congenital fibrinogen deficiency and reports in the USA testing the efficacy of HFC in correcting acquired fibrinogen deficiency in aortic surgery are lacking. As such, the primary aim of this study was to test the hypothesis that use of HFC at the US Food and Drug Administration (FDA) approved dose of 70 mg/kg increases the fibrinogen level in the high-risk setting for coagulopathic bleeding of proximal aortic reconstruction with hemiarch replacement after DHCA. Additionally, as an exploratory secondary aim, we performed a post hoc analysis investigating whether HFC administration reduced perioperative bleeding and allogeneic blood product transfusion compared to a contemporary propensity-matched cohort.
Patients and Methods
Study design and Patient Population
This was a single-center, prospective, pilot, off-label study designed to determine whether HFC (RiaSTAP®; CSL Behring) increases fibrinogen levels when administered as a one-time 70 mg/kg dose upon separation from cardiopulmonary bypass during non-emergent proximal aorta/hemi-arch reconstruction with DHCA. The study protocol was approved by the Duke University Medical Center (Durham, NC) Institutional Review Board, and informed consent was obtained from each patient who was enrolled. Patients ≥18 years of age undergoing non-emergent proximal thoracic aortic reconstruction (ascending aorta +/− aortic valve or root) with hemi-arch replacement and DHCA from December 2010 to April 2012 were eligible for the study. Exclusion criteria were as follows: concomitant coronary artery bypass grafting, coronary artery stenting within the last three years, refusal of blood transfusion, myocardial infarction within the past three months, pregnancy, an International Normalized Ratio >1.5, thienopyridines within five days of surgery, aspirin (325 mg) within 48 hours of surgery (aspirin 81mg was acceptable), platelet count of less than 100,000/mm3, inability to obtain written informed consent, and known coagulopathy.
Conduct of Surgery
Surgical techniques and the conduct of operation utilized for proximal aortic repair and hemi-arch replacement were as described previously.17 Porcine heparin was administered as a 300 U/kg bolus and then supplemented to maintain an activated clotting time longer than 480 seconds during CPB. Additionally, a 5000-unit bolus of heparin was given before circulatory arrest. Before the portion of the aortic reconstruction requiring DHCA, the patient was cooled on CPB until electrocerebral inactivity was detected by electroencephalography as described18, 19
Transfusion and Coagulopathy Management
An institutional transfusion algorithm has been developed for the management of bleeding and coagulopathy when separating from CPB in cases of aortic reconstruction with DHCA (Figure 1). This algorithm is based on our previously published experience on transfusion requirements during these procedures1 as well as societal perioperative transfusion guidelines.2, 3 In brief, antifibrinolytic therapy with epsilon-aminocaproic acid is administered as a 5-g bolus followed by a 1-g/h infusion continued into the ICU. Prior to separation from CPB, upon rewarming and reperfusion, the bypass pump is primed with four units of plasma. This quantity has been selected based on the fact that it represented the 25th percentile plasma requirement after aortic reconstruction with DHCA in our pre-algorithm experience.1 Also prior to separation from CPB, hemofiltration is preformed to ameliorate coagulation factor dilution, and a set of laboratory tests are obtained to help guide management. Protamine sulfate is then administered until activated clotting time is normalized. At the time of separation from CPB, an additional 5-g bolus of epsilon-aminocaproic acid is given, and a 0.3 μg/kg dose of desmopressin acetate is administered to help correct platelet dysfunction and increase Factor VIII and von Willebrand factor levels. If hemostasis is not immediately achieved, one unit of platelets is transfused followed by a second if bleeding persists. At this point, labs are rechecked, and if bleeding persists an additional unit of platelets and two units of plasma are administered. Based on laboratory results, cryoprecipitate (if fibrinogen level <200 mg/dL), platelets (if less than 100,000/mm3 more than the pre-CPB separation platelet value), and/or 2 plasma units are then transfused. If bleeding continues, recombinant activated factor VII (rFVIIa) (1–2 mg) is administered.20 If hemostasis is still not obtained, packed red blood cells (PRBCs) and plasma are administered at a 1:1 ratio with additional cryoprecipate, platelets, and hemostatic adjuncts administered at clinician discretion, with guidance from laboratory and functional tests. With regard to red blood cell transfusion, serial hematocrits are drawn prior to and after separation from CPB. The return of washed, shed red blood cells (BRAT II blood cell salvage machine; Cobe Cardiovascular Inc., Arvada, CO) to the patient is used in all cases and additional PRBC transfusion is generally avoided if the hematocrit is greater than 0.20.
Figure 1.
