Abstract
Toxoplasmosis is caused by T. gondii and can create serious health problems in humans and also worldwide economic harm. Because of the clinical and veterinary importance of toxoplasmosis, its timely and accurate diagnosis has a major impact on disease-fighting strategies. T. gondii surface antigen 1 (SAG1), an immunodominant-specific antigen, is often used as a diagnostic tool. Therefore, the aim of this study was the optimization of novel fluorescein isothiocyanate (FITC) labeling of the SAG1-specific IgY antibody to show the potential for immunofluorescence imaging of T. gondii in vitro. The specificity of IgY antibodies was controlled by an enzyme-linked immunosorbent assay (ELISA), and the concentration of the IgY antibody was detected using a spectrophotometer. The optimum incubation time and FITC concentration were determined with a fluorescence spectrometer. The obtained FITC-labeled IgY was used for marking T. gondii tachyzoites, which were cultured in vitro and viewed using light microscopy. The interaction of the fluorescence-labeled antibody and the T. gondii tachyzoites was examined with a fluorescence microscope. In this study, for the first time, a FITC-labeled anti-SAG1 IgY antibody was developed according to ELISA, fluorescence spectrometer, and fluorescence imaging of cell culture. In the future, the obtained FITC-labeled T. gondii tachyzoites’ specific IgY antibodies may be used as diagnostic tools for the detection of T. gondii infections in different samples.
Introduction
Toxoplasma gondii is an obligate intracellular parasite that infects approximately 30% of the world’s population1. The toxoplasmosis disease is caused by T. gondii and produces health problems in humans as well as economic harm worldwide. The condition is generally asymptomatic, but it can cause local lymphadenopathy, mortality in immunodeficient patients2 and hydrocephalus, intracranial calcification, chorioretinitis, fever, anemia, hypothermia, microcephaly, microphthalmia, and deafness in newborns. Congenital toxoplasmosis can also cause spontaneous abortion, premature birth, and stillbirth in pregnancy3. Toxoplasmosis is responsible for economic and health problems not only in humans but also in animals4–6, as the disease is widely present in many animal species5.
Because of the clinical and veterinary importance of toxoplasmosis, the diagnosis has a major impact on coping strategies of clinicans. Many diagnostic methods exist for the detection of toxoplasmosis (biologic, histological, or some combination of these methods)5, with each having its own advantages and disadvantages regarding cost, sensitivity, specificity, ease of use, etc.7.
Antibodies are useful tools for many diagnostic tests8, especially serological tests. Among antibody production methods, specific yolk antibody production has recently gained importance with its numerous advantages over monoclonal antibody production methods9. There are a limited number of studies in the literature about Toxoplasma-specific IgY production. Among these, Hassl et al. purified eggs to obtain IgY antibodies directed against the soluble proteins of T. gondii from immunized hens. They compared the specificity of IgY and IgG antibodies obtained from immunized hens and hyper-immunized rabbits, respectively10. In another study, Ferreira et al. also obtained polyclonal anti-T. gondii IgY antibodies against soluble tachyzoite antigens from immunized hens, and they highlighted the diagnostic applications of polyclonal IgY antibodies11. In one of the most recent studies, SAG1-specific antibodies were obtained from immunized hens12.
However, in the literature, there is no study of the development of fluorescent-labeled specific IgY antibodies against the surface antigen of T. gondii (SAG1) or the application of a direct immunofluorescence assay. Therefore, the aim of this study was the optimization of the fluorescent dye-labeling procedure between the SAG1-specific IgY antibody and FITC to develop a novel dye to detect T. gondii parasites.
