Abstract
The purpose of this review article is to understand the current literature on obesity, diabetes and therapeutic avenues across the world. Diabetes is a chronic lifestyle condition that affects millions of people worldwide and it is a major health concern in our society. Diabetes and obesity are associated with various conditions, including non-modifiable and modifiable risk factors. Early detectable markers are not well established to detect pre-diabetes and as a result, it becomes diabetes. Several published epidemiological studies were assessed and the findings were summarized. Resources from published studies were used to identify criteria used for pre-diabetes, the role of diet in pre-diabetics and potential risks and characteristics associated with pre-diabetes. Preventive strategies are needed to combat diabetes. Individuals diagnosed with pre-diabetes need detailed education, need to fully understand the risk factors and have the ability to manage diabetes. Interventions exist that include chronic disease self-management programs, lifestyle interventions and pharmacological strategies. Obesity plays a large role in causing pre-diabetes and diabetes. Critical analysis of existing epidemiological research data suggests that additional research is needed to determine the efficacy of interventions.
Keywords: Alzheimer’s disease, diabetes, obesity, FTO
Introduction
When an individuals’ blood glucose levels are higher than normal levels can be termed as pre-diabetes in that individual; however, blood glucose levels do not meet the standard to be classified as diabetes. In the United States, 37% of the population or 86 million adults have pre-diabetes [1–3]. Of those that have pre-diabetes, only 11% of the population is aware of the condition [4]. Individuals diagnosed with pre-diabetes need to understand the difference between a diagnosis of pre-diabetes and diabetes. Education is essential to help prevent persons that are in the pre-diabetes stage from having diabetes [5]. Understanding the long-term effects of diabetes and the types of diabetes an individual can develop can help individuals better understand why prevention of the disease is important [6].
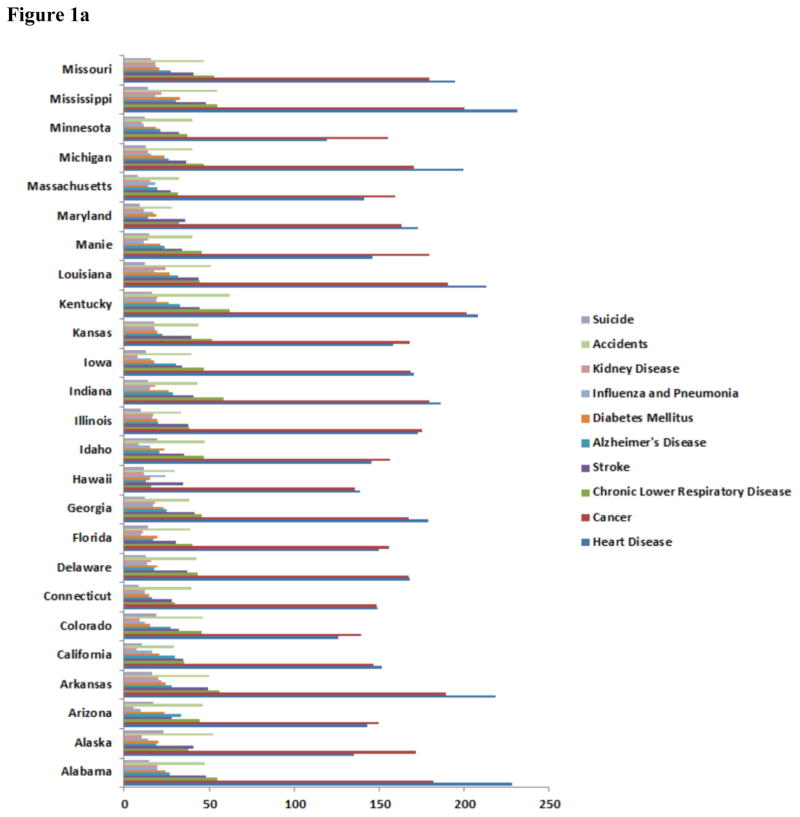
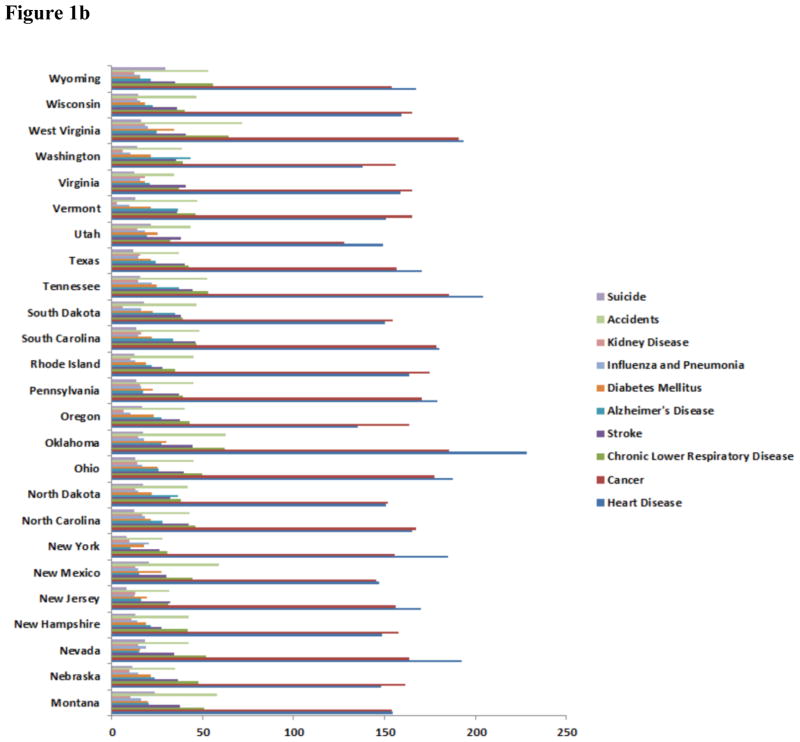
According to the International Diabetes Foundation (IDF) every six seconds one person dies of diabetes [7, 11]. In 2012, diabetes caused 1.5 million deaths (WHO Executive Summary) and ranked 6th overall in related deaths worldwide [8] (CDC; see Figure 1). In Texas, diabetes ranked 6th overall in related deaths (Figure 1). Worldwide 415 million people have been diagnosed with diabetes according to the IDF. It is projected to increase to over 600 million people by 2040. In the United States, alone, there are 29.1 million people have diabetes mellitus (DM) while there are 8.1 million people are undiagnosed. In Texas, 10.8% of adults were diagnosed with diabetes [7–11]. The statistics are staggering and based on the projections medical costs associated with treatment and hospital stays will also rise. Diabetes complications also are costly. They account for more than 35% of the estimated $91.8 billion in direct medical expenditures [12]. Over, $670 billion will be spent on diabetes healthcare costs and 12% of the health expenditure will be spent on diabetes [12–16].
Figure 1.
Major disease conditions and incidents for mortality in the US. Either diabetes mellitus or Alzheimer’s disease is 5th or 6th leading cause of death in these states. Mortality rate represented per 1000 population. (Data adopted from all the states departmental health websites as on January, 2016).
National Institute of Health and MedlinePlus defined DM as a chronic disease in which the body unable to regulate sugar levels in the blood.” DM results from several complex pathogenic processes among various organs in the body and is of two types such as Type 1 DM and Type 2 DM (T2DM). The pathology of DM is complex pathogenic process where autoimmune destruction of the β-cells of the pancreas causes insulin deficiency and the attributed abnormalities resulting to insulin resistance (IR) [17–19, 24]. Metabolic derangements that are associated with IR are based on outcome of metabolic function of insulin on carbohydrate, fat, and protein that are being metabolized in living organisms. The resistance to insulin action in DM is due to reduced insulin action on target tissues such as skeletal muscle and adipose tissues. Reduced metabolic function of insulin is due to decreased or inadequate production of insulin by the beta cells of the islets of Langerhans of the pancreas. Both decreased or inadequate production of insulin occurs simultaneously and/or separately in persons with diabetes. [17–19]. The normal effect of insulin on carbohydrate metabolism is hypoglycemia, on lipids it favors its synthesis (lipogenesis) and decreases its breakdown by favoring cholesterol biosynthesis by using glucose as substrate and also favors protein synthesis by decreasing protein catabolism. The dysregulation of insulin on carbohydrate, protein and lipid metabolism causes the clinical condition ‘hyperglycemia’. Still it is highly debatable about the events that are causing hyperglycemia [21–23].
Due to the metabolic complexity of the disease, diabetes, a heterogeneous metabolic disorder and it is difficult to classify. As mentioned above, diabetes is characterized by elevated blood glucose concentration levels. The elevated blood glucose concentration is due to failure in the production of insulin by the beta cells of the pancreas or insufficient production or both or insulin resistance [21].