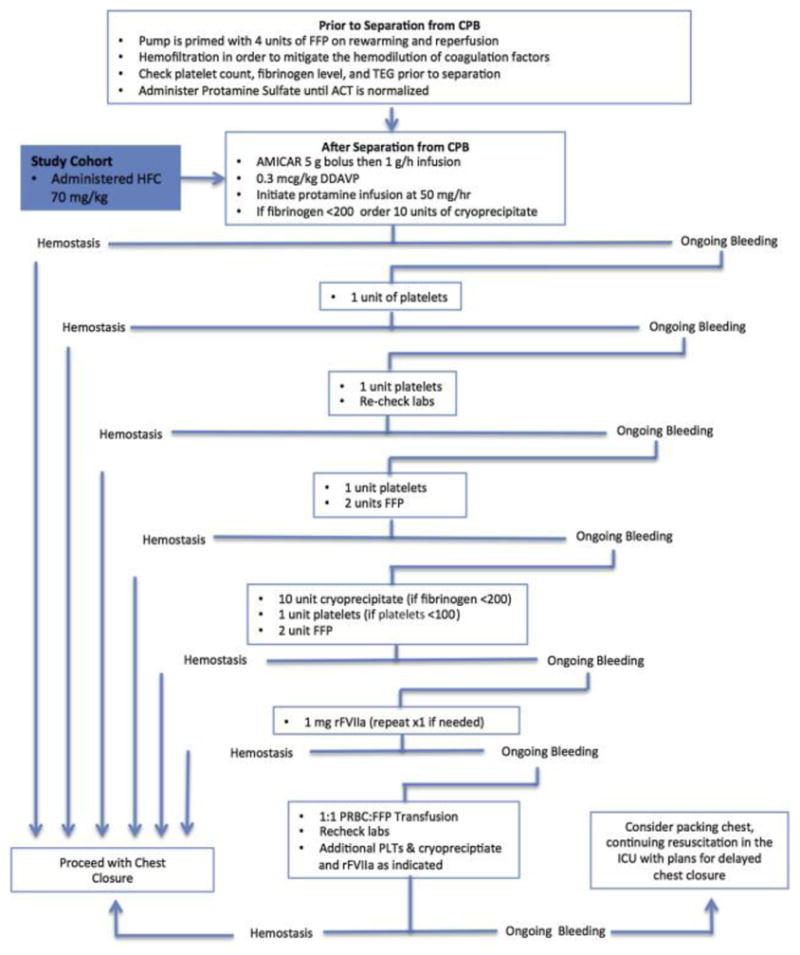
Institutional Transfusion Algorithm Schematic. In addition to standard treatment with the transfusion algorithm, the study cohort received 70 mg/kg of human fibrinogen concentrate upon separation from cardiopulmonary bypass (as depicted by the blue shaded box). CPB: cardiopulmonary bypass; TEG: thromboelastogram; DHCA: deep hypothermic circulatory arrest; HFC: human fibrinogen concentrate; FFP: fresh frozen plasma; rFVIIa: recombinant activated Factor VII; PRBC: packed red blood cells.
Institutional transfusion algorithm schematic. In addition to standard treatment with the transfusion algorithm, the study cohort received 70 mg/kg human fibrinogen concentrate (HFC) upon separation from cardiopulmonary bypass (CPB) (blue shaded box)...
In total, while this algorithmic approach for the management of bleeding and coagulopathy after CPB with DHCA is mostly empirical, it was developed to allow the reliable and timely management of the severe coagulopathic bleeding that often occurs in aortic reconstruction with DHCA. Although intraoperative laboratory testing, including thormoboelastography and platelet agglutination (MEA), are employed when available, our approach is not ultimately reliant on such tests. While these intraoperative tests can be useful in directing intraoperative transfusion when coming off CPB,21–23 they often do not provide information rapidly enough to guide therapy with acute intraoperative bleeding. Therefore, the supplemental information provided by intraoperative laboratory testing is used in conjunction with clinical judgment to modify the protocol when appropriate. After leaving the operating room, practices for postoperative transfusion were carried out according to societal guidelines.2, 3
Human Fibrinogen Concentrate Administration and Fibrinogen Level Monitoring
Patients enrolled in the study were administered 70 mg/kg of HFC at the time of separation from CPB (Figure 1). Fibrinogen levels were measured at five time points: (A) baseline at anesthesia induction, (B) after separation from cardiopulmonary bypass when protamine is given, (C) ten minutes following HFC administration, (D) on admission to the intensive care unit, and (E) 24 hours after anesthesia induction. Fibrinogen activity was determined using a modified Clauss assay (detection limit of 0.2 g/L). In a subset of patients, the whole blood, multiplate electroaggregometer (MEA, Diapharma, West Chester, OH) was used to evaluate platelet aggregation in response to adenosine diphosphate (ADP) and thrombin receptor activating peptide (TRAP) agonists.
The primary study outcome was the difference in fibrinogen level immediately after separation from cardiopulmonary bypass (B), when HFC was administered, and the fibrinogen level 10 minutes following HFC administration (C). No formal power calculation was performed in order to determine the number of patients that would need to be enrolled to detect a statistically significant difference for a given increase in fibrinogen level. Instead, the enrollment in this pilot study was determined largely by practical factors, such as funding and personnel resources.
Safety Outcomes
In addition to our primary outcome, a number of secondary outcomes were assessed to evaluate the safety of HFC and the extent of bleeding and transfusion requirement in these patients. Thromboembolic events and post-operative complications were defined according to Society of Thoracic Surgeons definitions.24 The study data safety and monitoring board, composed of a hematologist and transfusion medicine pathologist not otherwise involved in the study, reviewed all inpatient complications and adverse events to determine whether they may have been related to HFC administration. Additionally, bleeding and transfusion data collected included the following: cell saver on and post-CPB; intra-operative PRBC, plasma, platelet, and cryoprecipitate requirements pre- and post-CPB; post-operative PRBC, plasma, platelet, and cryoprecipitate requirements through post-operative day two, and chest tube drainage 24 hours postoperatively.
