Abstract
The first epidemic of cotton leaf curl disease (CLCuD) in early 1990’s in the Indian subcontinent was associated with several distinct begomoviruses along with a disease-specific betasatellite. Resistant cotton varieties were introduced in late 1990’s but soon resistance was broken and was associated with a single recombinant begomovirus named Burewala strain of Cotton leaf curl Kokhran virus that lacks a full complement of a gene encoding a transcription activator protein (TrAP). In order to understand the ongoing changes in CLCuD complex in Pakistan, CLCuD affected plants from cotton fields at Vehari were collected. Illumina sequencing was used to assess the diversity of CLCuD complex. At least three distinct begomoviruses characterized from the first epidemic; Cotton leaf curl Multan virus, Cotton leaf curl Kokhran virus and Cotton leaf curl Alabad virus, several distinct species of alphasatellites and cotton leaf curl Multan betasatellite were found associated with CLCuD. These viruses were also cloned and sequenced through Sanger sequencing to confirm the identity of the begomoviruses and that all clones possessed a full complement of the TrAP gene. A new strain of betasatellite was identified here and named CLCuMuBVeh. The implications of these findings in efforts to control CLCuD are discussed.
Introduction
Cotton is the largest fiber producing and important cash crop in Pakistan and India1. Cotton leaf curl disease (CLCuD) is the major biotic constraint of cotton, transmitted by the insect vector Bemisia tabaci (whitefly)2, 3. CLCuD affects cotton crops across Pakistan and northwestern India causing severe yield losses4. Cotton plants affected by CLCuD exhibit specific symptoms such as upward and downward leaf curling, vein thickening and swelling, stunted growth and development of leaf-like enations. In Pakistan, CLCuD was observed for the first time on cotton near Multan in 1967. The disease spread widely and some more cotton varieties were affected by late 1970, but were not considered significant5. In 1989, the disease was noted on a newly released variety S12 grown at Kokhran near Multan and became epidemic, spreading to all the growing areas of Pakistan. The first epidemic of the disease was associated with the “Multan strain” of begomoviruses6. By introduction of resistant varieties developed through conventional breeding, cotton production in Pakistan was restored to pre-epidemic levels in the late 1990s7. Unfortunately, in 2001 it became evident that resistance was broken and symptoms of CLCuD were observed in all previously resistant varieties at Burewala, Pakistan8. This signaled a second epidemic of CLCuD which spread to all the cotton growing areas of Pakistan8, 9.
The CLCuD complex is caused by monopartite begomoviruses (genus Begomovirus, family Geminiviridae) transmitted by B. tabaci 10. Begomoviruses are of two types, bipartite and monopartite; bipartite consists of two genomic components DNA-A and DNA-B equal in size (~2.7 kb). Monopartite begomoviruses have a single genomic component similar to DNA-A of bipartite begomoviruses and are often associated with satellite molecules called betasatellite and alphasatellite. Betasatellite (~1.4 kb in size) is a pathogenicity determinant, encodes a single βC1 protein and depends on the helper virus for movement, replication and encapsidation11. Alphasatellites (~1.4 kb in size) encode their own replication associated (Rep) protein, replicate independently from their helper virus and are usually not required for pathogenicity11.
During the first epidemic, CLCuD was caused by begomovirus-betasatellite complex. CLCuD was associated with several distinct monopartite begomoviruses – Cotton Leaf curl Multan virus (CLCuMuV), Cotton leaf curl Kokhran virus (CLCuKoV), Cotton leaf curl Alabad virus (CLCuAV) and Papaya leaf curl virus (PaLCuV)4, 6, 12, and a single betasatellite (Cotton leaf curl Multan betasatellite [CLCuMuB])13, 14. Following the appearance of the second CLCuD epidemic in cotton, to present time, a predominant single recombinant begomovirus named as Cotton leaf curl Kokhran virus- Burewala (CLCuKoV-Bu), previously known as Cotton leaf curl Burewala virus (CLCuBuV), was associated with CLCuD in Pakistan and India9. However, the occasional occurrence of four other virus species and strains [CLCuKoV-Ko (2005), CLCuKoV-La (2008), CLCuKoV-Sha (2004, 2005) and CLCoMuV-Dar (2006)] was also observed on cotton in Pakistan (Table S3). CLCuKoV-Bu is a recombinant virus, consisting of sequences encoding complementary-sense genes derived from CLCuMuV and sequences encoding virion-sense genes and origin of replication derived fromCLCuKoV9, 15. The most interesting feature of CLCuKoV-Bu isolates, associated with resistance breaking, was the lack of a full-length transcriptional activator protein (TrAP) and a mutated C2 protein of only 35 amino acids (aa)9. Recently CLCuKoV-Bu, with a full complement TrAP, has also been identified showing severe symptoms of CLCuD16. The betasatellite associated with CLCuKoV-Bu was also found to be recombinant with most of its sequence from CLCuMuB, but also containing a small fragment of SCR region derived from tomato leaf curl betasatellite16.
The CLCuD complex is in a state of continuous change, evolving rapidly to overcome resistance by component capture, recombination and mutation17. Recently a bipartite begomovirus, Tomato leaf curl New Delhi virus (ToLCNDV) was identified associated with CLCuD in Pakistan18, 19. Interestingly, ToLCNDV isolated from cotton maintained a full complement of TrAP. Another study identified Chickpea chlorotic dwarf virus (CpCDV), a Mastrevirus in cotton showing leaf curl virus disease symptoms20. These results suggest that CLCuD complex has captured viruses that may have contributed to complete breakdown of resistance.
Here we have characterized begomoviruses and associated satellites from symptomatic samples of cotton collected in Vehari from lines that were being screened for virus resistance. In this study, we tried to understand the evolving nature and recent changes in begomovirus disease complexes by using rolling circle amplification (RCA) followed by next generation sequencing (NGS) and Sanger sequencing. Based on NGS and Sanger sequencing data, three distinct begomoviruses (CLCuMuV, CLCuKoV, CLCuAV) characterized from the first epidemic and several distinct alphasatellites and a single betasatellite species were identified associated with CLCuD. We also performed Southern blot hybridization for semi-quantification of begomoviruses and betasatellites. An important feature of the complex found in recent samples from Vehari is the absence of CLCuKoV-Bu in recent samples. Implications of these findings on begomovirus disease complexes are discussed.
