Abstract
Background
Treatments for squamous cell carcinoma of the head and neck (HNSCC) are associated with toxicities that lead to emergency department (ED) presentation.
Methods
We utilized data from an ongoing prospective cohort of newly diagnosed, previously untreated patients (N=298) with HNSCC to evaluate the association between clinical and epidemiologic factors and risk and frequency of ED presentation. Time to event was calculated from the date of treatment initiation to ED presentation, date of death, or current date. Frequency of ED presentation was the sum of ED visits during the follow-up time.
Results
History of hypertension, normal/underweight body mass index (BMI), and probable depression predicted increased risk of ED presentation. BMI and severe pain were associated with higher frequency of ED presentation.
Conclusions
Clinical and epidemiologic factors can help predict HNSCC patients that will present to the ED to improve treatment-related patient outcomes and quality of life.
Keywords: chief complaints, emergency department, head and neck cancer, cohort study, treatment toxicity
INTRODUCTION
Head and neck cancer is the 6th most common malignancy globally.1 Nearly 90% of these tumors are classified as squamous cell carcinoma of the head and neck (HNSCC) and include cancer of the oral cavity, pharynx, and larynx. Survival rates for HNSCC are quite high when treated in early stages (5-year survival of approximately 75%),1 however many patients present with metastatic disease at diagnosis, resulting in a reduced survival of approximately 35%.1, 2
Advances in HNSCC treatment have improved survival even at advanced stages.3 However, these treatments are associated with significant toxicities that compromise patient functionality and quality of life and present significant management challenges for the clinical staff.2, 4 Emergency departments (EDs) are now a frequent site of care for many cancer patients.5 Visiting the ED may potentially reflect inadequacy in addressing or managing expected side effects or complications during routine care.5 Additionally, ED visits increase cost of care, result in unplanned hospitalizations and breaks in treatment, and are associated with poor overall survival and other cancer-related outcomes.5, 6
In the present study, we used an ongoing prospective cohort of patients (N=298) with HNSCC to determine the chief complaints during ED visits, risk of ED visit after treatment initiation, and frequency of ED visits. We assessed the importance of clinical, epidemiological, and behavioral factors associated with their ED presentation with the aim of identifying modifiable risk factors associated with overall cancer-related outcomes.
MATERIALS AND METHODS
Study Population
The study population consists of participants in an ongoing prospective cohort study of HNSCC at The University of Texas MD Anderson Cancer Center. These patients are newly diagnosed, histologically confirmed, HNSCC being treated at MD Anderson. This study was approved by the Institutional Review Board at MD Anderson, and informed consent was obtained from each patient.
Exclusions and Eligibility
The study includes newly diagnosed patients (with no prior history of cancer or previous cancer treatment) over the age of 18 with loco-regional HNSCC being treated at MD Anderson. Patients were English or Spanish speaking and able to provide written informed consent. Patients were excluded from the study if they had distant metastasis or were participating in clinical trials for pain. Additionally, 9 individuals withdrew from the study to receive treatment outside MD Anderson and were excluded from the present analysis. A total of 298 individuals are included in the present study.
Study Setting
The Multidisciplinary Head and Neck Cancer Center: Patients first present to the center for treatment evaluation. A multidisciplinary team of surgical, medical, radiation oncologists, dental oncology, speech language pathology, nutrition, and social work consult to recommend the best treatment option. Trained MD Anderson staff administered informed consent and questionnaires to patients prior to evaluation by clinicians. Data were collected at baseline, weekly during treatment, and during clinic visits.
Emergency Department: Established in 2010 as the first academic department of emergency medicine at a comprehensive cancer center, the MD Anderson ED has 44 beds. In 2014, the department received more than 20,000 patient visits. The data related to each ED visit include primary and secondary presenting symptoms/complaints, number and frequency of emergency visits, symptom severity, disposition (discharged or admitted to hospital/intensive care unit) and discharge diagnosis.
Epidemiology and Clinical Data Collection at Presentation to the Cancer Center
Trained MD Anderson staff administered questionnaires to patients presenting at the Head and Neck Center, prior to being seen by clinicians. The questionnaire was developed by an interdisciplinary team of epidemiologists, behavioral scientists, and medical oncologists in order to understand the epidemiology of the different types and cancers and the underlying factors associated with cancer risk, progression and survival. Clinical data was abstracted from patients’ charts.
Outcome Variables
Chief Complaints and Discharge Diagnoses
The chief complaint is the stated reason for the ED visit captured when the patient arrives in the ED. There is no standard nomenclature or coding mechanism for ED chief complaints. In order to clean and standardize the chief complaint fields, we utilized previously described categories for ED visits in cancer patients to categorize the chief complaints into 11 broad categories (see Table 1) 5; gastrointestinal (GI), bleeding, cardiovascular, fever, injury, malaise, neurologic, pain, respiratory, syncope and other.
Table 1.