Propensity-Matched Comparison with a Contemporary Patient Cohort
As an exploratory secondary aim of this study we performed a post hoc propensity-matched comparison of the study cohort with a contemporary cohort of patients undergoing non-emergent hemiarch replacement with DHCA over the same time period (who had not received HFC) to evaluate the possibility that HFC may reduce perioperative bleeding and transfusion. Covariables accounted for in the propensity match included age, sex, race, body mass index, hypertension, hyperlipidemia, current or previous smoking history, diabetes, coronary artery disease, history of stroke/transient ischemic attack, chronic obstructive pulmonary disease, renal insufficiency (creatinine > 1.5 mg/dL), peripheral vascular disease, redo-sternotomy, root replacement (Bentall and valve-sparing), ascending aortic replacement (supracoronary ascending and Wheat), concomitant procedure, previous aortic surgery, atherosclerotic disease, bicuspid aortic valve syndrome, acute or previous aortic dissection, presenting aortic symptoms, ejection fraction, grade of aortic insufficiency, maximum aortic diameter, American Society of Anesthesiologists (ASA) classification, and use retrograde or antegrade cerebral perfusion. Propensity score matching was implemented using one-to-one or pair matching without replacement using the nearest neighbor matching method. Optimal matching was used to minimize total within-pair difference of the propensity score. Each patient in the HFC group was matched to a patient in the contemporary no-HFC group with the closest propensity score; the maximum difference in propensity score for a match was less than 0.015.
Statistical Analysis
Fibrinogen levels, laboratory assessments, bleeding and transfusion data were summarized descriptively. Repeated measures analysis of variance with the Bonferroni adjustment for multiple comparisons was used to test for change in fibrinogen level with time. Comparisons between the HFC study group and the no-HFC contemporary group were performed using the Wilcoxon rank-sum test for continuous variables and Fisher's exact test (cell counts <5) or Pearson’s chi-squared test for categorical variables. A P value < 0.05 was considered statistically significant. Statistical analyses were performed with SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Study Population
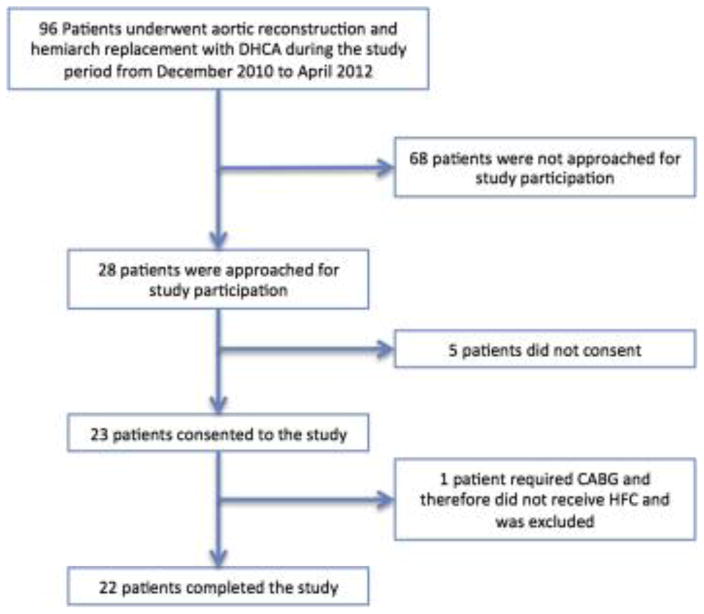
Twenty-two patients were enrolled and completed the study (Figure 2). Five patients approached for participation in the study did not give consent, and an additional patient who was enrolled required coronary artery bypass grafting as part of his procedure and was therefore excluded. In total, the final study cohort represented 23% (22/96) of all patients undergoing hemi-arch aortic reconstruction with DHCA during the study interval.
Figure 2.
CONSORT diagram
Consolidated Standards of Reporting Trials diagram. DHCA, Deep hypothermic circulatory arrest; CABG, coronary artery bypass grafting; HFC, human fibrinogen concentrate.
Fibrinogen Activity
Mean, median, maximum, and minimum fibrinogen plasma activity levels at the measured time points are displayed in Table 1. On average, fibrinogen plasma activity levels decreased from 317±49 mg/dL preoperatively to 235±39 (26±9%) at separation from CPB. Ten minutes following the administration of HFC, the mean fibrinogen plasma activity levels increased significantly to a peak of 331±41 mg/dL, or a mean increase of 97±33 mg/dL (43±17%). Subsequent to this time point the average fibrinogen level remained approximately at the baseline level or greater.
Table 1.
Fibrinogen Plasma Activity Levels and Hematocrit at Specified Time Points.
