Abstract
BACKGROUND
Evidence suggests that systemic inflammation may adversely impact HDL function. In this study we sought to evaluate the independent and incremental predictive performance of glycoprotein acetylation (GlycA)—a novel serum inflammatory biomarker that is an aggregate measure of enzymatically glycosylated acute phase proteins—and HDL subclasses on adverse events in a retrospective observational study of a secondary prevention population and to understand a priori defined potential interactions between GlycA and HDL subclasses.
METHODS
GlycA and HDL subclasses were measured using proton nuclear magnetic resonance spectroscopy in 7617 individuals in the CATHGEN (CATHeterization GENetics) cardiac catheterization biorepository.
RESULTS
GlycA was associated with presence [odds ratio (OR) 1.07 (1.02–1.13), P = 0.01] and extent [OR 1.08 (1.03, 1.12) P < 0.0005] of coronary artery disease and with all-cause mortality [hazard ratio (HR) 1.34 (1.29–1.39), P < 0.0001], cardiovascular mortality [1.37 (1.30–1.45), P < 0.0001] and noncardiovascular mortality [1.46 (1.39–1.54) P <0.0001] in models adjusted for 10 cardiovascular risk factors. GlycA and smaller HDL subclasses had independent but opposite effects on mortality risk prediction, with smaller HDL subclasses being protective [HR 0.69 (0.66–0.72), P < 0.0001]. There was an interaction between GlycA and smaller HDL subclasses—increasing GlycA concentrations attenuated the inverse association of smaller HDL subclasses with mortality. Adding GlycA and smaller HDL subclasses into the GRACE (Global Registry of Acute Coronary Events) and Framingham Heart Study Risk Scores improved mortality risk prediction, discrimination and reclassification.
CONCLUSIONS
These findings highlight the interaction of systemic inflammation and HDL with clinical outcomes and may increase precision for clinical risk assessment in secondary prevention populations.
Predicting and modifying risk of adverse events is a fundamental goal of precision care delivery in cardiovascular disease (CVD).5 Evolving molecular profiling technologies now enable measurement of a wide variety of circulating biomarkers that will potentially enhance predictive precision. Protein glycosylation, a posttranslational modification process whereby a glycan (carbohydrate) moiety is added to a protein, is an important mechanism of control and enhancement of functional heterogeneity of a protein. Partially because of their low plasma concentrations and thus difficulty with measurement, glycans have not been extensively evaluated as potential biomarkers of health or disease. Proton nuclear magnetic resonance (NMR) spectroscopy has sufficient sensitivity to detect circulating concentrations of plasma glycoproteins (1). Glycoprotein acetylation (GlycA) is a novel inflammatory biomarker that consists of the NMR signal from the N-acetyl methyl groups of the N-acetylglucosamine residues on enzymatically glycosylated acute phase proteins (primarily α1-antichymyotrypsin, α1-acid glycoprotein, haptoglobin, α1-antitrypsin, and transferrin) (2). Serum GlycA concentrations correlate with those of existing inflammatory biomarkers—C-reactive protein (CRP), fibrinogen, and interleukin-6—but exhibit less intraindividual variability over time (2). Moreover, GlycA independently predicted incident cardiovascular events and mortality in the Women’s Health Study, MESA (Multi-Ethnic Study of Atherosclerosis), and JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) trial (3–5), even after adjustment for high-sensitivity CRP (hsCRP) and other inflammatory biomarkers (5).
Lipoprotein subclass particle concentrations and size are also measurable by NMR spectroscopy and are independent markers of CVD (6, 7). Emerging preclinical and clinical data suggest a biological interaction between inflammation and HDL. HDL from healthy individuals is antiinflammatory with higher concentrations protecting against CVD, whereas HDL derived from patients with systemic inflammatory disorders such as CVD, metabolic syndrome, infection, or rheumatologic disease is proinflammatory (8, 9). Several explanations for this transition of HDL functionality have been described. For instance, the inflammatory enzyme myeloperoxidase selectively targets apolipoprotein A-I, the primary protein of HDL, resulting in retention of cholesterol in macrophages in atherosclerotic lesions (10, 11). Further, inflammatory processes impair the antioxidant capacity of HDL leading to enhanced oxidation of LDL in the arterial wall (12). Finally, a recent study demonstrated that the acute phase protein, serum amyloid A, modifies the biological effect of HDL-cholesterol (HDL-C) in several different clinical conditions (13).
The strong associations of inflammatory biomarkers with clinical outcomes in individuals with established CVD combined with the emerging evidence that systemic inflammation may adversely impact HDL function led us to explore GlycA in a secondary prevention cardiovascular cohort [CATHeterization GENetics (CATHGEN)]. In this study, we sought to do the following: (a) define the role of GlycA as a potential biomarker of adverse events in this population; (b) evaluate the independent and incremental predictive performance of GlycA and HDL subclasses; and (c) understand a priori defined potential interactions between HDL subclasses and GlycA.