Materials
Phosphate-buffered saline (PBS) and polyethylene glycol (PEG) 6000 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were used to purify the eggs. Na2HPO4(7 H2O) (Merck, Kenilworth, NJ, USA), NaH2PO4(2 H2O) (Duchefa Biochemie B.V., Haarlem, Netherlands), NaCl (Duchefa Biochemie B.V.), HCl (Merck), and NaOH (Merck) were utilized for the PBS buffer. Na2CO3 (Sigma-Aldrich, St. Louis, MO, USA) and HCl (Merck) or NaOH (Merck) were used to prepare the sodium carbonate buffer. PBS buffer, coupling buffer, PBS-T solution and blocking buffer were used for the ELISA. PBS buffer for the ELISA was formed from Na2HPO4 (12 H2O) (Merck), (KH2PO4) (Merck), KCl (Merck), HCl (Merck), NaOH (Merck), and NaCl (Duchefa Biochemie B.V.). The coupling solution was made from Na2CO3 (Sigma-Aldrich), NaHCO3 (Merck), NaN3 (Merck), HCl (Merck), and NaOH (Merck). Na2HPO4 (7H2O) (Merck), NaH2PO4(2H2O) (Duchefa Biochemie B.V.), NaCl (Duchefa Biochemie B.V.), HCl (Merck), NaOH (Merck), and Tween-20 (Merck) were used for the PBS-T solution. Also, BSA (Sigma-Aldrich) was added to the PBS for the blocking buffer. P-nitrophenyl was provided by Sigma-Aldrich. Fluorescein 5-isothiocyanate (FITC, SIGMA7250), dimethylsulfoxide (DMSO), and ethanolamine were obtained from Sigma-Aldrich.
Fluorescence Measurements
Fluorescence emission spectra were obtained using a QM-4/2003 Quanta Master Steady State Spectrofluorimeter (Photon Technology International, London, ON, CAN) operating in quanta-counting mode. The slits of the excitation and emission monochromators were adjusted to 2 nm. The excitation was performed at 280 and 495 nm. Emission spectrums was obtained at 324 nm and 520 nm, respectively.
Methods
Production of the IgY Antibody
Lohmann Brown hens that were 20 weeks old were immunized with 20 µg of recombinant SAG1 three times and 10 µg of rSAG1 one time every two weeks. Eggs that contained the highest level of the antibody were given booster doses and used in the study12. Control eggs were obtained from hens injected with phosphate buffered saline (PBS). The egg yolk antibody was isolated according to Polson et al.’s precipitation procedure. Briefly, the eggs were first cleaned with ethanol. The precipitation method followed; polyethylene glycol 6000 (PEG 6000; Sigma-Aldrich, St. Louis, MO, US) was used three times, and dialysis was then applied to the eggs to purify the IgY antibodies13. PBS, 3.5% PEG 6000, and the egg yolk were combined and then centrifuged. This procedure was repeated on the obtained supernatant with 8.5% and 12% PEG 6000 so that the pellet containing the IgY antibodies was dialyzed12. All of the experiments used in animals were approved by the Animal Ethics Committee of the University of Osmangazi in Eskisehir, Turkey. All methods were carried out in accordance with the ethical standards and guidelines of the responsible committee.
Protein Activity and Concentration
Protein activity was determined using the enzyme-linked immunosorbent assay (ELISA) method. Briefly, 96-well plates were coated with SAG1 protein and incubated overnight at +4 °C. After the incubation, these plates were washed three times with PBS-T. Then, the plates were coated with the blocking buffer (10 mg/ml bovine serum albumin (BSA) and incubated for 1 h at 37 °C. After the incubation time, three washings were again performed. A 1:100 dilution of the obtained IgY antibody was added to the wells (n = 5) with PBS-T and incubated for 1 hour at 37 °C12. The plates were then washed with PBS-T, and alkaline phosphatase conjugated anti-chicken IgY antibody was added at 1:1000 dilutions before the plates were incubated once more. Finally, 100 µl of the substrate solution (SIGMAFAST p-Nitrophenyl phosphate tablets) were added to each well and incubated for 45 min in the dark, and then the absorbance was measured at 405 nm with a microtiter plate reader (Thermo Labsystems Multiskan Ascent 354 Microplate Photometer).
The protein concentration of the purified eggs was calculated with the BioSpec-nano Small-volume UV spectrophotometer (Shimadzu, Kyoto, Japan). The absorbance of the protein solution exceeded 1.4. At 280 nm, the absorbance value of a protein solution should be between 0.2 and 1.4; therefore, the antibody solution was appropriately diluted. The antibody concentration of the diluted samples was measured three times at 280 nm with the spectrophotometer. The diluted protein solution was used in the fluorescence experiments.