Material and Methods
Sample collection, DNA extraction and rolling circle amplification
Cotton leaf samples from a total of six lines showing the typical CLCuD disease symptoms were collected from the Cotton Research Station (CRS) Vehari (Punjab province, Pakistan) in July 2015 (Fig. 1). Genomic DNA was extracted from infected samples using Cetyl trimethyl ammonium bromide (CTAB) method21, followed by ethanol precipitation and DNA quantification. To amplify circular molecules RCA22 was performed using phi 29 DNA polymerase (Thermo Fisher Scientific, Waltham, MA USA). RCA product was purified, enriched and processed for NGS.
Figure 1.

Cotton (Gossypium hirsutum) plants (A–F) from Vehari exhibiting typical symptoms of cotton leaf curl disease.
Library preparation and Illumina sequencing
The Illumina NeoPrep automation system (Illumina, San Diego, CA) was used with library kit, Illumina #NP-101-1001, “TruSeq Nano DNA Library Kit for NeoPrep”, which includes the adapter set “TruSeq LT”. The target insert size was 350 bp, with size selection performed by the NeoPrep instrument. Actual lower size limit of the libraries was ~300 bp as measured by the Agilent 2200 TapeStation. Sequencing was performed on the Illumina MiSeq, v2 chemistry, 2 × 150 bp.
Nucleotide sequence assembly and analysis
The MiSeq Reporter software was set to automatically trim the adaptors. These short sequences were processed using CLC Genomics Work Bench 7.5. The paired-end reads obtained from the Illumina MiSeq Sequencer pipeline were subjected to quality filtering using quality score 0.001 and Phred quality score of 30. De novo as well as reference-guided assemblies were made. Reference-guided mapping was performed using begomovirus and satellite sequences present in Genbank. Based on good quality of data, twelve sequences from six plant samples (MW 6, MW 7, MW 8, MW 9, MW 10 and MW 11) were selected as shown in Table 1. All sequences were searched for similarity in NCBI non-redundant nucleotides database (nt), using BLASTn tool, provided by NCBI.
Table 1.
Begomoviruses, betasatellites and alphasatellites obtained from infected cotton samples through NGS.
| Sample | Sequence Name | Begomovirus/alpha/betas | Accession No | Size (nt) | No. of reads |
|---|---|---|---|---|---|
| MW6 | MZ-50 | CLCuMuV | KX603682 | 2738 | 1849 |
| MZ-51 | CLCuAlV | KX656789 | 2737 | 1066 | |
| MZ-77 | CLCuMuB | KX656816 | 1340 | 4191 | |
| MZ-78 | CLCuMuB | KX656817 | 1410 | 1081 | |
| MW7 | MZ-52 | CLCuMuV | KX603683 | 2738 | 3916 |
| MZ-53 | CLCuAlV | KX656790 | 2737 | 2517 | |
| MZ-79 | CLCuMuB | KX656818 | 1342 | 18760 | |
| MZ-80 | CLCuMuB | KX656819 | 1414 | 6982 | |
| MZ-81 | CLCuMuB | KX656820 | 1371 | 12405 | |
| MZ-63 | GDarSLA | KX656836 | 1372 | 3075 | |
| MZ-64 | GDarSLA | KX656837 | 1374 | 17579 | |
| MZ-65 | GDarSLA | KX656838 | 1370 | 1631 | |
| MZ-67 | CLCuMA | KX656839 | 1373 | 6371 | |
| MW8 | MZ-54 | CLCuMuV | KX603681 | 2738 | 1442 |
| MZ-55 | CLCuAlV | KX656791 | 2737 | 2517 | |
| MZ-82 | CLCuMuB | KX656821 | 1340 | 4890 | |
| MZ-83 | CLCuMuB | KX656822 | 1358 | 3804 | |
| MW9 | MZ-56 | CLCuMuV | KX656786 | 2738 | 4776 |
| MZ-57 | CLCuAlV | KX656792 | 2737 | 2981 | |
| MZ-84 | CLCuMuB | KX656823 | 1356 | 22044 | |
| MZ-85 | CLCuMuB | KX656824 | 1348 | 18284 | |
| MZ-86 | CLCuMuB | KX656825 | 1413 | 6082 | |
| MW10 | MZ-58 | CLCuMuV | KX656787 | 2738 | 2256 |
| MZ-59 | CLCuAlV | KX656793 | 2737 | 1300 | |
| MZ-87 | CLCuMuB | KX656825 | 1413 | 6082 | |
| MZ-88 | CLCuMuB | KX656826 | 1370 | 1553 | |
| MZ-68 | GDarSLA | KX656840 | 1372 | 653 | |
| MZ-69 | GDavSLA | KX656847 | 1223 | 361 | |
| MZ-71 | GDavSLA | KX656848 | 1224 | 108 | |
| MZ-73 | GDavSLA | KX656849 | 1224 | 137 | |
| MW11 | MZ-60 | CLCuMuV | KX656788 | 2738 | 3895 |
| MZ-61 | CLCuAlV | KX656794 | 2737 | 2299 | |
| MZ-91 | CLCuMuB | KX656828 | 1416 | 5303 | |
| MZ-92 | CLCuMuB | KX656829 | 1356 | 8829 | |
| MZ-74 | GDarSLA | KX656841 | 1372 | 2541 | |
| MZ-76 | GDavSLA | KX656842 | 1363 | 761 | |
| Begomoviruses obtained through NGS with low percent coverage | |||||
| Sample | Sequence Name | Begomoviruses | % age coverage | Size (nt) | No. of reads |
| MW6 | MZ-100 | CLCuKoV-Ko | 50 | 2750 | 1131 |
| MZ-101 | CLCuMuV-Raj | 43 | 2740 | 114 | |
| MW7 | MZ-102 | CLCuKoV-Ko | 55 | 2748 | 2277 |
| MZ-103 | CLCuMuV-Raj | 56 | 2738 | 237 | |
| MW8 | MZ-104 | CLCuKoV-Ko | 50 | 2748 | 2816 |
| MZ-105 | CLCuMuV-Raj | 49 | 2736 | 212 | |
| MW9 | MZ-106 | CLCuKoV-Ko | 60 | 2750 | 2816 |
| MZ-107 | CLCuMuV-Raj | 64 | 2738 | 356 | |
| MW10 | MZ-108 | CLCuKoV-Ko | 51 | 2748 | 1397 |
| MZ-109 | CLCuMuV-Raj | 49 | 2738 | 137 | |
| MW11 | MZ-110 | CLCuKoV-Ko | 56 | 2748 | 2350 |
| MZ-111 | CLCuMuV-Raj | 53 | 2736 | 210 | |
PCR amplification, cloning and Sanger sequencing of begomoviruses, alphasatellites and betasatellites
To amplify the begomovirus-complex from genomic DNA, primer pairs Begom-F/Begom-R23, Beta01/Beta0224 and DNA 101/DNA 10225 were used. For amplification of NGS-assembled CLCuAlV new sets of primers were designed (Table S4). The PCR products of ~2.8 kb, ~1.4 kb partial fragments for virus, and ~1.4 kb for alphasatellite and betasatellite, were cloned in a TA cloning vector ((pTZ57R/T; Thermo Fisher Scientific, Waltham, MA USA). An average of five clones per plants were selected for sequencing. Plasmids of the desired clones were purified using AxyPrep™ plasmid miniprep kit (Axygen, USA) and sequenced commercially on an Applied Biosystems 3730XL DNA sequencer (USA). The sequences were assembled and analyzed using Lasergene software (DNAStar Inc., Madison, USA). All the sequences were analyzed and compared to the sequences available in the data bank (NCBI) using BLASTn search tool.
