Abstract
Salvia fruticosa (SF) Mill. is traditionally used for its antihypertensive actions. However, little is known about its pharmacologic and molecular mechanisms of action. Here we determined the effects of an ethanolic extract of SF leaves on rings of isolated thoracic aorta from Sprague-Dawley rats. Our results show that SF extract increased nitric oxide production and relaxed endothelium-intact rings in a dose-dependent (0.3 µg/ml–1 mg/ml) manner, and the maximum arterial relaxation (Rmax) was significantly reduced with endothelium denudation. Pretreatment of endothelium-intact rings with L-NAME (a non-selective inhibitor of nitric oxide synthase, 100 µM), or ODQ (an inhibitor of soluble guanylyl cyclase, 10 µM) significantly diminished SF-mediated vasorelaxation. Furthermore, SF induced Akt phosphorylation as well as increased cGMP levels in rings treated with increasing doses of SF. Prior exposure to PI3K inhibitors, wortmannin (0.1 µM) or LY294002 (10 µM), decreased cGMP accumulation and attenuated the SF-induced vasorelaxation by approximately 50% (Rmax). SF-evoked relaxation was not affected by indomethacin, verapamil, glibenclamide, tetraethylammonium, pyrilamine or atropine. Taken together, our results indicate that SF induces endothelium-dependent vasorelaxation through the PI3K/Akt/eNOS/NO/sGC/cGMP signaling pathway. Our data illustrate the health-orientated benefits of consuming SF which may act as an antihypertensive agent to reduce the burden of cardiovascular complications.
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in the world1. Along with many other risk factors, hypertension continues to be a major contributor to this mortality. Not only does hypertension kill one in every eight people, but it also threatens as many as 1 billion people worldwide2. Despite the tremendous therapeutic advances made in recent decades, current cardiovascular drugs remain inefficient at treating a significant proportion of patients3. Therefore, there is an increasing need for other approaches that could provide new avenues to combat CVD. Especially during the last 10 years, herbal medicine has emerged as a significant alternative for the treatment of several diseases including CVD4–6.
Herbs and other medicinal plants have been at the foundation of drug development from the very inception of global pharmaceutical industry, and continue to attract focus of attention for research, worldwide7, 8. Moreover, the public from both developed and developing nations hanker for alternative, cheaper and safer drugs, which may be used for prolonged duration with minimal side-effects7. Our knowledge regarding the beneficial constituents of plants, particularly related to ethnomedicine and ethnobotanicals, remains at the stage of infancy. However, the present interest in herbal medicine will certainly lead to an expansion in newer classes of botanical-based drugs during the next decade or thereafter. This action is urgently required, as many of the currently available drugs are not without serious undesired side effects3. Moreover, herbal remedies and their constituents are associated with amelioration of a number of global endemics linked to high morbidities and mortalities, including cardiovascular disease5, 6, 9, 10, metabolic syndrome11, 12, cancer13–16 and neurodegenerative diseases17–19.
There are a multitudinous number of medicinal herbs belonging to the genus Salvia (sage). Indeed, sage has a worldwide distribution with approximately 1000 species, and is the largest genus in the family Lamiaceae. Several species of Salvia have demonstrable physiological and pharmacological attributes associated with improvement and prevention in vascular dysfunction, including blood pressure-lowering effects20–24. Interestingly, culinary herbs such as sages are important components of diet in the Mediterranean basin, where the demographics of cardiovascular-associated morbidity and mortality is low25.
Salvia fruticosa Mill. (Fig. 1) (also referred to as S. libanotica Boiss. & Gaill., S. triloba L.f., and S. cyrpia Unger & Kotschy) is commonly known as the East Mediterranean sage and is widely used in the gastronomy of the Levant26. It is a perennial herb with trifoliate hairy leaves that are grey to green in color. Its flowers are lavender-pinkish in color and are held in a reddish five-pointed hairy calyx27. Accumulating evidence reveals a remarkable array of therapeutic properties for this herb. In addition to its many beneficial biological activities in its arsenal, sage is also endowed with anti-inflammatory28, anti-oxidant29, 30 and anti-proliferative31 effects, as well as the inhibition of smooth muscle contraction32.
Figure 1.

Salvia fruticosa Mill. (Sage). A photograph showing the aerial parts of SF. For medicinal uses, leaves are the most commonly consumed part of this plant.
Traditionally, as part of the armamentarium of ethnomedicine of the Eastern Mediterranean basin and the hinterland beyond, leaves of this herb have been used for their anti-hypertensive effects26, 33. An ethno-botanical study has divulged the ethno-pharmaceutical use of Salvia fruticosa Mill. by British Turkish-speaking Cypriots residing in London (United Kingdom) for amelioration of high blood pressure (BP)34. Furthermore, in Cyprus, the aerial parts of this sage are commonly used for its hypotensive effects35, 36. All of these remedial homeostatic effects are bestowed by a rich and diverse population of phytochemicals. The principle quantitative components of polyphenols isolated from SF are: hydroxycinnamic acid derivatives: rosmarinic acid (caffeic acid dimer), salvianolic acids (caffeic acid polymers), caffeic acid phenethyl ester (caffeic acid derivative); phenolic diterpenes: carnosic acid and carnosol; and flavonoids: luteolin-7-O-glucoside and rutin37, 38. In framework of the present study, rosmarinic acid exerts an arterio-relaxant effect in rat isolated thoracic aorta39. Moreover, rosmarinic acid was reported to lower BP in fructose-fed hypertensive rats. The drop in BP arose through a mechanism entailing a fall in circulating levels of endothelin-1, suppression in angiotensin-converting enzyme activity, and augmentation of nitric oxide synthesis40.
Despite all these common uses, the mechanism by which this medicinal herb elicits its therapeutic effects on the vasculature remains unknown. This study was therefore undertaken to investigate the vasorelaxant function of SF in the rat aorta, and to establish the underlying pharmaco-biochemical mechanism of action.
Results
Effect of SF on relaxation of rat isolated aortic rings
First, we determined the vasorelaxing effect of SF on endothelium-intact rat aortic rings. Figure 2 provides definitive evidence for SF-induced, dose-dependent relaxation of rat isolated aortic rings pre-contracted with norepinephrine, yielding a pED50 value of 4.78 ± 0.08 g/ml with 95% confidence interval: 4.93–4.63 g/ml, whereas the Rmax is 88 ± 4%. The highest percentage volume of vehicle (ethanol) used did not cause a significant relaxation effect on rat aortic ring.
Figure 2.
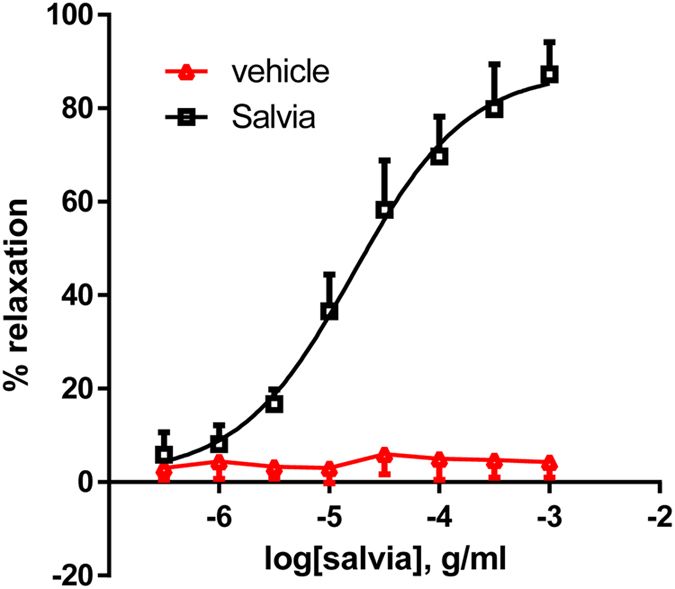
Effect of SF extract on vasorelaxation of aortic rings. Cumulative dose-dependent curves for SF-induced relaxation and of the vehicle (ethanol) in rat aortic rings. n = 7.
Role of the endothelium in SF-induced relaxation
In this series of experiments, we examined the vasorelaxing effect of SF on vascular smooth muscle using endothelium-denuded rat aortic rings. As illustrated in Fig. 3, SF induced relaxation in a dose-dependent manner for endothelium-intact and denuded vessels; however, removal of the endothelium causes a significant decrease (p < 0.01) in the vasorelaxation produced by SF from a dose of 10 µg/ml to 1 mg/ml. The relevant parameter values are: pED50 = 4.78 ± 0.08 g/ml with 95% confidence interval: 4.93–4.63 g/ml (with endothelium), 4.76 ± 0.13 with 95% confidence interval: 5.02–4.50 g/ml (without endothelium). Similarly, Rmax is 88 ± 4% for endothelium-dependent relaxation and 51 ± 3% for endothelium independent relaxation (p < 0.01). It is important to note that while endothelium-dependent relaxation is obvious, endothelial denudation did not seem to abolish the vasorelaxant effect completely. This suggests that there could be an endothelium-independent effect of SF.