Methods
STUDY POPULATION
The CATHGEN biorepository has been previously described (14–16). Briefly, for this study, consecutive patients undergoing cardiac catheterization at Duke University Medical Center and enrolled in the CATHGEN biorepository between 2001 and 2011, with sufficient available frozen EDTA plasma and nonmissing clinical and lipoprotein variables were identified (n = 7617). Demographics, medical history, angiographic data, and longitudinal follow-up were collected through the Duke Databank for Cardiovascular Disease. Follow-up included determination of mortality, myocardial infarction (MI), and coronary revascularization procedures; vital status was further confirmed through the National Death Index and the Social Security Death Index. This study and the CATHGEN biorepository were approved by the Duke University Institutional Review Board and were performed in accordance with the current revision of the Helsinki Declaration. Before collection of blood samples, all study participants provided written informed consent. For this study, time-to-event (all-cause mortality) was defined as time from enrolling CATHGEN cardiac catheterization to time of death at any point after enrollment. For cause-specific death, cardiovascular death was defined as death from 1 of the following causes: MI, congestive heart failure, sudden death, postresuscitation, vascular cause, during or post cardiac surgery, or during cardiac catheterization. Noncardiovascular death was defined as death due to a noncardiac medical cause or a noncardiac cause related to a procedure. Unknown causes of death were defined as unobserved or unknown cause of death. Hyperlipidemia was defined as a previous diagnosis and/or treatment of hypercholesterolemia. Diabetes was defined as a history of physician-diagnosed diabetes. Coronary artery disease (CAD) was defined as the presence of at least 1 epicardial coronary vessel with clinically significant stenosis (≥75%) at time of the index cardiac catheterization. CAD controls were defined as not having any clinically significant stenosis in any epicardial coronary artery at the time of cardiac catheterization; no prior or future history of CAD, percutaneous coronary intervention (PCI), and/or coronary artery bypass grafting (CABG), and no prior or future history of cardiac transplant. Extent of CAD was determined by calculation of the CADindex from the index CATHGEN catheterization; CADindex is an ordinal, 13 category variable that incorporates the extent and location of coronary artery stenosis (17).
LABORATORY METHODS
GlycA signals and lipoprotein particle concentrations and sizes were quantified in the CATHGEN frozen fasting plasma samples at LipoScience, Inc., using the LipoProfile-3 algorithm from NMR spectra obtained from the automated NMR Profiler system as described previously (3, 18). Measurements were performed in 2 batches of samples from individuals sequentially enrolled in CATHGEN: an initial cohort (n = 1738) from early in the study and a later cohort (n = 5879). Diameter range estimates of the measured HDL subclasses were 8.2–9.4 nm for the medium HDL particle subclass, and 7.3–8.2 nm for the small HDL particle subclass. Thus, the diameters of the particles comprising the combined medium plus small HDL-particle subclasses were ≤9.4 nm. The intra- and interassay CV for the various concentration measures are as follows: GlycA, 1.9 and 2.6%; small HDL particle concentration (HDL-P), 6.1 and 6.3%; medium HDL-P, 12.0 and 15.1%; small and medium HDL-P (MS-HDL-P), 1.8 and 4.2%.
STATISTICAL ANALYSIS
Measured GlycA values were standardized to approximate a normal distribution using z scores, thus odds ratios (ORs) and hazard ratios (HRs) are expressed as a function of a 1 SD change in GlycA. The relationship of GlycA concentrations with time-to-death (all-cause mortality) was tested using Cox proportional hazards models. Covariates included age, race, sex, and measurement batch in a basic model and age, race, sex, batch, smoking, hypertension, hyperlipidemia, body mass index (BMI), LDL particle concentration (LDL-P), left ventricular ejection fraction, CAD, and diabetes in a full model. The assumption of proportional hazards was tested by including time-varying covariates in the models. Multivariable logistic regression adjusted for age, race, sex, and measurement batch in a basic model and age, race, sex, batch, smoking, hypertension, hyperlipidemia, BMI, LDL-P, and diabetes in a full model was used to test the association of GlycA concentrations with presence of CAD and with extent of CAD (CADindex). Pearson correlation coefficients were used to determine the correlation between MS-HDL-P and GlycA. To determine improvement in model fit, the Akaike Information Criterion (AIC) and the χ2 value from the likelihood ratio tests were calculated for addition of GlycA, MS-HDL-P, and their interaction term in Cox models inclusive of the variables from the full model above. C statistics were calculated from multivariable logistic regression models of 2-year death adjusted for the same clinical covariates, for the Global Registry of Acute Coronary Events (GRACE) Risk Score or for the Framingham Risk Score with and without addition of GlycA, MS-HDL-P, and their interaction term. The GRACE Risk Score is a registry-based clinical risk prediction tool developed to estimate the cumulative 6-month risk of death or MI in individuals presenting with acute coronary syndrome (19, 20). All analyses were performed using SAS V9.4 software.