According to World Health Organization (WHO) Director, Dr. Margaret Chan, a report was generated that indicates that the prevalence of diabetes has doubled since 1980. The WHO estimated that globally approximately 422 million adults had diabetes in 2014. In 1980, 108 million adults were living with diabetes. The prevalence of DM has nearly doubled that means rising from 4.7% to 8.5% in the adult population since 1980 across the globe [8, 16, 20]. The purpose of the article is to provide a summary of an epidemiological review of diabetes–1) causal factors, 2) types of diabetes, 3) statistics, 4) risk factors and 5) therapeutic avenues.
Types of Diabetes mellitus
Diabetes is heterogeneous metabolic disorder and is difficult to classify. The maintenance of glucose level in blood within narrow limits is a very finely and efficiently regulated system. This is important, because it is essential to have continuous supply of glucose to the brain. Even though it can utilize ketone bodies to some extent, brain has an obligatory requirement for glucose. The glucose homeostasis is majorly regulated by insulin. The dysregulation of insulin causes diabetes, and it is characterized by elevated blood glucose concentration levels. These increased blood glucose concentration levels are due to the failure of insulin action on the target tissue or insufficient production of insulin, or failure of both actions [21], finally it causes hyperglycemia [22].
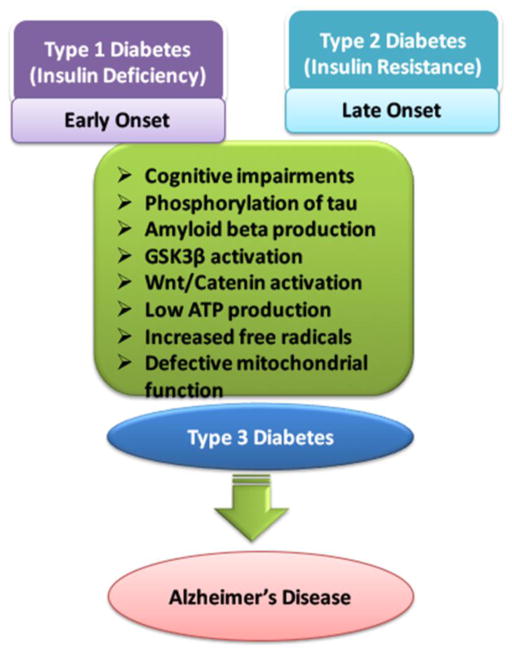
There are different types of diabetes. Below is a short overview of diabetes classifications. Figure 2 also details a basic overview of type 1 diabetes mellitus (T1DM), insulin deficiency that normally occurs early in life, and type 2 diabetes mellitus (T2DM) is an insulin resistance disease that normally occurs later in life [23].
Figure 2.
T1DM (insulin deficiency), T2DM (insulin resistance) causes cognitive impairments, phosphorylation of tau, amyloid beta production, GSK3β activation, Wnt/catenin activation, low ATP production, increased free radicals, defective mitochondrial function. All these symptoms are similar to the Alzheimer’s dementia and can be termed as ‘type 3 diabetes’.
Type 1 diabetes mellitus (T1DM) or Juvenile DM or Early onset DM
It is formerly known as insulin-dependent diabetes mellitus. Worldwide 5% of total diabetic patients are of T1DM and are deficient in circulated insulin levels. It is subdivided in to immune mediated and idiopathic DM. T1DM patients are below the age of 30 and dependent on insulin injections. They are prone to develop ketosis. In the immune mediated T1DM (early onset), circulating antibodies against insulin can be seen in 50% cases, and antibodies against islet cell cytoplasmic proteins can be seen in 80% cases. T1DM is an autoimmune disease where autoreactive T cells of the immune system attack the insulin secreting pancreatic islets of Langerhans. Cytotoxic T cells, with CD8 protein on their membrane, kill islets, thereby leading to lifelong dependence on insulin for affected patients. Eventually poorly controlled blood glucose levels inevitably result in early illness and early death. Insulin and the isoform of glutamic acid decarboxylase (GAD) are major autoantigens in patients with T1DM. GAD is a naturally occurring protein found in the brain and in insulin-secreting islets of the pancreas. It is a self-protein that functions as an autoantigen in patients with T1DM. The self-proteins can be subjected to attack not only by autoreactive T cells but also by autoantibodies to GAD, thus the β-cells of the pancreas will be destroyed. Genetics play a large role in causing T1DM and early-onset condition [23, 24].
In accordance with American Diabetes Association (ADA), the rate at which the β-cells destruction occurs varies. In some individuals with type 1 diabetes the rate may be fast, while in others it may be slow. The rapid destruction of β-cells normally occurs in infants, while in adults the rate is much slower [23–26].
T1DM in adolescents and children is characterized as ketoacidosis in its initial observation. Whereas other T1DM patients have mild fasting hyperglycemia and eventually changes to severe hyperglycemia with and without ketoacidosis. This type of diabetes majorly observed in the presence of infection or other stress. However, it varies and β-cells function may be retained for several years. So T1DM patients ultimately lead their life by taking intramuscular insulin injections for survival [24, 27, 28].
Type 2 diabetes mellitus (T2DM) or late onset DM
This type of diabetes, also known as adult-onset diabetes or late onset DM as it occurs in the late life. It comprises of 95% of the total population [29, 30]. T2DM encompasses individuals who have blood sugar levels that are higher than normal, may lead to increased insulin resistance and insulin deficiency [21]. According to the ADA, researchers, scientists and health professionals are not aware of the exact causes of T2DM. The development of this T2DM is associated with several risk factors. The risk factors for this T2DM are either modifiable or non-modifiable risk factors. Modifiable risk factors include high blood pressure obesity (BMI >30kg/m2), change in cholesterol levels and lack physical exercise. The non-modifiable risk factors associated with T2DM are history of hyperglycemia, pre-diabetes, and/or gestational diabetes, genetics, family history, race, ethnicity and age [31–33]. T2DM often goes undiagnosed for several years, and it results in long-term effects because the hyperglycemia develops gradually. Obviously, at the early stage the effects are not severe. Eventually the effects begin to take a toll on the body resulting in higher risks and resulting in other diseases such as micro-vascular complications. These T2DM patients may have normal insulin concentration levels or that would be elevated but the higher blood glucose levels in these T2DM patients can be expected to even higher insulin when they have normal function of β-cells of islets of Langerhans [34]. All these led to decrease or defect insulin secretion along with insufficient to compromise for IR [23, 35–38].
Type 3 Diabetes
Previous research studies have shown links between metabolic derangements of carbohydrates, lipids, proteins and brain dysfunction and cognitive impairment [39]. Because of brain dysfunction, cognitive impairment, oxidative stress and effects of glucose metabolism by IR on mitochondria leading to the “type 3 diabetes” which is classified as another form Alzheimer’s Disease (AD) [40–44]. Diabetics with this type of diabetes are at higher risk of cognitive decline when the age advances [45–48]. The mechanistic link between AD and diabetes is still unknown and recent literature suggesting inflammatory response, IR insulin growth factor (IGF) signaling, ApoEε4 allele on synaptic plasticity, acetylcholine esterase activity and vascular dysregulation of brain capillaries would play crucial role in forming pathophysiologic changes relating diabetes to dementia, including white matter disease, breakdown of the blood brain barrier, inflammation, and others [49–58]. Type 3 diabetes also effects glycogen synthase kinase 3β (GSK3β) signaling on Tau phosphorylation, and thus effect on mitochondria in terms to decrease or loss of ATP production [51–54] (Figure 2).
Other types of T2DM
There are other types of T2DM that can be seen in and around 25 years of age and is characterized by onset of hyperglycemia. It includes obese, non-obese and maturity onset diabetes of young (MODY). MODY is characterized by monogenetic defects in β-cell function of islets of Langerhans. The genetic defects which are inherited in autosomal dominant pattern of the β-cell are characterized by impaired insulin secretion with minimal or no defects in insulin action [21, 59, 60–62].
Genetic defects in insulin action
Normal glucose metabolism is regulated by hypoglycemic hormone i.e., insulin hormone which is produced by the pancreas that regulates plasma glucose. Genetic abnormalities of insulin may also cause diabetes mellitus. Major genetic and environmental factors along with increased calorie intake and lack of exercise causes insulin related abnormalities. All these sequential events cause metabolic syndrome [36].
Hojlund and colleagues studied effects of insulin action at the molecular level on glucose and lipid metabolism. Insulin usually facilitates the membrane transport of glucose. Facilitated diffusion of glucose in muscle is enhanced by insulin. In diabetes mellitus, the transporter, GIuT4 is reduced. However, glucose uptake in liver by GluT2 is independent of insulin. These authors demonstrated insulin signaling for glucose transport and glycogen synthesis in skeletal muscle in healthy individuals, persons with obesity, polycystic ovary syndrome (PCOS) and T2DM [36, 130, 131]. Further, they demonstrated postprandial hyperinsulinemic hypoglycemia and IR. Postprandial hyperinsulinemic hypoglycemia is due to the mutation in the insulin receptor gene especially in in the tyrosine kinase domain.