Figure 3.
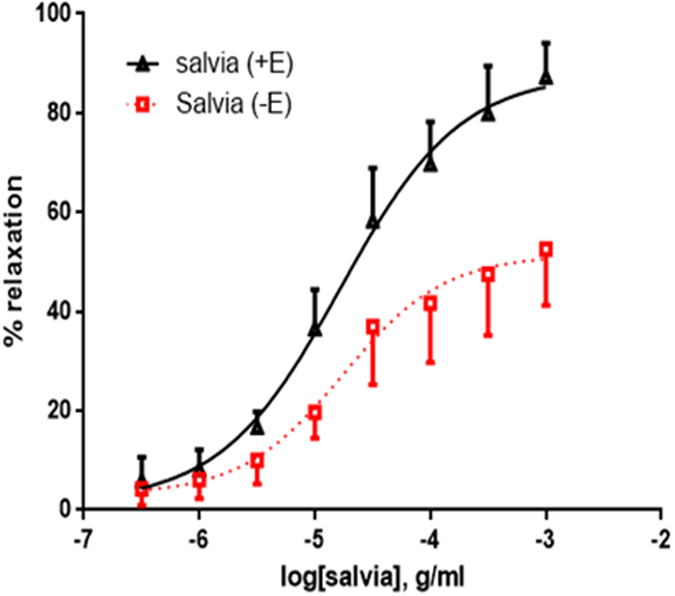
Role of endothelium in SF-induced relaxation. Cumulative dose-response curves for SF in isolated norepinephrine-precontracted rat aortic rings either with intact (+E; triangles) or denuded endothelium (−E; squares). n = 7; p < 0.01 for +E versus −E.
Aortic responses to SF in the absence and presence of L-NAME or ODQ
Next, we determined the role of nitric oxide and soluble guanylate cyclase in aortic relaxation responses to SF. The SF-induced vasorelaxation was significantly attenuated by the presence of L-NAME, an inhibitor of endothelial nitric oxide synthase (Fig. 4A). This confirms a role for NO in SF-induced aortic relaxation. The pED50 values are 4.72 ± 0.07 g/ml with 95% confidence interval: 4.86–4.58 g/ml (without L-NAME), 4.59 ± 0.09 with 95% confidence interval: 4.78–4.40 g/ml (with L-NAME). Similarly Rmax is 90 ± 4% prior to L-NAME exposure and 52 ± 3% in the presence of L-NAME (p < 0.01).
Figure 4.
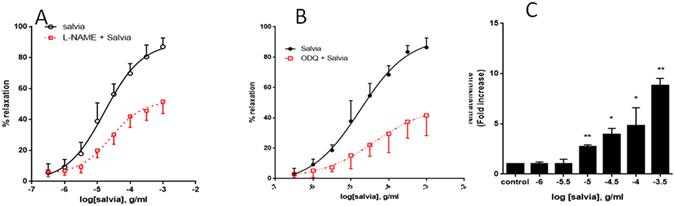
Effect of L-NAME or ODQ on SF-induced relaxation. (A) Endothelium-intact rings were incubated with cumulative doses of SF in the absence (circles) or presence of L-NAME (100 µM; squares. Data represent mean ± SEM (n = 7; p < 0.01 for Salvia versus L-Name plus Salvia). (B) Endothelium-intact rings were incubated with cumulative doses of SF in the absence (circles) or presence of ODQ (1 µM; squares). n = 7; p < 0.01 for Salvia versus ODQ plus Salvia. (C) Endothelium-intact rings were incubated with increasing doses of SF and levels of NO determined. n = 3; *denotes a p < 0.05 and **a p < 0.01 (compared to control).
In the presence of ODQ (sGC inhibitor, Fig. 4B), the SF-dependent vasorelaxation was significantly inhibited by approximately 50%, and there was no change in potency. The pED50 values = 4.76 ± 0.10 g/ml with 95% confidence interval: 4.97–4.55 g/ml (no ODQ exposure) and 4.38 ± 0.37 with 95% confidence interval: 5.14–3.63 g/ml (with ODQ) to increasing doses of SF-mediated relaxation. Similarly Rmax is 93 ± 6% for ODQ-independent relaxation and 48 ± 12% for ODQ-dependent relaxation (p < 0.01).
To further confirm the involvement of NO, rings were incubated with increasing doses of SF and the level of NO determined. As shown in Fig. 4C, SF caused a significant and dose-dependent increase in the production of nitrate/nitrite, indicative of increased NO production. This suggests that SF elicits its effects via increasing NO levels.
Effect of SF on production of cGMP in aortic rings
Since SF-induced vasorelaxation was significantly decreased by L-NAME and ODQ, these results suggested we address the question if SF modulates the level of cGMP. This nucleotide monophosphate is released from GTP by soluble guanylyl cyclase when nitric oxide docks onto its binding domain on the enzyme. Treatment with cumulative doses of SF evoked a significant and dose-dependent sigmoidal increase in the production of cGMP (pED50: 4.26 ± 0.14 g/ml; Rmax: 33 ± 5 pmol/mg protein) (Fig. 5A). In vehicle-treated rings, the cGMP content was 2.82 ± 0.87 (mean ± SEM) pmole/mg protein. However, in rings treated with 0.3 mg/ml SF, the cGMP level was 30.50 ± 3.31 (mean ± SEM) pmole/mg protein (p < 0.001).
Figure 5.
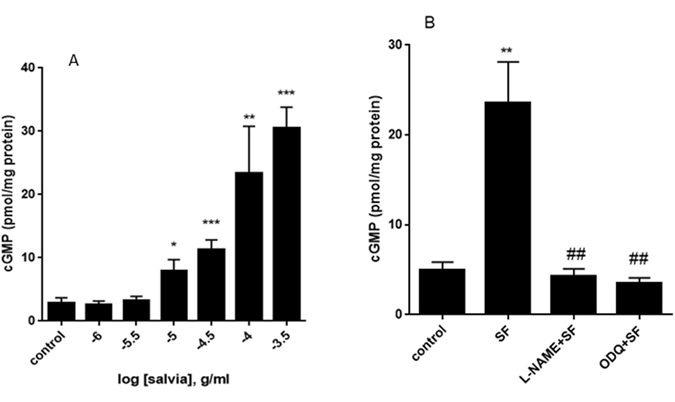
Modulation of cGMP levels by SF, L-NAME and ODQ. (A) Rings were incubated in the absence (control) or presence of increasing concentrations of SF. cGMP immunoassay followed and levels of cGMP were determined. n = 6; *denotes a p < 0.05, **a p < 0.01 and ***a p < 0.001 compared to control. (B) Rings were pretreated for 30 minutes with L-NAME (100 µM) or ODQ (1 µM) followed by SF (0.67 mg/ml) and cGMP levels determined. n = 5; **denotes a p < 0.01 compared to control; ##denotes a p < 0.01 compared to SF).
To confirm if this SF-increased cGMP accumulation is mediated by eNOS and/or sGC, rings were pre-treated with either L-NAME (100 µM) or ODQ (1 µM) followed by SF. Indeed, pretreatment with either inhibitor significantly inhibited the SF-induced cGMP accumulation (Fig. 5B). Our results also show that 8-(4-Chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate, a potent and selective activator of cGMP dependent protein kinase (PKG), evokes relaxation of aortic rings (data not shown).
Involvement of phosphoinositide 3-kinase(PI3K)/Akt (protein kinase B) signaling pathway axis in the SF-induced relaxation of aortic rings
The role of PI3K-Akt signaling axis in endothelium-dependent aortic relaxation is well-established41, 42. To determine if this signaling cascade is involved in the SF-induced relaxation, we utilized two structurally incongruent inhibitors of PI3K, wortmannin and LY294002. Indeed, pretreatment with either inhibitor caused a significant decrease in SF-induced vasorelaxation, with the maximum relaxation mitigated with no effect on potency. In control rings, pED50 was 4.72 ± 0.08 g/ml with 95% confidence interval of 4.89–4.55 g/ml. In wortmannin-preincubated rings, pED50 was 4.29 ± 0.34 with 95% confidence interval of 4.98–3.60 g/ml. On the other hand, Rmax was 90.0 ± 4.3% for wortmannin-independent relaxation and 49.1 ± 11.5% in the presence of wortmannin (p < 0.01, Fig. 6A). Similar results were obtained with LY294002 (10 µM) pre-treatment (p < 0.01, Fig. 6B). In LY294002-preincubated rings, pED50 was 4.36 ± 0.13 with 95% confidence interval of 4.64–4.08 g/ml, and Rmax was 77.4 ± 7.5% for wortmannin-independent relaxation and 44.5 ± 2.4% in the presence of LY294002.