Results
GlycA and HDL subclass measurements were performed in 7617 CATHGEN individuals. Table 1 presents baseline characteristics of the study population. There was a high prevalence of cardiometabolic risk factors including type 2 diabetes mellitus (28.7%), hyperlipidemia (61.4%), obesity [mean BMI 30.0 (SD 7.1)] and clinically significant CAD (65%) as defined at enrollment cardiac catheterization.
Table 1.
Baseline clinical characteristics of the CATHGEN study population.a
| Overall cohort (n = 7617) | Death cases (n = 2257) | Alive (n = 5299) | P Value | |
|---|---|---|---|---|
| Age | 61.6 ± 11.8 | 66.5 ± 11.8 | 59.5 ± 11.2 | <0.0001 |
| Sex (% female) | 2865 (37.6) | 831 (35.9) | 2034 (38.4) | 0.04 |
| Race (% white) | 5728 (75.2) | 1761 (76) | 3967 (74.9) | 0.003 |
| Hypertension (%) | 5233 (68.7) | 1645 (71) | 3588 (67.7) | 0.005 |
| Diabetes (%) | 2186 (28.7) | 816 (35.2) | 1370 (25.9) | <0.0001 |
| Family history of CVD | 2639 (34.7) | 833 (35.9) | 1806 (34.1) | 0.12 |
| Hyperlipidemia (%) | 4675 (61.4) | 1396 (60.2) | 3279 (61.9) | 0.18 |
| Smoking (%) | 3731 (49) | 1277 (55.1) | 2454 (46.3) | <0.0001 |
| CAD on cath (%)a | 4949 (65) | 1723 (74.3) | 3226 (60.9) | <0.0001 |
| BMI | 30 ± 7.1 | 29 ± 7.0 | 30.5 ± 7.1 | <0.0001 |
| Ejection fraction | 55.5 ± 13.6 | 51.4 ± 15.1 | 57.3 ± 12.5 | <0.0001 |
| GlycA, μmol/L | 379.9 ± 86.9 | 402.9 ± 99.9 | 369.9 ± 78.5 | <0.0001 |
| Lipoprotein parameters | ||||
| LDL-P, nmol/L | 1161.2 ± 392.9 | 1133.5 ± 404.0 | 1173.3 ± 387.4 | <0.0001 |
| HDL-P, μmol/L | 28.9 ± 6.3 | 27.1 ± 6.4 | 29.7 ± 6.1 | <0.0001 |
| Large HDL-P, μmol/L | 4.5 ± 2.8 | 4.8 ± 2.9 | 4.3 ± 2.7 | <0.0001 |
| Medium HDL-P, μmol/L | 9.9 ± 5.9 | 9.5 ± 5.4 | 10.1 ± 6.1 | 0.001 |
| Small HDL-P, μmol/L | 14.5 ± 6.2 | 12.8 ± 6.2 | 15.3 ± 6.1 | <0.0001 |
| MS-HDL-P, μmol/L | 24.4 ± 6.0 | 22.3 ± 6.2 | 25.4 ± 5.6 | <0.0001 |
| HDL size, nm | 9.2 ± 0.5 | 9.3 ± 0.6 | 9.1 ± 0.5 | <0.0001 |
Continuous variables presented as mean ± SD, categorical variables presented as n (%), P values result from χ2 test (categorical) or Wilcoxon rank sum test (continuous).
Defined as at least 1 epicardial coronary vessel with ≥75% stenosis.
GlycA concentrations were associated with presence and extent (as measured by CADindex) of CAD on index cardiac catheterization in both basic and multivariable models (Table 2).
Table 2.
Association between GlycA, coronary artery disease and mortality, stratified by diabetes status.a
| CAD
|
All-cause mortality
|
|||
|---|---|---|---|---|
| Basic OR (CI) | Adjusted OR (CI)b | Basic HR (CI) | Multivariable HR (CI) a | |
| Full population | 1.14 (1.09–1.20), P <0.0001 | 1.07 (1.02–1.13), P = 0.013 | 1.39 (1.34–1.44), P <0.0001 | 1.34 (1.29–1.39), P <0.0001 |
|
| ||||
| Diabetes | 1.01 (0.92–1.11), P = 0.078 | 1.03 (0.93–1.14), P = 0.58 | 1.30 (1.24–1.37), P <0.0001 | 1.28 (1.21–1.36), P <0.0001 |
|
| ||||
| No diabetes | 1.14 (1.07–1.21), P <0.0001 | 1.09 (1.02–1.16), P = 0.009 | 1.42 (1.36–1.48), P <0.0001 | 1.38 (1.32–1.45), P <0.0001 |
|
| ||||
| Cardiovascular mortalityc | ||||
|
| ||||
| 1.37 (1.30–1.45) P <0.0001 | ||||
|
| ||||
| Noncardiovascular mortality | ||||
|
| ||||
| 1.47 (1.39–1.54) P <0.0001 | ||||
|
| ||||
| Other mortality | ||||
|
| ||||
| 1.23 (1.10–1.38) P <0.001 | ||||
HR per 1 SD increase in GlycA concentrations.