Optimization of the Incubation Time and Concentration of FITC for Labeling with SAG1-specific IgY
The IgY antibody solution was prepared in a 0.1-M sodium carbonate buffer (pH: 9.0). Amine reactive dye FITC was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 1 mg/ml as suggested in the literature14 and then was prepared in the buffer stock with a ratio of 1:100. After a single addition of dye from the buffer stock to the antibody solution, the excitation wavelength was adjusted to 495 nm. The measurement intervals were every 1 min. The optimum incubation time was determined as the time when the fluorescence intensity ceased (Fig. 3).
Figure 3.
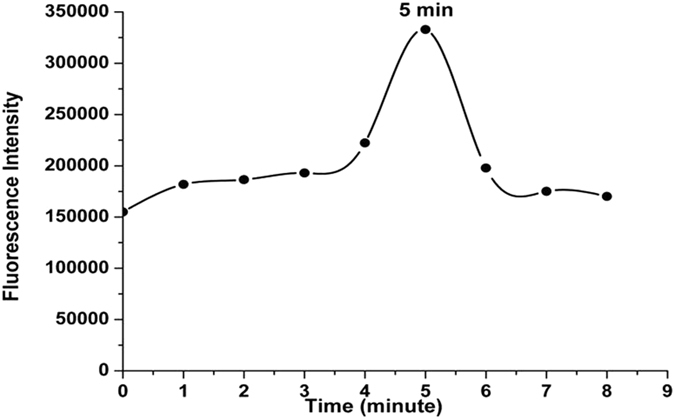
The fluorescence intensity of FITC-added anti-SAG1 IgY antibody against time (λex = 495 nm, λem = 520 nm).
Next, the optimum amounts of FITC concentration for anti-SAG1 IgY antibodies labeled with FITC were studied; a titration experiment was carried out by increasing the concentration from 10 µl to 500 µ with 10-µl intervals of FITC (2 mg/ml IgY antibody in each solution). The reaction was terminated by adding 0.1 M ethanol amine (Sigma-Aldrich). The FITC-labeled anti-SAG1 IgY was incubated at room temperature for 15 min in darkness and stored at −20 °C with BSA (Fig. 4).
Figure 4.
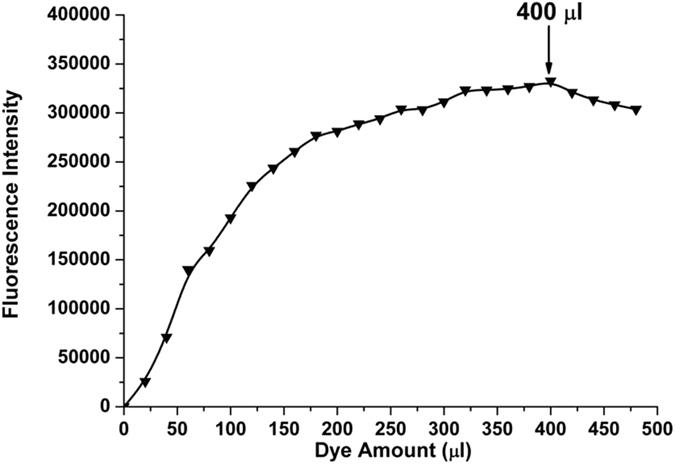
The fluorescence intensity of FITC-added anti-SAG1 IgY antibody against increasing amounts of FITC dye (λex = 495 nm, λem = 520 nm).
Conjugation of the FITC and IgY Antibodies Obtained from the Control Eggs
The FITC and IgY antibodies (control) were conjugated using the same method as applied for labeling with SAG1-specific IgY. The concentration (400 µl of 10 µg/ml FITC for 2 mg/ml IgY) and incubation time (4 min) were selected according to the results obtained regarding the optimization of the incubation time and the concentration of FITC experiments. The reaction was terminated by adding 0.1 M ethanol amine (Sigma-Aldrich). The FITC-labeled anti-SAG1 IgY was incubated at room temperature for 15 min in dark conditions and then used in experiments termed “Staining of T. gondii Parasites with FITC-labeled IgY Antibody Obtained from Control Eggs.”