Figure 6.
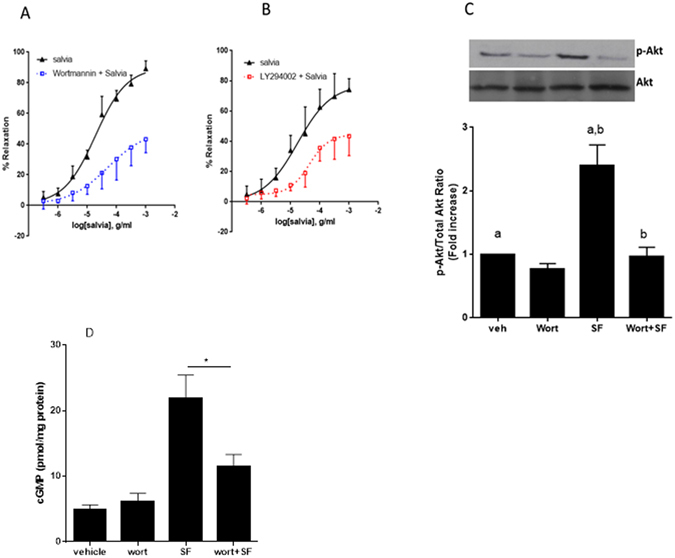
Interplay between SF and PI3K pathway. Endothelium-intact rings were incubated with cumulative doses of SF in the absence (triangles) or presence of PI3K inhibitors: (A) Wortmannin (0.1 µM; squares) or (B) LY29400 (10 µM; squares). Data represent mean ± SEM (n = 5; p < 0.01 for Salvia versus Wortmannin + Salvia or LY29400 + Salvia). (C) Modulation of Akt phosphorylation by SF. Aortic rings were incubated in either the absence (veh) or presence of wortmannin (wort) for 30 minutes. Rings were then pre-contracted with NE (3 µM) followed by treatment with SF (0.67 mg/ml) for 15 minutes. Proteins were extracted and subjected to SDS-PAGE against phosphorylated and total Akt levels. Bar graph represents normalization of the phosphorylated Akt levels to total ones. Bars with similar letters are significantly different; p < 0.05); n = 4 rings. (D) Rings were incubated with or without SF (0.67 mg/ml) after having been pre-treated without (vehicle) or with wortmannin (wort; 0.1 µM) for 30 minutes. Then, cGMP levels were determined. n = 5; *denotes a p < 0.05.
To further confirm the participation of Akt in the SF-induced vasorelaxant pathway, we determined the level of Akt phosphorylation in SF-treated rings that were pre-incubated with or without wortmannin. Indeed, SF evoked significant increase in phosphorylation of Akt which was significantly inhibited by wortmannin (n = 3; p < 0.05) (Fig. 6C). Furthermore, pre-treatment with tricirbine (10 µM), which selectively inhibits Akt but not PI3K, abolished the SF-induced relaxation (data not shown). Taken together, the above data clearly implicates activation of the PI3-K/Akt signaling axis in SF-induced aortic relaxation.
To further validate the role of PI3K/Akt cascade in SF-induced relaxation, we pre-incubated rings with wortmannin followed by SF treatment and then we assessed cGMP levels. In line with the above results, pre-treatment with wortmannin (0.1 µM) significantly abolished SF-induced cGMP accumulation (Fig. 6D)
Role of Potassium Channels in SF-induced relaxation of aortic rings
Increase in the bioavailability of NO is known to cause vasorelaxation via the activation of calcium-dependent potassium channels43. Interestingly, prior inhibition of ATP-dependent or calcium-activated potassium channels caused no effect on relaxant responses to SF. Indeed, in vehicle-treated rings, pED50 was 4.77 ± 0.07 g/ml with 95% confidence interval of 4.91–4.63 g/ml. Comparable results were obtained with the same rings pre-treated with glibenclamide, an ATP-sensitive potassium channels blocker, where pED50 was 4.69 ± 0.17 with 95% confidence interval of 5.03–4.34 g/ml. Similarly, Rmax was 93.4 ± 3.8% for inhibitor-independent relaxation and 79.4 ± 7.7% relaxation in the presence of glibenclamide (p > 0.05) (Fig. 7A). Similar results were obtained when Ca++-activated potassium channels were inhibited with tetraethylammonium, TEA (Fig. 7B). In vehicle-treated rings, pED50 was 4.78 ± 0.1 with 95% confidence interval of 4.98–4.58 g/ml, whereas TEA-pretreatment of the rings produced a pED50 value of 4.80 ± 0.15 with 95% confidence interval of 5.10–4.49 g/ml. Similarly, Rmax was 97.9 ± 5.9% for inhibitor-independent relaxation and 84.06 ± 6.8% relaxation in the presence of TEA (p > 0.05) (Fig. 7B).
Figure 7.
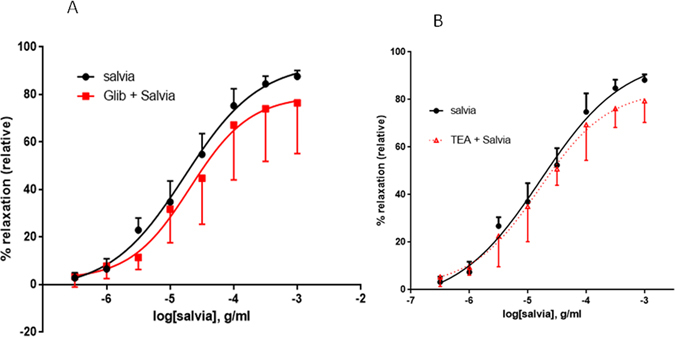
Role of potassium channels in SF-induced relaxation of aortic rings. Endothelium-intact rings were incubated with cumulative doses of SF in the absence (Salvia plus vehicle; filled circles) or presence of (A) 10 µM of Glibenclamide (Glib + Salvia; squares), or (B) Tetraethylammonium (100 µM) (TEA + Salvia; triangles). n = 6 or 5 for glibenclamide or TEA experiments, respectively. In both cases, no difference was noted between inhibitor treated and vehicle administered curves; p > 0.05 for Salvia alone versus either Glib + Salvia or Salvia + TEA.
Involvement of calcium channels
To establish if calcium channels participate in SF-induced relaxation, we exposed the aortic rings to verapamil, a L-type calcium channel blocker, and there was no significant difference between the control and treated vessels (p > 0.05) (Fig. 8). In fact, results in vehicle-treated rings (pED50 was 4.81 ± 0.15 g/ml with 95% confidence interval of 5.11–4.50 g/ml) were similar to verapamil-incubated rings (pED50 was 5.03 ± 0.28 with 95% confidence interval of 5.61–4.46 g/ml). Likewise, Rmax was 86.6 ± 7.4% or 89.5 ± 11.5% in the absence or presence of verapamil, respectively (Fig. 8).
Figure 8.
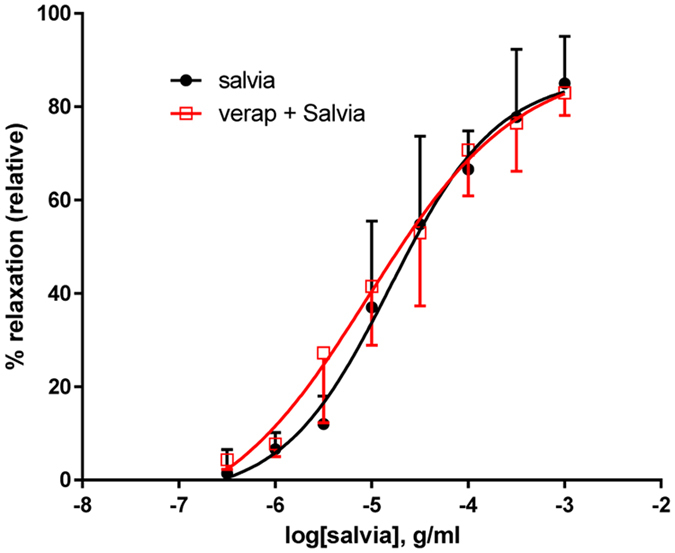
Role of Calcium Channels in SF-induced relaxation of aortic rings. Endothelium-intact rings were incubated with cumulative doses of SF in the absence (Salvia; circles) or presence of verapamil (1 µM; verap + Salvia; squares). n = 5; p > 0.05.