Adjusted for age, sex, race, BMI, diabetes, hypertension, smoking, hyperlipidemia, and LDL-P, and additionally for presence of CAD and ejection fraction in mortality models.
Multivariable model HR for CV mortality in the full population.
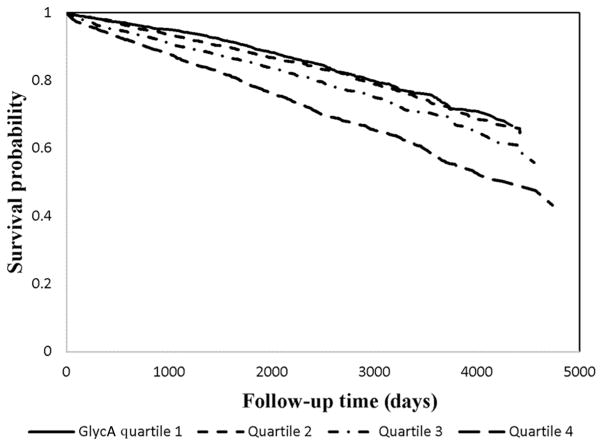
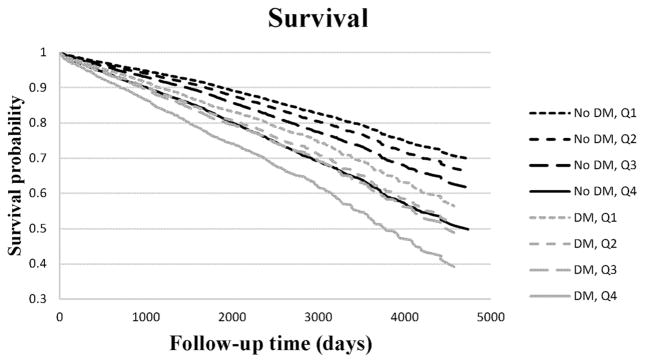
Over a mean time-to-event of 7.0 years (SD 3.2), 2257 individuals (29.6%) suffered from the primary endpoint of all-cause mortality. Of these 2257 individuals, 878 died from cardiovascular causes, 1069 died from non- cardiovascular causes, and 310 died from unknown causes. In a basic Cox regression model every 1 SD increase in GlycA concentration was associated with an HR of 1.39 (95% CI 1.34, 1.44, P < 0.0001). In a multivariable model the HR remained similar [HR 1.34 (95% CI 1.29–1.39), P <0.0001; Table 2]. Performing a sensitivity analysis with systolic blood pressure as a continuous variable instead of hypertension (n =7162), revealed similar results for CAD [HR 1.08 (1.02–1.14), P = 0.005] and all-cause mortality [HR 1.34 (1.29–1.39), P < 0.0001]. Similar magnitudes of associations in multivariable models were observed with GlycA and cardovascular, noncardiovascular, and unknown causes of death (Table 2). When evaluating GlycA concentrations by quartiles, a trend effect in the magnitude of the association with increased all-cause mortality was observed across the greatest 3 quartiles of GlycA concentration compared to the lowest quartile (Fig. 1). GlycA and diabetes were not associated in this population (interaction P = 0.22). However, because of the recently described relationship of GlycA to incident diabetes (21), we also stratified our analyses by diabetes status. In these models, the greatest quartile of GlycA concentration in individuals without diabetes was associated with a higher risk of mortality than individuals with diabetes who had GlycA concentrations in the lowest quartile (Fig. 2).
Fig. 1.
GlycA and all-cause mortality. Adjusted Kaplan-Meier curve demonstrating the relationship between GlycA quartiles and all-cause mortality in fully adjusted models.
HRs when compared to quartile 1 were as follows: quartile 2 HR 1.19 [1.05–1.35], P = 0.007; quartile 3 HR 1.42 [1.26–1.61], P = 0.004; quartile 4 HR 2.13 [1.89–2.40] P < 0.0001.
Fig. 2.
GlycA and all-cause mortality according to diabetes status. Adjusted Kaplan–Meier curves demonstrating the relationship between GlycA quartiles and all-cause mortality in fully adjusted models, stratified by diabetes status.
DM, diabetes mellitus.
There was a deviation of the assumption of proportional hazards in our models (P < 0.0001), which visually appeared to be related to deviation in later years of follow-up for individuals with the greatest GlycA concentrations. Therefore, we performed a sensitivity analysis that limited the endpoint to all-cause mortality within 2 years of enrollment and excluded outliers in the highest 5th percentiles of GlycA concentration. In this sensitivity analysis, the assumption of proportional hazards was not violated (P = 0.28 for linear hypothesis test) and the magnitude of the effect of GlycA concentrations on mortality was not decreased [multivariable models HR 1.50 (1.35–1.66), P =0.0001]. Adjudicated review by a rheumatologist of the medical records of the individuals in the top 1 percentile of GlycA concentrations confirmed the absence of any inflammatory diseases such as rheumatoid arthritis in these individuals.