| A | B | C | D | E | |
|---|---|---|---|---|---|
| Fibrinogen Activity Level (mg/dL) | |||||
| Mean ± SD | 317±49 | 235±(39)* | 331±41 | 312±41 | 372±45 |
| Median (IQR) | 301 (280–341) | 224 (217–256) | 337 (302–363) | 311 (291–333) | 357 (343–388) |
| Maximum | 414 | 345 | 404 | 382 | 480 |
| Minimum | 255 | 154 | 257 | 210 | 316 |
|
| |||||
| Hematocrit (%) | |||||
| Mean ± SD | 42±6 | 28±5 | 28±4 | 28±2 | 30±3 |
| Median (IQR) | 43 (40–45) | 27 (24–32) | 27 (26–31) | 29 (27–30) | 31 (28–32) |
| Maximum | 50 | 37 | 38 | 34 | 34 |
| Minimum | 25 | 20 | 21 | 23 | 25 |
SD=standard deviation; IQR=interquartile range; A=baseline at anesthesia induction; B=after separation from CPB when protamine is given; C=10 minutes following HFC administration; D=on admission to the intensive care unit; E=24 hours after anesthesia induction;
Significantly different value by ANOVA with Bonferroni correction for multiple comparisons (p<0.0001)
Safety Outcomes
There were no thromboembolic complications postoperatively. 30-day/in-hospital rates of death, stroke, and renal failure were 0%, and there were no reoperations for bleeding. There were 10 (43%) patients who experienced postoperative complications, including focal axillary artery dissection related to axillary arterial cannulation (n=1), postoperative atrial fibrillation (n=6), other dysrhythmia (n=3) (left bundle branch block, transient complete heart block, and atrial flutter), and left recurrent laryngeal nerve palsy (n=2). None of these complications were deemed related to the administration of HFC. There was one 30-day readmission in the cohort for atrial flutter, which was also unrelated to HFC administration.
Laboratory Assessment, Bleeding, and Transfusion
The mean and median hematocrits were highest at baseline (42% and 43%, respectively) (Table 1). After an initial decrement due to intra-operative blood loss, hematocrit remained stable from the initial postoperative value through post-operative day one. Following separation from CPB (during and immediately after administration of HFC) a large but highly variable volume of blood was processed through the cell saver as evidenced by a median value of 505 [IQR:242–725] mL of cell saver product being re-infused (Table 2). Generally, subjective evaluation of the surgical field by the attending surgeon following separation from CPB and HFC administration indicated persistence of microvascular bleeding, as evidenced by lack visible clot and absence of surgically correctable bleeding sites. Additionally, over 90% of the cohort required platelet transfusion (median 2 [IQR:2–3] units) to achieve resolution of microvascular bleeding. This was in the setting of an over 50% decrease in ADP stimulated platelet aggregation (within our locally determined range for clopidogrel maintenance therapy) in a subset of 9 patients where MEA data were available.
Table 2.
Transfusion at Specified Time Points.
| N=22 | During CPB | After CPB* | ICU arrival thru POD2 |
|---|---|---|---|
| Cell Saver (mL), Median (IQR) | 178 (18–303) | 505 (242–725) | - |
|
| |||
| PRBC | |||
| Number of Patients Transfused (%) | 2 (9.1%) | 9 (40.9%) | 6 (27.3%) |
| Median (IQR) Units Among Patients Transfused | 1.5 (1, 2) | 1 (1, 2) | 1 (1, 2) |
|
| |||
| FFP | |||
| Number of Patients Transfused (%) | 17 (77.3%) | 7 (31.8%) | 1 (4.5%) |
| Median (IQR) Units Among Patients Transfused | 4 (4, 4) | 2 (1, 4) | 5 |
|
| |||
| Platelets | |||
| Number of Patients Transfused (%) | 4 (18.2%) | 20 (90.9%) | 1 (4.5%) |
| Median (IQR) Units Among Patients Transfused | 1.5 (1, 2) | 2 (1, 2) | 1 |
|
| |||
| Cryoprecipitate | |||
| Number of Patients Transfused (%) | 0 | 3 (13.6%) | 0 |
| Median (IQR) Units Among Patients Transfused | NA | 1 (1, 2) | NA |
CPB=cardiopulmonary bypass; SD=standard deviation; IQR=interquartile range; ICU=intensive care unit; POD=post-operative day.
After CPB includes before, during, and after administration of HFC until the end of surgery.
Occasionally PRBCs and plasma were given during instances of rapid, high volume blood loss following separation from CPB (Table 2). In addition, less than half of the cohort required PRBC transfusion before or after CPB, and all transfusion requirements were minimal after chest closure.
Propensity Matched Comparison with a Contemporary Cohort
Baseline characteristics, comorbidities, and operative variables of the HFC (study cohort) and no-HFC (contemporaneous cohort that did not receive HFC) groups are shown in Table 3. After propensity matching, there were no significant differences in the baseline characteristics of these groups (Table 3). In review of perioperative transfusion requirements of these matched groups, a lower proportion of the HFC group received cryoprecipitate intraoperatively (13.6% vs. 54.5%, p<0.001) (Table 4).In addition, while the proportion of patients transfused with PRBCs did not differ significantly between groups, HFC patients compared to no-HFC patients who were transfused PRBCs received a significantly lower median number of transfusion units (1.5 [IQR: 1–2] vs. 3 [IQR 2–3] units, p=0.02), Otherwise, there were no significant differences in the rate or volume of specific blood products transfused between these groups intraoperatively or post-operatively.
Table 3.