Involvement of cyclooxygenases
Prostanoids are known to regulate vascular tone44, 45. Here, we show that indomethacin, a non-selective cyclooxygenase blocker, did not significantly alter relaxant responses of rat aortic rings to SF (p > 0.05). Indeed, in salvia-treated rings, pED50 was 4.78 ± 0.10 g/ml with 95% confidence interval: 4.98–4.58 g/ml. And in indomethacin-treated rings, pED50 was 4.75 ± 0.11 g/ml with 95% confidence interval of 4.97–4.53 g/ml. Similarly Rmax is 89.3 ± 4.6% for untreated relaxation and 82.4 ± 4.7% for indomethacin-exposed relaxation (Fig. 9).
Figure 9.
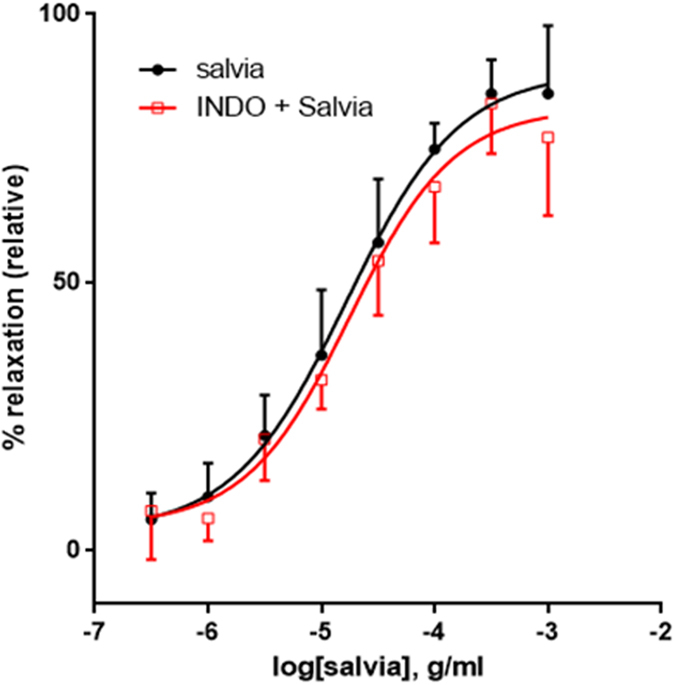
Role of cyclooxygenases in SF-induced relaxation of aortic rings. Endothelium-intact rings were incubated with cumulative doses of SF in the absence (Salvia; circles) or presence of indomethacin (10 µM; Indo + Salvia; squares). Data represent mean ± SEM (n = 5; p > 0.05).
Involvement of histaminergic or muscarinic receptors
Penultimately, we investigated the influence of pyrilamine, a blocker of histamine H1-receptors on SF-generated aortic relaxation. In rings treated with salvia alone, pED50 was 4.84 ± 0.13 g/ml with 95% confidence interval of 5.11–4.57 g/ml. These results are analogous to those obtained in the presence of pyrilamine, where pED50 was 5.06 ± 0.15 with 95% confidence interval of 5.36–4.76 g/ml, to increasing doses of SF-mediated relaxation. Similarly, Rmax is 85 ± 6% for rings treated with salvia alone, versus92 ± 6% for pyrilamine exposed ones, Fig. 10A). Pyrilamine did not have a significant effect on SF-induced relaxation (p > 0.05).
Figure 10.
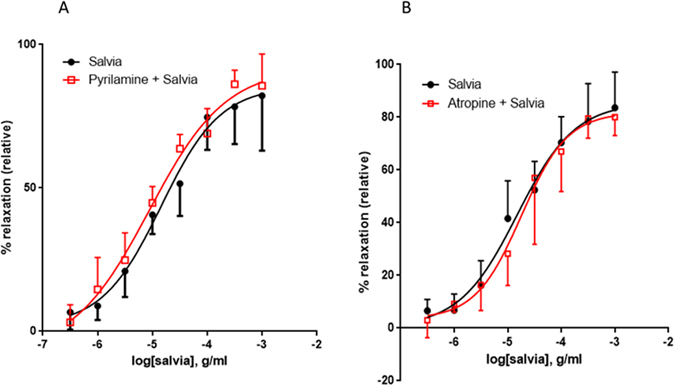
Involvement of histaminergic or muscarinic receptors in SF-induced vasorelaxation. Endothelium-intact rings were exposed to cumulative doses of SF in the absence (Salvia; circles) or presence of (A) 10 µM of pyrilamine (pyrilamine + Salvia; squares), or (B) 10 µM of atropine (atropine + Salvia; squares). Data represent mean ± SEM (n = 6 or 5 for pyrilamine or atropine experiments, respectively). p > 0.05 for Salvia alone versus either pyrilamine + Salvia or Salvia + atropine.
Lastly, we also determined the effect of atropine, a natural alkaloid that acts as a non-selective blocker of muscarinic receptors. In control rings, pED50 value was 4.82 ± 0.14 g/ml with 95% confidence interval of 5.11–4.54 g/ml. By comparison, in atropine-treated rings, pED50 was 4.75 ± 0.13 with 95% confidence interval of 5.01–4.49 g/ml to increasing doses of SF-mediated relaxation. Similarly, Rmax is 86 ± 6% for inhibitor-independent relaxation and 82 ± 6% for atropine-treated relaxation, and magnitude of maximal relaxation is similar in both groups, Fig. 10B). Therefore, atropine does not have any significant effect on SF-induced relaxation (p > 0.05).
Discussion
The objective of the current experiments was to determine the pharmacological effects of ethanolic extract of Salvia fruticosa Mill. leaves on rat isolated thoracic aorta. From these observations, the salient feature of our investigation points to the key role of the PI3K/Akt, endothelial nitric oxide synthase, nitric oxide, soluble guanylyl cyclase and cGMP pathway (Fig. 11). To our knowledge, this is the first investigation to examine the detailed effects of extracts from any Salvia species activating the PI3K through to cGMP pathway. Additionally,our results show that the dose-dependent relaxation responses to S. fruticosa Mill. are contingent on endothelial cells. This is reflected by the observations that relaxation was significantly diminished both upon denudation of luminal endothelial layer and by inhibition of eNOS by L-NAME.
Figure 11.
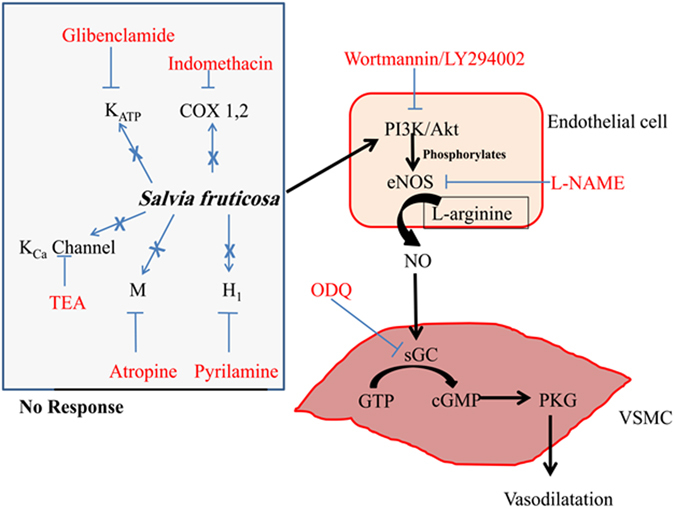
Schematic representation of the mechanism of Salvia fruticosa-induced relaxation of rat isolated aorta. The diagram displays the central role of nitric oxide, and its upstream activators and downstream effectors. Pharmacologic inhibitors are shown in red font color. Blue crosses reveal no involvement in the mechanism of SF-induced relaxation. The pathways for SF mechanism of action are drawn in black arrows.
In native, healthy endothelia, there is a choreographed balance between the actions of vasoconstrictors (pro-inflammatory) and vasodilators (anti-inflammatory). When this equilibrium is shifted, a derangement in vascular homeostasis ensues. The vascular dysfunction of the arterial tree is a prelude to hypertension. Ample evidence exists to support the notion of vascular abnormalities (functional, mechanical and structural – vascular remodeling) in hypertension affecting not only small vessels, but also large calibre arteries5, 6, 46–48. Moreover, the homeostatic disturbance results in augmentation of vascular tone, activation of inflammatory responses, proliferation of VSMCs, and eventually precipitates an atherothrombogenic milieu9, 49, 50. Hence, a healthy life-style, embracing the intake of protective nutrients, such as S. fruticosa Mill., is imperative to ensure that endothelial cell homeostasis is optimally maintained.