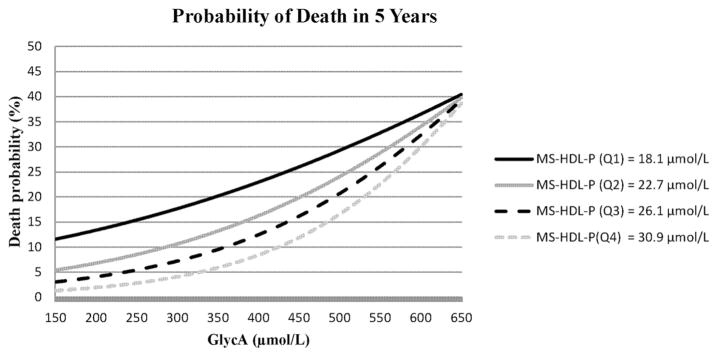
HDL-C is a known epidemiological risk factor for cardiovascular events. In our cohort, HDL particle size is associated with mortality risk—larger HDL size confers a higher risk of mortality and a sum of small- and medium-size HDL particles (MS-HDL-P) confers a lower risk (7). In studies from other groups the antiinflammatory properties of HDL become proinflammatory during the acute-phase response (22); this suggests a potential interaction between HDL and inflammation that could influence mortality risk. We evaluated models for such interactions in our data. MS-HDL-P and GlycA were not correlated (r = −0.02, P = 0.19). In a multivariable model including MS-HDL-P, both GlycA [HR 1.28 (1.24–1.32), P < 0.0001], and MS-HDL-P [HR 0.69 (0.66–0.72), P < 0.0001] were associated with mortality. An interaction term between GlycA and MS-HDL-P was found significant in the fully adjusted model after adjusting for the main effects of the covariates (P < 0.0001); with increasing deciles of GlycA concentrations there was an attenuation of the inverse association of MS-HDL-P concentrations with mortality, but these MS-HDL-P associations remained significant. To better visualize this interaction, we performed a sensitivity analysis to examine HRs for all-cause mortality of MS-HDL-P at the median of different deciles of GlycA (see the Figure in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol63/issue1). At the lowest decile of GlycA, MS-HDL-P was strongly inversely associated with mortality; this inverse association was attenuated at the highest decile of GlycA. A similar relation was not observed with large HDL-P (large HDL-P*GlycA, interaction P = 0.32, see online Supplemental Fig.). The interaction of GlycA and MS-HDL-P on mortality risk for a typical individual, representing average demographic and clinical risk factors, from the CATHGEN cohort is visually depicted in Fig. 3.
Fig. 3.
Effect of GlycA on the relationship between MS-HDL-P and mortality risk for a representative individual from CATHGEN. Mortality risk for a 62 year-old white male with hypertension, hyperlipidemia, CAD, and a BMI of 30 according to MS-HDL-P and GlycA concentrations.
The curves are the probability of death at the median quartile values of MS-HDL-P estimated at GlycA concentrations.
To further assess the independent and incremental effects of GlycA and MS-HDL-P on mortality, we compared model performance characteristics including the c-statistic, AIC, and the χ2 value from the likelihood ratio tests for addition of GlycA, MS-HDL-P, and their interaction term on top of our full clinical model, as well as in the GRACE and the Framingham Heart Study (FHS) Risk Scores (Table 3). This analysis demonstrated a monotonically increased improvement of model fit characteristics with incremental addition of these variables.
Table 3.
Comparison of clinical model fit with addition of GlycA, MS-HDL-P, and GlycA*MS-HDL-P interactions.
| AIC | Likelihood ratio Χ2a | C statisticb | |
|---|---|---|---|
| Clinical model | 38221 | 1178 | 0.72 |
| Clinical model + GlycA | 37997 | 1404 | 0.75 |
| Clinical model + GlycA + MS-HDL-P | 37726 | 1677 | 0.78 |
| Clinical model + GlycA + MS-HDL-P + GlycA*MS-HDL-P | 37685 | 1720 | 0.79 |
| GRACE | 26171 | 686 | 0.69 |
| GRACE + GlycA | 26042 | 818 | 0.73 |
| GRACE + GlycA + MS-HDL-P | 25825 | 1037 | 0.78 |
| GRACE + GlycA + MS-HDL-P + GlycA*MS-HDL-P | 25782 | 1081 | 0.78 |
| FHS + GlycA | 41266 | 589 | 0.64 |
| FHS + GlycA + MS-HDL-P | 40712 | 1145 | 0.74 |
| FHS + GlycA + MS-HDL-P + GlycA*MS-HDL-P | 40664 | 1195 | 0.75 |
From Cox models: clinical model includes age, sex, race, BMI, diabetes, hypertension, smoking, hyperlipidemia and LDL-P, presence of CAD, and ejection fraction.
From linear regression models for 2-year death.