Baseline and operative characteristics of the HFC study cohort and a contemporary no-HFC cohort undergoing elective proximal aortic surgery including hemi-arch replacement with DHCA before and after propensity matching.
| Variable | HFC (N=22) | Before Matching | After Matching | ||
|---|---|---|---|---|---|
| No-HFC (N=74) | p-value | No HFC (N=22) | p-value | ||
| Age (yrs, mean±SD) | 51.9±13.7 | 58.2 ± 13.2 | 0.04 | 52.4 ± 13.5 | 0.25 |
| Male | 15 (68.2) | 46 (62.2) | 0.69 | 15 (68.2) | >0.99 |
| White Race | 18 (81.8) | 52 (70.3) | 0.26 | 17 (77.3) | 0.71 |
| BMI (kg/m2, mean±SD) | 29.9 ± 6.6 | 29.0 ± 6.4 | 0.47 | 29.2 ± 4.9 | 0.74 |
| Hypertension | 16 (72.7) | 63 (85.1) | 0.22 | 17 (77.3) | 0.73 |
| Hyperlipidemia | 13 (59.1) | 47 (63.5) | 0.67 | 11 (50.0) | 0.54 |
| Smoker | 12 (54.5) | 40 (54.1) | 0.97 | 12 (54.5) | >0.99 |
| Diabetes | 2 (9.1) | 10 (13.5) | 0.57 | 5 (18.2) | 0.22 |
| Coronary Artery Disease | 6 (27.3) | 23 (31.1) | <0.001 | 7 (31.8) | 0.74 |
| History of Stroke/TIA | 2 (9.1) | 4 (5.4) | 0.57 | 1 (4.5) | 0.55 |
| COPD | 1 (4.5) | 12 (16.2) | 0.03 | 2 (9.1) | 0.55 |
| Renal Insufficiency (Cr>1.5mg/dL) | 1 (4.5) | 11 (14.8) | 0.11 | 2 (9.1) | 0.55 |
| Peripheral Vascular Disease | 0 (0) | 5 (6.8) | 0.009 | 0 (0) | >0.99 |
| Redo-Sternotomy | 3 (13.6) | 20 (27.0) | 0.16 | 4 (18.2) | 0.68 |
| Root Replacement | 9 (40.9) | 31 (41.9) | 0.93 | 12 (54.5) | 0.37 |
| Ascending Aortic Repair | 12 (54.5) | 43 (58.1) | 0.94 | 10 (45.5) | 0.55 |
| Concomitant Procedure | 5 (22.7) | 32 (43.2) | <0.001 | 6 (27.3) | 0.73 |
| Previous Aortic Surgery | 3 (13.6) | 22 (29.7) | 0.10 | 5 (22.7) | 0.43 |
| Atherosclerotic Disease | 8 (36.4) | 48 (64.9) | 0.02 | 9 (40.9) | 0.76 |
| Bicuspid Aortic Valve Syndrome | 13 (59.1) | 25 (33.8) | 0.03 | 13 (59.1) | >0.99 |
| Chronic Type A Dissection | 1 (4.5) | 7 (9.5) | <0.001 | 2 (9.1) | 0.55 |
| Previous Aortic Dissection | 2 (9.1) | 8 (10.8) | 0.86 | 3 (13.6) | 0.63 |
| Presenting Aortic Symptoms | 2 (9.1) | 28 (37.8) | <0.001 | 3 (13.6) | 0.63 |
| Ejection Fraction (%, mean±SD) | 54.3 ± 1.8 | 51.9 ± 8.2 | 0.01 | 51.4 ± 9.7 | 0.18 |
| Aortic Insufficiency | 10 (45.5) | 42 (56.8) | 0.91 | 10 (45.5) | >0.99 |
| Max Aortic Diameter (cm, mean±SD) | 5.6 ± 0.7 | 5.6 ± 1.3 | 0.80 | 5.9 ± 1.4 | 0.54 |
| ASA Class | <0.001 | 0.55 | |||
| Class 2 | 1 (4.5) | 4 (5.4) | 2 (9.1) | ||
| Class 3 | 21 (95.5) | 47 (63.5) | 20 (90.1) | ||
| Retrograde Cerebral Perfusion | 5 (22.7) | 12 (16.2) | 0.52 | 4 (18.2) | 0.71 |
| Antegrade Cerebral Perfusion | 17 (77.3) | 62 (83.8) | 0.31 | 18 (81.8) | 0.71 |
| *Minimum Core Temp. (°C, mean±SD) | 16.7±1.5 | 18.3±2.7 | 0.0009 | 17.9±2.3 | 0.05 |
| *Minimum Nasopharyngeal Temp. (°C, mean±SD) | 14.7±2.0 | 14.6±1.9 | 0.83 | 14.3±1.5 | 0.39 |
| *Operative Time (minutes, mean±SD) | 310±44.1 | 346.3±71.7 | 0.006 | 336.5±70.3 | 0.15 |
| *Cardiopulmonary Bypass Time (minutes, mean±SD) | 202.1±30.1 | 210.7±37.1 | 0.27 | 214.6±36.4 | 0.22 |
| *Circulatory Arrest Time (minutes, mean±SD) | 16.5±4.1 | 17.3±5.1 | 0.45 | 16.2±4.0 | 0.85 |
SD: standard deviation; BMI: body mass index; TIA: transient ischemic attack; Cr: creatinine; ASA: American Society of Anesthesiologists;
not included as matching variables
Table 4.