Hojlund and colleagues investigated subjects that harboring insulin receptor gene mutation as a model for inherited IR. Interestingly these authors found the association of obesity T2DM and PCOS with the defects in the insulin-stimulated glucose uptake and promote majorly glycogen synthesis by glycogenesis and to a lesser extent it allows glucose oxidation. They also demonstrated in the reduction of lipid β-oxidation by insulin. These authors further discussed that in inherited IR subjects’ insulin effects not only on glucose uptake but also glycogenesis is reduced or impaired. All these findings indicate that impairment in lipid as well as glucose oxidations are common secondary metabolic disorders associated with this mutation [36, 130, 131].
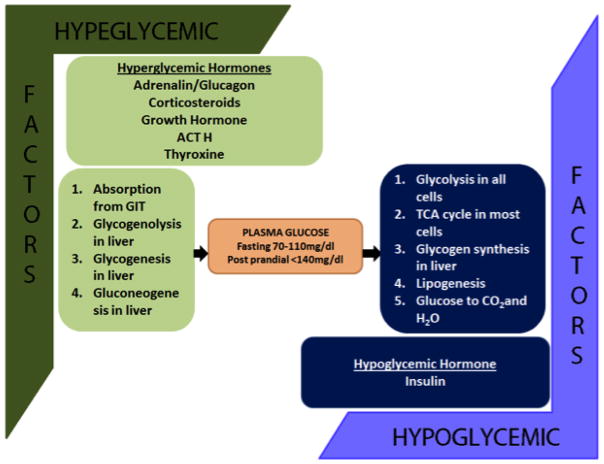
Furthermore, hyperglycemic hormones such as glucagon, epinephrine or adrenaline, glucocorticoids, adreno-cortico trophic hormone (ACTH), growth hormone (GH), thyroxine antagonizes the hypoglycemic action of insulin action. Excess production of these hyperglycemic hormones causes acromegaly (GH), cushing’s syndrome (ACTH), glucagonoma (glucagon), pheochromocytoma (adrenaline). All these hyperglycemic hormones can cause DM [21] (Figure 3).
Figure 3.
Overview of plasma glucose concentration regulation in the body. Hyperglycemic and hypoglycemic hormones play crucial role on the regulation plasma glucose concentration.
Viral infections associated with DM
Certain viruses such as cytomegalovirus, adenovirus, coxsackievirus B, rubella virus and mumps have been linked with DM. These viruses destroy β-cells of islets of Langerhans. DM occurs in congenital rubella patients even though these patients have immune markers and Human leucocyte antigen (HLA) antigens (characteristic of T1DM) [63–66].
Unusual immune response directed DM
One type of autoimmune disorder is stiff-man syndrome which patients show stiffness in the axial muscles. This stiffness syndrome is characterized by painful spasms and ultimately affects the brain function. Approximately one-third of these syndrome patients eventually may develop DM [27, 61–71].
Diabetes and genetic syndromes
The chances of developing diabetes are greatly increased, if individuals’ have genetic syndromes. Multiple conditions, including Down syndrome, Wolfram syndrome, Turner’s syndrome and Klinefelter’s syndrome are associated with changes in genome. Wolfram’s syndrome is an autosomal recessive disorder which is characterized with insulin-deficient diabetes along with the absence of β-cells [27, 71–86].
Gestational diabetes mellitus (GDM): An abnormal GTT curve
This term is used when carbohydrate intolerance is noticed, for the first time, during a pregnancy. The definition applies regardless either with insulin biological action on glucose or with diet modification as therapy or GDM exist after pregnancy [87–93]. Labeling this DM type with someone is not as important as treatment. It remains unknown what type of mechanism causes diabetes [94], and pre-diabetes is a status given to individuals that are borderline diabetic. In all antenatal women, a glucose challenge test is done between 22 and 24 weeks of pregnancy by giving an oral glucose load of 50 g of glucose regardless of the time. If the 2-hour post-glucose value is more than 140 mg/dl, the test is positive. An oral glucose tolerance test (OGTT) with 75 g glucose load should be done to confirm or exclude GDM. Some obstetricians prefer to do an OGTT without a screening test with 75 gm of glucose. In these cases, three blood samples are drawn, fasting,1 hour and 2-hour post-glucose load. Women with GDM are at increased risk for subsequent development of frank diabetes. GDM is associated with an increased incidence of neonatal mortality. Maternal hyperglycemia causes the fetus to secrete more insulin, causing stimulation of fetal growth and increased birth weight. After the child birth, the women should be re-assessed. [95–98]. Preventing diabetes is important; as a result, once an individual is diagnosed as pre-diabetic, education and other interventions may prevent diabetes and other types of diabetes.
Criteria to detect diabetes
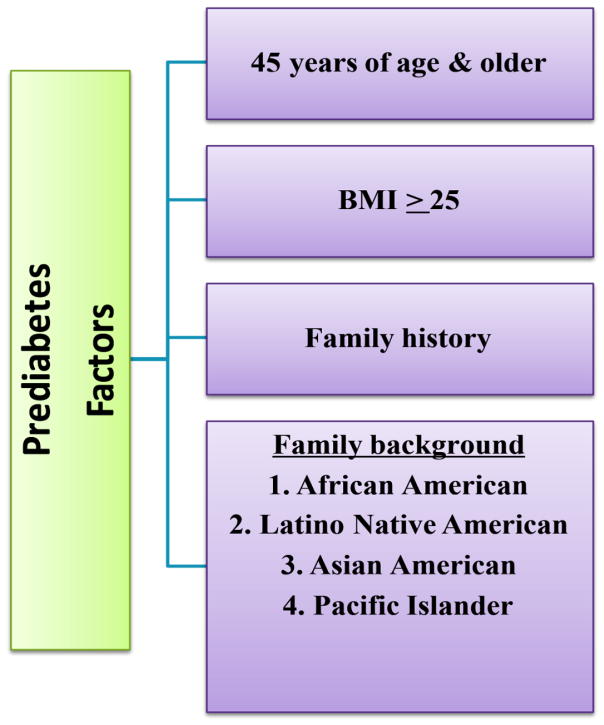
In 2106, Bansal, stated pre-diabetes identified criteria is not identical across the globe in various platforms in international organizations, institutes, labs and university hospitals [1]. Chawla et al (2016) also suggests an association of observational evidence between pre-diabetes and symptoms of DM i.e., retinopathy, neuropathy and nephropathy [34]. In addition, previous research does not indicate why some people develop pre-diabetes, there are factors that increase the risk. The factors include (Figure 4):
Figure 4.
Deciphering pre-diabetes factors such as age, BMI, family history and family back ground of different ethnic population
Weight. An increase in fatty tissue results in more resistance the cells become to insulin. If a person’s body mass index (BMI) is 25% or higher, the risk is greater [99].
Inactivity. A decrease in activity results in an increase in weight, decrease in glucose use as energy and a sensitivity to insulin. If a person does any type of physical activity less than three times per week, the risk is greater [99].
Family history. There is an increased risk for an individual if someone closely related to them, such as immediate family, has type 2 diabetes. It has been found that there is a genetic alternative, rs9939609, of the fat mass and obesity-associated (FTO) gene that has repeatedly been found to have a firm correlation with obesity, based on an experiment performed by the Finnish Diabetes Prevention Group [100]. This experiment also brought to the light the correlation between the FTO variant and BMI, although no correlation was found between rs9939609 and the degree of weight loss managed by changing everyday living habits. Also, the FTO gene was discovered to be contributing to energy consumption opposed to energy expenditure [100].
In another study performed by Sonestedt et al (2012), the authors explored the relationship between Gastric Inhibitory Polypeptide Receptor (GIPR) genetic alternative rs10423928, macronutrients, and fiber consumption on body mass index and type 2 diabetes risk [129]. The experiment had a sum on 24,840 subjects between the ages of 45 and 74. Out of the total, 1541 subjects were found to have type 2 diabetes. During this study that included twelve years of follow-up information, dietary consumption was monitored using a diet history method. Sonestedt et al. (2012) found no evidence that dietary consumption greatly changes the correlation with the GIPR genotype and body mass index. However, they observed the compelling relationship between the GIPR genotype and quintiles of carbohydrate and fat consumption on type 2 diabetes. When the highest carbohydrate quintile was contrasted with the lowest, it was noted that the subjects in the highest quintile were found to be at a 23% decreased risk of type 2 diabetes risk. This experiment proposes that the GIPR genotype may be directly related to the type 2 diabetes risk that is cause by carbohydrate and fat consumption [129].