We then evaluated the usefulness of GlycA, MS-HDL-P, and their interaction term in risk reclassification when added to established clinical risk prediction models, the GRACE and FHS Risk Scores. Based on availability of GRACE Risk Score variables, 5099 individuals from the full cohort were included in this analysis. The net reclassification index (NRI) and integrated discrimination improvement (IDI)—2 indices of risk reclassification—after addition of these variables to the GRACE model were 0.24 (P < 0.0001) and 0.06 (P < 0.0001), respectively. Similar results were found using the FHS Risk Score (see online Supplemental Table) (23).
Discussion
In these analyses, we validated and extended previous findings for the role that the protein glycan derived inflammatory biomarker, GlycA, contributes to CVD risk. Specifically, we report novel findings of the strong independent relationship between GlycA concentrations and presence and extent of CAD, and with mortality in a secondary prevention population. GlycA was incrementally additive to validated clinical models of mortality in a secondary prevention population and showed robust reclassification characteristics. Equally as important, we report for the first time on statistical interactions between HDL subclasses and inflammation that are supported by prior biological data and recent epidemiological studies (13). Thus our study yields new insight into the biological interactions between inflammation and HDL. These interactions suggest that the protective effects of HDL are modified and attenuated by inflammation and provide epidemiological evidence of a proposed biological effect. Additionally, these results could explain in part the lack of efficacy of HDL-modifying therapeutic agents, such as the cholesterylester transfer protein or CETP (cholesteryl ester transfer protein) inhibitors, which may target the wrong end of the HDL spectrum.
Analyses stratified by diabetes status, a CAD “risk equivalent,” highlighted the magnitude of the potential clinical implications of GlycA in risk prediction. In fact, individuals without diabetes who had the greatest quartile of GlycA concentration actually had a greater risk than patients with diabetes who had lower GlycA concentration. The relationship between diabetes and GlycA concentrations was incremental; patients with diabetes with the greatest quartile of GlycA concentration had the greatest mortality risk (Fig. 2).
Of particular interest, we observed a significant interaction between GlycA concentrations and HDL subclass concentrations on mortality risk. A strong foundation of laboratory-based research has established that HDL is a key player in a complex relationship between cholesterol efflux, inflammation, oxidation, and endothelial function (24). Large human studies have shown a consistent relationship between overall HDL-C concentrations and cardiovascular risk (25). Although the associations of HDL subclasses in relation to clinical outcomes are conflicting (26), more recent evidence suggests that for many protective mechanisms, the smaller HDL subclasses appear to be more functional (7). Smaller HDL subclasses have greater antioxidant activity, greater antiinflammatory activity, greater cholesterol efflux capacity, and offer more cytoprotection (27–29). Moreover, several recent studies, using varying methods of HDL subclass measurement in diverse populations, have shown that smaller HDL subclasses are associated with reduced cardiovascular risk (7, 30–35). Unfortunately, large HDL particles have a greater influence on the traditionally-measured HDL-C concentration (because larger HDL particles carry more cholesterol). Thus, HDL-C concentrations may not be linked as closely to cardiovascular risk protection as HDL subclasses.
HDL particles become proinflammatory during acute-phase responses (12). Despite these compelling biological data linking inflammation and changes in HDL function, before now no study has evaluated potential interactions between inflammatory biomarkers and HDL subclasses on cardiovascular risk. Using robust epidemiological models, we have observed that higher concentrations of systemic inflammation can modify the association of HDL subclasses with clinical outcomes. Additionally, the inclusion of GlycA and MS-HDL-P in commonly used risk prediction tools improved risk assessment (see online Supplemental Table). The NRIs of 0.24 and 0.34 in the GRACE and FHS Risk Score models, respectively, indicate a strong ability of these biomarkers to reclassify risk in populations similar to ours. For comparison, although performed in a lower risk population and using different risk models, hsCRP has an NRI of 0.057 in the Women’s Health Study (36).
These results are consistent with studies examining the relation of inflammatory biomarkers and HDL-C, in which increased concentrations of systemic inflammation attenuate, or even reverse, the protective associations of HDL with cardiovascular outcomes. For instance, in the setting of increased CRP, high HDL-C is associated with increased risk of recurrent cardiovascular events in a postinfarction cohort and predicts the incidence of first cardiovascular event in a primary prevention population (37, 38). Similar observations have been made with HDL-C and increased concentrations of circulating serum amyloid A (13). Interestingly, new data suggest a potential mechanistic link between glycosylation of HDL components and HDL function (39); this may partially explain the relation of GlycA—an inflammatory biomarker composed of glycosylated acute phase reactants—to HDL subclasses.
