Abstract
Background
Most methods for assessing microvascular function are not readily available in the cardiac catheterization laboratory. The aim of this study is to determine whether the Index of Microcirculatory Resistance (IMR), measured at the time of primary percutaneous coronary intervention (PCI) is predictive of death and rehospitalization for heart failure.
Methods and Results
IMR was measured immediately after primary PCI in 253 patients from 3 institutions using a pressure-temperature sensor wire. The primary endpoint was the rate of death or rehospitalization for heart failure. The prognostic value of IMR was compared to coronary flow reserve, TIMI myocardial perfusion grade and clinical variables. The mean IMR was 40.3 ±32.5. Patients with an IMR>40 had a higher rate of the primary end point at one year compared to patients with an IMR≤40 (17.1% vs. 6.6%, p=0.027). During a median follow-up period of 2.8 years, 13.8% suffered the primary end point and 4.3% died. An IMR>40 was associated with an increased risk of death or rehospitalization for heart failure (HR 2.1, p=0.034) and of death alone (HR 3.95, p=0.028). On multivariate analysis, independent predictors of death or rehospitalization for heart failure included IMR>40 (HR 2.2, p=0.026), fractional flow reserve ≤0.8 (HR 3.24, p=0.008) and diabetes (HR 4.4, p<0.001). An IMR>40 was the only independent predictor of death alone (HR 4.3, p=0.02).
Conclusions
An elevated IMR at the time of primary PCI predicts poor long term outcomes.
Keywords: microvascular dysfunction, myocardial infarction, physiology
Coronary microvascular dysfunction resulting from ST segment elevation myocardial infarction (STEMI) portends a poor prognosis.1,2 Noninvasive methods are considered the reference standard for diagnosing acute microvascular dysfunction in this setting.3,4 However, these methods are not readily available at the time of primary percutaneous coronary intervention (PCI), the preferred initial therapy for STEMI. The Index of Microcirculatory Resistance (IMR) is a readily available, quantitative and reproducible, wire-based method for invasively assessing coronary microvascular function independent of the epicardial artery in the cardiac catheterization laboratory.5-7 We and others have demonstrated that IMR measured at the time of STEMI correlates with infarct size and predicts recovery of left ventricular function.8-10 The long-term prognostic value of IMR measured in this setting is unknown.
The aim of this study is to determine whether IMR predicts mortality and rehospitalization for heart failure when measured immediately after primary PCI in patients suffering from STEMI and to compare it to other commonly used methods for invasively assessing the coronary microvasculature such as coronary flow reserve (CFR) and the thrombolysis in myocardial infarction (TIMI) myocardial perfusion grade (TMPG).
Methods
This is a prospective, multicenter, international study including hemodynamically stable patients presenting with STEMI within 12 hours of onset of symptoms or after failed fibrinolytic therapy, who had persistent ST segment elevation ≥ 1 mm in contiguous leads on the electrocardiogram and who provided informed, written consent. Patients who required pressor support or intraaortic balloon counterpulsation were not included. The study was approved by each site's Internal Review Board.
Coronary Physiology Assessment
After primary PCI was completed, intracoronary nitroglycerin (100-200 micrograms) was administered and a coronary pressure wire (St. Jude Medical) was calibrated, equalized to the guide catheter pressure with the pressure wire sensor positioned at the tip of the catheter, and then advanced to the distal two-thirds of the culprit vessel. Three milliliters of room temperature saline were briskly injected through the guide catheter and the mean transit time was measured using a previously described thermodilution technique.11,12 Three measurements were made and averaged. Maximal hyperemia was then induced by infusing intravenous adenosine at 140 μg/kg/min or by injecting intracoronary papaverine (10-20 mg). During maximal hyperemia, the mean transit time was measured again as described above. The mean distal coronary pressure measured with the pressure wire and mean proximal coronary pressure measured with the guide catheter were recorded during maximal hyperemia.