Transfusion and chest tube output data in HFC group (n=22) and contemporary propensity matched controls (n=22) undergoing elective proximal thoracic aortic surgery including hemi-arch replacement with DHCA and not receiving study drug.
| Outcome | HFC (n=22) | No HFC (n=22) | p-value |
|---|---|---|---|
| Total Cell Saver (mL) | 643 (474–879) | 500 (288–750) | 0.20 |
|
| |||
| Intraoperative PRBC | |||
| Number of Patients Transfused (%) | 10 (45.5%) | 11 (50.0%) | 0.99 |
| Median (IQR) Units Among Patients Transfused | 1.5 (1, 2) | 3 (2, 3) | 0.02 |
|
| |||
| Intraoperative FFP | |||
| Number of Patients Transfused (%) | 19 (86.4%) | 21 (95.5%) | 0.61 |
| Median (IQR) Units Among Patients Transfused | 4 (4, 6) | 5 (4, 8) | 0.15 |
|
| |||
| Intraoperative Platelets | |||
| Number of Patients Transfused (%) | 20 (90.9%) | 22 (100%) | 0.49 |
| Median (IQR) Units Among Patients Transfused | 2 (2, 3) | 2 (2, 3) | 0.73 |
|
| |||
| Intraoperative Cryoprecipiate | |||
| Number of Patients Transfused (%) | 3 (13.6%) | 12 (54.5%) | 0.01 |
| Median (IQR) Units Among Patients Transfused | 1 (1, 1.5) | 1 (1, 1) | 0.33 |
|
| |||
| Postoperative PRBC | |||
| Number of Patients Transfused (%) | 6 (27.3%) | 1 (4.5%) | 0.09 |
| Median (IQR) Units Among Patients Transfused | 1 (1, 1.8) | 1 | 0.53 |
|
| |||
| Postoperative FFP | |||
| Number of Patients Transfused (%) | 1 (4.5%) | 0 | 0.99 |
|
| |||
| Postoperative Platelets | |||
| Number of Patients Transfused (%) | 1 (4.5%) | 0 | 0.99 |
|
| |||
| Postoperative Cryoprecipitate | |||
| Number of Patients Transfused (%) | 0 | 0 | NA |
|
| |||
| 24hr Postoperative Chest Tube Drainage (mL) | 525 (388–720) | 570 (493–795) | 0.16 |
|
| |||
| Intraoperative Factor VIIa Administration | 2 (9.1) | 4 (18.2) | 0.37 |
|
| |||
| Postoperative Factor VIIa Administration | 0 | 0 | NA |
DHCA=deep hypothermic circulatory arrest; SD=standard deviation; PRBCs=packed red blood cells; FFP=fresh frozen plasma; POD=post-operative day; NA=not applicable
Discussion
Here we present the first prospective study on the use of HFC as a hemostatic adjunct during aortic surgery in the United States. We found that, when HFC was administered at the FDA approved dose of 70 mg/kg at the time of separation from CPB after aortic reconstruction with DHCA, fibrinogen levels increased by approximately 100 mg/dL within 10 minutes of administration. Additionally, there were no adverse events related to the administration HFC observed in the study cohort. Thus, the findings from this pilot study suggest that HFC may safely and rapidly raise fibrinogen levels in the high-risk setting for coagulopathic bleeding associated with aortic reconstruction and DHCA.
By rapidly restoring fibrinogen levels during these procedures HFC may offer several advantages over conventional methods for fibrinogen repletion, which entail transfusion of plasma and cryoprecipitate. First, administration of HFC may be time-saving by precluding the need for thawing and cross-matching. Second, since the dose of fibrinogen in HFC is known, it can be given in an intelligible fashion to raise fibrinogen to a targeted level, which is not the case with the administration of plasma or cryoprecipitate. Finally, administration of HFC avoids some of the risks associated with allogeneic blood product transfusion, including infectious disease transmission, hypersensitivity reactions, and adverse events related to volume overload and other issues that arise with large volume blood product transfusion.3–5, 27–29
Consistent with the putative benefits of HFC, when compared to a contemporary propensity-matched cohort that had not received HFC, a significantly lower portion of the HFC cohort received cryoprecipitate intraoperatively. Furthermore, while the rate of transfusion of PRBCs was similar between groups, the volume of transfusion among patients who received PRBCs was slightly lower in the HFC group. While these findings suggest that the HFC administration may help to reduce transfusion requirement and obtain hemostasis more rapidly in aortic surgery with DHCA, there is potential for bias in the comparison of these two non-randomized groups and additional study will be needed to fully discern the efficacy of HFC in controlling coagulopathy and bleeding during these procedures.
In addition, despite the potential benefit that HFC may hold as a hemostatic adjunct during aortic surgery with DHCA, it was also clear from this study that HFC does not completely reverse the coagulopathy incurred with these procedures. This was evidenced by the considerable platelet transfusion requirement and the microvascular bleeding observed after separation from CPB in patients who had received HFC as well as the severe platelet dysfunction was demonstrated in the subset of patients who had platelet aggregometery data. This suggests that platelet dysfunction, which is a well-known occurrence after DHCA,30 was a major contributing factor to the persistent coagulopathy observed in the patients who had received HFC, indicating that adjunctive HFC may not address this aspect of coagulopathy after aortic reconstruction with hypothermia.