The endothelium plays a critical role in determining vasotone, and receptors located on the endothelial surface are primary targets for initiating vasodilatory effects51 Activation of muscarinic M3 subtype52 and histaminergic H153 receptors will induce relaxation of blood vessels. From our observations, we can conclude that SF–induced aortic relaxation is not mediated by muscarinic or histaminergic receptors. Accordingly, these results are consistent with a previous work showing their lack of involvement in relaxant effect of Danshen on rat isolated femoral arteries54.
Stimulation of the PI3K/Akt signaling pathway axis triggers a network of multiple outcomes. Accumulated evidence shows PI3K phosphorylates Akt at Ser473 causing the kinase to be activated, and to mediate downstream signaling processes associated with cell growth and migration44, 55–58, but has also been implicated in endothelium-dependent relaxation41, 42. More pertinently, eNOS has been found to be activated via the PI3K/Akt pathway by natural compounds like epigallocatechin-3-gallate59. Recently, in an ischemia/reperfusion injury model, it was found that ginsenosides, the major bioactive components of species panax ginseng, increase coronary arterial flow through stimulation of PI3K/Akt/eNOS mechanistic route. This demonstrates that the application of isolated extracts of ginseng bestowed protection against myocardial damage60. Similarly, in another study, activation of PI3K/Akt/NO pathway by the principle metabolite of ginsenosides evoked cardioprotection in myocardial ischemia-reperfusion injury61.
Stimulation of Akt enhances the activity of eNOS by phosphorylating serine at 117762, 63. This, in turn, triggers increased release of nitric oxide (NO)62–64. NO then diffuses from the endothelial layer to the VSMCs wherein it activates the soluble guanylyl cyclase (sGC). Active sGC catalyzes the subsequent conversion of GTP to cGMP, which phosphorylates protein kinase G (PKG), a major intermediary in the vasorelaxation process45. Evidently, the results of our SF-induced aortic relaxation and dissection of its endothelium-reliant mechanistic route complements the aforementioned signaling mechanism through the PI3K/Akt/eNOS/NO/cGMP pathway. Notably, of all the Salvia species examined to date, this is the first study to show vasorelaxant effect from PI3K to cGMP in healthy normotensive vessels. In a corresponding manner, our results are in accord with a previous report demonstrating that extracts from the species Salvia miltiorrhiza (the dried root of which is commonly known as danshen) evoked endothelium-dependent relaxation that was reliant on NO and cGMP in rat aortic helical strips21. Conversely, endothelium-independent relaxation of rat coronary arteries responding to an aqueous extract of danshen has also been reported65.
Nitric oxide, a gaso-transmitter, is not only a potent vasodilator66–68, but also demonstrates antagonistic proliferative and thrombogenic behavior69. This endogenous intervention ensures vasculoprotection of the circulatory network by promoting homeostatic blood flow. In contrast, diminished NO bioavailability leads to a derangement in vasodilatory control, resulting in the onset and progression of hypertension as well as atherosclerosis among other vascular pathologies49.
Potassium channels play a critical role in regulating plasma membrane potential, and hence determining vascular tone70, 71. Increased bioavailability of nitric oxide is known to activate calcium-gated potassium channels43, which induce vasorelaxation by a mechanism involving hyperpolarization72. Our results suggest that neither Ca2+-dependent nor ATP-driven potassium channels are involved in SF-induced aortic relaxation. On the other hand, tanshinone IIA (a lipophilic diterpene quinone isolated from danshen) activates ATP-dependent potassium channels to decrease intracellular calcium concentration in rat aortic smooth muscle cells, and leading to relaxation73. Similarly, by using a 1000-fold higher concentration of TEA (100 mM) than that administered in the present study (100 µM), other researchers have demonstrated that danshen-induced participation of TEA-responsive potassium channels in endothelium-independent relaxation of rat coronary arteries65. These examples clearly illustrate the dichotomy in the members of genus Salvia, possibly due to differences in phytochemical profiles. Interestingly, ginsenoside-Re, a constituent of Panax ginseng, activates PI3K/Akt and NO signaling network to promote the opening of Ca2+-stimulated potassium channels in aortic smooth muscle cells74. The discrepancy that exists between our results and those outlined from the three different groups of investigators may be explained by highlighting the interplay of multiple contributory factors. Namely, disparity in Genus (Salvia versus Panax), dissimilarity in Saliva species (fruticosa versus miltiorrhiza), as well as the differences in parts of the plant (root versus leaves), each of these attributes will likely yield a highly variable phytochemical fingerprint. In this regard, some phytochemicals are exclusive to danshen (such as lipophilic tanshinones), whereas other constituents may share a commonality between the botanicals. An important point to take into account is that the concentration of each component will be highly variable due to location of harvesting, soil conditions, weather and time of harvest (season). In addition, there are methodological differences, for instance the applied concentration of antagonists (as is the case with TEA discussed above), extraction solvent and concentration of the herbal extract, and location of segment of vessel acquired from the arterial network (aorta versus coronary artery) will all contribute to disparity between the results.
Our data reveal that Ca2+ channels do not contribute to SF-induced relaxation of rat aortas. Interestingly, the blockage of calcium channels by danshen induces endothelium-independent relaxant activity in rat coronary arteries65. While this may appear to conflict with our study, it is important to note that different vascular beds behave differently even to the same stimulus75, 76. This apparent difference in response may also be explained by the points discussed above.
Cyclooxygenases (isoforms COX-1 and COX-2) are crucial enzymes in the conversion of arachidonic acid to biologically active prostanoids. This group of lipophilic compounds is known to play multiple roles in vascular biology, including vasodilation67, 77. One such potent vasodilator released by the endothelium is prostacyclin (PGI2), which acts via its corresponding G-protein coupled hepta-helical transmembrane domain receptors (IP)78. As a matter of fact, in the present investigation, the cyclooxygenase pathway does not appear to participate in SF-mediated relaxant activity. This concurs with an earlier report of a non-contributory function of prostaglandins to danshen-induced relaxant responses in rat femoral arteries54. This is not surprising as both herbs, danshen79 and SF37, are characterized by the presence of rosmarinic acid. More to the point, rosmarinic acid is a non-selective inhibitor of the enzyme isoforms COX-1 and COX-280. Furthermore, the authors highlighted the property of inhibitory activity being comparable to the non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, ibuprofen and naproxen80. Apparently, SF will be unable to recruit the prostanoid metabolites for regulation of vascular tone.
The molecular mechanism of action for SF in rat aorta is summarized in Fig. 11. Collectively, our investigation supports the health orientated benefits of SF as a vasodilator. All blood vessels contribute to the overall systemic resistance and consequent blood pressure. The aorta itself affects and is affected by changes in blood pressure. However, it is the resistance vessels that impart the biggest effect on vascular resistance. Therefore, the present studies are to be extended to resistance-size arteries (<300 µm internal diameter). If the results from these microvessels mirrored those obtained from aortic vessels, then this would confirm the potential for SF as a regulator for blood pressure. These studies may underscore and provide concrete confirmation for what we have observed in a large conduit vessel; and raison d’etre for its inclusion in ethnomedicine cabinet pertaining to inhabitants of the Levant. As such, these future investigations could substantiate the notion of Salvia fruticosa Mill. as an anti-hypertensive agent.
Materials and Methods
Preparation of the extract
Leaves of Salvia fruticosa Mill. were harvested from Yanouh area (East of Tyre, Lebanon) (GPS coordinates: 33°15′52 N and 35°17′53 E). No specific permission is required for collecting this plant or for using the land where it is located. This plant is neither an endangered nor a protected species; it is readily and commercially available in the market.
The SF extract was prepared as in our recent publications of two other herbs16, 81, 82. Leaves were rinsed in distilled water to remove any dust or dirt and air-dried in the dark at room temperature. After grinding the leaves with a mortar and pestle, the powder was extracted three times in 70% aqueous ethanol and the mixture kept in the dark for 72 hours at 4 degrees without stirring. The mixture was then filtered through a sintered glass funnel and the filtrate evaporated to dryness using rotary evaporator at room temperature. The obtained residue was collected and kept at −20 °C until further use, when it would be dissolved in aqueous ethanol.
Preparation of rat isolated thoracic aortae
Ethics Statement
All procedures used within the present study were performed in strict compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Institutional approval was obtained for all protocols and procedures (Scientific Committee in the Faculty of Public Health in the Lebanese University; (Permit number for Samaha Salvia Project: UL/FSPIV/07/2011)). All efforts were made to minimize suffering.