IMR was defined as the mean distal pressure multiplied by the mean hyperemic transit time, as previously described.5 CFR was calculated by dividing the mean resting transit time by the mean hyperemic transit time and fractional flow reserve (FFR) was defined as the mean distal pressure divided by the mean proximal pressure during maximal hyperemia.11,12 The coefficient of variation for the IMR and CFR were previously reported to be 6.9 ± 6.5% and 18.6 ± 9.6% respectively.6
Other Measures of Microvascular Function
The TMPG was assessed from the final recorded cine images after completion of the procedure as previously described.2 If necessary, the view was adjusted so that the culprit vessel territory was not superimposed on non-infarcted regions. The duration of cine filming was prolonged by at least 3 cardiac cycles to make sure that the entire washout phase was included. TMPG was assessed during the same phase of the cardiac cycle. The images were analyzed offline independently by two interventional cardiologists blinded to the IMR result. Any discrepancies were resolved by consensus. The corrected TIMI frame count (cTFC) was defined as the number of frames necessary for the dye to reach standardized distal landmarks, as previously described.13 The left anterior descending coronary artery frame counts were corrected by dividing by 1.7. The previously reported mean difference between two separate measurements for the corrected TIMI frame count was 4.7 ± 3.9 and the overall agreement for TIMI perfusion grade was 0.59 ± 0.04.13
Clinical Follow-Up
The prespecified primary end point was the incidence of death or rehospitalization due to congestive heart failure, with a secondary endpoint of all cause death alone. Rehospitalization for congestive heart failure was defined as hospitalization because of signs and symptoms of heart failure in conjunction with noninvasive imaging findings and/or a discharge diagnosis of congestive heart failure. Follow-up was performed by clinic visit, medical record review and telephone contact.
Statistics
Results are expressed as mean ±standard deviation unless otherwise stated. Categorical variables were compared using the chi-square test. Cox proportional-hazard regression models were used to determine predictors of the clinical endpoints. All individual variables listed in Table 1 with p-value of <0.1 were considered for inclusion into multivariable forward stepwise models to determine the independent predictors. A two-sided p-value of 0.05 was considered significant. Statistical analyses were performed with the use of SPSS v.15 (SPSS, Chicago, IL). Figures were generated using Graphpad Prism v.5.01 (Graphpad Software, La Jolla, CA).
Table 1. Baseline Clinical and Angiographic Characteristics.
| Variable | Whole Cohort n = 253 | IMR <40N = 173 | IMR >40 n = 80 | P value |
|---|---|---|---|---|
| Age, mean – years | 56.8 ± 10.6 | 56.2 ± 10.6 | 58.2 ± 12.0 | 0.495 |
| Male sex – no. (%) | 216 (85.4) | 151 (87.3) | 65 (81.3) | 0.142 |
| Body mass index, mean– kg/m2 | 25.0 ± 3.7 | 24.6 ± 2.9 | 26.0 ± 5.1 | 0.230 |
| Co-morbidities – no. (%) | ||||
| Diabetes | 61 (24.1) | 42 (24.2) | 19 (23.8) | 0.927 |
| Hypertension | 114 (45.1) | 75 (43.4) | 39 (48.8) | 0.497 |
| Dyslipidemia | 169 (66.8) | 119 (68.8) | 50 (62.5) | 0.389 |
| Smoking | 118 (46.6) | 88 (50.9) | 30 (37.5) | 0.056 |
