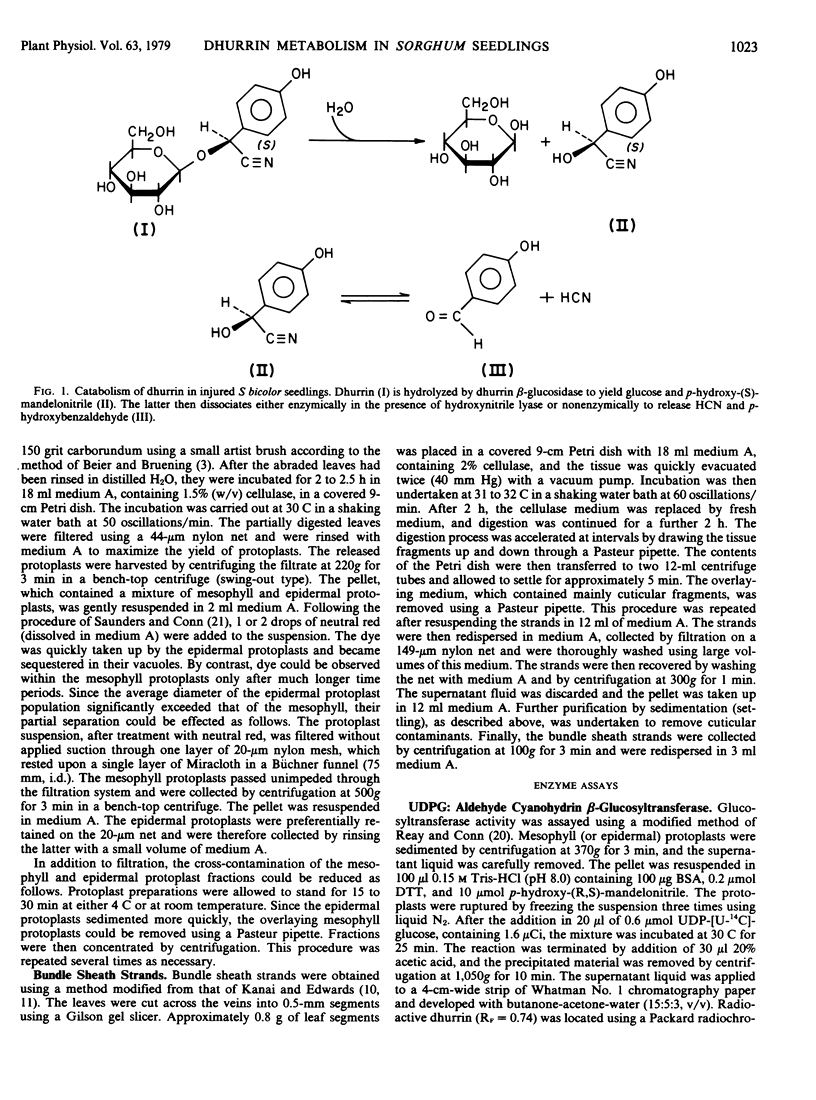
Abstract
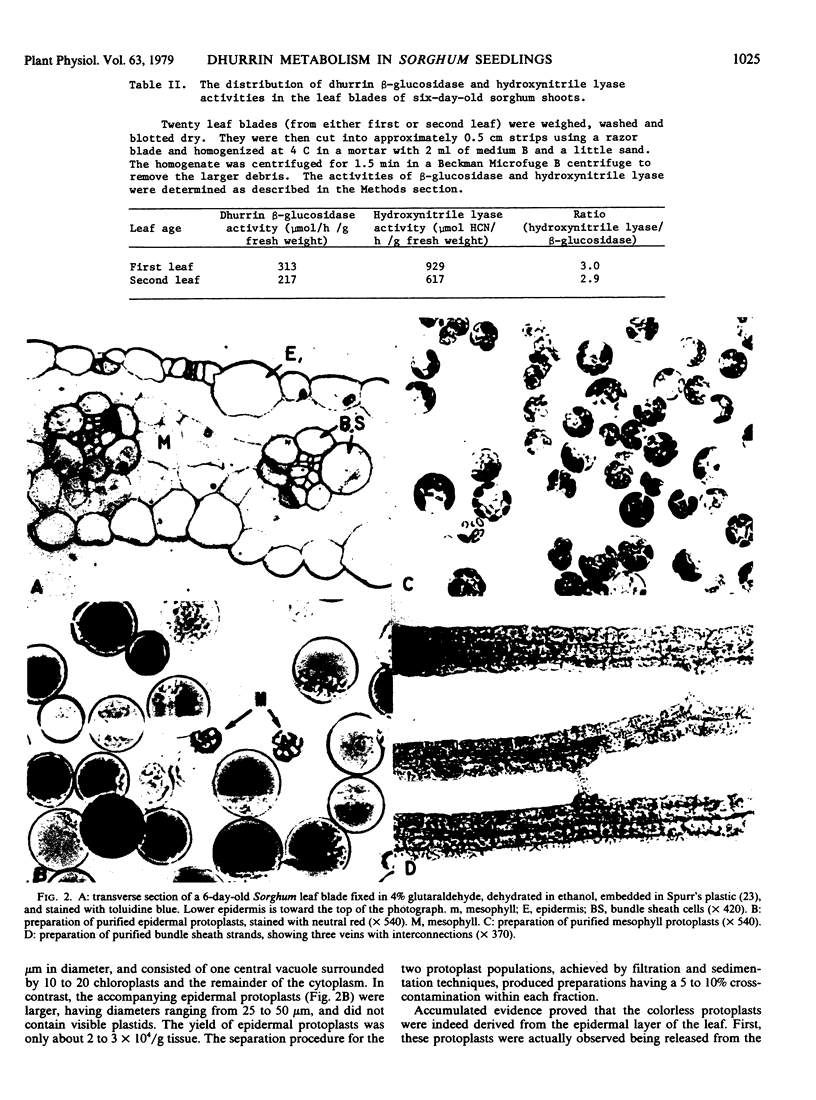
The tissue distributions of dhurrin [p-hydroxy-(S)-mandelonitrile-β-d-glucoside] and of enzymes involved in its metabolism have been investigated in leaf blades of light-grown Sorghum bicolor seedlings. Enzymic digestion of these leaves using cellulase has enabled preparations of epidermal and mesophyll protoplasts and bundle sheath strands to be isolated with only minor cross-contamination. Dhurrin was located entirely in the epidermal layers of the leaf blade, whereas the two enzymes responsible for its catabolism, namely dhurrin β-glucosidase and hydroxynitrile lyase, resided almost exclusively in the mesophyll tissue. The final enzyme of dhurrin biosynthesis, uridine diphosphate glucose:p-hydroxymandelonitrile glucosyltransferase, was found in both mesophyll (32% of the total activity of the leaf blade) and epidermal (68%) tissues. The bundle sheath strands did not contain significant amounts of dhurrin or of these enzymes. It was concluded that the separation of dhurrin and its catabolic enzymes in different tissues prevents its large scale hydrolysis under normal physiological conditions. The well documented production of HCN (cyanogenesis), which occurs rapidly on crushing Sorghum leaves, would be expected to proceed when the contents of the ruptured epidermal and mesophyll cells are allowed to mix.