Animals
Experiments in this study were conducted on male Sprague-Dawley rats weighing 230–290 gm. Animals were kept under a 12:12 hours light: dark cycle at a temperature of 23 ± 2 °C and had access to food and water ad libitum.
Drugs and Chemicals
Norepinephrine, 3-isobutyl-1-methylxanthine (IBMX), acetylcholine, Nω-nitro-L-arginine methyl ester (L-NAME), atropine, pyrilamine, verapamil, indomethacin, tetraethylammonium (TEA), 1H-[1,2,4]oxadiazolo[4,3-alpha]quinoxalin-1-one (ODQ) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Wortmannin and LY294002 were obtained from Alexis Biochemicals (USA) and Tocris (USA), respectively.
Quantification of NO production
As we recently described82, NO production was determined by calculating the amount of nitrate/nitrite produced based on the Griess reaction. A colorimetric assay kit (Cayman Chemicals, Michigan, USA) was used as per the manufacturer’s instructions83. Briefly aortic homogenates and Griess reagents were mixed and incubated at room temperature for 10 min and the absorbance was measured at 540 nm wavelength. The amount of nitrite formed was normalized to the protein content of the respective aortic rings. A standard curve was constructed using known concentrations of sodium nitrite. The absorbance value of blank wells were subtracted from the values of the wells with the rings.
Preparation of Aortic Rings and Tension Measurement
On the day of experimentation, the animals were euthanized by an overdose of phenobarbital (50 mg/kg body weight) and the thoracic aorta was rapidly dissected and isolated, and placed in cold (4 °C) modified Krebs-Henseleit buffer (KHB) pre-equilibrated with 5% CO2 in oxygen. The aorta was carefully cleaned of adhering connective and fat tissues, and cut into ring segments of 2 to 3 mm in length. Each aortic ring was suspended by a pair of stainless steel hook (stationary) and stirrup (attached to isometric force transducer) in a pre-warmed (37 ± 2 °C) organ bath chamber containing a modified Krebs-Henseleit buffer (KHB, pH 7.4, 37 ± 2 °C) with the composition previously described84 (mM): NaCl (130), Mg2SO4 ·7H2O (1.17), NaHCO3 (14.9), KCl (4.7), KH2PO4 (1.18), CaCl2 ·2H2O (1.6) and glucose (5.5). This solution was aerated with 95% O2 and 5% CO2 to maintain the pH of KHB at 7.4. During the initial equilibration period of 1 hr, the rings were subjected to isometric resting tension of 2 g in a step-wise manner as previously described85. Aortic ring tension responses were recorded by a computerized data acquisition system (AD Instruments, UK) and Chart software (AD Instruments, UK). Subsequently, the vessel integrity was determined by application of 3 µM NE, followed by 2 exposures to 80 mM KCl, with thorough washes with KHB in between each activation of the aorta. If the tension recordings to the two KCl challenges were similar, the protocols below were followed.
Experimental protocols
Endothelium-intact aortic rings were precontracted with norepinephrine (NE) (3 µM). Cumulative concentrations (0.3 µg/ml to 1 mg/ml, at half-log doses) of SF were added to determine the rings’ reactivity. At each dose of SF, the curve was allowed to reach a plateau before the addition of subsequent dose. To ensure that integrity of the endothelium was maintained, vascular relaxant response to Ach (10 µM) was assessed in each vessel following precontraction with NE (>80% relaxation of NE-dependent contraction). When the involvement of the endothelium in the observed relaxation was desired, vessels were denuded of the endothelial layer. Failure of Ach to produce a vasorelaxant response greater than 25% was assumed as an indication of effective endothelium denudation. Cumulative dose response curves (0.3 µg/ml to 1 mg/ml, at half-log doses) of SF were then determined in these endothelial-stripped rings.
The concentrations of inhibitors/antagonists used in the proceeding experiments have already been described previously86, each blocker was incubated for 30 min as appropriate, and followed SF’s ability to relax aortic segments. The solvents used for dissolving the blockers are indicated below (following each inhibitor). None of the vehicles/solvents used has any vasorelaxing effects on its own at all the doses used.
To determine the role of NO, rings with intact endothelium were pretreated with L-NAME (100 μM), a nitric oxide (NO) synthase inhibitor, for 30 minutes. Then, aortic rings were contracted with NE, followed by treatment with SF.
To investigate the role of guanylate cyclase, prostaglandins, potassium channels, muscarinic receptors, histamine H1-receptors or calcium channels in SF-induced relaxation, the following inhibitors were used: oxadiazole quinoxalin (ODQ, 10 µM), a soluble guanylate cyclase inhibitor; Indomethacin (10 µM), a non-selective cyclooxygenase inhibitor; Glibenclamide (10 µM), an ATP-sensitive potassium channels blocker, or Tetraethylammonium (100 µM), a non-selective inhibitor of Ca++-activated potassium channels; pyrilamine (10 μM), a histamine H1-receptor antagonist or atropine (10 μM), a muscarinic receptor antagonist; and Verapamil hydrochloride (1 µM), a L-type Calcium channel blocker.
The role of the PI3K/Akt signaling was assessed by pre-incubating the rings with wortmannin (100 nM) or LY294002 (10 µM) for 30 minutes. This was followed by cumulative dose response curve for treatments with SF.
To validate that no significant irreversible or residual effect on vascular responsiveness to SF extract has taken place, the aortic rings that had been exposed to cumulative concentrations of SF were washed for 45 to 60 minutes, followed by new exposure to NE (3 μM) and Ach (10 μM). Finally, the rings were challenged again with KCl (80 mM) to confirm the viability of the vessels.
cGMP assay
Pre-equilibrated rings were incubated in 3-isobutyl-1-methylxanthine (IBMX, a competitive nonselective phosphodiesterase inhibitor; 0.1 mM) for 30 minutes before addition of NE. Vessels were then allowed to equilibrate for an additional 30 minutes before addition of SF. The reaction was then quickly stopped by freezing the tissues in liquid nitrogen. After homogenization in trichloroacetic acid, samples were centrifuged at 10000 × g for 10 min and the supernatant was extracted five times with water-saturated diethylether. The cGMP and protein content in the extract were determined by a specific immunoassay (Amersham Biosciences (now GE Healthcare Life Sciences), USA) and by the use of Bradford’s method, respectively. Results are expressed as pmoles of cGMP per milligram of protein.
Western blotting
Rings were homogenized in a sample buffer containing 2% SDS and 60 mM Tris ⋅ HCl (pH = 6.8) at room temperature. After clearing the homogenate by centrifugation (14,000 g, 10 min, 4 °C), the supernatant was collected. Protein concentrations were determined using the bincinchoninic acid assay (Pierce). Equal amounts of proteins were loaded and resolved on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinyldifluoride membrane (Millipore). After blocking with 10% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20, membranes were washed then incubated with primary antibodies for total or phosphorylated Akt (Cell Signaling, USA) at 4 °C, overnight. The membrane was then washed three times and incubated with HRP-conjugated secondary antibody for 1 hour. After extensive washings, blots were developed using enhanced chemiluminescence (Amersham Biosciences, USA).
Statistical analysis
Data are presented as means ± SEM. Relaxations to SF are expressed as a percentage decrease of the NE contraction. Each cumulative dose-effect curve for SF-induced relaxation was plotted, contingent on application of sigmoidal curve fitting and non-linear regression, using Prism version 6 (GraphPad Software Inc., San Diego, CA., U.S.A.) to generate the parameters Rmax (maximal relaxant response) and pED50 (the negative logarithm of SF dose required to give 50% of the maximum aortic-relaxant response, Rmax). Statistical analysis of the data was performed by Student’s t-test. When more than two means were compared, Analysis of Variance (ANOVA) was used: either a one-way ANOVA with Tukey’s post hoc test (data represented as bar graphs) or a two-way ANOVA with Sidak’s multiple comparison post hoc test (data represented as sigmoidal curves) (Prism, GraphPad Software, San Diego, CA). A p value of less than 0.05 was considered statistically significant.
Acknowledgements
The authors would like to thank Dr. Ali Al-Khatib (Lebanese International University, Beirut Lebanon) for his help in the identification/classification of the plant.
Author Contributions
A.H.E. conceived of the project. A.H.E. and R.I. designed the experiments. M.A.A. helped in the design of some experiments. M.A.A., A.I.S., R.I. and A.H.E. performed data analysis. A.A.S. and S.B. performed the experiments. M.A.A., A.I.S. and A.H.E. wrote the manuscript. All authors read and approved the submitted version.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
M. Akhtar Anwar and Ali A. Samaha contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rabah Iratni, Email: r_iratni@uaeu.ac.ae.