| Discharge Medications – no. (%) | ||||
| Aspirin | 247 (97.6) | 168 (97.1) | 79 (98.8) | 0.569 |
| Clopidogrel | 253 (100) | 173 (100) | 80 (100) | - |
| Statin | 238 (94.1) | 167 (96.5) | 71 (88.8) | 0.074 |
| ACE-inhibitor | 204 (80.6) | 142 (82.1) | 62 (77.5) | 0.852 |
| Beta-blocker | 216 (85.4) | 149 (86.1) | 67 (83.8) | 0.792 |
| Coronary physiology, mean | ||||
| IMR | 40.3 ± 32.5 | 24.3 ± 8.8 | 74.8 ± 37.8 | <0.001 |
| Coronary flow reserve | 1.9±0.9 | 2.0 ± 1.0 | 1.6 ±0.6 | <0.001 |
| Fractional flow reserve | 0.89 ± 0.09 | 0.88 ±0.1 | 0.91±0.08 | 0.036 |
| Corrected TIMI frame count | 20.3 ± 13.1 | 17.7 ± 7.8 | 25.7 ± 18.8 | 0.001 |
| TMPG – no. (%) | ||||
| 0/1 | 4 (1.6) | 0 (0) | 4 (5.0) | <0.001 |
| 2 | 40(15.8) | 17(9.8) | 23 (28.8) | |
| 3 | 209 (82.6) | 139 (80.3) | 70 (87.5) | |
| Vascular territory – no. (%) | ||||
| Left anterior descending | 138 (54.5) | 95 (54.9) | 43 (53.8) | 0.943 |
| Left circumflex | 23 (9.1) | 15 (8.7) | 8 (10.0) | |
| Right coronary artery | 92 (36.4) | 63 (36.4) | 29 (36.3) | |
| Proximal culprit lesion – no. (%) | 146 (57.7) | 101 (58.4) | 45 (56.3) | 0.416 |
| Stents deployed – no. (%) | ||||
| 0 | 8 (3.2) | 5 (2.9) | 3 (3.8) | 0.705 |
| 1 | 194 (76.7) | 127 (73.4) | 67 (83.8) | |
| 2 | 44(17.4) | 33 (19.1) | 11 (13.8) | |
| >2 | 6 (2.8) | 5 (2.9) | 1 (1.3) | |
| Patients with DES – no. (%) | 137 (54.2) | 95 (54.9) | 42 (52.5) | 0.683 |
| IIb-IIIa inhibitor use – no. (%) | 183 (72.3) | 126 (72.8) | 57 (71.3) | 0.761 |
Results
Two hundred and fifty-three patients were enrolled from three centers. There was 100% follow-up of all patients. Baseline characteristics are displayed in Table 1. Papaverine was administered to obtain maximal hyperemia during the IMR measurement 177 patients. There was one case of ventricular fibrillation, which required electrical cardioversion. In one case IMR could not be calculated because of inadequate thermodilution curves. This patient was not included in the analysis. The median and mean IMR at the end of the procedure were 31 (interquartile range, 20.8 – 49.4) and 40 ±32, respectively. Eighty patients (31.6%) had an IMR > 40, while 31 (12.3%) had an FFR ≤ 0.80. Ejection fraction on echocardiography performed during the initial hospitalization correlated with IMR (r=-0.31, p<0.001). The median follow-up duration was 2.8 years, and there were 11 (4.3%) deaths and 24 (9.5%) hospitalizations for heart failure.
At one year, patients with an IMR ≤ 40 had a significantly lower rate of the primary endpoint, death or rehospitalization for heart failure compared to patients with an IMR > 40 (6.6% vs. 17.1%, p=0.027). Over the entire follow-up period, the rate of death or rehospitalization for heart failure was significantly lower in those patients with an IMR ≤ 40 compared to those with an IMR > 40 (11.0 vs. 20.0%, p=0.04) (Figure 1A). The rate of death was significantly lower in those patients with an IMR ≤ 40 compared to those with an IMR > 40 (2.3 vs. 8.8%, p=0.04) (Figure 1B). In the patients who had both an IMR > 40 and an FFR ≤ 0.80, the rate of death was 25.0%, compared to 3.7% in those who did not meet these criteria (p=0.04). The Kaplan-Meier curves displaying the relationship between IMR > 40 and survival free of death or rehospitalization for heart failure, and between IMR > 40 and survival free of death are shown in Figure 2A and Figure 2B.
Figure 1.
