Abstract
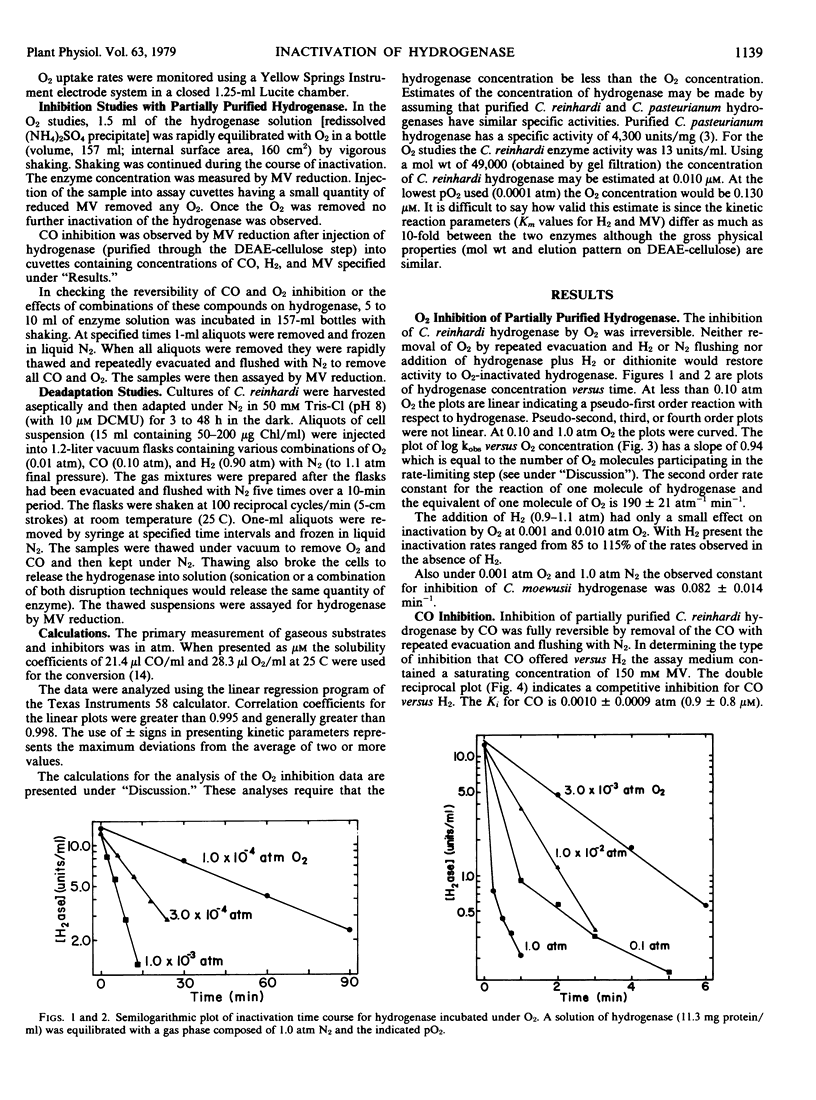
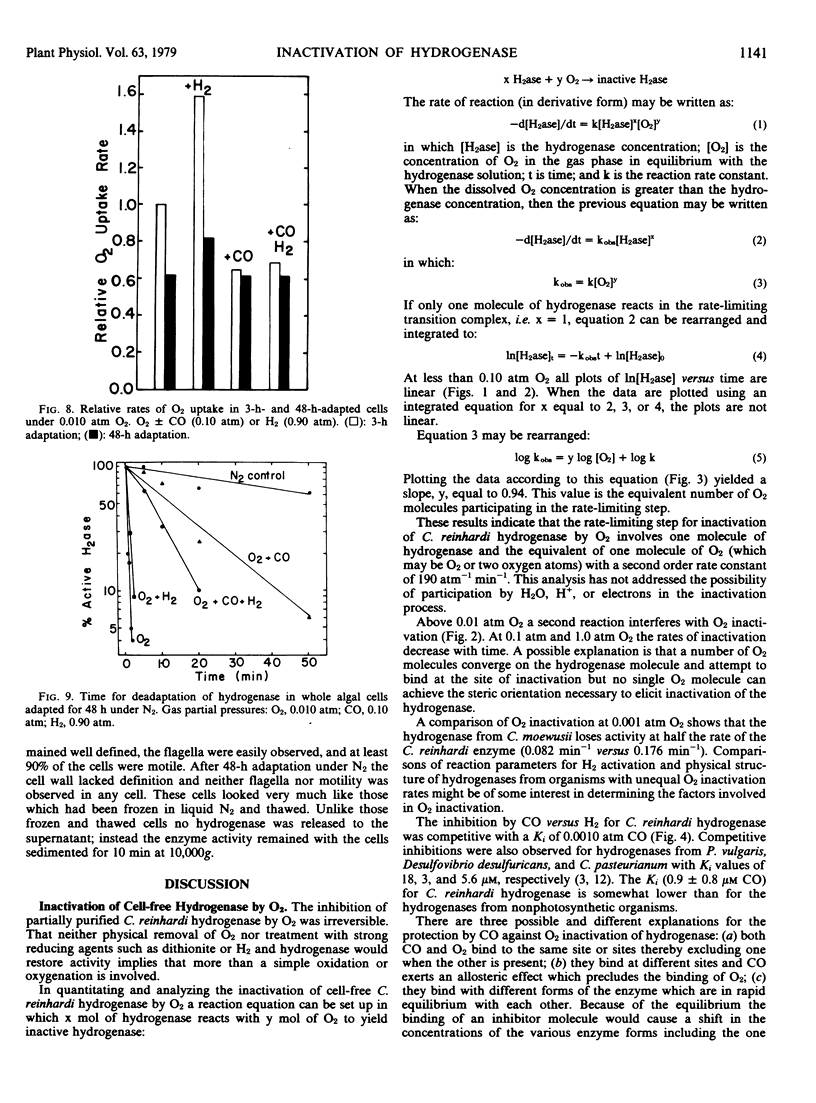
O2 irreversibly inactivates hydrogenase from Chlamydomonas reinhardi. The mechanism for the inactivation involves the reaction of one molecule of hydrogenase with one molecule of O2 (or two oxygen atoms) in the transition complex of the rate-limiting step. The second order rate constant for this reaction is 190 atmospheres−1 minute−1 (1.4 × 105 molar−1 minute−1). At levels above 0.01 atmosphere O2, the increased numbers of O2 molecules may compete for the site of inactivation hindering the proper orientation for inactivation of any one O2 molecule and resulting in lowered rates of inactivation.
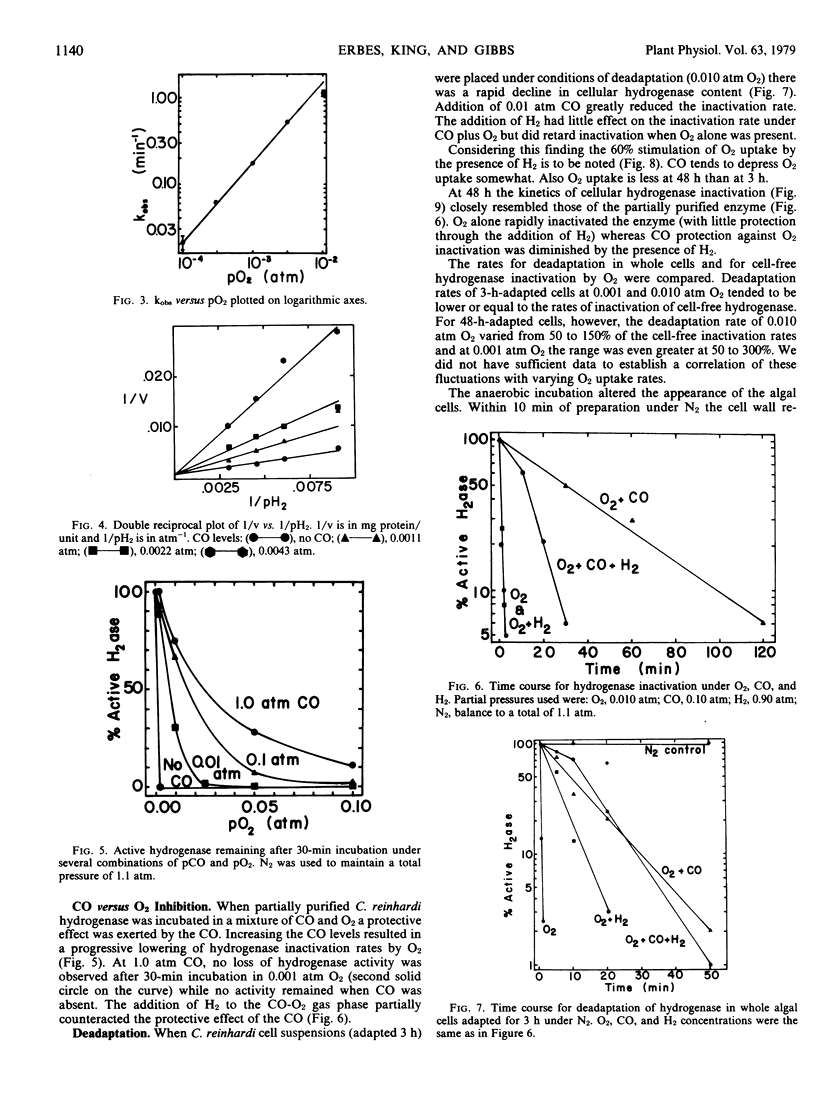
CO is a reversible inhibitor of hydrogenase acting competitively against H2. The Ki for CO is 0.0010 atmosphere. CO antagonizes O2 inactivation. In a period when complete inactivation by O2 would usually occur, the presence of CO greatly reduces the inactivation rate.