In a different investigation by Moleres et al. (2012), the authors observed the relationship between dietary fatty acid consumption distribution, FTO gene, and obesity in a 354 Spanish minors between the ages of 6–18 (49% male) [101]. The patients were genotyped for the rs9939609 alternative of the FTO gene. The authors examined to see if there was a relationship between the consumption of saturated fatty acids (SFA), which is a percentage of total energy, and poly unsaturated fatty acids (PUFA) as a ratio of PUFA:SFA. Along with this ratio, obesity risk was monitored to see if there was a link between it and rs9939609 SNP of the FTO gene. Moleres et al. (2012) observed that TT genotype carriers had more of an obesity risk. Similarly, A allele carriers with an intake ratio lower than 0.43 PUFA:SFA had a higher obesity risk than TT genotype carrier subjects. This evidence shows that the rs9939609 polymorphism of the FTO gene is effected by the dietary fatty acid on minors’ obesity risk [101].
In a different experiment, the relationship between the −174G/C polymorphism of the IL6 gene and a Mediterranean-style diet to document body weight changes after 3 years of a diet change in a high cardiovascular risk population was performed by Razquin et al [102]. There were 737 participants involved in this experiment who were aged 55–80 years that were assigned to either a low-fat diet or to a Mediterranean-style diet group with high consumption of virgin olive oil (VOO) or nuts. Anthropometric measurements were taken at the beginning of the study and again after a 3-year span had passed. The -174G/C polymorphism of the IL6 gene was genotyped and the minor allele frequency (C) was 0.39. The investigation observed that when there was evidence of higher measures of fat tissue, also the CC genotype was observed. After 3 years when the anthropometric measures were take again, a relationship was observed between the polymorphism (GG+GC versus CC) and the diet change. The CC genotype participants consuming the MD+VOO were observed as gaining the least amount of weight. Razquin et al concluded that at beginning of the study, the CC subjects for the -174G/C polymorphism of IL6 had the highest body weight and BMI. But after the diet change and 3 years had passed with MD+VOO, these participants had the greatest reduction in body weight [102].
In a study accounting for different variables, Ahmad et al (2011) analyzed the FTO polymorphism, rs8050136, gene when taking into account physical activity, caloric intake, and body measurements in 21,675 healthy Caucasian women. This study showed that the outcome of the risk allele (A) on BMI was larger among inactive or higher caloric consumption women [103]. Among inactive/high caloric consumption women, each A allele was associated with mean BMI. Specifically, each A allele was associated with mean BMI difference of +0.73 among inactive women, compared with +0.31 among active women. Similarly as seen in the comparison of active vs. inactive women, each A allele was correlated with mean BMI difference of +0.65 among high caloric intake women compared with +0.38, among low caloric intake women (≤1,679 kcals/day). The findings of this study suggest that life habit changes the genetic risk of FTO on obesity as each A allele carried increased risk of obesity and diabetes, particularly among women who were both inactive and had high consumption. Healthier habits that include physical activity and lower caloric consumption only reduced but did not completely eliminate the associated genetic risk [103].
Race. People’s ethnicity and race play a major role in health disparities that exist all over the world along with many factors that influence them. There is an increased risk if a person’s family background includes any of the African American, Hispanics, American Indians, and Asian Americans races [104, 105]. Other factors for health disparities include social, biological and/or clinical factors. In 2007, diabetes was listed as an underlying and contributing cause of death in 231,404 death certificates in the United States [106]. According to Spanakis and Golden (2013), the highest observed total percentage of diabetes is among Native Americans at 33 % and lowest among Alaska natives. Hispanic Americans also have a fairly high rate of 12.6 % and when adjusted for age for diagnosed diabetes in the US for Hispanic men was 16.7% and 17.2% for Hispanic women [104]. When compared to Native Hispanic Whites in different parts of the world, Native Americans, Alaskan Natives, Hispanic Americans, Native Hawaiians, and Filipinos living in Hawaii are between 2 and almost 6 times more likely to die from diabetes [104, 106, 107].
Age. There is an increase of risk if an individual is 45 years of age or older. Unlike the risk factors mentioned above, age is not a modifiable risk trait much like that of weight and activity. In terms of weight, an individual’s diet plays a large part in risk associated with diabetes and obesity. Therefore, anyone diagnosed with pre-diabetes should change their diet, which is associated with obesity which in turn contributes to pre-diabetes, which could be a critical change for ones’ health [99]. If someone feels they are at risk for any of these reasons, cost effective clinical screenings of pre-diabetes exist [108, 109]. The types of screenings that can be conducted to determine if someone is pre-diabetic include FPG, OGTT and HbA1c [109, 110]
Potential risks and characteristics associated with diabetes and pre-diabetes
Out of the many people that are diagnosed with pre-diabetes, as many as 70% will develop full blown diabetes in their lives according to the ADA. By the year 2030, it is projected that over 470 million people will have pre-diabetes [111–113]. There are many terrible complications that are correlated to pre-diabetes. There is a link between the damage to organs such as eyes, kidneys, blood vessels and the heart that people with pre-diabetes. Also, additional complications that are particularly relevant to pre-diabetes include: (1) nephropathies and chronic kidney disease, (2) neuropathies, (3) diabetic retinopathy, and (4) macrovascular diseases [111–113].
Over the past years, methods and standards used for pinpointing pre-diabetes have changed. Recently, Pantalone et al. (2015) studied clinical characteristics, complications, comorbidities, and treatment patterns in subjects diagnosed with type 2 diabetes and using an electronic health record system. This system was used to create 2 data sets of all patients with type 2 diabetes, one starting on July 1st, 2008 and the other July 1st, 2013. When comparing the resulting two data sets, the gathered information was adjusted for age, gender, race, and household income [132]. Pantalone et al. (2015) observed these experiments performed in 2008 and 2013 that 24,493 and 41,582 patients with type 2 diabetes were identified, respectively. From the total of patients found with type 2 diabetes in 2008 and 2013, a majority were male (52.3% and 50.1%) and Caucasian (79% and 75.2%), respectively. The mean ages of the patients found with diabetes in the study was 64.8 in 2008 and 64.3 in 2013. In the analyzes of the study, authors found most common type 2 diabetes related comorbidities in 2008 and 2013 were hypertension (82.5% and 87.2%) and cardiovascular disease (26.9% and 22.3%), respectively [132].
There is some promising statistics that shows that it is possible to have some improvements with diabetes related complications over the years. This previously mentioned research includes that using the USA National Health and Nutrition Examination Survey (NHANES) database. The NHANES reported that from 2003 to 2006, only 57% of people with diabetes had a glycosylated HbA1C<7%. More recently in the last decade from 2007–2010, the percentage of patients with glycemic control was found to be even lower with only 52.5% of people with diabetes achieving an A1C<7.0%. But in trying to have glycemic control, one must be cautious. Poor glycemic control can result in blindness, kidney failure, and no-traumatic amputations, which could result in cardiovascular disease. Even with promising, recent reports, continued education and improvements need to be made in attaining glycemic targets and managing multiple conditions [114].
Current Status of Diabetes/Obesity Treatment and Biomolecules
The available treatment for diabetes is variable and is based on the type of diabetes an individual had been diagnosed. Other factors that are important to consider are a person’s life habits, diet, and prescription regiment. To help a person with their lifestyle, diet and medicine intake choices, self-management programs are available in several areas. In a study performed by Norris et al. (2002), self-management education increases glycated hemoglobin (GHb) levels in a short period of time but the positive GHb effect returns to normal after 1–3 after the attainment of education ceases [115]. In addition to Norris et al’ observations, the Diabetes Prevention Program Research Group found that taking the prescription pill of metformin was less effective than making life habit changes. Metformin is a prescriptive drug, used along with an oral antidiabetic medicine called sulfonylurea or with insulin that is used to treat high blood sugar levels or regulate dysfunction of energy gain from food that is caused by type 2 diabetes [116]. Any lifestyle changes that are used for treatment to battle type 2 diabetes should be specific to the individual and systematic, much like those performed in this investigation as participants received specialized one-on-one counseling. Persons with type 2 diabetes that got to participate in the lifestyle reconciliation had a much greater ability to lose weight and a more positive approach to exercise than did participants assigned to receive metformin or placebo.