While this is the first prospective study on HFC conducted in the USA, several pilot studies from European centers have evaluated HFC in aortic and cardiac surgery previously. Rahe-Mayer and colleagues found that administration of HFC resulted in reduced postoperative bleeding and allogeneic transfusion, including platelets, for patients undergoing ascending aorta replacement and aortic valve surgery with moderate hypothermia (n=15).14 In addition, Solomon and colleagues evaluated the impact of HFC in coronary artery bypass grafting (CABG) and observed a reduction in PRBC transfusion.31 Our study is consistent with these prior studies in several respects, including the findings that HFC increases fibrinogen levels and may reduce allogeneic blood product transfusion. However, our findings differ from those of Rahe-Mayer and colleagues in that patients who received HFC continued to require platelet transfusion and demonstrated ongoing coagulopathy early after separation from CPB that was due, at least in part, to platelet dysfunction. Several factors may explain this discrepancy. First, Rahe-Meyer et al. cooled patients to 24°C on average,14 whereas our study included only patients with DHCA, which amounts to nadir temperatures typically less than 18°C.19 This deep degree of hypothermia has been recognized to seriously impair platelet function for many hours after rewarming30 and may explain the reliance on allogeneic platelet transfusion in our study cohort. Second, Rahe-Meyer used HFC with the objective of achieving upper normal fibrinogen levels (mean 3.6 g/L). This fact limits direct comparisons with the current study where no specific fibrinogen level was targeted.
Our pilot study has several limitations, the main one being the lack of a randomized control group. Although the contemporary propensity-matched group was well balanced in the baseline and operative characteristics considered, we acknowledge that such an approach cannot control for potential confounding and bias as well as a prospective randomized trial. Additionally, the multi-faceted nature of our algorithmic approach towards the management of intraoperative bleeding and coagulopathy makes it difficult to delineate the impact of any single intervention, as variations in the approach to bleeding and coagulopathy management are dictated by case-specific factors. Carefully controlling for such variations will be important considerations for future prospective evaluation of HFC efficacy. We also did not have robust data on intraoperative coagulopathy assessment, which will be important in future studies in order to objectively determine the extent to which HFC administration improves coagulation system function and clot strength. Finally, while we did not observe any thromboembolic events associated with the administration of HFC, the small sample size of this study leaves a high chance for type II error in regard to safety outcomes as well as endpoints compared between the HFC and non-HFC matched groups. Therefore, definitive conclusions on the safety and efficacy of HFC should not be drawn from this pilot study. Larger, prospective studies will be needed to firmly establish the safety of HFC and to determine whether it is efficacious in the management of coagulopathy during aortic reconstruction with DHCA.
Despite these limitations, we conclude that, when administered at the FDA-approved dose of 70 mg/kg at the time of separation from CPB, HFC increases fibrinogen levels by approximately 100 mg/dL and may potentially reduce transfusion requirements during procedures involving aortic reconstruction with DHCA. These findings support that HFC may prove to be a valuable hemostatic adjunct for the correction of coagulopathy associated with aortic reconstruction and DHCA. Further prospective study will be needed to verify these results and to definitively determine the safety and efficacy of HFC in a larger cohort of patients.
Acknowledgments
Funding: Financial support for this study was received from CSL Behring (Marburg, Germany). CSL Behring also donated human fibrinogen concentrate (RiaSTAP®) for the purposes of conducting this study.
Footnotes
Disclosures: Dr. Ian Welsby serves as a consultant for CSL Behring. All authors had full freedom of investigation before, during, and after the study in regards to control of the design of the study, acquisition, analysis, and interpretation of data, and freedom to fully disclose all results.
References
- 1.Williams JB, Phillips-Bute B, Bhattacharya SD, Shah AA, Andersen ND, Altintas B, et al. Predictors of massive transfusion with thoracic aortic procedures involving deep hypothermic circulatory arrest. The Journal of thoracic and cardiovascular surgery. 2011;141:1283–8. doi: 10.1016/j.jtcvs.2010.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. The Annals of thoracic surgery. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 4.Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. The Annals of thoracic surgery. 2002;74:1180–6. doi: 10.1016/s0003-4975(02)03766-9. [DOI] [PubMed] [Google Scholar]
- 5.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. The New England journal of medicine. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 6.Callum JPP. Clinical Guide to Transfusion. 4. Canadian Blood Services; 2007. Adverse Reactions; pp. 82–111. [Google Scholar]
- 7.Groner A. Reply. Pereira A. Cryoprecipitate versus commercial fibrinogen concentrate in patients who occasionally require a therapeutic supply of fibrinogen: risk comparison in the case of an emerging transfusion-transmitted infection. Haematologica 2007;92:846–9. Haematologica. 2008;93:e24–6. doi: 10.3324/haematol.11072. author reply e7. [DOI] [PubMed] [Google Scholar]