In Vitro Culture of T. gondii Tachyzoites
T. gondii tachyzoites were kindly supplied by Dr. Cahit Babür of the Public Health Institution of Turkey. The tachyzoites were washed three times with PBS containing penicillin-streptomycin and then centrifuged for five minutes at 1500 rpm. The supernatant was poured off, and the number of tachyzoites was counted using hemocytometry. The tachyzoites were added to the cultured L929 cells in vitro (the ratio of L929 cells to tachyzoites was 1:10) and incubated at 37 °C in 5% CO2. The tachyzoites were released from the cells three to five days after the co-culture began. Proliferated tachyzoites were used for direct immunofluorescence assay.
Identification of T. gondii by Direct Microscopic Examination and Giemsa Staining
Tachyzoites cultured with L929 were directly observed using an inverted microscope with x40 lens magnification during the culture. After three to five days of culture, the released tachyzoites were collected and placed in a thin layer on microscopic slides. Next, the tachyzoites were fixed with methanol and dried at room temperature. Giemsa stain was added to the slides, which were incubated at room temperature for 15 min. Finally, the prepared slides were washed, and the stained tachyzoites were observed using a light microscope15.
Staining of T. gondii Parasites with FITC-labeled SAG1-specific IgY Antibody
The released tachyzoites from the L929 cells were fixed with methanol onto microscopic slides. An FITC-labeled IgY antibody solution was added to the slides, and they were incubated in the dark at room temperature for 30 min. A fluorescence microscope (INV100-FL; BEL Engineering®, Milan, Italy) was used for the imaging interaction between parasites and the FITC-labeled SAG1-specific IgY antibody.
Staining of T. gondii Parasites with FITC-labeled IgY Antibody Obtained from the Control Eggs
The methanol-fixed tachyzoites on the microscope slides were added to the FITC-labeled control IgY antibody solution. After incubation in the dark at room temperature for 30 min, the slides were observed under a fluorescence microscope.
Staining of T. gondii Parasites with FITC
This experiment was conducted to show whether there is an unspecific interaction between the FITC dye and tachyzoites. For the next step, 400 µl of 10-µg/ml FITC and 0.1 M ethanol amine (Sigma-Aldrich) were mixed at room temperature and added to a thin layer of tachyzoites on a microscopic slide. A fluorescence microscope (INV100-FL; BEL Engineering®, Milan, Italy) was used for the imaging interaction between the tachyzoites and the FITC.
Results and Discussion
ELISA was used to show the specificity of the obtained IgY antibody for the SAG1 protein by comparing it with IgY obtained from control hens. As shown in Fig. 1, the absorbance value of antibodies from the control eggs was 0.4, while the absorbance value of the eggs from immunized chickens was nearly 3.2. These results indicated that the IgY antibodies specific to SAG1 were obtained and purified from the immunized hens. There is a specific type of binding that takes place between the SAG1 protein and IgY antibodies from immunized hens. This obtained anti-SAG1 IgY antibody was used for FITC-labeling studies after the amount of protein was detected.
Figure 1.
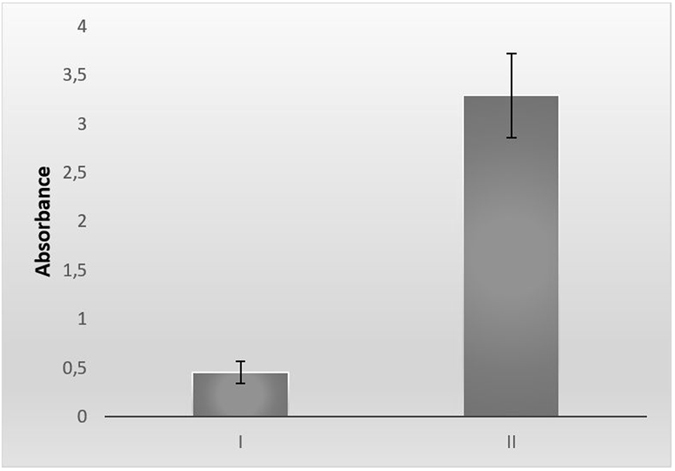
Absorbance values of ELISA. I: The anti-SAG1 IgY antibody amount of eggs in the control group. II: The anti-SAG1 IgY antibody amount of immunized chicken eggs with SAG1.