SDT and phylogenetic analysis
Sequence demarcation tool (SDT) v1.226 was used for identification of species and strains of begomoviruses based on muscle alignment and identity score matrixes. Full length viruses and satellites sequences were aligned using pairwise multiple MUSCLE alignment algorithm in MEGA627. This alignment was used to construct phylogenetic trees supported with 1000 bootstrap values through a neighbor-joining algorithm. Phylogenetic trees were edited and labelled in MEGA 6.
Recombination analysis
Recombination among viruses and satellites were detected using RDP4 Beta 4.7428. RDP using 9 recombination analysis methods, RDP29, GENECONV30, BOOTSCAN31, MAXIMUM CHI SQUARE32, CHIMAERA33, SISCAN34, 3SEQ35, PHYLPRO36, LARD37 and VisRD38 for possible recombination breakpoints within query sequences. Recombination events identified by multiple recombination methods with high p-values and good phylogeny were considered potential targets for recombination.
Southern blot hybridization
For Southern blot hybridization an agarose gel (1.2% [w/v]) stained in ethidium bromide was cast in a tray and an equal amount of DNA (~10 µg) per sample was resolved on the gel. After washing in depurination (0.25 M HCl), denaturation (1.5 M NaCl and 0.5 M NaOH) and neutralization buffer [1 M Tris (pH 7.4) & 1.5 M NaOH] the gel was blotted on Hybond-N+ membrane (GE Healthcare, UK) and UV cross-linked. The blot was pre-hybridized at 42 °C for 2–3 h in DIG Easy Hyb solution (Roche, Germany) and hybridized overnight at 42 °C in DIG-labelled probe. For begomoviruses, primers CLCV1/CLCV2. (5′-CCGTGCTGCTGCCCCCATTGTCCGCGTCAC-3′/5′-CTGCCACAACCATGGATTCACGCACAGGG-3′) and for betasatellites Beta01/Beta02 (5′-GTACCGGCTGCTGCGTAGCGTAGT-3′/5′-GGTACCTACCCTCCCAGGGGTACAC-3′) primers were used for synthesis of probes. Genomic DNA of infected cotton plants was used as insert to amplify DIG labeled probes. Hybridization signals were detected on blot after treatment with Nitro blue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3-indolyl phosphate [(BCIP, Thermo Scientific™, USA)].
Results
Begomoviruses, alphasatellites and betasatellites obtained through NGS
The de novo assembly of the high-throughput Illumina sequencing data was carried out on CLC Genomics Work bench 7.5 (CLC bio) software. Resulting contigs of size ~2.8 kb and 1.4 kb, based on putative genome size of begomoviruses and alpha/betasatellites respectively, were selected from the data. The selected contigs were searched by using the BLASTn search tool for closely related begomoviruses and satellites molecules available at NCBI-GenBank database. From the NGS data, BLASTn indicated three kinds of begomoviruses, Cotton leaf curl Multan virus -Pakistan (CLCuMuV-PK), Cotton leaf curl Alabad virus -Multan (CLCuAlV-Mu) and cotton leaf curl Kokhran virus-Kokhran (CLCuKoV-Ko) were present. However, CLCuKoV-Ko sequences identified here with NGS have low percent (50–60%) coverage as shown in Table 1.
The BLASTn searches for satellite molecules identified three types of alphasatellites, Gossypium darwinii symptomless alphasatellite (GDarSLA), Gossypium davidsonii symptomless alphasatellite (GDavSLA) and cotton leaf curl Burewala alphasatellite (CLCuBuA) and one type of betasatellite, cotton leaf curl Multan betasatellite (CLCuMuB) in the collected infected samples (Table 1).
Analysis and ORF identification of begomovirus, alphasatellites and betasatellites obtained through Sanger sequencing
The 2.8 kb and 1.4 kb clones obtained here were checked for the presence of putative open reading frames (ORF) using ORF finder at (www.ncbi.nlm.nih.gov/gorf). The ORF analysis shows that 2.8 kb clones resembled and had an arrangement of genes typical of the DNA-A component of begomoviruses (Table 2). Similarly, 1.4 kb clones resembled to alpha/betasatellites molecules respectively. The BLASTn and SDT results of Sanger sequencing data showed that 2 strains of Multan virus, CLCuMuV-PK and Cotton leaf curl Multan virus-Rajasthan (CLCuMuV-Raj) and one strain of CLCuKoV, Cotton leaf curl Kokhran virus-Shadadpur (CLCuKoV-Sha) were identified (Table 2). However, CLCuAlV identified with NGS was not amplified by universal begomo primers. Primer sets (Table S4) were designed according to the assembled sequences of NGS for detection of CLCuAlV in the diseased samples. PCR with the new sets of primers confirmed the presence of CLCuAlV in the diseased samples.