Strengths of our study include the size of the cohort, the duration of follow-up, and the clinical characteristics and outcomes available including detailed angiographic data. Our results are generalizable to high-risk individuals with established CVD or with multiple cardiovascular risk factors. Important confounding variables that have an effect on HDL subclasses and systemic inflammation may not have been measured or considered in our analyses. Other inflammatory biomarkers, such as hsCRP, are not available in CATHGEN and therefore the performance of GlycA compared to these measurements was not possible. However, it is noteworthy that in the Women’s Health Study, GlycA and hsCRP had similar associations with incident CVD (3). Static measures of HDL particles, such as HDL subclasses used here, do not fully capture the complexity of HDL metabolism and composition. Though not feasible in this study, the integration of HDL subclass data along with HDL functional measurements is likely to provide the biggest utility in cardiovascular risk assessment in the future (40). Future areas of investigation should include the effect of therapeutic interventions on GlycA concentrations in relation to cardiovascular outcomes and the measurement of HDL function in individuals with increased GlycA.
In conclusion, GlycA, a novel protein glycan-derived inflammatory biomarker, was strongly associated with mortality and CAD, modified the association of HDL subclasses with mortality risk, and improved mortality risk prediction and reclassification in a high-risk cardiovascular cohort. These findings provide insight into the possible biological interaction of systemic inflammation and HDL and offer new tools for clinical risk assessment in secondary prevention populations.
Supplementary Material
Acknowledgments
Role of Sponsor: The funding organizations played a direct role in review and interpretation of data.
We thank all of the CATHGEN participants; Z. Elaine Dowdy and Melissa Hurdle for study recruitment and enrollment; and Jim Otvos and Irina Shalaurova from LipoScience, Inc. (now LabCorp, Inc.) which developed the NMR assays used in this study, for helpful discussions regarding the scientific interpretations underlying the findings.
Footnotes
Previous presentation of manuscript: Results from this study have been presented in abstract form at the American Heart Association Scientific Sessions, November 2015.
Nonstandard abbreviations: CVD, cardiovascular disease; NMR, nuclear magnetic resonance; GlycA, glycoprotein acetylation; CRP, C-reactive protein; hsCRP, high-sensitivity CRP; HDL-C, HDL-cholesterol; CATHGEN, CATHeterization GENetics: MI, myocardial infarction; CAD, coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; HDL-P, HDL particle concentration; MS-HDL-P, medium plus small HDL-P subclasses; OR, odds ratio; HR, hazard ratio; BMI, body mass index; LDL-P, LDL particle concentration; AIC, Akaike Information Criterion; GRACE, Global Registry of Acute Coronary Events; FHS, Framingham Heart Study; NRI, net reclassification index; IDI, integrated discrimination improvement.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
- Employment or Leadership: None declared.
- Consultant or Advisory Role: None declared.
- Stock Ownership: None declared.
- Honoraria: None declared.
- Research Funding: J.P. Kelly, grant T32HL7101–39 from the NIH; W.E. Kraus, Institutional support from Liposcience, Incorporated; S.H. Shah, Sponsored research agreement between BMS and Duke.
- Expert Testimony: None declared.
- Patents: None declared.
References
- 1.Bell JD, Brown JC, Nicholson JK, Sadler PJ. Assignment of resonances for “acute-phase” glycoproteins in high resolution proton NMR spectra of human blood plasma. FEBS Lett. 1987;215:311–5. doi: 10.1016/0014-5793(87)80168-0. [DOI] [PubMed] [Google Scholar]
- 2.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, Tracy RP. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015;61:714–23. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 3.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc. 2014;3:e001221. doi: 10.1161/JAHA.114.001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawler PR, Akinkuolie AO, Chandler PD, Moorthy MV, VanDenburgh MJ, Schaumberg DA, et al. Circulating N-linked glycoprotein acetyls and longitudinal mortality risk. Circ Res. 2016;118:1106–15. doi: 10.1161/CIRCRESAHA.115.308078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR., Jr Comparison of the predictive value of GlycA and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clin Chem. 2016;62:1020–31. doi: 10.1373/clinchem.2016.255828. [DOI] [PubMed] [Google Scholar]
- 6.Williams PT, Zhao XQ, Marcovina SM, Otvos JD, Brown BG, Krauss RM. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis. 2014;233:713–20. doi: 10.1016/j.atherosclerosis.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGarrah RW, Craig DM, Haynes C, Dowdy ZE, Shah SH, Kraus WE. High-density lipoprotein subclass measurements improve mortality risk prediction, discrimination and reclassification in a cardiac catheterization cohort. Atherosclerosis. 2016;246:229–35. doi: 10.1016/j.atherosclerosis.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansell BJ, Fonarow GC, Fogelman AM. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol. 2007;18:427–34. doi: 10.1097/MOL.0b013e3282364a17. [DOI] [PubMed] [Google Scholar]
- 9.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–32. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 10.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in patients with cardiovascular disease. J Clin Invest. 2004;114:529–41. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009;284:30825–35. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–67. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zewinger S, Drechsler C, Kleber ME, Dressel A, Riffel J, Triem S, et al. Serum amyloid A: high-density lipoproteins interaction and cardiovascular risk. Eur Heart J. 2015;36:3007–16. doi: 10.1093/eurheartj/ehv352. [DOI] [PubMed] [Google Scholar]