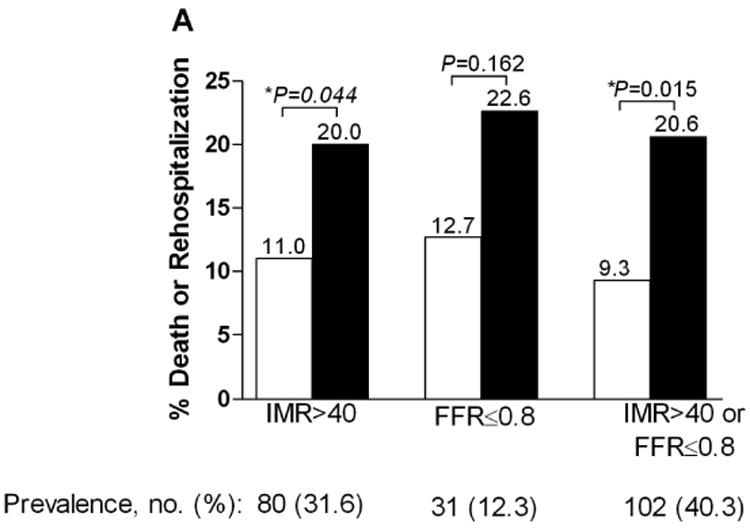
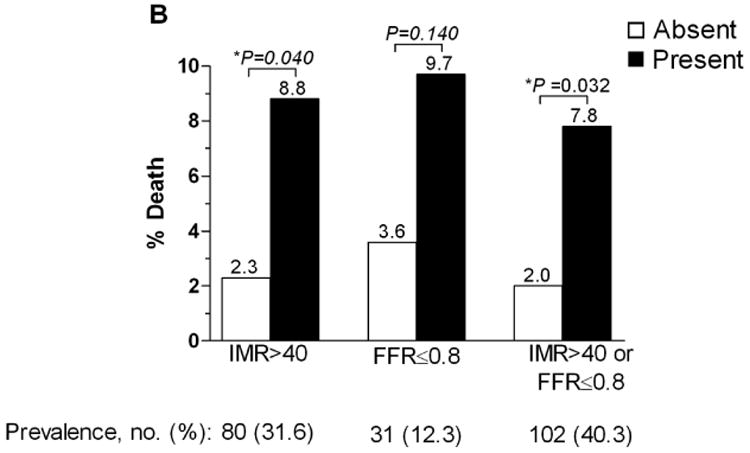
A. The percentage of patients suffering death or rehospitalization for heart failure based on presence or absence of an IMR > 40, FFR < 0.80, and an IMR > 40 or FFR < 0.80. B. The percentage of patients suffering death based on presence or absence of an IMR > 40, FFR < 0.80, and an IMR > 40 or FFR < 0.80.
Figure 2.
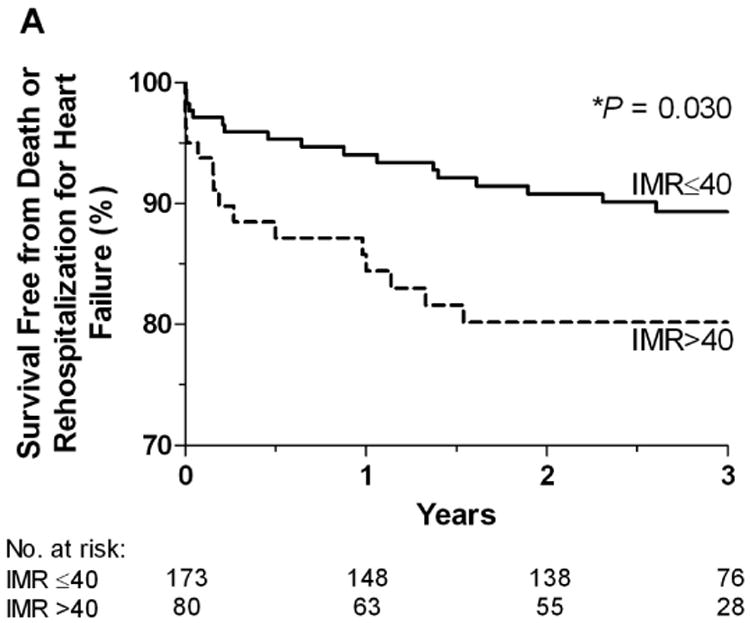
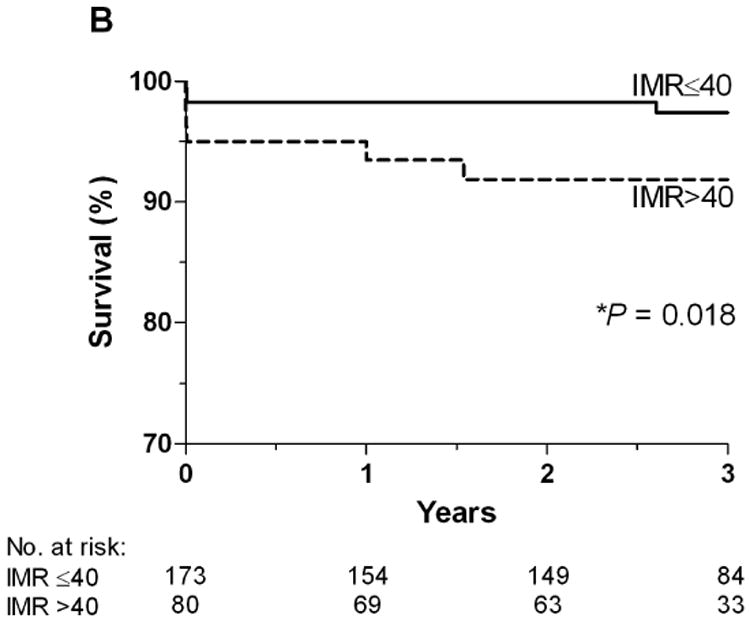
A. The Kaplan-Meier curves displaying the relationship between IMR > 40 and survival free of death or rehospitalization for heart failure. B. The Kaplan-Meier curves displaying the relationship between IMR > 40 and survival free of death.