Table 3.
Features of betasatellites and alphsatellites obtained through Sanger sequencing.
| Betasatellites obtained through Sanger sequencing | ||||||
|---|---|---|---|---|---|---|
| Clone | Satellite/Original/Recombinant | Location (province/district) | Accession No | Size (nt) | [Coordinates/no. of amino acids] | |
| βC1 | Rep | |||||
| MZ-32 | CLCuMuB/NA | Punjab/Vehari | KX697597 | 1356 | 194–550/118 | — |
| MZ-33 | CLCuMuB/Original | Punjab/Vehari | KX697598 | 1369 | 194–550/118 | — |
| MZ-34 | CLCuMuB/Original | Punjab/Vehari | KX697599 | 1374 | 194–550/118 | — |
| MZ-35 | CLCuMuB/Recombinant | Punjab/Vehari | KX697600 | 1370 | 194–550/118 | — |
| MZ-36 | CLCuMuB/Original | Punjab/Vehari | KX697601 | 1410 | 194–550/118 | — |
| MZ-37 | CLCuMuB/Original | Punjab/Vehari | KX697602 | 1358 | 194–550/118 | — |
| Alphasatellites obtained through Sanger sequencing | ||||||
| MZ-38 | CLCuBuA | Punjab/Vehari | KX656851 | 1394 | — | 77–1024/315 |
| MZ-40 | AConSLA | Punjab/Vehari | KX656850 | 1361 | — | 82–1029/315 |
| MZ-41 | GDarSLA | Punjab/Vehari | KX656852 | 1375 | — | 70–1017/315 |
Table 2.
Features of begomoviruses obtained from infected cotton samples through Sanger sequencing.
| Sample | Clone Name | Virus Component | Location (province/District | Accession no. | Size (nt) | Coding sequences [coordinates/no. of amino acids] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CP | V2 | Rep | TrAP | REn | C4 | ||||||
| MW6 | MZ-4 | CLCuMuV | Punjab/Vehari | KX656795 | 2739 | 276–1046/256 | 116–481/121 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 |
| MZ-1 | CLCuMuV-Raj | Punjab/Vehari | KX656814 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MZ-2 | CLCuMuV-Raj | Punjab/Vehari | KX656805 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MW7 | MZ-7 | CLCuMuV-Raj | Punjab/Vehari | KX656806 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 |
| MZ-5 | CLCuMuV | Punjab/Vehari | KX656796 | 2739 | 276–1046/256 | 116–481/121 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MZ-9 | CLCuMuV-Raj | Punjab/Vehari | KX656807 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MZ-11 | CLCuKoV-Sha | Punjab/Vehari | KX656802 | 2748 | 292–1062/256 | 132–488/118 | 1505–2593/362 | 1156–1608/150 | 1059–1463/134 | 2137–2439/100 | |
| MW8 | MZ-15 | CLCuMuV | Punjab/Vehari | KX656797 | 2739 | 276–1046/256 | 116–481/121 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 |
| MZ-10 | CLCuMuV-Raj | Punjab/Vehari | KX656808 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MZ-19 | CLCuKoV-Sha | Punjab/Vehari | KX656803 | 2748 | 292–1062/256 | 132–488/118 | 1505–2593/362 | 1156–1608/150 | 1059–1463/134 | 2137–2439/100 | |
| MW9 | MZ-17 | CLCuMuV | Punjab/Vehari | KX656799 | 2739 | 276–1046/256 | 116–481/121 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 |
| MZ-13 | CLCuMuV-Raj | Punjab/Vehari | KX656809 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MZ-14 | CLCuMuV-Raj | Punjab/Vehari | KX656810 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MZ-25 | CLCuKoV-Sha | Punjab/Vehari | KX656804 | 2748 | 292–1062/256 | 132–488/118 | 1505–2593/362 | 1156–1608/150 | 1059–1463/134 | 2137–2439/100 | |
| MW10 | MZ-20 | CLCuMuV | Punjab/Vehari | KX656899 | 2739 | 276–1046/256 | 116–481/121 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 |
| MZ-24 | CLCuMuV | Punjab/Vehari | KX656800 | 2739 | 276–1046/256 | 116–481/121 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MZ-21 | CLCuMuV-Raj | Punjab/Vehari | KX656811 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MW11 | MZ-29 | CLCuMuV | Punjab/Vehari | KX656801 | 2739 | 276–1046/256 | 116–481/121 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 |
| MZ-22 | CLCuMuV-Raj | Punjab/Vehari | KX656812 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
| MZ-28 | CLCuMuV-Raj | Punjab/Vehari | KX656813 | 2737 | 274–1044/256 | 114–470/118 | 1495–2583/362 | 1146–1598/150 | 1049–1453/134 | 2127–2429/100 | |
Analysis of CLCuMuV-PK isolates showed that they have 92–96%, CLCuMuV-Raj isolates have 96–99% and CLCuKoV-Sha isolates have 96–98% sequence identity with sequences of their respective viruses available in the NCBI database (Figure S1A–C). Three types of alphasatellites, GDarSLA, CLCuBuA and Ageratum conyzoides symptomless alphasatellite (AConSLA) and one type of betasatellite, CLCuMuB were also identified from Sanger sequencing as shown in (Table 3).
The BLASTn searches for satellite molecules identified three types of alphasatellites, Gossypium darwinii symptomless alphasatellite (GDarSLA), Gossypium davidsonii symptomless alphasatellite (GDavSLA) and cotton leaf curl Burewala alphasatellite (CLCuBuA) and one type betasatellite, cotton leaf curl Multan betasatellite (CLCuMuB) in the collected infected samples (Table 2).
Analysis of TrAP gene of begomoviruses
A detailed analysis of TrAP encoding gene showed that isolates, CLCuMuV-PK, CLCuMuV-Raj and CLCuKoV-Sha obtained here through Sanger sequencing encoded a putatively full-length TrAP of 150 amino acids as shown in Table 2.