Because the amount of antibodies is an important parameter for the optimization and standardization of fluorescence labeling, the protein amount of anti-SAG1 IgY antibodies was measured using a BioSpec-nano small-volume ultraviolet (UV) spectrophotometer at 280 nm. The absorbance values of the diluted protein solution are given in Table 1. The optical density (OD) values of the diluted protein solution were between 0.2–1.4. The data of the diluted protein solution were obtained as a result of measurements produced by the BioSpec-nano small-volume UV spectrophotometer.
Table 1.
Absorbance values of specific IgY antibodies obtained from the eggs of immunized hens.
| Number of measurement | OD | Molarity (10−5 ) | Concentration of protein (mg/ml) |
|---|---|---|---|
| 1st | 1.213 | 1.213 | 2.2 |
| 2nd | 1.192 | 1.192 | 2.15 |
| 3rd | 1.224 | 1.224 | 2.2 |
The protein concentrations were determined as the mean value of three measurement results, which was approximately 2.2 mg/ml. The final protein concentration was calculated by using 25 as the dilution coefficient. As a result, the anti-SAG1 IgY antibody concentration was calculated as 54.575 mg/ml. Appropriate dilutions were made according to the concentration detection results.
The typical wavelength of anti-SAG1 IgY antibody showed that the maximum intensity was detected before the FITC labeling studies. As shown in Fig. 2, the maximum fluorescence intensity of the anti-SAG1 IgY antibody was detected at 324 nm.
Figure 2.
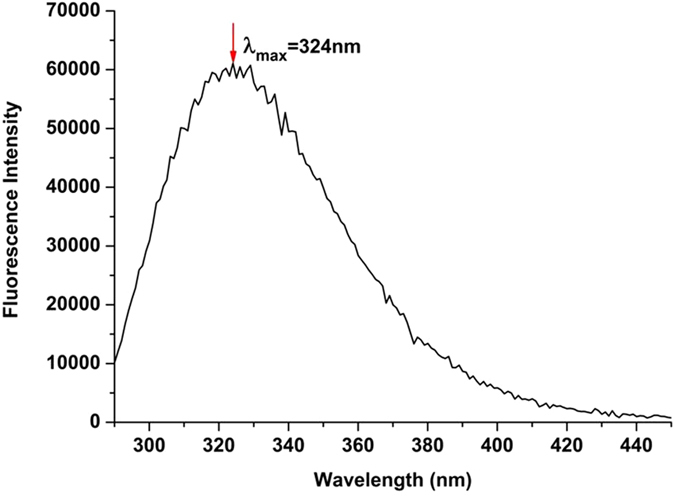
Fluorescence spectra of anti-SAG1 IgY antibody (λex = 280 nm).
In the IgY antibody and FITC-labeling experiments, the different incubation times were first examined to define the optimum time required for the interaction between the reactive FITC dye and the primary amine groups of the IgY antibody. Figure 3 shows the fluorescence intensity over time, which increased until five minutes had passed; no additional increases were detected after this time. During this period, entire FITC molecules probably interacted with the primary amine groups of the IgY antibody. As a result, the optimum incubation time was five minutes at room temperature in the dark compartment of a fluorescence spectrometer, as noted in the literature16 (Fig. 3).
The following experiments were conducted to examine the effect of increasing concentrations of FITC on the fluorescence intensity while the amount of IgY antibody remained constant. As shown in Fig. 4, the maximum fluorescence intensity was 400 µl of FITC; additional FITC did not increase the density value because the IgY antibodies were probably saturated with the 400 µl of FITC. Therefore, the optimum FITC concentration and incubation times were determined for labeling specific IgY antibodies against SAG1 with FITC.