Chief Complaints by Category
| Chief Complaint Category | Types of Chief Complaints Included in Category |
|---|---|
| Bleeding | Bleeding, bleed, blood |
| Cardiovascular | Hypotension, tachycardia |
| Fever | Fever, chills |
| Gastrointestinal | Diarrhea, vomiting, nausea, constipation, can’t/won’t eat, dehydration, unable to eat, feed tube complication, unable to swallow, dysphagia |
| Injury | Fall, fall with laceration |
| Malaise | Weak, weakness, fatigue, failure to thrive |
| Neurologic | Altered mental status, vision changes, confusion |
| Pain | pain, abdominal pain, back pain, headache, chest pain, epigastric pain, mouth sores/pain oral pain, leg pain, pain and swelling |
| Respiratory | Shortness of breath, cough, trouble breathing |
| Syncope | Syncope, dizziness, fainting |
| Other | Neck tightness, rash, neck rash, multiple complaints, Inability to urinate, eye irritation |
The International Classification of Diseases, Ninth Revisions (ICD-9) is the system of assigning codes to diagnoses and procedures associated with hospital use in the United States. Generally, ED visits are assigned more than one ICD-9 per visit. For this study, we evaluated ICD-9 codes in positions 1 to 5 for the purpose of describing the top discharge diagnoses associated with the primary chief complaints. ICD-9 codes associated with the tumor (ICD-9 codes 140-239 “Neoplasms”) were not included as all the study subjects were known to have head and neck cancer.
Risk and Frequency of ED visits
For the descriptive analysis, individuals were coded as 0 if they had no ED visits, and 1 if they had at least one ED visit after treatment initiation. Follow-up time was defined as time from treatment initiation to first ED visit (for those who presented to the ED at least once), time from treatment initiation to the current date (for individuals with no ED visits) and time from treatment initiation to death (for individuals that died during the follow-up period, but did not present to the ED). Frequency of the ED visit was defined as number of ED visits during the follow-up time.
Independent Variables
Epidemiologic factors include age, sex, race/ethnicity (defined as white versus non-white), education (less than or greater than high school education) and marital status (married versus not married). Body mass index (BMI) was categorized according to the standard categorizations of the World Health Organization (underweight: BMI < 18.5 kg/m2, normal: BMI 18.6–25 kg/m2, overweight: BMI 25–29.9 kg/m2, and obese: BMI>30 + kg/m2). Due to the small number of patients in the underweight category (N=9), underweight and normal weight participants were combined. Previous studies and our preliminary analysis suggested that higher BMI is protective against adverse cancer-related outcomes.7 Therefore, we dichotomized BMI as overweight/obese versus normal/underweight, and used the overweight/obese category as the reference in the analysis. Depression, alcohol use, smoking status (never/ever), fatigue, and pain were also assessed. We used a single item measure to assess fatigue (“during the past 4 weeks, did you have a lot of energy?”). Depression was assessed using the Center for Epidemiologic Studies Depression (CESD) scale which is a 20 question self-report instrument, scored on a scale of 0–60, used to measure several functional domains commonly linked to depression.8 The CESD score was calculated and a cut off of 23 was used to define “probable depression” according to the literature.9 Alcohol intake was categorized according to number of drinks per week (0, 1–6, 7–13, or 14+). Self-reported pretreatment pain was assessed on a scale of 0–10 (0 representing no pain). Since normality was not met, we used the National Comprehensive Cancer Network cutoff score of ≥7 for severe pain. Treatment modality was coded as surgery, radiation, or chemotherapy/chemoradiation. Cancer stage was divided into categories according to the Surveillance, Epidemiology, and End Results (SEER) program (in situ, localized, regional, distant site, unknown/unstaged). Due to the collinearity between treatment modality and cancer stage, we only included treatment in the present analysis. Analysis including stage of disease instead of cancer treatment are consistent with the findings described below.
Statistical Analysis
We performed descriptive analyses of patient characteristics of those with no ED visits versus those with at least 1 ED visit. Chief complaints were assessed by time to first ED visit (<14 days, 14–29 days, 30–89 days, 90–179 days, 180+ days). Top discharge diagnoses by ICD-9 category and top ICD-9 codes associated with the first ED visit were also described for those individuals with at least 1 ED visit.
We evaluated the role of the clinical and epidemiologic factors in risk of ED visit using Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Predictors with a p-value of <0.1 in the univariate analysis and a priori selected predictors with suspected associations with clinical outcomes (age, gender, ethnicity) were included in the multivariable analysis. Adherence to the Cox proportional hazards assumption was confirmed by plotting the Schoenfled residuals.
Linear regression models were used to evaluate the association between clinical and epidemiologic factors and frequency of ED visit. We took the natural log of the frequency of ED visits to account for the non-normal distribution of the ED visit data. In order to take into account individuals with 0 ED visits, “1” was added to the frequency of the ED visit for every subject. Univariate analysis including follow-up time was conducted to determine inclusion in the multivariable model. Variable selection was conducted in a similar manner to the time-to-event analysis.
Finally, we tested for multiplicative interactions between treatment type and the variables with significant associations in each analysis to account for potential residual confounding due to treatment. We included the cross product term of the treatment variable (chemotherapy/chemoradiation versus other) and the predictors of interest in the multivariable model. The statistical significance of the interaction term was determined using the Wald statistic. All analyses were conducted using Stata statistical software (version 14.0; StataCorp LP, College Station, Texas).
RESULTS
A total of 97 patients presented to the ED at least once in this study. Median time to first ED presentation was 30 days with a mean time of approximately 52 days. Individuals that visited the ED during the study period presented to the ED between 1–6 times. The median frequency of ED presentation was 1 ED visit, with a mean of approximately 2 ED (1.86) visits.