Recombination analysis of begomoviruses
To determine recombination among begomoviruses isolates identified here, RDP4 Beta 4.7428 analysis was conducted. The isolates of CLCuMuV-Raj, CLCuKoV-Sha and CLCuMuV identified here were aligned with 260 full genome sequences of begomoviruses available and retrieved from the database (Table S1). The RDP result for begomoviruses is shown in Fig. 2 and further details including p-values are provided in Table S2.
Figure 2.

Recombination analyses of begomoviruses with RDP-4. Recombination analysis of Cotton leaf curl Multan virus-Rajasthan (CLCuMuV-Raj), Cotton leaf curl Kokhran virus-Shadadpur (CLCuKoV-Sha) and Cotton leaf curl Multan virus-Pakistan (CLCuMuV-PK). The isolates are grouped into species (marked on the right). A linear genome map of begomoviruses is plotted on top of figure, with position of genes and their orientation indicated by arrows to show recombinant fragments.
Recombination analysis of CLCuMuV-Raj isolates show that CLCuMuV-Raj is a recombinant of two viruses CLCuMuV and CLCuKoV-Ko as reported previously40. Recombination analysis shows that CLCuKoV-Sha isolates contain recombinant regions of CLCuKoV-Ko in both virion-sense and complementary-sense strands. The CLCuMuV isolates contain a small fragment of CLCuMuV-Fai[PK:DGK26:95] and CLCuMuV-Raj[IN:Sri:94] in the coat protein region. The CLCuMuV isolates also contain fragments of Pedilanthus leaf curl virus (PeLCV-[IN:Cn:11]) in the coat protein and Tomato leaf curl Palampur virus (ToLCPalV-[IN:Rau:TC238:09]) in Rep in the complementary sense.
Phylogenetic analysis of begomoviruses
A phylogenetic dendrogram of begomoviruses was constructed on the basis of pairwise multiple MUSCLE alignment (MEGA6) of CLCuMuV, CLCuMuV-Raj and CLCuKoV-Sha and their similar isolates available in the database. The phylogenetic tree of CLCuMuV, CLCuMuV-Raj and CLCuKoV-Sha isolates shows that they are separated with closely related begomoviruses interspersed with the three begomoviruses as shown in Fig. 3.
Figure 3.

Phylogenetic tree of monopartite begomoviruses and DNA-A component. Cotton leaf curl Multan virus-Rajasthan (CLCuMuV-Raj) is colored sky blue, and Cotton leaf curl Kokhran virus-Shadadpur (CLCuKoV-Sha) is colored magenta and Cotton leaf curl Multan virus-Pakistan (CLCuMuV-PK) is colored orange. The tree arbitrarily rooted on Tomato golden mosaic virus (ToMoV) was taken as outgroup is colored red. The tree was generated with 1000 bootstrap value represented along each root.
Analysis of alphasatellites
Three types of alphasatellites GDarSLA, CLCuBuA and AConSLA were identified from Sanger sequencing data. The phylogenetic tree of alphasatellites indicated that they clustered with closely related GDarSLA, CLCuBuA and AConSLA as shown in Fig. 4. RDP analysis of alphasatellites shows that sequence (MZ-40) consists of recombinant fragments of CLCuBuA and Croton yellow vein mosaic alphasatellite (CYVMA) sequences. RDP analysis of alphasatellites is shown with detail in Figure S2.
Figure 4.

Phylogenetic tree of alphasatellites. Gossypium darwinii symptomless alphasatellite (GDarSLA) is colored orange, Ageratum conyzoides symtomless alphasatellite (AConSLA) is colored magneta and cotton leaf curl Burewala alphasatellite (CLCuBuA) is colored sky-blue. Cotton leaf curl Gezira alphasatellite (CLCuGeA) is taken as outgroup and colored red. The tree is supported by 1000 bootstrap value.
Betasatsallites identified and analysis of SCR region
A single type of betasatellite, CLCuMuB was obtained from Sanger sequencing data. Analysis of the SCR region of these betasatellites showed that three types of betasatellites separated into three groups as shown in Fig. 5. The first group of betasatellites consisting of 3 clones (MZ-33, MZ-34, MZ-36) segregated with CLCuMuB which was associated with CLCuD in Pakistan during the 1990s. It was first identified by Briddon et al.41, and known as Multan betasatellite (CLCuMuBMul). The second group consisted of a single clone (MZ-35) and is closely related to recombinant CLCuMuB associated with CLCuBuV known as (CLCuMuBBur). The clone (MZ-35) contains approximately 95 nt derived from Tomato leaf curl betasatellite (ToLCuB) within the SCR region17, 42. The third group consisted of two clones (MZ-32, MZ-37) and their sequence in SCR does not match either CLCuMuBMul or ToLCuB. Instead they contain approximately 90 nt within the SCR derived from cotton leaf curl virus betasatellite defective interfering DNA (KT228331), recently identified from India, and here we designate it as Vehari betasatellite (CLCuMuBVeh). Phylogenetic trees of full length betasatellites and their SCR regions is shown in Fig. 5.
Figure 5.
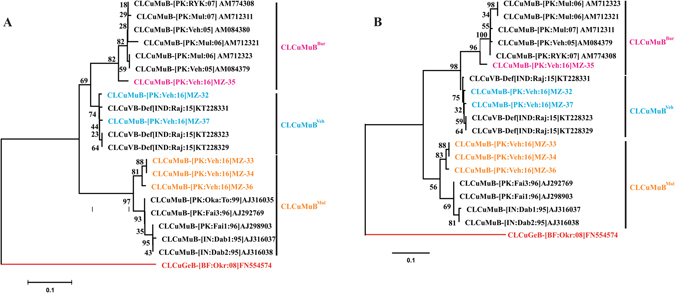
Phylogenetic tree of full length and SCR of betasatellite clones from cotton. (A) Phylogenetic tree of full length betasatellites. Cotton leaf curl Gezira betasatellite (CLCuGeB) was taken as outgroup. The tree is supported by 1000 bootstrap value. (B) Phylogenetic tree of SCR region of betasatellites. Three types of betasatellites were identified from both full and SCR alignments.
Southern blot hybridization of begomoviruses and betasatellites
To determine relative titer of begomoviruses and betasatellites, Southern blot hybridization of the infected cultivated cotton samples was performed. Southern blot analysis of DNA samples extracted from symptomatic leaves showed weak hybridization signals for virus probe in sample 1 and 2 indicating that virus titer was low in these samples, while virus titer was high in samples 3, 4, 5 and 6 showing good hybridization signals for virus probe as shown in (Fig. 6A). The titer of betasatellites was high in all the samples showing good hybridization signals with beta probe (Fig. 6B).