FITC is the most popular amine-reactive fluorophore that is commonly used for fluorescence labeling of antibodies. FITC can bind with the side-chain of amino acids, including primary amines as lysines in the structure of the antibody. Antibodies have many different functional groups, but only their primary amine groups are capable of reacting with FITC. In our study, IgY did not appear on the fluorescence spectrum at 520 nm because IgY has a maximum fluorescence intensity of 324 nm (Fig. 2). After the addition of FITC to the IgY solution, FITC reacted with the primary amine groups of IgY and FITC-labeled IgY showed fluorescence emission at 520 nm, which is the characteristic emission wavelength of FITC.
For the cell-imaging experiments, L929 cells were used as the host for cultivation of T. gondii tachyzoites. The presence of T. gondii tachyzoites was indicated by both the Giemsa-stained preparations (Fig. 5A) and also those without staining (Fig. 5B) under light microscopy.
Figure 5.
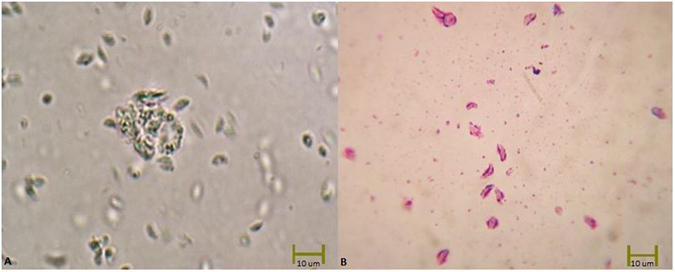
Microscopic images of T. gondii tachyzoites (A). Unstained (x40) (B). Stained with Giemsa (x40).
The interaction of the FITC (alone) and FITC-labeled IgY antibodies (SAG1-specific and control) with T. gondii tachyzoites was examined under a fluorescence microscope. Figure 6 reveals that the FITC-labeled SAG1-specific IgY antibodies specifically reacted with T. gondii. Fluorescence signals were absent in both the tachyzoites with FITC-labeled control IgY and tachyzoites with FITC dye (Fig. 6C and D). As a result of the fluorescent microscopic examination, only FITC-labeled, SAG1-specific IgY antibodies interacted with T. gondii, and there were no non-specific interactions between T. gondii and the FITC-labeled control eggs or FITC alone. Because SAG1 is a surface protein of T. gondii tachyzoites, the fluorescence-labeled antibodies specific to SAG1 covered the surface of the parasites, and the fluorescence images were compatible with the natural shape of the parasites (Fig. 6).
Figure 6.
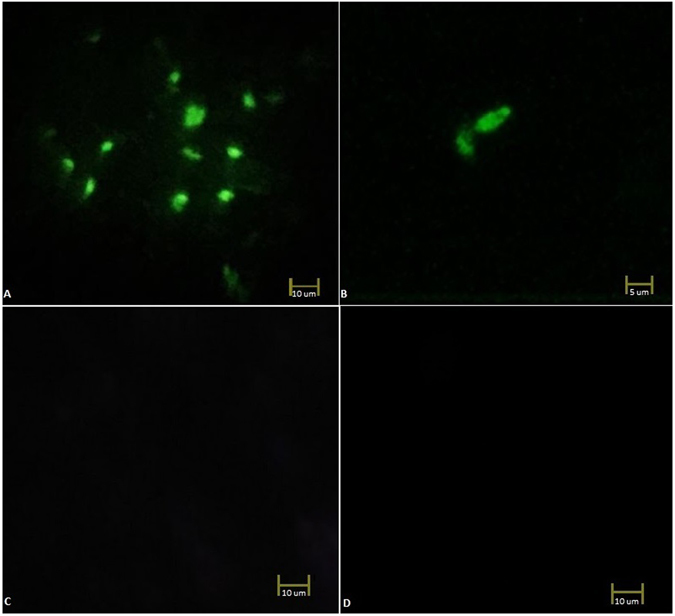
Microscopic images T. gondii tachyzoites (x40) stained with (A,B). FITC-conjugated anti-SAG1 IgY antibody, (C). FITC-labeled IgY Antibody Obtained from the Control Eggs, (D). and FITC (without antibody).