Ali H. Eid, Email: ae81@aub.edu.lb
References
- 1.Laslett LJ, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60:S1–49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global status report on noncommunicable diseases 2010. http://apps.who.int/iris/handle/10665/44579 (2011).
- 3.August P. Overview: mechanisms of hypertension: cells, hormones, and the kidney. J Am Soc Nephrol. 2004;15:1971–1973. doi: 10.1097/01.ASN.0000133197.23478.76. [DOI] [PubMed] [Google Scholar]
- 4.Frishman WH, Beravol P, Carosella C. Alternative and complementary medicine for preventing and treating cardiovascular disease. Dis Mon. 2009;55:121–192. doi: 10.1016/j.disamonth.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Anwar MA, Al Disi SS, Eid AH. Anti-Hypertensive Herbs and Their Mechanisms of Action: Part II. Front Pharmacol. 2016;7:50. doi: 10.3389/fphar.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Disi SS, Anwar MA, Eid AH. Anti-hypertensive Herbs and their Mechanisms of Action: Part I. Front Pharmacol. 2015;6:323. doi: 10.3389/fphar.2015.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winslow LC, Kroll DJ. Herbs as medicines. Arch Intern Med. 1998;158:2192–2199. doi: 10.1001/archinte.158.20.2192. [DOI] [PubMed] [Google Scholar]
- 8.Kleiner SM. The True Nature of Herbs. Physician Sportsmed. 1995;23:13–14. doi: 10.1080/00913847.1995.11947846. [DOI] [PubMed] [Google Scholar]
- 9.Heber D. Herbs and atherosclerosis. Curr Atheroscler Rep. 2001;3:93–96. doi: 10.1007/s11883-001-0016-9. [DOI] [PubMed] [Google Scholar]
- 10.Shouk R, Abdou A, Shetty K, Sarkar D, Eid AH. Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr Res. 2014;34:106–115. doi: 10.1016/j.nutres.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Yin J, Zhang H, Ye J. Traditional chinese medicine in treatment of metabolic syndrome. Endocrine, metabolic & immune disorders drug targets. 2008;8:99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jungbauer, A. & Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas71, 227–239, doi:10.1016/j.maturitas.2011.12.009. [DOI] [PubMed]
- 13.Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. Journal of ethnopharmacology. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Sung B, Prasad S, Yadav VR, Lavasanifar A, Aggarwal BB. Cancer and diet: How are they related? Free radical research. 2011;45:864–879. doi: 10.3109/10715762.2011.582869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitagishi Y, Kobayashi M, Matsuda S. Protection against Cancer with Medicinal Herbs via Activation of Tumor Suppressor. Journal of oncology. 2012;2012:236530. doi: 10.1155/2012/236530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Dhaheri Y, et al. Anti-metastatic and anti-tumor growth effects of Origanum majorana on highly metastatic human breast cancer cells: inhibition of NFkappaB signaling and reduction of nitric oxide production. PLoS One. 2013;8:e68808. doi: 10.1371/journal.pone.0068808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imanshahidi M, Hosseinzadeh H. The pharmacological effects of Salvia species on the central nervous system. Phytotherapy research: PTR. 2006;20:427–437. doi: 10.1002/ptr.1898. [DOI] [PubMed] [Google Scholar]
- 18.Perry E, Howes MJ. Medicinal plants and dementia therapy: herbal hopes for brain aging? CNS neuroscience & therapeutics. 2011;17:683–698. doi: 10.1111/j.1755-5949.2010.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Jo D-G, Park D, Chung HY, Mattson MP. Adaptive Cellular Stress Pathways as Therapeutic Targets of Dietary Phytochemicals: Focus on the Nervous System. Pharmacological Reviews. 2014;66:815–868. doi: 10.1124/pr.113.007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Kamata K, Iizuka T, Nagai M, Kasuya Y. Endothelium-dependent vasodilator effects of the extract from Salviae Miltiorrhizae radix. A study on the identification of lithospermic acid B in the extracts. Gen Pharmacol. 1993;24:977–981. doi: 10.1016/0306-3623(93)90176-X. [DOI] [PubMed] [Google Scholar]
- 22.Lin TH, Hsieh CL. Pharmacological effects of Salvia miltiorrhiza (Danshen) on cerebral infarction. Chin Med. 2010;5:22. doi: 10.1186/1749-8546-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulubelen A, et al. Cardioactive diterpenes from the roots of Salvia eriophora. Planta medica. 2002;68:818–821. doi: 10.1055/s-2002-34408. [DOI] [PubMed] [Google Scholar]
- 24.Ulubelen A, Oksuz S, Kolak U, Birman H, Voelter W. Cardioactive terpenoids and a new rearranged diterpene from Salvia syriaca. Planta medica. 2000;66:627–629. doi: 10.1055/s-2000-8629. [DOI] [PubMed] [Google Scholar]
- 25.Trichopoulou A, Critselis E. Mediterranean diet and longevity. Eur J Cancer Prev. 2004;13:453–456. doi: 10.1097/00008469-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Gali-Muhtasib H, Hilan C, Khater C. Traditional uses of Salvia libanotica (East Mediterranean sage) and the effects of its essential oils. J Ethnopharmacol. 2000;71:513–520. doi: 10.1016/S0378-8741(99)00190-7. [DOI] [PubMed] [Google Scholar]
- 27.Clebsch, B. The New Book of Salvias: Sages for Every Garden. Second edn (Timber Press, 2008).
- 28.Kaileh M, Vanden Berghe W, Boone E, Essawi T, Haegeman G. Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity. Journal of ethnopharmacology. 2007;113:510–516. doi: 10.1016/j.jep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Al-Mustafa AH, Al-Thunibat OY. Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pakistan journal of biological sciences: PJBS. 2008;11:351–358. doi: 10.3923/pjbs.2008.351.358. [DOI] [PubMed] [Google Scholar]
- 30.Senol FS, et al. Evaluation of cholinesterase inhibitory and antioxidant activities of wild and cultivated samples of sage (Salvia fruticosa) by activity-guided fractionation. Journal of medicinal food. 2011;14:1476–1483. doi: 10.1089/jmf.2010.0158. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Dahab R, Afifi F, Kasabri V, Majdalawi L, Naffa R. Comparison of the antiproliferative activity of crude ethanol extracts of nine salvia species grown in Jordan against breast cancer cell line models. Pharmacognosy magazine. 2012;8:319–324. doi: 10.4103/0973-1296.103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todorov S, et al. Experimental pharmacological study of three species from genus Salvia. Acta physiologica et pharmacologica Bulgarica. 1984;10:13–20. [PubMed] [Google Scholar]
- 33.Duke, J. Handbook of Medicinal Herbs. Second edn (CRC Press, 2002).