Another form of therapy is called Pharmacotherapy, which is a type of treatment based on the administration of antidiabetic drugs. Metformin, as previously described, has been in use for many years to help treat diabetes and has positive results when it comes to BMI and lipid levels, with only minor side effects [117]. Another group of antidiabetic drugs called the glitazones, which include troglitazone, rosiglitazone and pioglitazone, act through the peroxisome proliferator-activated receptor gamma (PPAR-γ) receptor by increasing hepatic and peripheral insulin sensitivity and preserving insulin secretion [118]. A third group of antidiabetic medicines include the α-glucosidase inhibitors, which reduce the rate of polysaccharide digestion from the proximal small intestine. Two examples of α-glucosidase inhibitors include exenatide and liraglutide, who were both found to produce sustained weight loss among obese subjects and were associated with increased reversion from prediabetes to normoglycemia over 1–2 years. The most frequent minor side effects of the α-glucosidase inhibitors were nausea and vomiting [119]. Lastly, there is a type of treatment that uses a drug that is not an antidiabetic. These types of drugs are gastrointestinal lipase inhibitors and renin-angiotensin-aldosterone system blockers, such as orlistat [113]. In a desperate last effort for morbidly obese people to have weight loss, bariatric surgery is an option that has a positive effect in reduction for type 2 diabetes [120].
As discussed above, multiple molecules, including 1) metformin, 2) α-glucosidase inhibitors, such as exenatide and liraglutide, and 3) renin-angiotensin-aldosterone system blockers, such as orlistat are potential anti-diabetic drugs that can be used to treat persons with diabetes.
The treatment for diabetes mirrors the treatment associated with obesity in many ways. Long-term lifestyle adjustments, including dietary modifications, behavioral changes and incorporating exercises to daily living must be adopted. Recent therapies that are invasive include gastric bypass surgery, laparoscopic adjustable gastric banding, biliopancreatic diversion with duodenal switch and gastric sleeve. One new treatment that received Food and Drug Administration (FDA) approval in 2014 is vagal nerve blockade [135]. This treatment involves the implanting a device in the abdomen that sends signals to the brain when the stomach is empty or full [135]. Along with current and available surgeries, FDA-approved biomolecules in the obesity treatment includes, phentermine (Adipex-P®), orlistat (Xenical®), lorcaserin (Belviq®) and liraglutide (Saxenda®). However, for the treatment of obesity, these FDA therapies have limited efficacy due to limitation in the regulatory pathways [135].
Factors that may reduce diabetes
Based on previous research, the factors that may reduce diabetes include being educated on the subject of diabetes, becoming physically active, and controlling ones’ diet. These factors may reduce diabetes are known to be associated with the modifiable risk factors (Figure 4). In a previous study, The Finnish Diabetes Prevention Study was the primary source to show that a long-time lifestyle change prevented type 2 diabetes for people with an impaired glucose tolerance (IGT) [121, 122]. In education, written material provided in self-management classes are resources and forms of education that make positive impacts on diabetes management for individuals [123–125]. For physical activity to play a role, a person needs to exercise about five times a week for 30 minutes each time, optimally. The minimum amount of exercise would include exercising more than 3 times per week and averaging 150 minutes of physical activity for every week. For better results and contributing to diet control along with the other two modifiable risk factors mentioned, combining the understanding of the role of diet and exercise in order to prevent diabetes is criterial to taking the right steps for a long-term health status change.
Role of education
Educational resources play a major role in diabetes and can be effective for people diagnosed with medical conditions such as diabetes. While the educational material may be obtainable, it is up to the individual with the condition to decide to go forward with providing themselves with the proper education to care for them. In some instances, there are obstacles that prevent individuals from obtaining or understanding the education provided to them about their personal condition. These obstacles include a possible language barrier and not being able to obtain the resources due to a lack of transportation or lack of computer access. Even though these obstacles are reasonable based on an individual’s own personal status, a person with a medical condition that strives to learn more about their condition must make this self-management education a top priority.
In 1986, an independent organization known as the National Certification Board for Diabetes (NCBDE) was created to train Certified Diabetes Educators (CDE), who through written examinations, are health professionals qualified to help those seek education and awareness of diabetes management, pre-diabetes, and diabetes prevention (http://www.ncbde.org). A CDE helps individuals with diabetes by providing education and support to promote optimal health outcomes and also with self-management courses. These self-management classes provide information to the individuals so they are able to maintain the best quality of life not only in the classes, but at home as well. The main objective for these self-management classes is teaching individuals to use problem solving skills through self-efficacy. Through this training, a person has the ability to assess a situation and respond in the most efficient manner to accomplish their goal. Research based on evidence from controlled clinical trials offers three bits of information that pertains to the success of self-management courses: (1) that programs promoting self-management skills are more useful for a patient than just providing education on a condition through information only; (2) in a few instances, the information learned in the self-management classes enhance results and cut costs; and (3) in early investigations, bringing together individuals with an array of conditions in a self-management program and improve results and cut costs. Self-management education is an effective tool in reducing diabetes in individuals currently at risk or already diagnosed [123–125].
Role of exercise
In 2002, ADA released a statement explaining how physical activity and exercise could be used as a beneficial tool for individuals dealing with pre-diabetes and diabetes. These health benefits associated with physical activity are rapidly, positively growing (Figure 4). Exercise improves a number of elements and reduces the chances of tragic events inside the body, including increasing blood circulation, reducing the risk of heart disease, reducing the risk of stroke, improves self-esteem, and it improves whole-body blood glucose levels by keeping them at appropriate levels [126]. Hypoglycemia during physical activity rarely occurs in non-diabetic individuals and the body maintains normoglycemia during exercise through hormone meditation. Specific exercise can also improve changes in skeletal muscle are considered important to this effect because this tissue is responsible for the majority of glucose disposal. Although skeletal muscle is a vital factor to the positive effects of physical activity on metabolic homeostasis, exercise training also changes several other tissues, including white adipose tissue [126].
Physical activity obviously is a core factor in diabetes self-management courses. According to the ADA, it is recommended that an individual include 30 minutes of moderate-to-vigorous intensity aerobic exercise at least 5-day a week or a total of 150 minutes per week. But if the individual has not performed any physical activity over a long period of time, it is critical to start into an exercise routine slowly and gradually work up to more intense activity. An individual in this case should consider starting with 5 to 10 minutes of exercise initially and gradually increase their minutes of physical activity each week. While the amount of time that an individual is active for in a session is important, the intensity of exercise is also important but is often overlooked. When aiming to do moderate-to-vigorous exercises, individuals can be described as being able to talk while exercising, but not being able to sing. When trying to achieve a vigorous exercise, an individual should be able to only barely speak a couple of words while exercising.
There are two different types of exercise that should be used together to gain the maximum potential for your condition through an exercise program. The first type of exercise is known as aerobic exercise that improves your cardiovascular system in your body. The following are aerobic exercises to consider: playing tennis, walking, jogging, running, cycling, swimming, dancing, rowing, and group exercises such as Zumba, Yoga, TaiChi, and even gardening at a moderate to high intensity level that can be considered [134]. Strength training is the other type of exercise to incorporate into your exercise routine throughout the week [133]. According to the ADA, strength training makes your body more sensitive to insulin, can lower blood glucose, and obviously strengthens bones and muscles. To go along with strengthening bones, this type of training also reduces the risk of osteoporosis and bone fractures in the body. But even with these numerous benefits, strength training is not a recommended daily physical activity. It is recommended only about twice a week in addition to a person’s other weekly exercises. Individuals can use resistance bands, free weights and use their own body weight in push-ups, sit-ups, or squats to exercise their muscles through strength training [133–134].
Role of diets
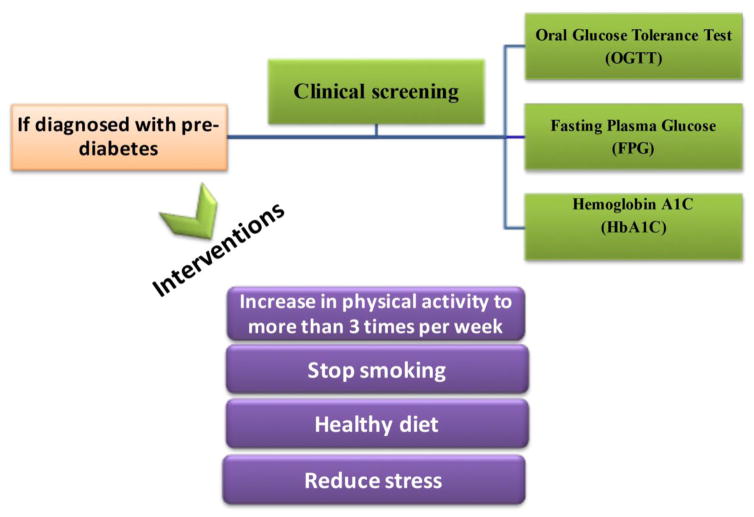
Diet recommendations for diabetics are often based on personal judgment or the viewpoint of the medical treatment provider [127]. Evidence has shown that nutrition therapy is practical and useful for improving glycemic control and metabolism. Preventive methods and therapy of diabetes should include a controlled diet and should focus on healthy eating [128]. Often diet recommendations are confusing as shown in previous years’ recommendations included a low-carbohydrate diet while other diets for diabetics propose a diet with high carbohydrates, high-protein or low-fat diet. New research does provide information for the importance of using evidence based approach when choosing a diet [127–129]. Nutritional education in diabetes self-management programs is a critical component in a therapeutic plan for seeking treatment. But individuals should also seek assistance from healthcare professionals. It is vital for diabetics to consult closely with a healthcare person(s) such as a nutritionist to find the correct amount the nutritional change that will provide the best positive impact for each person. A reduced energy intake strategy of food consumption should be used for people that suffer T2DM (Figure 5). People that have T1DM should seek a diet strategy plan based on an adjustment that is insulin based on carbohydrate counting.