Figure 6.

Southern blot hybridization analysis of begomovirus and betasatellite. (A) Southern blot hybridization with begomovirus probe. Genomic DNA from cotton samples were loaded in lane 1–6 respectively. Agro-infiltrated plants genomic DNA was used as positive control and healthy plant genomic DNA as negative control represented as +Ve and −Ve, loaded in lane 7 and 8 respectively. (B) Southern blot with betasatellite probe. Genomic DNA from cotton samples were loaded in lane 1–6 respectively. Positive control and negative control represented as +Ve and −Ve, loaded in lane 7 and 8 respectively. The different replicative forms of viral DNA are shown as open circular (oc), linear (lin), supercoiled (sc) and single stranded (ss).
Discussion
The begomovirus disease complex can severely affect yields of cultivated cotton in Pakistan and India, and previously often occurred as an infection of multiple begomoviruses. The CLCuKoV-Bu strain, with a truncated TrAP, is the only begomovirus strain associated with the resistance breaking of CLCuD currently found throughout Pakistan, with the occasional identification of other strains (Table S3)9, 43. We have found that some recent isolates of CLCuKoV-Bu encode a full length TrAP (Hassan et al., unpublished data). Our current study shows that the CLCuD complex can occur as result of infection of multiple begomoviruses. The begomovirus disease complex can easily evolve due to component capture, recombination and mutation in order to overcome disease resistance and to expand its host range17, 44.
To understand ongoing changes in the CLCuD complex, symptomatic leaves of previously resistant cotton varieties were collected from Vehari. Vehari is a major cotton growing area of Pakistan and exhibits a high diversity of begomoviruses. To evaluate the samples collected, a highly sensitive method of RCA coupled with NGS was used followed by confirmation with Sanger sequencing. Our NGS data showed that the three distinct begomoviruses, CLCuMuV, CLCuAlV and CLCuKoV-Ko associated with the first epidemic of CLCuD in early 1990s, a betasatellite, CLCuMuB and 3 alphasatellites GDarSLA, GDavSLA and CLCuBuA were identified in infected samples from Vehari. The reason for the low coverage (50–60%) of CLCuKoV-Ko in NGS data is not known. To confirm our NGS data results, genomic DNA from the collected samples were amplified with universal begomo, alpha and beta primers, cloned and dideoxy nucleotide chain termination sequencing was performed. From the Sanger sequencing data, two strains of Multan, CLCuMuV-Mu, CLCuMuV-Raj and one strain of Kokhran, CLCuKoV-Sha associated with the first epidemic of CLCuD, were identified. Our results from both NGS and Sanger sequencing data shows that CLCuKoV-Bu was not identified in infected cotton samples from Vehari. The presence of previously identified begomoviruses and the absence of CLCuKoV-Bu which was the only begomovirus found in infected cotton plants, indicate a potential major change in the begomovirus complex associated with CLCuD in Pakistan. A single species of betasatellite (CLCuMuB) and three species of alphasatellites (GDarSLA, CLCuBuA and AConSLA) were identified in these samples. Sanger sequencing data complementing our NGS data, identified and confirmed the presence of CLCuMuV, CLCuMuV-Raj and CLCuKoV-Sha. However, CLCuAlV identified with NGS was not amplified with universal begomo primers. The CLCuAlV was amplified from infected samples with CLCuAlV specific primers designed according to the assembled sequences of NGS (Table S4).
A closer detailed analysis of the TrAP gene of CLCuMuV, CLCuMuV-Raj and CLCuKoV-Sha isolates identified here encode a full length TrAP protein of 150 aa. The CLCuKoV-Bu which is the dominant strain associated with CLCuD from 2000 onward in cultivated cotton, encodes a truncated C2 protein of 35 amino acids (aa)9, 14. TrAP encoded by begomoviruses is a multi-functional protein, functioning as a pathogenicity determinant44 and also a strong suppressor of post transcriptional gene silencing activity (PTGS) of the host45. Full length and truncated TrAP both exhibited PTGS activity but truncated ones have lower PTGS activity as compared to full length TrAP46.
Recombination is a major process of evolution in begomoviruses47, 48. The recombination analysis shows that diversification of begomoviruses identified here occurs as a result of recombination between CLCuMuV and CLCuKoV. Most of begomoviruses identified here are recombinant between CLCuMuV and CLCuKoV exchanged as either virion-sense or complementary-sense genes but some isolates of CLCuMuV contain fragments of PeLCV and ToLCPalV in virion and complementary sense. Three types of betasatellites, replicating with begomoviruses, were identified from infected samples. A new type of recombinant betasatellite named as CLCuMuBVeh, has a recombinant region within the SCR region different from the previously identified CLCuMuBMul and recombinant CLCuMuBBur.
The emergence of viruses associated with the first epidemic of CLCuD in cultivated cotton indicate that the begomovirus complex may be changing to resemble the original CLCuD complex found during the first epidemic of the 1990s before the introduction of resistant varieties derived from LRA5166 and CP15/2 as a source of resistance. The arrival CLCuMuV, CLCuAlV, and CLCuKoV back into cultivated cotton (Table S3) is a sign of changes in the CLCuD complex and might be a sign that a new epidemic is possible. A further study of begomovirus diversity at Vehari and surrounding areas using RCA and NGS will help to understand CLCuD in a broader sense. The changing scenario indicates the need for identification and incorporation of new sources of resistance with several recently developed technologies, into the cultivated cotton49–51.
Electronic supplementary material
Acknowledgements
We are thankful to Higher Education Commission (HEC), Pakistan and “Pakistan-U.S. Cotton Productivity Enhancement Program” of ICARDA funded by the United States Department of Agriculture (USDA), Agricultural Research Service (ARS) for their financial support. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the USDA or ICARDA.