Conclusion
In this study, for the first time in the literature, FITC-labeled anti-SAG1 IgY antibody was used to detect T. gondii tachyzoites in vitro, and parameters including the concentration of the dye and the incubation time were optimized for the IgY-labeling experiments.
The detection of specific antibodies in serum samples using serological assays is generally used for the diagnosis of toxoplasmosis17. However, directly identifying the presence of a parasite is very important because it is frequently difficult to define whether the infection was recent or old using only the detected antibodies18 in addition, antibody detection methods cannot differentiate between vaccine-induced and infection-induced antibodies17. While the polymerase chain reaction method can also be used to show the presence of parasites, it cannot distinguish between viable intact and non-viable, fragmented parasites19. Therefore, the direct detection of parasites using different methods, such as fluorescent-labeled antibodies, retains its importance in clinical practice.
Our results showed that fluorescent-labeled SAG1 antibodies interacted with the tachyzoites and produced fluorescent images. Until recently, tachyzoites were not considered important sources of infection because they are sensitive to the proteolytic enzymes of milk and gastric juices and usually break down. However, previous studies have shown that tachyzoites can survive in a solution with pepsin, and orally administered tachyzoites could infect cats20, 21. Under these circumstances, the detection of T. gondii tachyzoites gains further importance, and the fluorescence-labeled antibody against T. gondii tachyzoites developed in this study may be useful for the identification of T. gondii tachyzoites in humans, animals, foods, and water. SAG1 is a tachyzoite-specific antigen of and is highly conserved between strains and is therefore indicated as a good candidate for diagnosis22. Some studies also showed that the rSAG1 does not have any cross reactivity against proteins of other microorganisms23.
This study showed the potential of the developed antibody-FITC conjugate for the detection of tachyzoites in different sources, such as milk, contaminated water, and body fluids. In the future, this FITC-labeled SAG1-specific IgY antibody may be commercially used for diagnostic purposes after extensive cross reactivity, validation, and specificity tests have been conducted with different samples.
Acknowledgements
The authors thanks to Dr. Cahit BABUR from the Public Health Institution of Turkey for providing us T. gondii tachyzoites. The authors also thank the TURKEY GENERAL DIRECTORATE OF AGRICULTURAL RESEARCH AND POLICIES (Project Number: TAGEM/15/AR-GE/41) for financial support.
Author Contributions
R.C.K. wrote the abstract, introduction, discussion, M.S. and Y.B.K. wrote the material method section, R.C.K. reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Foe IT, et al. Global Analysis of Palmitoylated Proteins in Toxoplasma gondii. Cell host & microbe. 2015;18:501–511. doi: 10.1016/j.chom.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akarsu GA. Toksoplazmoz tanısı. Ankara Üniversitesi Tıp Fakültesi Mecmuası. 2008;61:180–190. doi: 10.1501/Tipfak_0000000670. [DOI] [Google Scholar]
- 3.Güngör, S. et al. Bir üçüncü basamak hastanede Toxoplasma gondii IgG avidite test istem ve sonuçlarının değerlendirilmesi. Journal of Clinical and Experimental Investigations5 (2014).
- 4.Hill DE, Chirukandoth S, Dubey J, Lunney JK, Gamble HR. Comparison of detection methods for Toxoplasma gondii in naturally and experimentally infected swine. Veterinary parasitology. 2006;141:9–17. doi: 10.1016/j.vetpar.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay, D. S. & Weiss, L. M. Opportunistic infections: toxoplasma, sarcocystis, and microsporidia Vol. 9 (Springer Science & Business Media, 2004).