Figure 5.
Schematic approach in pre-diabetes clinical screening and interventions. The suggested interventions are increase in physical activity, stop smoking, diet (antioxidant, low carbo hydrate, low SFA: High PUFA) and management for stress.
Conclusions and Future Directions to Control Diabetes
Either T1DM or T2DM is a chronic metabolic syndrome that affects millions of people worldwide and it is a major health concern in our society. There are different risk factors such as modifiable and non-modifiable are associated with diabetes and obesity. Early detectable markers are not well established to detect pre-diabetes and as a result, it becomes diabetes. Recent on epidemiological research revealed that lifestyle activities, diet, lack of physical activity and family factors, including genetic factors play a major role in DM and obesity. Incorporating positive lifestyle changes that include a healthy diet and physical activity can result in preventing diabetes. Although, there is research that does identify that diabetes can occur based on some non-modifiable risk factors such as genetics, understanding ways to prevent or delay diabetes through diet and exercise needs to be placed on the priority list in educational sessions that are provided to individuals with pre-diabetes. There are several important issues to consider in diabetes control:
It is important to reconsider altering baseline values of blood sugar −82 to 110 mg/dL. These numbers based on young adult group – these numbers change with –1) age and 2) gender.
Baseline values vary from ethnic group to ethnic group and genetics and family history may have stronger implications.
It is important to consider and/or reconsider other factors such as cardiovascular, cognitive, obesity and culture–when clinicians determine–someone is chronic diabetes/uncontrollable diabetes – because same medication may not work for all.
In the future, Alzheimer’s and Diabetes together may be called “Alzbetes” because of sharing cellular and molecular mechanisms between these two diseases.
Highlights.
Diabetes is a chronic lifestyle condition that affects millions of people worldwide and it is a major health concern in our society.
Diabetes and obesity are associated with non-modifiable and modifiable risk factors.
Obesity plays a large role in causing pre-diabetes and diabetes.
Critical analysis of existing epidemiological research data suggests that additional research is needed to determine the efficacy of interventions.
Individuals diagnosed with pre-diabetes need detailed education, need to understand the risk factors and have the ability to manage diabetes.
Acknowledgments
NIH grants (AG042178, AG047812) & the Garrison Family Foundation supports this work.
Abbreviations
- AD
Alzheimer's disease
- Aβ
beta amyloid
- ApoE4
apolipoprotein E4 genotype
- T2DM
type 2 diabetes mellitus
- GSK3β
Glucose synthase kinase 3 beta
- Drp1
Dynamin-related protein1
- ROS
Reactive oxygen species
- WHO
World Health Organization
- ADA
American Diabetes Association
- PCOS
polycystic ovary syndrome
- HLA
Human leukocyte antigen
- GDM
Gestational diabetes mellitus
- FTO
Fat mass and obesity
- PUFA
Poly unsaturated fatty acids
- SFA
Saturated fatty acids
- GIPR
gastric inhibitory polypeptide receptor
- VOO
Virgin olive oil
- IL-6
Interleukin 6
- BMI
Body mass index
- HbA1C
Glycosylated hemoglobin A1C
- FPG
Fasting blood glucose
- OGTT
Oral glucose tolerance test
- PPAR-γ
Peroxisome proliferator-activated receptor gamma
- IGT
Impaired glucose tolerance test
- WAT
White adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bansal N. Prediabetes diagnosis and treatment: A review. World J Diabetes. 2016;6:296–303. doi: 10.4239/wjd.v6.i2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faerch K, Hulman A, Solomon TP. Heterogeneity of Pre-diabetes and Type 2 Diabetes: Implications for Prediction, Prevention and Treatment Responsiveness. Curr Diabetes Rev. 2016;12:30–41. doi: 10.2174/1573399811666150416122903. [DOI] [PubMed] [Google Scholar]
- 3.Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55:3536–3549. doi: 10.2337/db06-0319. [DOI] [PubMed] [Google Scholar]
- 4.Babey SH, Wolstein J, Diamant AL, Goldstein H. Prediabetes in California: Nearly Half of California Adults on Path to Diabetes. Policy Brief UCLA Cent Health Policy Res. 2016:1–8. [PubMed] [Google Scholar]
- 5.http://www.niddk.nih.gov/health-information/health-topics/Diabetes/insulin-resistance-prediabetes/Pages/index.aspx
- 6.Funnell MM, Brown TL, Childs BP, Haas LB, Hosey GM, Jensen B, Maryniuk M, Peyrot M, Piette JD, Reader D, Siminerio LM, Weinger K, Weiss MA. National standards for diabetes self-management education. Diabetes Care. 2010;33(Suppl 1):S89–96. doi: 10.2337/dc10-S089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.http://www.diabetesatlas.org/
- 8.www.who.int/diabetes/glocal-report
- 9.http://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html
- 10.http://wwwn.cdc.gov/CommunityHealth/profile/currentprofile/TX/Lubbock/125
- 11.Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim Biophys Acta. 2016 May 6; doi: 10.1016/j.bbadis.2016.04.017. pii: S0925–4439(16)30097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, Everson-Stewart S, Kinder L, Oliver M, Boyko EJ, Katon WJ. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 13.da Rocha Fernandes J, Ogurtsova K, Linnenkamp U, Guariguata L, Seuring T, Zhang P, Cavan D, Makaroff LE. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract. 2016;117:48–54. doi: 10.1016/j.diabres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res. 2016;118:1723–1735. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Diabetes Mellitus: Report of a WHO Study Group. Geneva: World Health Org; 1985. (Tech. Rep. Ser., no. 727) [PubMed] [Google Scholar]
- 17.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 18.Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982;298:167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- 19.Catalano KJ, Maddux BA, Szary J, Youngren JF, Goldfine ID, Schaufele F. Insulin resistance induced by hyperinsulinemia coincides with a persistent alteration at the insulin receptor tyrosine kinase domain. PLoS One. 2014;9:e108693. doi: 10.1371/journal.pone.0108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf
- 21.Triplitt C, Solis-Herrera C, Cersosimo E, Abdul-Ghani M, Defronzo RA. Empagliflozin and linagliptin combination therapy for treatment of patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2015;16:2819–2833. doi: 10.1517/14656566.2015.1114098. [DOI] [PubMed] [Google Scholar]
- 22.Falciglia M. Causes and consequences of hyperglycemia in critical illness. Curr Opin Clin Nutr Metab Care. 2007;10:498–503. doi: 10.1097/MCO.0b013e3281a3bf0a. [DOI] [PubMed] [Google Scholar]
- 23.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 24.Poudel A, Savari O, Striegel DA, Periwal V, Taxy J, Millis JM, Witkowski P, Atkinson MA, Hara M. Beta-cell destruction and preservation in childhood and adult onset type 1 diabetes. Endocrine. 2015;49:693–702. doi: 10.1007/s12020-015-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wojcik JL, Devassy JG, Wu Y, Zahradka P, Taylor CG, Aukema HM. Protein source in a high-protein diet modulates reductions in insulin resistance and hepatic steatosis in fa/fa Zucker rats. Obesity (Silver Spring) 2016;24:123–131. doi: 10.1002/oby.21312. [DOI] [PubMed] [Google Scholar]
- 26.Wojcik M, Malek J, Janus D, Fijorek K. The association between metabolic complications and arterial hypertension in obese adolescents. Neuro Endocrinol Lett. 2015;36:583–588. [PubMed] [Google Scholar]
- 27.Diabetes Care. 2009 Jan;32(Supplement 1):S62–S67. [Google Scholar]
- 28.Diabetes Care. 2014 Jan;37(Supplement 1) [Google Scholar]
- 29.Fradkin JE. Confronting the urgent challenge of diabetes: an overview. Health Aff (Millwood) 2012;31:12–19. doi: 10.1377/hlthaff.2011.1150. [DOI] [PubMed] [Google Scholar]