Chief Complaints and Discharge Diagnoses
Distribution of the chief complaints according to time to first ED visit from treatment initiation are shown in Figure 1. The most common chief complaints within 14 days of treatment initiation were GI (29.03%) and pain (19.35%). Pain persisted as a top chief complaint associated with presentation to the ED past 180 days.
Figure 1.
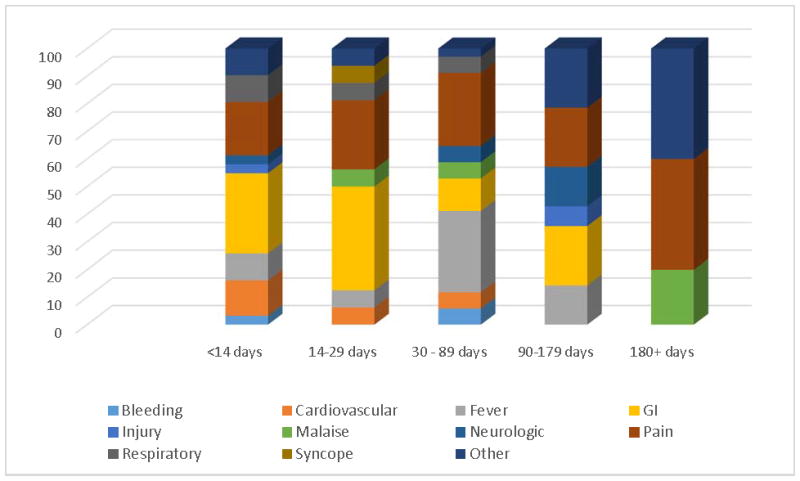
Chief Complaints for First ED visits and Time from Treatment Initiation
Table 2 describes the top diagnosis categories, ICD-9 codes, and descriptions starting with the most frequently occurring, of the overall population with at least 1 ED visit and the subset of those admitted to the hospital or intensive care unit. The top diagnosis categories and ICD-9 codes were consistent between the two groups with “Symptoms” and “Endocrine, Nutritional, Metabolic, Immunity” categories being the most common discharge diagnosis categories.
Table 2.
Diagnoses for all ED Presentations
| Category | Top ICD-9 codes | |
|---|---|---|
| Code | Description | |
| Symptoms | 787.01 | Nausea and vomiting |
| 786.2 | Cough; chronic cough | |
| 786.05 | Shortness of breath | |
| Endocrine, Nutritional, Metabolic, Immunity | 276.51 | Dehydration |
| 275.2 | Disorders of Magnesium metabolism | |
| 276.8 | Hypokalemia | |
| Digestive System | 528 | Oral soft tissue disease |
| 528.01 | Mucositis (ulcerative) due to antineoplastic therapy | |
| 530.5 | Dyskinesia of esophagus | |
| Nervous System and Sense Organs | 338.3 | Neoplasm related pain (acute) (chronic) |
| 338.19 | other acute pain | |
| 346.93 | Migraine, unspecified, with intractable migraine, so stated, with status migrainosus | |
| Respiratory | 486 | Pneumonia; community-acquired pneumonia (CAP); nosocomial pneumonia (hospital-acquired) |
| 482.9 | Bacterial Pneumonia, unspecified | |
| 482.89 | Pneumonia due to other specified bacteria | |
Abbreviations: ED, Emergency Department; ICD-9, International Statistical Classification of Diseases and Related Health Problems, 9th revision.
Factors Associated with Emergency Department Presentation
Distribution of the patient characteristics between those that presented to the ED and those that did not are shown in Table 3. Individuals with at least one ED visit were more likely to fall in the normal weight category according to BMI, be treated with chemotherapy/chemoradiation, present with severe pain at baseline, have a history of hypertension, and be classified as having depression according to a CESD score of greater than or equal to 23 (p-value <0.03 for each). Distribution of gender was different between those that presented to the ED at least once and those that did not (27% female versus 18% male) however the difference was not statistically significant (P-value =0.08).
Table 3.