Author Contributions
M.Z. and S.S.Z. contributed equally to this work. S.M., S.S.Z., M.Z. and I.A. designed the study. M.Z., S.S.Z., S.S. and M.F. performed experiments, analyzed data and wrote first draft. B.E.S. provided N.G.S. All authors read and approved the manuscript for submission.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Muhammad Zubair and Syed Shan-e-Ali Zaidi contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00727-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anthony VM, Ferroni M. Agricultural biotechnology and smallholder farmers in developing countries. Curr. Opin. Biotechnol. 2012;23:278–285. doi: 10.1016/j.copbio.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Briddon RW. Cotton leaf curl disease, a multicomponent begomovirus complex. Mol Plant Pathol. 2003;4:427–434. doi: 10.1046/j.1364-3703.2003.00188.x. [DOI] [PubMed] [Google Scholar]
- 3.Briddon RW, Markham PG. Cotton leaf curl virus disease. Virus Res. 2000;71:151–159. doi: 10.1016/S0168-1702(00)00195-7. [DOI] [PubMed] [Google Scholar]
- 4.Mansoor S, Briddon RW, Zafar Y, Stanley J. Geminivirus disease complexes: an emerging threat. Trends Plant Sci. 2003;8:128–134. doi: 10.1016/S1360-1385(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 5.T. Hussain MAli. A review of cotton diseases of Pakistan. Pakistan Cotton. 1975;19:71–86. [Google Scholar]
- 6.Briddon R, Markham P. Cotton leaf curl virus disease. Virus Res. 2000;71:151–159. doi: 10.1016/S0168-1702(00)00195-7. [DOI] [PubMed] [Google Scholar]
- 7.Rahman M, Hussain D, Zafar Y. Estimation of genetic divergence among elite cotton cultivars–genotypes by DNA fingerprinting technology. Crop Sci. 2002;42:2137–2144. doi: 10.2135/cropsci2002.2137. [DOI] [Google Scholar]
- 8.Mansoor S, et al. Breakdown of resistance in cotton to cotton leaf curl disease in Pakistan. Plant Pathol. 2003;52:784. doi: 10.1111/j.1365-3059.2003.00893.x. [DOI] [Google Scholar]
- 9.Amrao L, et al. Cotton leaf curl disease in resistant cotton is associated with a single begomovirus that lacks an intact transcriptional activator protein. Virus Res. 2010;152:153–163. doi: 10.1016/j.virusres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, et al. Identification of the cryptic species of Bemisia tabaci transmitting Cotton leaf curl Multan virus. J. Plant Protect. 2016;43:91–98. [Google Scholar]
- 11.Zhou X. Advances in understanding begomovirus satellites. Annu. Rev. Phytopathol. 2013;51:357–381. doi: 10.1146/annurev-phyto-082712-102234. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Liu Y, Robinson DJ, Harrison BD. Four DNA-A variants among Pakistani isolates of cotton leaf curl virus and their affinities to DNA-A of geminivirus isolates from okra. J. Gen. Virol. 1998;79:915–923. doi: 10.1099/0022-1317-79-4-915. [DOI] [PubMed] [Google Scholar]
- 13.Briddon RW, et al. Diversity of DNA b: a satellite molecule associated with some monopartite begomoviruses. Virology. 2003;312:106–121. doi: 10.1016/S0042-6822(03)00200-9. [DOI] [PubMed] [Google Scholar]
- 14.Briddon RW, et al. Effects of genetic changes to the begomovirus/betasatellite complex causing cotton leaf curl disease in South Asia post-resistance breaking. Virus Res. 2014;186:114–119. doi: 10.1016/j.virusres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Kumar J, et al. Cotton leaf curl Burewala virus with intact or mutant transcriptional activator proteins: complexity of cotton leaf curl disease. Arch. Virol. 2015;160:1219–1228. doi: 10.1007/s00705-015-2384-4. [DOI] [PubMed] [Google Scholar]
- 16.Amin I, et al. Mobilisation into cotton and spread of a recombinant cotton leaf curl disease satellite. Arch. Virol. 2006;151:2055–2065. doi: 10.1007/s00705-006-0773-4. [DOI] [PubMed] [Google Scholar]
- 17.Sattar MN, Kvarnheden A, Saeed M, Briddon RW. Cotton leaf curl disease - an emerging threat to cotton production worldwide. J Gen Virol. 2013;94:695–710. doi: 10.1099/vir.0.049627-0. [DOI] [PubMed] [Google Scholar]
- 18.Zaidi S, Iqbal Z, Amin I, Mansoor S. First report of Tomato leaf curl Gujarat virus, a bipartite begomovirus on cotton showing leaf curl symptoms in Pakistan. Plant Dis. 2015;99:1655–1656. doi: 10.1094/PDIS-02-15-0195-PDN. [DOI] [Google Scholar]
- 19.Zaidi SA, et al. Frequent occurrence of Tomato leaf curl New Delhi virus in cotton leaf Curl disease affected cotton in Pakistan. PLoS One. 2016;11:e0155520. doi: 10.1371/journal.pone.0155520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzoor MT, et al. A distinct strain of chickpea chlorotic dwarf virus (genus Mastrevirus, family Geminiviridae) identified in cotton plants affected by leaf curl disease. Arch. Virol. 2014;159:1217–1221. doi: 10.1007/s00705-013-1911-4. [DOI] [PubMed] [Google Scholar]
- 21.Doyle JJ. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- 22.Inoue-Nagata AK, Albuquerque LC, Rocha WB, Nagata TT. A simple method for cloning the complete begomovirus genome using the bacteriophage phi29 DNA polymerase. J. Virol Methods. 2004;116:209–211. doi: 10.1016/j.jviromet.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Briddon RW, Markham PG. Universal primers for the PCR amplification of dicot-infecting geminiviruses. Mol Biotechnol. 1994;1:202–205. doi: 10.1007/BF02921559. [DOI] [PubMed] [Google Scholar]
- 24.Briddon RW, et al. Universal primers for the PCR-mediated amplification of DNA beta - A molecule associated with some monopartite begomoviruses. Mol Biotechnol. 2002;20:315–318. doi: 10.1385/MB:20:3:315. [DOI] [PubMed] [Google Scholar]