- 34.Yoney A, Prieto JM, Lardos A, Heinrich M. Ethnopharmacy of Turkish-speaking Cypriots in Greater London. Phytother Res. 2010;24:731–740. doi: 10.1002/ptr.3012. [DOI] [PubMed] [Google Scholar]
- 35.Karousou R, Deirmentzoglou S. The herbal market of Cyprus: traditional links and cultural exchanges. J Ethnopharmacol. 2011;133:191–203. doi: 10.1016/j.jep.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Tejero MR, et al. Medicinal plants in the Mediterranean area: synthesis of the results of the project Rubia. J Ethnopharmacol. 2008;116:341–357. doi: 10.1016/j.jep.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 37.Cvetkovikj I, et al. Polyphenolic characterization and chromatographic methods for fast assessment of culinary Salvia species from South East Europe. J Chromatogr A. 2013;1282:38–45. doi: 10.1016/j.chroma.2012.12.068. [DOI] [PubMed] [Google Scholar]
- 38.Boukhary R, Raafat K, Ghoneim AI, Aboul-Ela M, El-Lakany A. Anti-Inflammatory and Antioxidant Activities of Salvia fruticosa: An HPLC Determination of Phenolic Contents. Evid Based Complement Alternat Med. 2016;2016:7178105. doi: 10.1155/2016/7178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ersoy S, et al. Endothelium-dependent induction of vasorelaxation by Melissa officinalis L. ssp. officinalis in rat isolated thoracic aorta. Phytomedicine. 2008;15:1087–1092. doi: 10.1016/j.phymed.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Karthik D, Viswanathan P, Anuradha CV. Administration of rosmarinic acid reduces cardiopathology and blood pressure through inhibition of p22phox NADPH oxidase in fructose-fed hypertensive rats. J Cardiovasc Pharmacol. 2011;58:514–521. doi: 10.1097/FJC.0b013e31822c265d. [DOI] [PubMed] [Google Scholar]
- 41.Isenovi E, et al. Phosphatidylinositol 3-kinase may mediate isoproterenol-induced vascular relaxation in part through nitric oxide production. Metabolism. 2002;51:380–386. doi: 10.1053/meta.2002.30525. [DOI] [PubMed] [Google Scholar]
- 42.Gu Y, Tang X, Xie L, Meng G, Ji Y. Aliskiren improves endothelium-dependent relaxation of thoracic aorta by activating PI3K/Akt/eNOS signal pathway in SHR. Clin Exp Pharmacol Physiol. 2016;43:450–458. doi: 10.1111/1440-1681.12550. [DOI] [PubMed] [Google Scholar]
- 43.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 44.Chen HF, Xie LD, Xu CS. The signal transduction pathways of heat shock protein 27 phosphorylation in vascular smooth muscle cells. Molecular and cellular biochemistry. 2010;333:49–56. doi: 10.1007/s11010-009-0203-5. [DOI] [PubMed] [Google Scholar]
- 45.Lucas KA, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 46.Park JB, Schiffrin EL. Effects of antihypertensive therapy on hypertensive vascular disease. Curr Hypertens Rep. 2000;2:280–288. doi: 10.1007/s11906-000-0011-5. [DOI] [PubMed] [Google Scholar]
- 47.Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007–1021. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 48.Anwar MA, Saleh AI, Al Olabi R, Al Shehabi TS, Eid AH. Glucocorticoid-induced fetal origins of adult hypertension: Association with epigenetic events. Vascul Pharmacol. 2016 doi: 10.1016/j.vph.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- 50.Zhang F, Ren X, Zhao M, Zhou B, Han Y. Angiotensin-(1-7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci Rep. 2016;6:34621. doi: 10.1038/srep34621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busse R, Trogisch G, Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985;80:475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- 52.Walch L, Brink C, Norel X. The muscarinic receptor subtypes in human blood vessels. Therapie. 2001;56:223–226. [PubMed] [Google Scholar]
- 53.Carrier GO, White RE, Kirby ML. Histamine-induced relaxation of rat aorta. Importance of H1 receptor and vascular endothelium. Blood Vessels. 1984;21:180–183. [PubMed] [Google Scholar]
- 54.Lam FF, Yeung JH, Cheung JH. Mechanisms of the dilator action of Danshen (Salvia miltiorrhiza) on rat isolated femoral artery. J Cardiovasc Pharmacol. 2005;46:361–368. doi: 10.1097/01.fjc.0000175439.94906.e9. [DOI] [PubMed] [Google Scholar]
- 55.Hu ZW, Shi XY, Lin RZ, Hoffman BB. Alpha1 adrenergic receptors activate phosphatidylinositol 3-kinase in human vascular smooth muscle cells. Role in mitogenesis. The Journal of biological chemistry. 1996;271:8977–8982. doi: 10.1074/jbc.271.15.8977. [DOI] [PubMed] [Google Scholar]
- 56.Goncharova EA, et al. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. American journal of physiology. Lung cellular and molecular physiology. 2002;283:L354–363. doi: 10.1152/ajplung.00010.2002. [DOI] [PubMed] [Google Scholar]
- 57.Isenovic ER, et al. Role of PI3K/AKT, cPLA2 and ERK1/2 signaling pathways in insulin regulation of vascular smooth muscle cells proliferation. Cardiovascular & hematological disorders drug targets. 2009;9:172–180. doi: 10.2174/187152909789007034. [DOI] [PubMed] [Google Scholar]
- 58.Pantan R, Tocharus J, Phatsara M, Suksamrarn A, Tocharus C. Synergistic effect of atorvastatin and cyanidin-3-glucoside against angiotensin II-mediated vascular smooth muscle cell proliferation and migration through MAPK and PI3K/Akt pathways. Arch Pharm Res. 2016 doi: 10.1007/s12272-016-0836-3. [DOI] [PubMed] [Google Scholar]
- 59.Lorenz M, et al. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. The Journal of biological chemistry. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 60.Yi XQ, et al. Total ginsenosides increase coronary perfusion flow in isolated rat hearts through activation of PI3K/Akt-eNOS signaling. Phytomedicine. 2010;17:1006–1015. doi: 10.1016/j.phymed.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Tsutsumi YM, et al. Compound K, a metabolite of ginsenosides, induces cardiac protection mediated nitric oxide via Akt/PI3K pathway. Life sciences. 2011;88:725–729. doi: 10.1016/j.lfs.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 63.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho DH, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) gamma-dependent and PPARgamma-independent signaling pathways. The Journal of biological chemistry. 2004;279:2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- 65.Lam FF, Yeung JH, Chan KM, Or PM. Relaxant effects of danshen aqueous extract and its constituent danshensu on rat coronary artery are mediated by inhibition of calcium channels. Vascular pharmacology. 2007;46:271–277. doi: 10.1016/j.vph.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 66.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 67.Gerber RT, Anwar MA, Poston L. Enhanced acetylcholine induced relaxation in small mesenteric arteries from pregnant rats: an important role for endothelium-derived hyperpolarizing factor (EDHF) British journal of pharmacology. 1998;125:455–460. doi: 10.1038/sj.bjp.0702099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anwar MA, Ford WR, Herbert AA, Broadley KJ. Signal transduction and modulating pathways in tryptamine-evoked vasopressor responses of the rat isolated perfused mesenteric bed. Vascular pharmacology. 2013;58:140–149. doi: 10.1016/j.vph.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michel T, Vanhoutte P. Cellular signaling and NO production. Pflugers Arch - Eur J Physiol. 2010;459:807–816. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.HYP.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sobey CG. Potassium channel function in vascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:28–38. doi: 10.1161/01.ATV.21.1.28. [DOI] [PubMed] [Google Scholar]
- 72.Archer SL, et al. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan P, Liu IM, Li YX, Yu WJ, Cheng JT. Antihypertension Induced by Tanshinone IIA Isolated from the Roots of Salvia miltiorrhiza. Evidence-based complementary and alternative medicine: eCAM. 2011;2011:392627. doi: 10.1093/ecam/nep056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakaya Y, et al. The phytoestrogen ginsensoside Re activates potassium channels of vascular smooth muscle cells through PI3K/Akt and nitric oxide pathways. The journal of medical investigation: JMI. 2007;54:381–384. doi: 10.2152/jmi.54.381. [DOI] [PubMed] [Google Scholar]
- 75.Archer SL. Diversity of phenotype and function of vascular smooth muscle cells. J Lab Clin Med. 1996;127:524–529. doi: 10.1016/S0022-2143(96)90142-0. [DOI] [PubMed] [Google Scholar]
- 76.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiological reviews. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 78.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 79.Cao J, et al. Determination of fifteen bioactive components in Radix et Rhizoma Salviae Miltiorrhizae by high-performance liquid chromatography with ultraviolet and mass spectrometric detection. Biomed Chromatogr. 2008;22:164–172. doi: 10.1002/bmc.911. [DOI] [PubMed] [Google Scholar]
- 80.Kelm MA, Nair MG, Strasburg GM, DeWitt DL. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- 81.Al Dhaheri Y, et al. Mitotic arrest and apoptosis in breast cancer cells induced by Origanum majorana extract: upregulation of TNF-alpha and downregulation of survivin and mutant p53. PLoS One. 2013;8:e56649. doi: 10.1371/journal.pone.0056649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El Hasasna H, et al. Rhus coriaria suppresses angiogenesis, metastasis and tumor growth of breast cancer through inhibition of STAT3, NFkappaB and nitric oxide pathways. Sci Rep. 2016;6:21144. doi: 10.1038/srep21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Passaglia P, et al. Angiotensin type 1 receptor mediates chronic ethanol consumption-induced hypertension and vascular oxidative stress. Vascul Pharmacol. 2015;74:49–59. doi: 10.1016/j.vph.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 84.de Oliveira LM, et al. Endothelium-Dependent Vasorelaxant Effect of Butanolic Fraction from Caryocar brasiliense Camb. Leaves in Rat Thoracic Aorta. Evid Based Complement Alternat Med. 2012;2012:934142. doi: 10.1155/2012/934142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anwar, M. A., F. E., Vedernikov, Y., Saade, G., Garfield, R. E. Roles for nitric oxide and potassium channels in the vascular effect of vasopressin in pregnancy. American journal of obstetrics and gynecology184(1), S76:(228) (2001).
- 86.Anwar MA, Ford WR, Herbert AA, Broadley KJ. Signal transduction and modulating pathways in tryptamine-evoked vasopressor responses of the rat isolated perfused mesenteric bed. Vascul Pharmacol. 2013;58:140–149. doi: 10.1016/j.vph.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