Descriptive Statistics for Cohort of Head and Neck Cancer Patients (N=298)*
| At least one ED visit | Chi-2 P-value | ||
|---|---|---|---|
| Yes (N=97) | No (N=201) | ||
| No. of patients (%) | No. of patients (%) | ||
| Male | |||
| Female | 26 (27) | 36 (18) | |
| Male | 71 (73) | 165 (82) | 0.08 |
| Age | |||
| <60 | 48 (50) | 114 (57) | |
| 60 + | 49 (50) | 87 (43) | 0.24 |
| BMI (kg/m2) | |||
| Underweight/Normal | 42 (44) | 54 (27) | |
| Overweight | 30 (32) | 91 (47) | |
| Obese | 23 (24) | 52 (26) | 0.01 |
| Race | |||
| White | 82 (85) | 171 (85) | |
| Non-White | 15 (15) | 30 (15) | 0.9 |
| Smoking Status | |||
| Never | 43 (57) | 86 (56) | |
| Ever | 33 (43) | 67 (44) | 0.96 |
| Alcohol (drinks/week) | |||
| 0 | 40 (63) | 69 (51) | |
| 1–6 | 16 (25) | 32 (24) | |
| 7–13 | 2 (3) | 15 (11) | |
| 14+ | 6 (9) | 20 (15) | 0.15 |
| Marital Status | |||
| Not-married | 23 (24) | 56 (28) | |
| Married | 73 (76) | 141 (72) | 0.42 |
| Education | |||
| Less Than HS | 30 (32) | 51 (26) | |
| HS or Higher | 64 (68) | 145 (74) | 0.3 |
| Treatment | |||
| Surgery only | 14 (14) | 56 (28) | |
| Radiation | 18 (19) | 56 (28) | |
| Chemo or Chemo-radiation | 65 (67) | 89 (44) | 0.001 |
| Severe Pain | |||
| No (Score < 7) | 70 (72) | 167 (83) | |
| Yes (Score 7+) | 27 (28) | 34 (17) | 0.03 |
| SEER Stage | |||
| In Situ | 1 (1) | 3 (2) | |
| Localized | 7 (7) | 41 (20) | |
| Regional | 63 (65) | 94 (47) | |
| Distant | 9 (9) | 11 (5) | |
| Unstaged/Unknown | 17 (18) | 52 (26) | 0.01 |
| CHD | |||
| No | 80 (87) | 173 (91) | |
| Yes | 12 (13) | 18 (9) | 0.35 |
| Stroke | |||
| No | 88 (97) | 185 () | |
| Yes | 3 (3) | 5 (3) | 0.72 |
| Hypertension | |||
| No | 35 (38) | 112 (58) | |
| Yes | 57 (62) | 80 (42) | 0.001 |
| Diabetes | |||
| No | 80 (87) | 173 (90) | |
| Yes | 12 (13) | 20 (10) | 0.5 |
| Lung Disease | |||
| No | 89 (96) | 184 (97) | |
| Yes | 4 (4) | 6 (3) | 0.63 |
| Depression (CESD) | |||
| <23 | 63 (72) | 167 (90) | |
| 23 + | 24 (28) | 19 (10) | <0.001 |
| Fatigue | |||
| No | 31 (33) | 79 (42) | |
| Yes | 63 (67) | 109 (58) | 0.14 |
missing values not included in the table so not all categories will add up to 298
Abbreviations: ED, Emergency Department; BMI, body mass index; HS, high school; SEER, Surveillance, Epidemiology, and End Results; CHD, coronary heart disease; CESD, the Center for Epidemiologic Studies Depression scale.
Risk for Emergency Department Presentation
Univariate and multivariable analysis for risk of ED presentation are shown in Table 4. In the univariate analysis, chemotherapy/chemoradiation (HR=2.41, 95% CI: 1.34–4.30), severe pretreatment pain (HR=1.82, 95% CI: 1.16–2.83), history of hypertension (HR=2.11, 95% CI: 1.38–3.21), normal BMI (HR=1.70 95% CI: 1.13–2.55), and depression (HR=2.71, 95% CI: 1.69–4.35) significantly associated increased risk of ED presentation.
Table 4.
Univariate and Multivariable Time to Event Analysis of First ED Presentation
| Univariate | Multivariable (Total No. multivariable analysis=252) | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender | ||||
| Female | 1(REF) | 1 (REF) | ||
| Male | 0.65 (0.42–1.02) | 0.06 | 0.67 (0.39–1.17) | 0.16 |
| Age | ||||
| <60 | 1(REF) | 1 (REF) | ||
| 60+ | 1.27 (0.85–1.89) | 0.24 | 1.21 (0.75–1.98) | 0.44 |
| BMI (kg/m2) | ||||
| Overweight/Obese | 1 (REF) | 1 (REF) | ||
| Normal | 1.70 (1.13–2.55) | 0.01 | 1.86 (1.18–2.94) | 0.01 |
| Race | ||||
| White | 1(REF) | 1(REF) | ||
| Non-White | 1.04 (0.60–1.81) | 0.85 | 1.61 (0.75–3.43) | 0.22 |
| Smoking | ||||
| Never | 1(REF) | --- | --- | |
| Ever | 0.98 (0.62–1.55) | 0.86 | --- | --- |
| Alcohol | ||||
| 0 | 1 (REF) | |||
| 1–6 | 0.87 (0.48–1.54) | 0.62 | --- | --- |
| 7–13 | 0.27 (0.06–1.10) | 0.07 | --- | --- |
| 14+ | 0.51 (0.22–1.21) | 0.13 | --- | --- |
| Marital Status | ||||
| Unmarried | 1(REF) | --- | --- | |
| Married | 1.12 (0.70–1.78) | 0.65 | --- | --- |
| Education | ||||
| < High School | 1(REF) | --- | --- | |
| High School + | 0.76 (0.49–1.17) | 0.21 | --- | --- |
| Treatment | ||||
| Surgery only | 1(REF) | 1 (REF) | ||
| Radiation | 1.23 (0.61–2.48) | 0.56 | 0.83 (0.38–1.80) | 0.64 |
| Chemo or Chemo-radiation | 2.41 (1.34–4.30) | 0.003 | 1.80 (0.94–3.45) | 0.07 |
| Severe Pain | ||||
| No (Score < 7) | 1 (REF) | 1 (REF) | ||
| Yes (Score 7+) | 1.82 (1.16–2.83) | 0.01 | 1.66 (0.96–2.87) | 0.07 |
| CHD | ||||
| No | 1(REF) | --- | --- | |
| Yes | 1.52 (0.83–2.79) | 0.18 | --- | --- |
| Stroke | ||||
| No | 1(REF) | --- | --- | |
| Yes | 1.37 (0.43–4.35) | 0.59 | --- | --- |
| Hypertension | ||||
| No | 1(REF) | 1 (REF) | ||
| Yes | 2.11 (1.38–3.21) | 0.001 | 2.12 (1.29–3.45) | 0.003 |
| Diabetes | ||||
| No | 1(REF) | --- | --- | |
| Yes | 1.37 (0.75–2.53) | 0.3 | --- | --- |
| Lung Disease | ||||
| No | 1(REF) | --- | --- | |
| Yes | 1.33 (0.49–3.64) | 0.57 | --- | --- |
| Probable depression (CESD) | ||||
| No | 1(REF) | 1 (REF) | ||
| Yes | 2.71 (1.69–4.35) | <0.001 | 2.06 (1.16–3.65) | 0.01 |
| Fatigue | ||||
| No | 1(REF) | 1 (REF) | ||
| Yes | 1.47 (0.96–2.26) | 0.08 | 0.90 (0.54–1.53) | 0.7 |
Missing excluded from multivariable analysis Total N = 264 N = EC visits = 80
Multivariable Analyses adjusting any variables with P<0.1 in the overall univariate analysis
Overall N for multivariate model= 252 ( Missing; Depression N =25, History of hypertension N=14, Fatigue N = 16, BMI N=6
Stage not included in multivariable analysis due to collinearity with treatment type.