- 25.Bull SE, Briddon RW, Markham PG. Universal primers for the PCR-mediated amplification of DNA 1: a satellite-like molecule associated with begomovirus-DNA β complexes. Mol Biotechnol. 2003;23:83–86. doi: 10.1385/MB:23:1:83. [DOI] [PubMed] [Google Scholar]
- 26.Muhire BM, Varsani A, Martin DP. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS One. 2014;9:e108277. doi: 10.1371/journal.pone.0108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura K, et al. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1:vev003. doi: 10.1093/ve/vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin D, Rybicki E. RDP: detection of recombination amongst aligned sequences. Bioinformatics. 2000;16:562–563. doi: 10.1093/bioinformatics/16.6.562. [DOI] [PubMed] [Google Scholar]
- 30.Padidam M, Sawyer S, Fauquet CM. Possible emergence of new geminiviruses by frequent recombination. Virology. 1999;265:218–225. doi: 10.1006/viro.1999.0056. [DOI] [PubMed] [Google Scholar]
- 31.Martin D, Posada D, Crandall K, Williamson C. A modified bootscan algorithm for automated identification of recombinant sequences and recombination breakpoints. AIDS Res. Hum. Retroviruses. 2005;21:98–102. doi: 10.1089/aid.2005.21.98. [DOI] [PubMed] [Google Scholar]
- 32.Smith JM. Analyzing the mosaic structure of genes. J. Mol. Evol. 1992;34:126–129. doi: 10.1007/BF00182389. [DOI] [PubMed] [Google Scholar]
- 33.Martin DP, Williamson C, Posada D. RDP2: recombination detection and analysis from sequence alignments. Bioinformatics. 2005;21:260–262. doi: 10.1093/bioinformatics/bth490. [DOI] [PubMed] [Google Scholar]
- 34.Gibbs MJ, Armstrong JS, Gibbs AJ. Sister-scanning: a Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics. 2000;16:573–582. doi: 10.1093/bioinformatics/16.7.573. [DOI] [PubMed] [Google Scholar]
- 35.Boni MF, Posada D, Feldman MW. An exact nonparametric method for inferring mosaic structure in sequence triplets. Genetics. 2007;176:1035–1047. doi: 10.1534/genetics.106.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiller GF. Phylogenetic profiles: a graphical method for detecting genetic recombinations in homologous sequences. Mol. Biol. Evol. 1998;15:326–335. doi: 10.1093/oxfordjournals.molbev.a025929. [DOI] [PubMed] [Google Scholar]
- 37.Holmes EC, Worobey M, Rambaut A. Phylogenetic evidence for recombination in dengue virus. Mol. Biol. Evol. 1999;16:405–409. doi: 10.1093/oxfordjournals.molbev.a026121. [DOI] [PubMed] [Google Scholar]
- 38.Lemey P, Lott M, Martin DP, Moulton V. Identifying recombinants in human and primate immunodeficiency virus sequence alignments using quartet scanning. BMC Bioinformatics. 2009;10:1. doi: 10.1186/1471-2105-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JK, et al. Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch Virol. 2015;160:1593–1619. doi: 10.1007/s00705-015-2398-y. [DOI] [PubMed] [Google Scholar]
- 40.Saleem H, et al. Diversity, mutation and recombination analysis of cotton leaf curl geminiviruses. PLoS One. 2016;11:e0151161. doi: 10.1371/journal.pone.0151161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briddon RW, et al. Identification of DNA components required for induction of cotton leaf curl disease. Virology. 2001;285:234–243. doi: 10.1006/viro.2001.0949. [DOI] [PubMed] [Google Scholar]
- 42.Briddon RW, et al. Diversity of DNA β, a satellite molecule associated with some monopartite begomoviruses. Virology. 2003;312:106–121. doi: 10.1016/S0042-6822(03)00200-9. [DOI] [PubMed] [Google Scholar]
- 43.Briddon RW, et al. Effects of genetic changes to the begomovirus/betasatellite complex causing cotton leaf curl disease in South Asia post-resistance breaking. Virus Res. 2014;186:114–119. doi: 10.1016/j.virusres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 44.van Wezel R, Liu H, Tien P, Stanley J, Hong Y. Gene C2 of the monopartite geminivirus tomato yellow leaf curl virus-China encodes a pathogenicity determinant that is localized in the nucleus. Mol Plant Microbe Interact. 2001;14:1125–1128. doi: 10.1094/MPMI.2001.14.9.1125. [DOI] [PubMed] [Google Scholar]
- 45.Van Wezel R, et al. Mutation of three cysteine residues in Tomato yellow leaf curl virus-China C2 protein causes dysfunction in pathogenesis and posttranscriptional gene-silencing suppression. Mol Plant Microbe Interact. 2002;15:203–208. doi: 10.1094/MPMI.2002.15.3.203. [DOI] [PubMed] [Google Scholar]
- 46.Akbar, F. et al. The 35-amino acid C2 protein of Cotton leaf curl Kokhran virus, Burewala, implicated in resistance breaking in cotton, retains some activities of the full-length protein. Virus Genes. 1–10 (2016). [DOI] [PubMed]
- 47.Lefeuvre P, Lett JM, Varsani A, Martin D. Widely conserved recombination patterns among single-stranded DNA viruses. J. Virol. 2009;83:2697–2707. doi: 10.1128/JVI.02152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefeuvre P, et al. Begomovirus ‘melting pot’ in the south-west Indian Ocean islands: molecular diversity and evolution through recombination. J. Gen. Virol. 2007;88:3458–3468. doi: 10.1099/vir.0.83252-0. [DOI] [PubMed] [Google Scholar]
- 49.Zaidi, S. S., Briddon, R. W. & Mansoor, S. Engineering dual begomovirus-Bemisia tabaci resistance in plants. Trends Plant. Sci. 22, 6-8 doi:10.1016/j.tplants.2016.11.005 (2016). [DOI] [PubMed]
- 50.Zaidi S, Mansoor S, Ali Z, Tashkandi M, Mahfouz M. Engineering plants for geminivirus resistance with CRISPR/Cas9 system. Trends Plant. Sci. 2016;21:279–281. doi: 10.1016/j.tplants.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 51.Zaidi SS, Tashkandi M, Mansoor S, Mahfouz MM. Engineering plant immunity: Using CRISPR/Cas9 to generate virus resistance. Front. Plant. Sci. 2016;7:1673. doi: 10.3389/fpls.2016.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