The univariable and multivariable predictors of death or rehospitalization for heart failure are shown in Table 2. An IMR > 40 was a significant predictor of death or rehospitalization for heart failure (hazard ratio [HR] 2.1; 95% confidence interval [CI] 1.1-4.1; p=0.03). On multivariable analysis, an IMR > 40 was an independent predictor of death or rehospitalization for heart failure (HR 2.2; 95% CI 1.1 - 4.5; p=0.03). The other independent predictors were an FFR ≤0.8 (HR 3.24, p=0.008) and diabetes (HR 4.4, p<0.001). The univariable predictors of death alone are shown in Table 3. An IMR > 40 was a significant univariable predictor of death (HR 4.0; 95% CI 1.2 - 13.5; p=0.03). On multivariable analysis, IMR > 40 was the only independent predictor of death (HR 4.3; 95% CI, 1.3 – 15.0; p=0.02) (Table 3).
Table 2. Univariable and Multivariable Predictors of Death or Rehospitalization due to Heart Failure (p<0.1).
| Univariable Predictors | p-value | Hazard ratio | 95% CI |
|---|---|---|---|
| Diabetes | <0.001 | 3.98 | 2.05 – 7.75 |
| CFR < 2 | 0.021 | 3.40 | 1.20 – 9.66 |
| Hypertension | 0.030 | 2.15 | 1.08 – 4.27 |
| IMR > 40 | 0.034 | 2.08 | 1.06 – 4.07 |
| Age | 0.058 | 1.03 | 1.00 – 1.06 |
| FFR≤ 0.8 | 0.072 | 2.15 | 0.93 – 4.94 |
| TIMI myocardial perfusion grade < 3 | 0.087 | 1.95 | 0.91 – 4.18 |
| Multivariable Predictors | |||
| Diabetes | <0.001 | 4.44 | 2.22 – 8.88 |
| FFR≤ 0.8 | 0.008 | 3.24 | 1.35 – 7.76 |
| IMR > 40 | 0.026 | 2.23 | 1.10 – 4.49 |
Table 3. Univariable and Multivarible Predictors of Death (p<0.1).
| Univariable Predictors | p-value | Hazard ratio | 95% CI |
|---|---|---|---|
| IMR > 40 | 0.028 | 3.95 | 1.16 – 13.50 |
| FFR ≤ 0.8 | 0.09 | 3.16 | 0.84 – 11.94 |
| TIMI myocardial perfusion grade < 3 | 0.084 | 2.96 | 0.87 – 10.14 |
| Multivariable Predictors | |||
| IMR > 40 | 0.020 | 4.34 | 1.26 – 15.00 |
An IMR value greater than the median value of 31 was a univariable predictor of death or rehospitalization for heart failure (HR 3.1; CI 1.4-6.6; p=0.004). On multivariable analysis, an IMR > 31 was an independent predictor of death or rehospitalization for heart failure (HR 3.1; 95% CI 1.4-6.7; p=0.005). The median IMR was not a predictor of death alone.
Discussion
The main finding in this study is that IMR, measured at the time of primary PCI for STEMI, is an independent predictor of long-term clinical outcome, including death alone and death or rehospitalization because of congestive heart failure, whereas other common invasive methods for assessing microvascular function are not. An interesting secondary finding is that FFR measured at the same time is also predictive of clinical outcome. These findings reinforce the importance of microvascular dysfunction in determining outcome after STEMI. They also highlight the independent effects of epicardial coronary artery disease, as assessed by FFR, and of microvascular dysfunction, as assessed by IMR, on patient outcomes after STEMI.
