Abstract
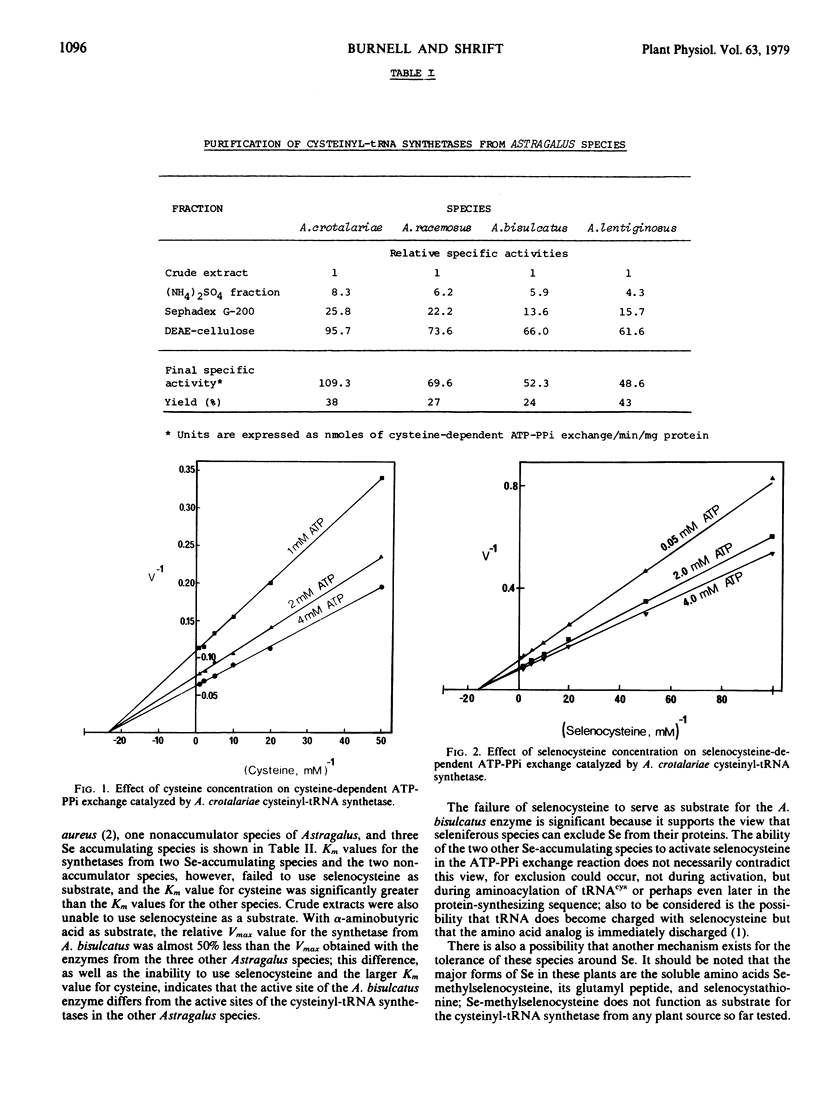
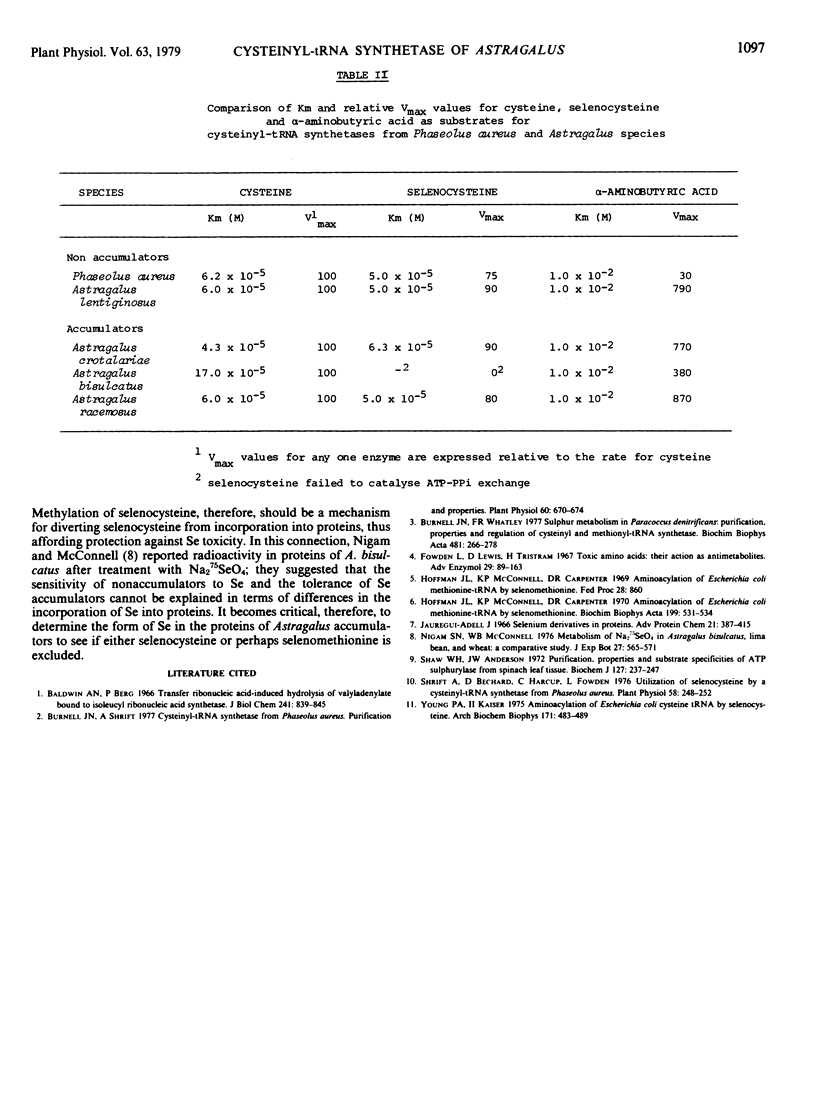
l-Cysteinyl-tRNA synthetases (EC 6.1.1.16) from four Astragalus species were partially purified. The substrate specificities of the cysteinyl-tRNA synthetase from three selenium accumulator species (A. crotalariae, A bisulcatus, and A. racemosus) were compared with those from two nonaccumulator species (A. lentigenosus and Phaseolus aureus). All species had similar Km values for cysteine, selenocysteine, and α-aminobutyric acid except A. bisulcatus which failed to use selenocysteine as a substrate and which had a Km for cysteine four times greater than the Km values for other species.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin A. N., Berg P. Transfer ribonucleic acid-induced hydrolysis of valyladenylate bound to isoleucyl ribonucleic acid synthetase. J Biol Chem. 1966 Feb 25;241(4):839–845. [PubMed] [Google Scholar]
- Burnell J. N. Cysteinyl-tRNA Synthetase from Phaseolus aureus: Purification and Properties. Plant Physiol. 1977 Nov;60(5):670–674. doi: 10.1104/pp.60.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Whatley F. R. Sulphur metabolism in Paracoccus denitrificans. Purification, properties and regulation of cysteinyl-and methionyl-tRNA synthetase. Biochim Biophys Acta. 1977 Mar 15;481(1):266–278. doi: 10.1016/0005-2744(77)90158-9. [DOI] [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- Hoffman J. L., McConnell K. P., Carpenter D. R. Aminoacylation of Escherichia coli methionine tRNA by selenomethionine. Biochim Biophys Acta. 1970 Feb 18;199(2):531–534. doi: 10.1016/0005-2787(70)90098-5. [DOI] [PubMed] [Google Scholar]
- Jáuregui-Adell J. Selenium derivatives in proteins. Adv Protein Chem. 1966;21:387–415. doi: 10.1016/s0065-3233(08)60130-8. [DOI] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Purification, properties and substrate specificity of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1972 Mar;127(1):237–247. doi: 10.1042/bj1270237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrift A., Bechard D., Harcup C. Utilization of Selenocysteine by a Cysteinyl-tRNA Synthetase from Phaseolus aureus. Plant Physiol. 1976 Sep;58(3):248–252. doi: 10.1104/pp.58.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]