Abbreviations: ED, Emergency Department; CI, confidence interval; HR, hazard ratio; BMI, body mass index; CHD, coronary heart disease; CESD, the Center for Epidemiologic Studies Depression scale.
In the multivariable analyses, normal BMI (HR = 1.86, 95% CI: 1.18–2.94), history of hypertension (HR=2.12, 95% CI: 1.29–3.45) and depression (HR =2.06, 95% CI: 1.16–3.65) remained significant predictors of increased risk of ED presentation. Treatment type (chemotherapy/chemoradiation HR= 1.80, 95% CI: 0.94–3.45) and severe pain (HR = 1.66 95% CI: 0.96–2.87) were marginally associated with risk of ED presentation in the multivariable model. There were no significant interactions between treatment type and the clinical/patient characteristics associated with risk of ED visit (not shown).
Frequency of Emergency Department Presentation
Univariate and multivariable linear regression models for frequency of ED visit are shown in Table 5. Treatment with chemotherapy/chemoradiation (P=0.02), severe pretreatment pain (P=0.04), history of hypertension (P=0.03), normal BMI (P=0.04) and depression (P=0.001) were all positively associated with frequency of ED visit in the univariate analysis. Associations between normal BMI (P=0.02) and severe pain (P=0.02) and frequency of ED visits persisted after mutual adjustment in the multivariable analysis. Treatment with chemotherapy/chemoradiation (P=0.09) and history of hypertension (P=0.09) were marginally significant. No interactions were observed between treatment type and the significant predictors of ED visit frequency (not shown).
Table 5.
Linear Model for Frequency of ED Visits Among Head and Neck Cancer Patients
| Univariate * | Multivariable ** | |||
|---|---|---|---|---|
| Beta (SD) | P-value | Beta (SD) | P-value | |
| Gender | ||||
| Female | REF | REF | ||
| Male | −0.02 | 0.81 | −0.02 | 0.83 |
| Age | ||||
| <60 | REF | REF | ||
| 60+ | 0.09 | 0.11 | 0.10 | 0.1 |
| BMI (kg/m2) | ||||
| Overweight/Obese | REF | REF | ||
| Normal | 0.12 | 0.04 | 0.15 | 0.02 |
| Race | ||||
| White | REF | REF | ||
| Non-White | 0.08 | 0.25 | −0.03 | 0.72 |
| Smoking | ||||
| Never | REF | -- | -- | |
| Ever | −0.12 | 0.1 | -- | -- |
| Alcohol | ||||
| 0 | REF | |||
| 1–6 | −0.05 | 0.53 | -- | -- |
| 7–13 | −0.16 | 0.23 | -- | -- |
| 14+ | −0.08 | 0.44 | -- | -- |
| Marital Status | -- | -- | ||
| Unmarried | REF | -- | -- | |
| Married | 0.05 | 0.39 | -- | -- |
| Education | -- | -- | ||
| < High School | REF | -- | -- | |
| High School + | −0.06 | 0.34 | -- | -- |
| Treatment | ||||
| Surgery only | REF | REF | ||
| Radiation | −0.02 | 0.98 | −0.02 | 0.72 |
| Chemo or Chemo-radiation | 0.17 | 0.02 | 0.13 | 0.09 |
| Severe Pain | ||||
| No (Score < 7) | REF | REF | ||
| Yes (Score 7+) | 0.14 | 0.04 | 0.19 | 0.02 |
| CHD | ||||
| No | REF | -- | -- | |
| Yes | 0.08 | 0.36 | -- | -- |
| Stroke | ||||
| No | REF | -- | -- | |
| Yes | 0.14 | 0.45 | -- | -- |
| Hypertension | ||||
| No | REF | REF | ||
| Yes | 0.13 | 0.03 | 0.10 | 0.09 |
| Diabetes | ||||
| No | REF | -- | -- | |
| Yes | −0.003 | 0.97 | -- | -- |
| Lung Disease | ||||
| No | REF | -- | -- | |
| Yes | −0.16 | 0.29 | -- | -- |
| Probable depression (CESD) | ||||
| No | REF | REF | ||
| Yes | 0.26 | 0.001 | 0.08 | 0.24 |
| Fatigue | ||||
| No | REF | -- | -- | |
| Yes | 0.09 | 0.12 | -- | -- |
Univariate models also include adjustment for follow-up time
Multivariable Analyses adjusting any variables with P<0.1 in the overall univariate analysis and follow-up time
Missing excluded from multivariable analysis; Total N = 264
Stage not included in multivariable analysis due to collinearity with treatment type
Abbreviations: ED, Emergency Department; SD, standard deviation; BMI, body mass index; CHD, coronary heart disease; CESD, the Center for Epidemiologic Studies Depression scale.