A number of previous studies have described the relationship between microvascular dysfunction occurring due to STEMI and adverse cardiac outcomes.1,2 Therapies aimed specifically at treating acute microvascular dysfunction secondary to STEMI are now being developed and tested.14 The benefit of any new therapy may be greatest if delivered acutely in patients with greater degrees of microvascular disruption. Unfortunately, previously reported methods for assessing microvascular dysfunction acutely in the cardiac catheterization laboratory such as CFR and TMPG are qualitative, lack reproducibility, are not independent of the epicardial coronary artery, and/or are unwieldy and difficult to apply in this setting.6,15-17
IMR is a quantitative, reproducible index which is independent of epicardial coronary disease and specific for the microcirculation and which can be measured relatively easily at the time of STEMI.5-8 A normal value is generally considered to be < 25.7,18 We and others have found that an elevated IMR at the time of STEMI predicts a larger degree of myocardial damage as assessed by cardiac enzyme elevation and noninvasive assessment with cardiac magnetic resonance imaging or positron emission tomography.8-10 In addition, there is less recovery of left ventricular function over time in patients with an elevated IMR.8-10
In the current study, we extend these findings by showing that in patients with an IMR greater than the mean value of 40, the rate of death or rehospitalization for congestive heart failure was significantly higher compared to patients with an IMR ≤ 40 (20 vs. 11%, p=0.04), and the rate of death alone was also significantly higher (8.8 vs. 2.3%, p=0.04). On multivariate analysis, IMR was an independent predictor of both survival alone and survival or rehospitalization for congestive heart failure, while other common invasive methods for assessing the microvasculature were not. The relationship between IMR and adverse long-term outcomes is likely explained by the correlation between an elevated IMR and increased myocardial damage with greater left ventricular dysfunction.8-10 The main clinical implication of these findings is that by measuring IMR at the time of STEMI, one can identify the highest risk patients who might benefit most from closer follow-up or early intervention with novel therapies aimed at microvascular recovery. For example, administration of intracoronary streptokinase has been shown to result in less microvascular damage based on IMR assessment at the time of primary PCI for STEMI.14 Moreover, delivery of autologous stem cells may have its greatest effect on the highest risk patients.19
FFR measurement in the culprit vessel at the time of STEMI is not advocated for assessing residual epicardial disease because the acute microvascular dysfunction that occurs with STEMI may be partially reversible resulting in an FFR value which may be lower if measured again at a later timepoint, when any reversible component of the microvascular dysfunction has resolved. Nevertheless it appears that those patients who have an FFR ≤ 0.80 in the culprit vessel at the time of STEMI are at increased risk for future adverse events. The association between an ischemic FFR after primary PCI and decreased survival may be due to the residual epicardial disease and its association with recurrent ischemic events.20 Likewise, the fact that diabetes is also an independent predictor of death or rehospitalization for heart failure may reflect the impact of diabetes on both epicardial coronary artery disease and microvascular dysfunction.
The limitations of this study include the lack of data regarding the extent of coronary disease in the patients included. The mortality rate in this study was lower than in other reports of patients with STEMI.21 This suggests that the patients included in this study may represent a lower risk population. One would expect, however, that if higher risk patients had been included, the negative prognostic value of a high IMR would only be more pronounced. Finally, the number of events in this study was relatively small; the findings will need to be confirmed in a larger study.
Conclusion
In conclusion, this study demonstrates that IMR measured at the time of primary PCI for STEMI predicts longer term clinical outcomes such as death and rehospitalization for heart failure. IMR may be a useful method for identifying a high risk cohort of patients whom would benefit most from novel therapies to salvage acutely damaged myocardium.
Acknowledgments
Funding Sources: This work was supported in part by grant 1 K23 HL072808-01 A1 (WFF) and R01 HL093475-01A1 (WFF) from the National Institutes of Health, Heart Lung and Blood Institute, Bethesda, MD.