- 30.Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241:211–218. doi: 10.1016/j.atherosclerosis.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Fukuoka Y, Choi J, SBM, Gonzalez P, Arai S. Family history and body mass index predict perceived risks of diabetes and heart attack among community-dwelling Caucasian, Filipino, Korean, and Latino Americans--DiLH Survey. Diabetes Res Clin Pract. 2015;109:157–163. doi: 10.1016/j.diabres.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.http://www.heart.org/HEARTORG/Conditions/Diabetes/UnderstandYourRiskforDiabetes/Understand-Your-Risk-forDiabetes_UCM_002034_Article.jsp#.V320s9IrK70
- 33.Lorber D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2014;7:169–183. doi: 10.2147/DMSO.S61438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab. 2016;20:546–551. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mari A, Tura A, Natali A, Laville M, Laakso M, Gabriel R, Beck-Nielsen H, Ferrannini E. Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia. 2010;53:749–756. doi: 10.1007/s00125-009-1647-6. [DOI] [PubMed] [Google Scholar]
- 36.Hojlund K. Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance. Dan Med J. 2014;61:B4890. [PubMed] [Google Scholar]
- 37.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pankov YA. Adipogenic function and other biologic effects of insulin. Biomed Khim. 62(92016):5–13. doi: 10.18097/PBMC20166201005. [DOI] [PubMed] [Google Scholar]
- 39.Stoeckel LE, Arvanitakis Z, Gandy S, Small D, Kahn CR, Pascual-Leone A, Pawlyk A, Sherwin R, Smith P. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Res. 2016;5:353. doi: 10.12688/f1000research.8300.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer's disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 42.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 43.Alam F, Islam MA, Sasongko TH, Gan SH. Type 2 Diabetes Mellitus and Alzheimer's Disease: Bridging the Pathophysiology and Management. Curr Pharm Des. 2016 doi: 10.2174/1381612822666160527160236. [DOI] [PubMed] [Google Scholar]
- 44.Mittal K, Katare DP. Shared links between type 2 diabetes mellitus and Alzheimer's disease: A review. Diabetes Metab Syndr. 2016 Feb 11; doi: 10.1016/j.dsx.2016.01.021. pii: S1871-4021(15)30070-9. [DOI] [PubMed] [Google Scholar]
- 45.Barbagallo M, Dominguez LJ. Type 2 diabetes mellitus and Alzheimer's disease. World J Diabetes. 2014;5:889–893. doi: 10.4239/wjd.v5.i6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawamura T, Umemura T, Hotta N. Cognitive impairment in diabetic patients: Can diabetic control prevent cognitive decline? J Diabetes Investig. 2012;3:413–423. doi: 10.1111/j.2040-1124.2012.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuljis RO, Salkovic-Petrisic M. Dementia, diabetes, Alzheimer's disease, and insulin resistance in the brain: progress, dilemmas, new opportunities, and a hypothesis to tackle intersecting epidemics. J Alzheimers Dis. 2011;25(9):29–41. doi: 10.3233/JAD-2011-101392. [DOI] [PubMed] [Google Scholar]
- 48.Jellinger KA. The Diabetic Brain and Dementia. J Alzheimers Dis Parkinsonism. 2015;5:193. [Google Scholar]
- 49.Hsu JL, Chen YL, Leu JG, Jaw FS, Lee CH, Tsai YF, Hsu CY, Bai CH, Leemans A. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage. 2012;59:1098–1105. doi: 10.1016/j.neuroimage.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu F, Sano Y, Tominaga O, Maeda T, Abe MA, Kanda T. Advanced glycation end-products disrupt the blood-brain barrier by stimulating the release of transforming growth factor-beta by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol Aging. 2012;34:1902–1912. doi: 10.1016/j.neurobiolaging.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Kandimalla R, Reddy PH. Multiple faces of dynamin-related protein 1 and its role in Alzheimer's disease pathogenesis. Biochim Biophys Acta. 2016;1862:814–828. doi: 10.1016/j.bbadis.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddy PH. Amyloid beta-induced glycogen synthase kinase 3beta phosphorylated VDAC1 in Alzheimer's disease: implications for synaptic dysfunction and neuronal damage. Biochim Biophys Acta. 2013;1832:1913–1921. doi: 10.1016/j.bbadis.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maqbool M, Mobashir M, Hoda N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer's disease. Eur J Med Chem. 2016;107:63–81. doi: 10.1016/j.ejmech.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Ma DL, Chen FQ, Xu WJ, Yue WZ, Yuan G, Yang Y. Early intervention with glucagon-like peptide 1 analog liraglutide prevents tau hyperphosphorylation in diabetic db/db mice. J Neurochem. 2015;135:301–308. doi: 10.1111/jnc.13248. [DOI] [PubMed] [Google Scholar]
- 55.Qu ZS, Li L, Sun XJ, Zhao YW, Zhang J, Geng Z, Fu JL, Ren QG. Glycogen synthase kinase-3 regulates production of amyloid-beta peptides and tau phosphorylation in diabetic rat brain. Scientific World Journal. 2014:878123. doi: 10.1155/2014/878123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel S, Doble B, Woodgett JR. Glycogen synthase kinase-3 in insulin and Wnt signalling: a double-edged sword? Biochem Soc Trans. 2004;32:803–808. doi: 10.1042/BST0320803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rooney B, O'Donovan H, Gaffney A, Browne M, Faherty N, Curran SP, Sadlier D, Godson C, Brazil DP, Crean J. CTGF/CCN2 activates canonical Wnt signalling in mesangial cells through LRP6: implications for the pathogenesis of diabetic nephropathy. FEBS Lett. 2011;585:531–538. doi: 10.1016/j.febslet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Voskas D, Ling LS, Woodgett JR. Does GSK-3 provide a shortcut for PI3K activation of Wnt signalling? F1000 Biol Rep. 2010;2:82. doi: 10.3410/B2-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haliloglu B, Hysenaj G, Atay Z, Guran T, Abali S, Turan S, Bereket A, Ellard S. GCK Gene Mutations are a Common Cause of Childhood-Onset MODY (Maturity-Onset Diabetes Of The Young) in Turkey. Clin Endocrinol (Oxf) 2016 Jun 3; doi: 10.1111/cen.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez AP, de Dios A, Chiesa I, Perez MS, Frechtel GD. Analysis of mutations in the glucokinase gene in people clinically characterized as MODY2 without a family history of diabetes. Diabetes Res Clin Pract. 2016;118:38–43. doi: 10.1016/j.diabres.2016.04.057. [DOI] [PubMed] [Google Scholar]
- 61.Heuvel-Borsboom H, de Valk HW, Losekoot M, Westerink J. Maturity onset diabetes of the young: Seek and you will find. Neth J Med. 2016;74:193–200. [PubMed] [Google Scholar]
- 62.Yang J, Jiang F, Guo H, Soniya T, Yan CX, Tian ZF, Shi BY. Studies of genetic variability of the hepatocyte nuclear factor-1alpha gene in an Indian maturity-onset diabetes of the young family. Cell Biosci. 2016;6:29. doi: 10.1186/s13578-016-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bian X, Wallstrom G, Davis A, Wang J, Park J, Throop A, Steel J, Yu X, Wasserfall C, Schatz D, Atkinson M, Qiu J, LaBaer J. Immunoproteomic Profiling of Antiviral Antibodies in New-Onset Type 1 Diabetes Using Protein Arrays. Diabetes. 2016;65:285–296. doi: 10.2337/db15-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dezayee ZM. The status of serum gamma-interferonand antiviral antibodies in patients with type I and type 2 diabetes: A comparative study. J Res Med Sci. 2012;17:855–858. [PMC free article] [PubMed] [Google Scholar]
- 65.Jun HS, Yoon JW. A new look at viruses in type 1 diabetes. Diabetes Metab Res Rev. 2003;19:8–31. doi: 10.1002/dmrr.337. [DOI] [PubMed] [Google Scholar]
- 66.Precechtelova J, Borsanyiova M, Sarmirova S, Bopegamage S. Type I diabetes mellitus: genetic factors and presumptive enteroviral etiology or protection. J Pathog. 2014;2014:738512. doi: 10.1155/2014/738512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goppert D, Gardill K, Beischer W, Wietholter H. The stiff-man syndrome with diabetes mellitus type 1 and autoimmune thyroiditis. Dtsch Med Wochenschr. 2000;125:826–829. doi: 10.1055/s-2000-7008. [DOI] [PubMed] [Google Scholar]