DISCUSSION
This is the first study to evaluate the association between clinical and epidemiologic factors and incidence of ED presentation in patients with HNSCC in a cohort study in the United States. A previous study in a Taiwanese population found that pain and gastrointestinal issues were primary complaints and for head and neck cancer (HNC) patients presenting to the ED, consistent with the findings from our study.10 This study identifies GI symptoms as the primary reason for ED presentation and one of the primary discharge diagnoses in this population, consistent with known symptoms of HNC treatment.4, 11, 12 Dysphagia (impairment of the swallowing process) is common in HNSCC patients, and is often under-diagnosed and improperly treated13, resulting in in dehydration and malnutrition 14 and may necessitate the use of non-oral nutritional support. A recent study aimed at understanding the management and prevention of acute and late effects due to HNC therapy, in particular treatment associated effects related to swallowing, suggested that precautions can be taken prior to, during and after treatment in order to minimize the impact of dysphagia13. Radiation can also lead to nausea, vomiting, and mouth ulcers, which are often a source of GI related symptoms in this cancer population.3, 11
Chemotherapy/chemoradiation was marginally associated with risk of and frequency of ED visit in this study. A majority (approximately 95%) of the chemotherapy/chemoradiation group was comprised of patients being treated with chemoradiation. Treatment for HNSCC is closely linked with stage of the disease and overall health of the patient.15 Treatment with chemoradiation has been associated with a variety of toxicities (severe fever, neutropenia, anemia, mucositis, hematologic toxicities).16 Our study suggests that treatment could be associated with risk or frequency of ED presentation and poorer overall cancer-related outcomes.
Pain is one of the first signs of HNC, and can be a result of the tumor itself or as a consequence of therapy. Up to 80% of patients with HNC report pain during treatment, and for nearly 40%, pain persists beyond treatment.17 We found that pain persisted as a top chief complaint associated with presentation to the ED past 180 days from treatment initiation. However, the role of pain in treatment naïve patient outcomes is less well understood. A recent study found that HNC patients with lymph node metastases were more likely to report severe pretreatment pain compared to patients without lymph node involvement, and that this was significantly correlated with measures of overall quality of life and increased symptom burden.4 Pre-treatment pain has been associated with poorer overall survival in patients with HNSCC18 thus suggesting the need for prompt pain treatment and management.
Depression occurs in over 10 % of cancer patients 19 and results in higher rates of mortality in cancer patients by up to 39%.20 The negative influence of depression on nutritional status and quality of life in patients with HNC has been previously demonstrated.21, 22 Baseline depression has been identified as a modifiable risk factor for malnutrition as a result of radiotherapy22 and the close correlation between psychosocial status and nutrition has been well documented in other cancer sites.23–26 Literature suggests that depression can be reliably diagnosed in oncology settings and that antidepressant medications and brief psychotherapy are effective for 60–80% of those affected.27, 28 Thus, the ED may be an important setting for screening for depression in this population.
The results of this study indicate that higher BMI may protect individuals from presentation to the ED, but history of hypertension, depression, and severe pain at diagnosis are positively associated with ED presentation and frequency. While high BMI has been linked with higher risk of several cancers, the association between pretreatment BMI and cancer-related outcomes and survival are not consistent across cancer sites.7, 29–31 A recent meta-analysis suggests that HNC patients with higher BMI prior to treatment have better survival rates, lower recurrence rates, and better distant failure or metastasis free survival rates compared to normal weight individuals.7 Our results are consistent with these findings. HNC patients experience significant weight loss throughout treatment, which is an important predictor of HNSCC outcomes.7, 32 Overweight and obese individuals may have higher nutritional reserves throughout therapy. Potentially, dietary interventions may be developed to maintain patient body weight throughout the course of treatment, in hopes of improving overall outcomes and reduce ED presentation in these patients.
Hypertension prior to cancer treatment has been associated with the development of myocardiopathy due to therapeutic agents used in cancer treatment.33 Cardiotoxicity of cancer treatment is an important concern, with a history of hypertension as a risk factor. A State of Science report on cancer treatment-related cardiotoxicity suggests a need for clinical assessment of cardiovascular risk prior to treatment initiation.34 Due to the association between history of hypertension and risk of ED presentation, we subsequently stratified each analysis by history of hypertension. These analyses yielded consistent results, however we found that the association between BMI and ED presentation only persisted in the subset of patients without a history of hypertension (no history of hypertension HR =3.47, 95% CI: 1.55–7.79 versus history of hypertension HR = 1.20, 95% CI: 0.64–2.26). These results suggest that history of hypertension is possibly a more important predictor of ED presentation than BMI. Further research is necessary to further elucidate the association between BMI, hypertension, and ED presentation.