- 14.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–14. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 15.Shah SH, Granger CB, Hauser ER, Kraus WE, Sun JL, Pieper K, et al. Reclassification of cardiovascular risk using integrated clinical and molecular biosignatures: design of and rationale for the Measurement to Understand the Reclassification of Disease of Cabarrus and Kannapolis (MURDOCK) Horizon 1 Cardiovascular Disease. Am Heart J. 2010;160:371–9. e2. doi: 10.1016/j.ahj.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Kraus WE, Granger CB, Sketch MH, Donahue MP, Ginsburg GS, Hauser ER, et al. A guide for a cardiovascular genomics biorepository: the CATHGEN Experience. J Cardiovasc Transl Res. 2015;8:449–57. doi: 10.1007/s12265-015-9648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mark DB, Nelson CL, Califf RM, Harrell FE, Lee KL, Jones RH, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–25. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 18.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–70. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) BMJ. 2006;333:1091. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox KA, Fitzgerald G, Puymirat E, Huang W, Carruthers K, Simon T, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open. 2014;4:e004425. doi: 10.1136/bmjopen-2013-004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akinkuolie AO, Pradhan AD, Buring JE, Ridker PM, Mora S. Novel protein glycan side-chain biomarker and risk of incident type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2015;35:1544–50. doi: 10.1161/ATVBAHA.115.305635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GHB, Rao VS, Kakkar VV. Friend turns foe: transformation of anti-inflammatory HDL to proinflammatory HDL during acute-phase response. Cholesterol. 2011;2011:274629. doi: 10.1155/2011/274629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 24.Rosenson RS, Brewer HB, Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon DJ, Rifkind BM. High-density lipoprotein–the clinical implications of recent studies. N Engl J Med. 1989;321:1311–6. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 26.Rizzo M, Otvos JD, Nikolic D, Montalto G, Toth PP, Banach M. Subfractions and subpopulations of HDL: an update. Curr Med Chem. 2014;21:1–11. doi: 10.2174/0929867321666140414103455. [DOI] [PubMed] [Google Scholar]
- 27.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17:594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Calabresi L, Gomaraschi M, Simonelli S, Bernini F, Franceschini G. HDL and atherosclerosis: insights from inherited HDL disorders. Biochim Biophys Acta. 2015;1851:13–8. doi: 10.1016/j.bbalip.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Du X, Kim MJ, Hou L, Le Goff W, Chapman MJ, van Eck M, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116:1133–42. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- 30.Martin SS, Khokhar AA, May HT, Kulkarni KR, Blaha MJ, Joshi PH, et al. HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur Heart J. 2015;36:22–30. doi: 10.1093/eurheartj/ehu264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duprez DA, Otvos J, Tracy RP, Feingold KR, Gross MD, Lima JA, et al. High-density lipoprotein subclasses and noncancer chronic inflammatory-related events versus cardiovascular events: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4:e002295. doi: 10.1161/JAHA.115.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ditah C, Otvos J, Nassar H, Shaham D, Sinnreich R, Kark JD. Small and medium sized HDL particles are protectively associated with coronary calcification in a cross-sectional population-based sample. Atherosclerosis. 2016;251:124–31. doi: 10.1016/j.atherosclerosis.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Joshi PH, Toth PP, Lirette ST, Griswold ME, Massaro JM, Martin SS, et al. Association of high-density lipoprotein subclasses and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. Eur J Prev Cardiol. 2016;23:41–9. doi: 10.1177/2047487314543890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albers JJ, Slee A, Fleg JL, O’Brien KD, Marcovina SM. Relationship of baseline HDL subclasses, small dense LDL and LDL triglyceride to cardiovascular events in the AIM-HIGH clinical trial. Atherosclerosis. 2016;251:454–9. doi: 10.1016/j.atherosclerosis.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGarrah RW. Refocusing the AIM on HDL in the metabolic syndrome. Atherosclerosis. 2016;251:531–3. doi: 10.1016/j.atherosclerosis.2016.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–9. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 37.Corsetti JP, Gansevoort RT, Sparks CE, Dullaart RP. Inflammation reduces HDL protection against primary cardiac risk. Eur J Clin Invest. 2010;40:483–9. doi: 10.1111/j.1365-2362.2010.02287.x. [DOI] [PubMed] [Google Scholar]
- 38.Corsetti JP, Ryan D, Rainwater DL, Moss AJ, Zareba W, Sparks CE. Cholesteryl ester transfer protein polymorphism (TaqIB) associates with risk in postinfarction patients with high C-reactive protein and high-density lipoprotein cholesterol levels. Arterioscler Thromb Vasc Biol. 2010;30:1657–64. doi: 10.1161/ATVBAHA.110.207977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang J, Lee H, Zivkovic AM, Smilowitz JT, Rivera N, German JB, et al. Glycomic analysis of high density lipoprotein shows a highly sialylated particle. J Proteome Res. 2014;13:681–91. doi: 10.1021/pr4012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.