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akazawa T., Miljanich P., Conn E. E. Studies on Cyanogenic Glycoside of Sorghum Vulgare. Plant Physiol. 1960 Jul;35(4):535–538. doi: 10.1104/pp.35.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Bruening G. The use of an abrasive in the isolation of cowpea leaf protoplasts which support the multiplication of cowpea mosaic virus. Virology. 1975 Mar;64(1):272–276. doi: 10.1016/0042-6822(75)90099-9. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- MacFarlane I. J., Lees E. M., Conn E. E. The in vitro biosynthesis of dhurrin, the cyanogenic glycoside of Sorghum bicolor. J Biol Chem. 1975 Jun 25;250(12):4708–4713. [PubMed] [Google Scholar]
- Reay P. F., Conn E. E. The purification and properties of a uridine diphosphate glucose: aldehyde cyanohydrin beta-glucosyltransferase from sorghum seedlings. J Biol Chem. 1974 Sep 25;249(18):5826–5830. [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E. Presence of the cyanogenic glucoside dhurrin in isolated vacuoles from sorghum. Plant Physiol. 1978 Feb;61(2):154–157. doi: 10.1104/pp.61.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E. Subcellular localization of the cyanogenic glucoside of sorghum by autoradiography. Plant Physiol. 1977 Apr;59(4):647–652. doi: 10.1104/pp.59.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]