The present study suggests that treatment modality, severe pretreatment pain, BMI, hypertension, and depression may be important predictors of ED presentation after treatment initiation and/or frequency of ED presentation. These factors have also been previously linked with HNSCC related outcomes, therefore ED presentation may be a potential mediator between these factors and HNSCC outcomes. The ED may act as the first patient care delivery site where intervention may be applied to remediate the factors that are associated with poor HNSCC outcomes. In addition to being the first of its kind, this study utilizes prospective data to evaluate the role of pretreatment risk factors and clinical characteristics.
This study has limitations. Firstly, the study has a relatively small sample size, which reduces the statistical power. However, a post-hoc sample size calculation using a failure (ED presentation) rate of approximately 33% suggests that 64 events and a total sample size of 200 are necessary to reach 80% power. Therefore, we believe the sample size of the present study is sufficient to detect meaningful associations that warrant further replication.
Information regarding human papillomavirus (HPV) status was missing, and previous studies have suggested differential cancer-related outcomes for patients with HPV-positive versus HPV-negative tumors. The study was also limited to HNSCC patients at one tertiary care cancer center, and therefore these results may not be generalizable to other head and neck cancer populations. Additionally, it is possible that this study did not capture all ED visits (i.e. if a patient went to an ED outside of MDACC). However, as these patients are being treated at MD Anderson, they are also likely to be referred back to MD Anderson ED for care. Therefore, we do not believe this to be a common occurrence in this population. Another limitation is the lack of data on the specific etiology of pain. Future studies on the cause of pain, the cause of dehydration, and other ED-related presentation as well as those that led to a hospital admission are ongoing. It is important to highlight integration of dental oncology in the overall multidisciplinary care of these patients. Routinely, patients are evaluated prior to therapy by our dental oncology team. This consultation is driven by site and planned treatment of the cancer. For example, all patients requiring radiation therapy as part of their planned treatment are evaluated by our dental oncology experts. In our dataset, 240 received oral care and 58 did not. When we analyzed data on the impact of oral care on the frequency of ED visits, we found that patients who received oral care were less likely to visit the ED (Mann-Whitney U test statistics=5766.00, two sided p =0.018).
This study provides a snapshot of the predictors of ED presentation in HNSCC patients in the United States. Further studies are necessary to validate our findings and provide mechanistic explanations for these associations. Efforts targeted at pain and GI distress in specific subgroups of the HNSCC patient population (those with pretreatment depression, hypertension, and low BMI) may improve overall quality of life and cancer-related outcomes by avoiding ED presentation in these patients.
Acknowledgments
This work is supported by the National Institutes of Health grant R01DE022891 (CCR, SS). This study, and Dr. Melkonian, are also funded in part by the Program in Oncologic Emergency Medicine. This research was also supported in part by the Barnhart Family Distinguished Professorship in Targeted Therapy (SS) and by the MD Anderson Cancer Center Support Grant (NIH grant P30 CA016672). We would like to thank our research team; Veronica Paredes, Guadalupe J Padilla, Mary Rose T Silva, Jazmin R. Menendez and Cindy K. Menendez and our patients.
Footnotes
The authors have no conflicts of interest to disclose
Author contributions; CRG designed the study and directed its implementation. SCM, CRG, SSS, designed and implemented the study’s analytic strategy and prepared the Materials and Methods and Discussion. Critical reviews and revisions were conducted by SCM, SSS, GBG, CL, MSC, SRB, EYH, SY and CRG.
References
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. Retrieved November 20, 2015. [Google Scholar]
- 2.Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer-part 1: epidemiology, presentation, and preservation. Clin Otolaryngol. 2011;36:65–68. doi: 10.1111/j.1749-4486.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- 3.Logan RM. Advances in understanding of toxicities of treatment for head and neck cancer. Oral Oncol. 2009;45:844–848. doi: 10.1016/j.oraloncology.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Hanna EY, Mendoza TR, Rosenthal DI, et al. The symptom burden of treatment-naive patients with head and neck cancer. Cancer. 2015;121:766–773. doi: 10.1002/cncr.29097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol. 2011;29:2683–2688. doi: 10.1200/JCO.2010.34.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys. 2007;68:654–661. doi: 10.1016/j.ijrobp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Hollander D, Kampman E, van Herpen CM. Pretreatment body mass index and head and neck cancer outcome: A review of the literature. Crit Rev Oncol Hematol. 2015;96:328–338. doi: 10.1016/j.critrevonc.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. J Youth Adolesc. 1991;20:149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- 9.Augustin LS, Gallus S, Negri E, La Vecchia C. Glycemic index, glycemic load and risk of gastric cancer. Ann Oncol. 2004;15:581–584. doi: 10.1093/annonc/mdh130. [DOI] [PubMed] [Google Scholar]