After 3 hours of adaptation in whole cells, the presence of H2 lowers the rate of deadaptation of hydrogenase. Inasmuch as H2 promotes increased O2 uptake the cellular concentration of O2 is likely to be lower. After 48 hours of adaptation O2 uptake is reduced even when H2 is present and the pattern of deadaptation under O2 with and without H2 and CO is qualitatively the same as observed for the inactivation of cell-free hydrogenase. The mechanism of inactivation of cell-free hydrogenase by O2 may be the same as the mechanism for loss of hydrogenase during deadaptation in whole algal cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B. Cell-free Hydrogenase from Chlamydomonas. Plant Physiol. 1964 Mar;39(2):169–176. doi: 10.1104/pp.39.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H., Orme-Johnson W. H. On the iron-sulfur cluster in hydrogenase from Clostridium pasteurianum W5. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4795–4799. doi: 10.1073/pnas.72.12.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H. The kinetics of methyl viologen oxidation and reduction by the hydrogenase from Clostridium pasteurianum. Biochim Biophys Acta. 1978 Jul 7;525(1):45–54. doi: 10.1016/0005-2744(78)90198-5. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN H., KRASNA A. I. Studies on the "adaptation" of hydrogenase in Scenedesmus. J Biol Chem. 1963 Feb;238:749–757. [PubMed] [Google Scholar]
- Lappi D. A., Stolzenbach F. E., Kaplan N. O., Kamen M. D. Immobilization of hydrogenase on glass beads. Biochem Biophys Res Commun. 1976 Apr 19;69(4):878–884. doi: 10.1016/0006-291x(76)90455-1. [DOI] [PubMed] [Google Scholar]
- PUREC L., KRASNA A. I., RITTENBERG D. The inhibition of hydrogenase by carbon monoxide and the reversal of this inhibition by light. Biochemistry. 1962 Mar;1:270–275. doi: 10.1021/bi00908a013. [DOI] [PubMed] [Google Scholar]