- 8.Kreuz W, Meili E, Peter-Salonen K, Haertel S, Devay J, Krzensk U, et al. Efficacy and tolerability of a pasteurised human fibrinogen concentrate in patients with congenital fibrinogen deficiency. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2005;32:247–53. doi: 10.1016/j.transci.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Danes AF, Cuenca LG, Bueno SR, Mendarte Barrenechea L, Ronsano JB. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox sanguinis. 2008;94:221–6. doi: 10.1111/j.1423-0410.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- 10.Fenger-Eriksen C, Jensen TM, Kristensen BS, Jensen KM, Tonnesen E, Ingerslev J, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. Journal of thrombosis and haemostasis : JTH. 2009;7:795–802. doi: 10.1111/j.1538-7836.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- 11.Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, Ingerslev J, Sorensen B. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. British journal of anaesthesia. 2008;101:769–73. doi: 10.1093/bja/aen270. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson M, Ternstrom L, Hyllner M, Baghaei F, Flinck A, Skrtic S, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thrombosis and haemostasis. 2009;102:137–44. doi: 10.1160/TH08-09-0587. [DOI] [PubMed] [Google Scholar]
- 13.Mittermayr M, Streif W, Haas T, Fries D, Velik-Salchner C, Klingler A, et al. Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesthesia and analgesia. 2007;105:905–17. doi: 10.1213/01.ane.0000280481.18570.27. table of contents. [DOI] [PubMed] [Google Scholar]
- 14.Rahe-Meyer N, Pichlmaier M, Haverich A, Solomon C, Winterhalter M, Piepenbrock S, et al. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. British journal of anaesthesia. 2009;102:785–92. doi: 10.1093/bja/aep089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahe-Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. The Journal of thoracic and cardiovascular surgery. 2009;138:694–702. doi: 10.1016/j.jtcvs.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 16.Solomon C, Pichlmaier U, Schoechl H, Hagl C, Raymondos K, Scheinichen D, et al. Recovery of fibrinogen after administration of fibrinogen concentrate to patients with severe bleeding after cardiopulmonary bypass surgery. British journal of anaesthesia. 2010;104:555–62. doi: 10.1093/bja/aeq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima B, Williams JB, Bhattacharya SD, Shah AA, Andersen N, Gaca JG, et al. Results of proximal arch replacement using deep hypothermia for circulatory arrest: is moderate hypothermia really justifiable? The American surgeon. 2011;77:1438–44. [PMC free article] [PubMed] [Google Scholar]
- 18.Husain AMAK, Hughes GC. In: A practical approach to neurophysiologic intraoperative monitoring. Husain AM, editor. New York: Demos Medical Publishing, LLC; 2008. [Google Scholar]
- 19.James ML, Andersen ND, Swaminathan M, Phillips-Bute B, Hanna JM, Smigla GR, et al. Predictors of electrocerebral inactivity with deep hypothermia. The Journal of thoracic and cardiovascular surgery. 2014;147:1002–7. doi: 10.1016/j.jtcvs.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen ND, Bhattacharya SD, Williams JB, Fosbol EL, Lockhart EL, Patel MB, et al. Intraoperative use of low-dose recombinant activated factor VII during thoracic aortic operations. The Annals of thoracic surgery. 2012;93:1921–8. doi: 10.1016/j.athoracsur.2012.02.037. discussion 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Essell JH, Martin TJ, Salinas J, Thompson JM, Smith VC. Comparison of thromboelastography to bleeding time and standard coagulation tests in patients after cardiopulmonary bypass. Journal of cardiothoracic and vascular anesthesia. 1993;7:410–5. doi: 10.1016/1053-0770(93)90161-d. [DOI] [PubMed] [Google Scholar]
- 22.Despotis GJ, Santoro SA, Spitznagel E, Kater KM, Cox JL, Barnes P, et al. Prospective evaluation and clinical utility of on-site monitoring of coagulation in patients undergoing cardiac operation. The Journal of thoracic and cardiovascular surgery. 1994;107:271–9. [PubMed] [Google Scholar]
- 23.Nuttall GA, Oliver WC, Ereth MH, Santrach PJ. Coagulation tests predict bleeding after cardiopulmonary bypass. Journal of cardiothoracic and vascular anesthesia. 1997;11:815–23. doi: 10.1016/s1053-0770(97)90112-9. [DOI] [PubMed] [Google Scholar]
- 24.http://www.sts.org/sts-national-database/database-managers/adult-cardiac-surgery-database/data-collection. [cited 2014 October 26, 2014 ]; v2.81 Data Specifications]. Available from:
- 25.Hiippala ST, Myllyla GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesthesia and analgesia. 1995;81:360–5. doi: 10.1097/00000539-199508000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Vinard E, Leseche G, Andreassian B, Costagliola D. In vitro endothelialization of PTFE vascular grafts: A comparison of various substrates, cell densities, and incubation times. Annals of vascular surgery. 1999;13:141–50. doi: 10.1007/s100169900232. [DOI] [PubMed] [Google Scholar]
- 27.Spiess BD, Royston D, Levy JH, Fitch J, Dietrich W, Body S, et al. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143–8. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 28.Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. British journal of haematology. 2004;126:139–52. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- 29.Vamvakas EC, Carven JH. RBC transfusion and postoperative length of stay in the hospital or the intensive care unit among patients undergoing coronary artery bypass graft surgery: the effects of confounding factors. Transfusion. 2000;40:832–9. doi: 10.1046/j.1537-2995.2000.40070832.x. [DOI] [PubMed] [Google Scholar]
- 30.Thomas R, Hessel EA, 2nd, Harker LA, Sands MP, Dillard DH. Platelet function during and after deep surface hypothermia. The Journal of surgical research. 1981;31:314–8. doi: 10.1016/0022-4804(81)90054-8. [DOI] [PubMed] [Google Scholar]
- 31.Solomon C, Schochl H, Hanke A, Calatzis A, Hagl C, Tanaka K, et al. Haemostatic therapy in coronary artery bypass graft patients with decreased platelet function: comparison of fibrinogen concentrate with allogeneic blood products. Scandinavian journal of clinical and laboratory investigation. 2012;72:121–8. doi: 10.3109/00365513.2011.643818. [DOI] [PubMed] [Google Scholar]