- 10.Tang PL, Cheng JS, Huang WC, Chang HS, Chen HC. Why do head and neck cancer patients visit the emergency department? Am J Emerg Med. 2015;33:1102–1105. doi: 10.1016/j.ajem.2015.04.077. [DOI] [PubMed] [Google Scholar]
- 11.Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer. 2004;91:447–452. doi: 10.1038/sj.bjc.6601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trotti A. Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47:1–12. doi: 10.1016/s0360-3016(99)00558-1. [DOI] [PubMed] [Google Scholar]
- 13.Schindler A, Denaro N, Russi EG, et al. Dysphagia in head and neck cancer patients treated with radiotherapy and systemic therapies: Literature review and consensus. Crit Rev Oncol Hematol. 2015;96:372–384. doi: 10.1016/j.critrevonc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Denaro N, Merlano MC, Russi EG. Dysphagia in Head and Neck Cancer Patients: Pretreatment Evaluation, Predictive Factors, and Assessment during Radio-Chemotherapy, Recommendations. Clin Exp Otorhinolaryngol. 2013;6:117–126. doi: 10.3342/ceo.2013.6.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Sarraf M. Treatment of locally advanced head and neck cancer: historical and critical review. Cancer Control. 2002;9:387–399. doi: 10.1177/107327480200900504. [DOI] [PubMed] [Google Scholar]
- 16.Givens DJ, Karnell LH, Gupta AK, et al. Adverse events associated with concurrent chemoradiation therapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1209–1217. doi: 10.1001/archoto.2009.174. [DOI] [PubMed] [Google Scholar]
- 17.Epstein JB, Hong C, Logan RM, et al. A systematic review of orofacial pain in patients receiving cancer therapy. Support Care Cancer. 2010;18:1023–1031. doi: 10.1007/s00520-010-0897-7. [DOI] [PubMed] [Google Scholar]
- 18.Reyes-Gibby CC, Anderson KO, Merriman KW, Todd KH, Shete SS, Hanna EY. Survival patterns in squamous cell carcinoma of the head and neck: pain as an independent prognostic factor for survival. J Pain. 2014;15:1015–1022. doi: 10.1016/j.jpain.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith HR. Depression in cancer patients: Pathogenesis, implications and treatment (Review) Oncol Lett. 2015;9:1509–1514. doi: 10.3892/ol.2015.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SA, Roh JL, Lee SA, et al. Pretreatment depression as a prognostic indicator of survival and nutritional status in patients with head and neck cancer. Cancer. 2015 doi: 10.1002/cncr.29693. [DOI] [PubMed] [Google Scholar]
- 22.Britton B, Clover K, Bateman L, et al. Baseline depression predicts malnutrition in head and neck cancer patients undergoing radiotherapy. Support Care Cancer. 2012;20:335–342. doi: 10.1007/s00520-011-1087-y. [DOI] [PubMed] [Google Scholar]
- 23.Daudt HM, Cosby C, Dennis DL, Payeur N, Nurullah R. Nutritional and psychosocial status of colorectal cancer patients referred to an outpatient oncology clinic. Support Care Cancer. 2012;20:1417–1423. doi: 10.1007/s00520-011-1224-7. [DOI] [PubMed] [Google Scholar]
- 24.Nho JH, Kim SR, Kwon YS. Depression and appetite: predictors of malnutrition in gynecologic cancer. Support Care Cancer. 2014;22:3081–3088. doi: 10.1007/s00520-014-2340-y. [DOI] [PubMed] [Google Scholar]
- 25.Tian J, Chen ZC, Hang LF. Effects of nutritional and psychological status of the patients with advanced stomach cancer on physical performance status. Support Care Cancer. 2009;17:1263–1268. doi: 10.1007/s00520-009-0579-5. [DOI] [PubMed] [Google Scholar]
- 26.Tian J, Chen ZC, Hang LF. Effects of nutritional and psychological status in gastrointestinal cancer patients on tolerance of treatment. World J Gastroenterol. 2007;13:4136–4140. doi: 10.3748/wjg.v13.i30.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta RD, Roth AJ. Psychiatric considerations in the oncology setting. CA Cancer J Clin. 2015;65:300–314. doi: 10.3322/caac.21285. [DOI] [PubMed] [Google Scholar]
- 28.Rodin G. Treatment of depression in patients with cancer. Lancet. 2008;372:8–10. doi: 10.1016/S0140-6736(08)60968-X. [DOI] [PubMed] [Google Scholar]
- 29.Taghizadeh N, Boezen HM, Schouten JP, Schroder CP, Elisabeth de Vries EG, Vonk JM. BMI and lifetime changes in BMI and cancer mortality risk. PLoS One. 2015;10:e0125261. doi: 10.1371/journal.pone.0125261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan P, Hu C, Quan C, et al. Body mass index and risk of lung cancer: Systematic review and dose-response meta-analysis. Sci Rep. 2015;5:16938. doi: 10.1038/srep16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Secord AA, Hasselblad V, Von Gruenigen VE, et al. Body mass index and mortality in endometrial cancer: A systematic review and meta-analysis. Gynecol Oncol. 2015 doi: 10.1016/j.ygyno.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McRackan TR, Watkins JM, Herrin AE, et al. Effect of body mass index on chemoradiation outcomes in head and neck cancer. Laryngoscope. 2008;118:1180–1185. doi: 10.1097/MLG.0b013e31816fca5c. [DOI] [PubMed] [Google Scholar]
- 33.Souza VB, Silva EN, Ribeiro ML, de Martins WA. Hypertension in patients with cancer. Arq Bras Cardiol. 2015;104:246–252. doi: 10.5935/abc.20150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelburne N, Adhikari B, Brell J, et al. Cancer treatment-related cardiotoxicity: current state of knowledge and future research priorities. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju232. [DOI] [PMC free article] [PubMed] [Google Scholar]