Footnotes
Conflict of Interest Disclosures: Dr. Fearon receives institutional research support from St. Jude Medical. The other authors have no conflicts of interest related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawamoto T, Yoshida K, Akasaka T, Hozumi T, Takagi T, Kaji S, Ueda Y. Can coronary blood flow velocity pattern after primary percutaneous transluminal coronary angioplasty [correction of angiography] predict recovery of regional left ventricular function in patients with acute myocardial infarction? Circulation. 1999;100:339–345. doi: 10.1161/01.cir.100.4.339. [DOI] [PubMed] [Google Scholar]
- 2.Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, McCabe CH, Van De Werf F, Braunwald E. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125–130. doi: 10.1161/01.cir.101.2.125. [DOI] [PubMed] [Google Scholar]
- 3.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 4.Camici PG, Crea F. Coronary microvascular dysfunction. New Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 5.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 6.Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–2061. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- 7.Aarnoudse W, Fearon WF, Manoharan G, Geven M, van de Vosse F, Rutten M, De Bruyne B, Pijls NH. Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation. 2004;110:2137–2142. doi: 10.1161/01.CIR.0000143893.18451.0E. [DOI] [PubMed] [Google Scholar]
- 8.Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, Schnittger I, Lee DP, Vagelos RH, Fitzgerald PJ, Yock PG, Yeung AC. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;51:560–565. doi: 10.1016/j.jacc.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 9.Lim HS, Yoon MH, Tahk SJ, Yang HM, Choi BJ, Choi SY, Sheen SS, Hwang GS, Kang SJ, Shin JH. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. Eur Heart J. 2009;30:2854–2860. doi: 10.1093/eurheartj/ehp313. [DOI] [PubMed] [Google Scholar]
- 10.McGeoch R, Watkins S, Berry C, Steedman T, Davie A, Byrne J, Hillis S, Lindsay M, Robb S, Dargie H, Oldroyd K. The index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2010;3:715–722. doi: 10.1016/j.jcin.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 11.De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–2006. doi: 10.1161/hc4201.099223. [DOI] [PubMed] [Google Scholar]
- 12.Pijls NH, De Bruyne B, Smith L, Aarnoudse W, Barbato E, Bartunek J, Bech GJ, Van De Vosse F. Coronary thermodilution to assess flow reserve: validation in humans. Circulation. 2002;105:2482–2486. doi: 10.1161/01.cir.0000017199.09457.3d. [DOI] [PubMed] [Google Scholar]
- 13.Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr, Alexander B, Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 14.Sezer M, Oflaz H, Goren T, Okçular I, Umman B, Nisanci Y, Bilge AK, Sanli Y, Meriç M, Umman S. Intracoronary streptokinase after primary percutaneous coronary intervention. N Engl J Med. 2007;356:1823–1834. doi: 10.1056/NEJMoa054374. [DOI] [PubMed] [Google Scholar]
- 15.Kern MJ. Coronary physiology revisited : practical insights from the cardiac catheterization laboratory. Circulation. 2000;101:1344–1351. doi: 10.1161/01.cir.101.11.1344. [DOI] [PubMed] [Google Scholar]
- 16.De Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94:1842–1849. doi: 10.1161/01.cir.94.8.1842. [DOI] [PubMed] [Google Scholar]
- 17.Ali A, Cox D, Dib N, Brodie B, Berman D, Gupta N, Browne K, Iwaoka R, Azrin M, Stapleton D, Setum C, Popma J. AIMI Investigators. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J Am Coll Cardiol. 2006;48:244–252. doi: 10.1016/j.jacc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Melikian N, Vercauteren S, Fearon WF, Cuisset T, MacCarthy PA, Davidavicius G, Aarnoudse W, Bartunek J, Vanderheyden M, Wyffels E, Wijns W, Heyndrickx GR, Pijls NH, de Bruyne B. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention. 2010;5:939–945. [PubMed] [Google Scholar]
- 19.Strauer BE, Steinhoff G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol. 2011;58:1095–1104. doi: 10.1016/j.jacc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Bech GJ, Pijls NH, De Bruyne B, Peels KH, Michels HR, Bonnier HJ, Koolen JJ. Usefulness of fractional flow reserve to predict clinical outcome after balloon angioplasty. Circulation. 1999;99:883–888. doi: 10.1161/01.cir.99.7.883. [DOI] [PubMed] [Google Scholar]
- 21.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz CB, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso JE, Tracy CM, Woo YJ, Zhao DX CF/AHA Task Force. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]