- 6.Ueno N, Lodoen MB, Hickey GL, Robey EA, Coombes JL. Toxoplasma gondii-infected natural killer cells display a hypermotility phenotype in vivo. Immunology and cell biology. 2015;93:508–513. doi: 10.1038/icb.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James GS, Sintchenko VG, Dickeson DJ, Gilbert GL. Comparison of cell culture, mouse inoculation, and PCR for detection of Toxoplasma gondii: effects of storage conditions on sensitivity. Journal of clinical microbiology. 1996;34:1572–1575. doi: 10.1128/jcm.34.6.1572-1575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrebaeck CA. Antibodies in diagnostics–from immunoassays to protein chips. Immunology today. 2000;21:379–382. doi: 10.1016/S0167-5699(00)01683-2. [DOI] [PubMed] [Google Scholar]
- 9.Mine Y, Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. Journal of medicinal food. 2002;5:159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- 10.Hassl A, Aspöck H, Flamm H. Comparative studies on the purity and specificity of yolk immunoglobulin Y isolated from eggs laid by hens immunized with Toxoplasma gondii antigen. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene. Series A: Medical Microbiology, Infectious Diseases, Virology, Parasitology. 1987;267:247–253. doi: 10.1016/S0176-6724(87)80009-3. [DOI] [PubMed] [Google Scholar]
- 11.Júnior ÁF, et al. Production, characterization and applications for Toxoplasma gondii-specific polyclonal chicken egg yolk immunoglobulins. Plos One. 2012;7:e40391. doi: 10.1371/journal.pone.0040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cakir-Koc, R. Production of anti-SAG1 IgY antibody against Toxoplasma gondii parasites and evaluation of antibody activity by ELISA method. Parasitology Research 1–6 (2016). [DOI] [PubMed]
- 13.Pauly, D., Chacana, P. A., Calzado, E. G., Brembs, B. & Schade, R. IgY technology: extraction of chicken antibodies from egg yolk by polyethylene glycol (PEG) precipitation. JoVE (Journal of Visualized Experiments) e3084–e3084 (2011). [DOI] [PMC free article] [PubMed]
- 14.Wisdom, G. B. Conjugation of antibodies to fluorescein or rhodamine. Immunochemical Protocols 131–134 (2005). [DOI] [PubMed]
- 15.Rashidi S, Sadraei J, Jafari-Modrek M. Comparison of Giemsa Staining, Intraperitoneal Injection and Oral Administration Methods in Rat’s Infected Brain with Toxoplasma Gondii. Zahedan Journal of Research in Medical Sciences. 2014;16:45–49. [Google Scholar]
- 16.Sack, U., Tárnok, A. & Rothe, G. Cellular diagnostics: basic principles, methods and clinical applications of flow cytometry (Karger Medical and Scientific Publishers, 2009).
- 17.Wang Y-H, et al. Development of an immunochromatographic strip for the rapid detection of Toxoplasma gondii circulating antigens. Parasitology international. 2011;60:105–107. doi: 10.1016/j.parint.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Dannemann BR, Vaughan WC, Thulliez P, Remington J. Differential agglutination test for diagnosis of recently acquired infection with Toxoplasma gondii. Journal of clinical microbiology. 1990;28:1928–1933. doi: 10.1128/jcm.28.9.1928-1933.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia JL, Gennari SM, Machado RZ, Navarro IT. Toxoplasma gondii: detection by mouse bioassay, histopathology, and polymerase chain reaction in tissues from experimentally infected pigs. Experimental parasitology. 2006;113:267–271. doi: 10.1016/j.exppara.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Tavassoli, M., Esmaeilnejad, B., Malekifard, F., Soleimanzadeh, A. & Dilmaghani, M. Detection of Toxoplasma gondii DNA in Sheep and Goat Milk in Northwest of Iran by PCR-RFLP. Jundishapur Journal of Microbiology6 (2013).
- 21.Powell CC, Brewer M, Lappin MR. Detection of Toxoplasma gondii in the milk of experimentally infected lactating cats. Veterinary parasitology. 2001;102:29–33. doi: 10.1016/S0304-4017(01)00521-0. [DOI] [PubMed] [Google Scholar]
- 22.Kotresha D, Noordin R. Recombinant proteins in the diagnosis of toxoplasmosis. Apmis. 2010;118:529–542. doi: 10.1111/j.1600-0463.2010.02629.x. [DOI] [PubMed] [Google Scholar]
- 23.Selseleh MM, et al. Production and evaluation of Toxoplasma gondii recombinant surface antigen 1 (SAG1) for serodiagnosis of acute and chronic Toxoplasma infection in human sera. Iranian journal of parasitology. 2012;7:1–9. [PMC free article] [PubMed] [Google Scholar]