- 68.Kosseifi SG, Mehta JB, Roy T, Byrd R, Jr, Farrow J. The occurrence of stiff person syndrome in a patient with thymoma: case report and literature review. Tenn Med. 2010;103:43–47. [PubMed] [Google Scholar]
- 69.Ziegler B, Schlosser M, Luhder F, Strebelow M, Augstein P, Northemann W, Powers AC, Ziegler M. Murine monoclonal glutamic acid decarboxylase (GAD)65 antibodies recognize autoimmune-associated GAD epitope regions targeted in patients with type 1 diabetes mellitus and stiff-man syndrome. Acta Diabetol. 1996;33:225–231. doi: 10.1007/BF02048548. [DOI] [PubMed] [Google Scholar]
- 70.Walikonis JE, Lennon VA. Radioimmunoassay for glutamic acid decarboxylase (GAD65) autoantibodies as a diagnostic aid for stiff-man syndrome and a correlate of susceptibility to type 1 diabetes mellitus. Mayo Clin Proc. 1998;73:1161–1166. doi: 10.4065/73.12.1161. [DOI] [PubMed] [Google Scholar]
- 71.Enuh H, Park M, Ghodasara A, Arsura E, Nfonoyim J. Stiff man syndrome: a diagnostic dilemma in a young female with diabetes mellitus and thyroiditis. Clin Med Insights Case Rep. 2014;7:139–141. doi: 10.4137/CCRep.S16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kota SK, Tripathy PR, Jammula S. Type 2 diabetes mellitus: An unusual association with Down's syndrome. Indian J Hum Genet. 2013;19:358–359. doi: 10.4103/0971-6866.120818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peiris H, Duffield MD, Fadista J, Jessup CF, Kashmir V, Genders AJ, McGee SL, Martin AM, Saiedi M, Morton N, Carter R, Cousin MA, Kokotos AC, Oskolkov N, Volkov P, Hough TA, Fisher EM, Tybulewicz VL, Busciglio J, Coskun PE, Becker A, Belichenko PV, Mobley WC, Ryan MT, Chan JY, Laybutt DR, Coates PT, Yang S, Ling C, Groop L, Pritchard MA, Keating DJ. A Syntenic Cross Species Aneuploidy Genetic Screen Links RCAN1 Expression to beta-Cell Mitochondrial Dysfunction in Type 2 Diabetes. PLoS Genet. 2016;12:e1006033. doi: 10.1371/journal.pgen.1006033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huttly WJ, Bestwick JP, Wald NJ. Insulin dependent diabetes mellitus (IDDM) and first trimester markers in prenatal screening for Down syndrome. Prenat Diagn. 2016;36(9):192–193. doi: 10.1002/pd.4722. [DOI] [PubMed] [Google Scholar]
- 75.Costa Gomes R, Cerqueira Maia J, Fernando Arrais R, Andre Nunes Jatoba C, Auxiliadora Carvalho Rocha M, Edinilma Felinto Brito M, Laissa Oliveira Nazion A, Marques Maranhao C, De Sousa Maranhao H. The celiac iceberg: from the clinical spectrum to serology and histopathology in children and adolescents with type 1 diabetes mellitus and Down syndrome. Scand J Gastroenterol. 2016;51:78–185. doi: 10.3109/00365521.2015.1079645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Panimolle F, Tiberti C, Granato S, Semeraro A, Gianfrilli D, Anzuini A, Lenzi A, Radicioni A. Screening of endocrine organ-specific humoral autoimmunity in 47,XXY Klinefelter's syndrome reveals a significant increase in diabetes-specific immunoreactivity in comparison with healthy control men. Endocrine. 2016;52:157–164. doi: 10.1007/s12020-015-0613-y. [DOI] [PubMed] [Google Scholar]
- 77.Nan HL, Zhang Y, Chen Z. A case of Klinefelter's syndrome with refractory asthma, diabetes mellitus and rib fracture. Chin Med J (Engl) 2013;126:196. [PubMed] [Google Scholar]
- 78.Jiang-Feng M, Hong-Li X, Xue-Yan W, Min N, Shuang-Yu L, Hong-Ding X, Liang-Ming L. Prevalence and risk factors of diabetes in patients with Klinefelter syndrome: a longitudinal observational study. Fertil Steril. 2012;98:1331–1335. doi: 10.1016/j.fertnstert.2012.07.1122. [DOI] [PubMed] [Google Scholar]
- 79.Cai XP, Zhao L, Mao M, Yang ZJ, Xing XY, Li GW. A case of Klinefelter's syndrome with type 1 diabetes mellitus. Chin Med J (Engl) 2012;125:937–940. [PubMed] [Google Scholar]
- 80.Gravholt CH, Jensen AS, Host C, Bojesen A. Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta Paediatr. 2011;100:871–877. doi: 10.1111/j.1651-2227.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- 81.Bojesen A, Host C, Gravholt CH. Klinefelter's syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol Hum Reprod. 2010;16:396–401. doi: 10.1093/molehr/gaq016. [DOI] [PubMed] [Google Scholar]
- 82.Obara–Moszynska M, Banaszak M, Niedziela M. Growth hormone therapy in a girl with Turner syndrome and diabetes type 1 - case report. Pediatr Endocrinol Diabetes Metab. 2016;20:75–81. doi: 10.18544/PEDM-20.02.0006. [DOI] [PubMed] [Google Scholar]
- 83.Navarro Moreno C, Canabate Reche F, Vicente Pintor A, Vela Enriquez FR, del Benavides Roman M. Ketoacidosis as a debut to type 1B diabetes mellitus in a patient with Turner's syndrome. Endocrinol Nutr. 2014;61:439–441. doi: 10.1016/j.endonu.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 84.Callea M, Radovich F, Cappa M, Clarich G. Turner's syndrome with mental retardation, microcephaly and type 1 diabetes in a 6 year old child. Case report and literature review. Minerva Pediatr. 2013;65:251–252. [PubMed] [Google Scholar]
- 85.Bakalov VK, Cheng C, Zhou J, Bondy CA. X-chromosome gene dosage and the risk of diabetes in Turner syndrome. J Clin Endocrinol Metab. 2009;94:3289–3296. doi: 10.1210/jc.2009-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lichiardopol C, Mota M, Braicu D, Militaru C, Mixich F. Diabetes mellitus and Turner syndrome. Rom J Intern Med. 2007;45:299–304. [PubMed] [Google Scholar]
- 87.Spencer L, Bubner T, Bain E, Middleton P. Screening and subsequent management for thyroid dysfunction pre-pregnancy and during pregnancy for improving maternal and infant health. Cochrane Database Syst Rev. 2015:CD011263. doi: 10.1002/14651858.CD011263.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tieu J, McPhee AJ, Crowther CA, Middleton P. Screening and subsequent management for gestational diabetes for improving maternal and infant health. Cochrane Database Syst Rev. 2014:CD007222. doi: 10.1002/14651858.CD007222.pub3. [DOI] [PubMed] [Google Scholar]
- 89.Tieu J, Middleton P, McPhee AJ, Crowther CA. Screening and subsequent management for gestational diabetes for improving maternal and infant health. Cochrane Database Syst Rev. 2010:CD007222. doi: 10.1002/14651858.CD007222.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feldman AZ, Brown FM. Management of Type 1 Diabetes in Pregnancy. Curr Diab Rep. 2016;16:76. doi: 10.1007/s11892-016-0765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adane AA, Mishra GD, Tooth LR. Diabetes in Pregnancy and Childhood Cognitive Development: A Systematic Review. Pediatrics. 2016;137:e20154234. doi: 10.1542/peds.2015-4234. [DOI] [PubMed] [Google Scholar]
- 92.Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292–301. doi: 10.1111/dme.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim C, Draska M, Hess ML, Wilson EJ, Richardson CR. A web-based pedometer programme in women with a recent history of gestational diabetes. Diabet Med. 2012;29:278–283. doi: 10.1111/j.1464-5491.2011.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Z, Zhang H, Shen XH, Jin KL, Ye GF, Qian L, Li B, Zhang YH, Shi GP. Immunoglobulin E and mast cell proteases are potential risk factors of human pre-diabetes and diabetes mellitus. PLoS One. 2011;6:e28962. doi: 10.1371/journal.pone.0028962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lorenzo C, Wagenknecht LE, Hanley AJ, Rewers MJ, Karter AJ, Haffner SM. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2010;33:2104–2109. doi: 10.2337/dc10-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vlaar EM, Admiraal WM, Busschers WB, Holleman F, Nierkens V, Middelkoop BJ, Stronks K, van Valkengoed IG. Screening South Asians for type 2 diabetes and prediabetes: (1) comparing oral glucose tolerance and haemoglobin A1c test results and (2) comparing the two sets of metabolic profiles of individuals diagnosed with these two tests. BMC Endocr Disord. 2013;13:8. doi: 10.1186/1472-6823-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okwechime IO, Roberson S, Odoi A. Prevalence and Predictors of Pre-Diabetes and Diabetes among Adults 18 Years or Older in Florida: A Multinomial Logistic Modeling Approach. PLoS One. 2015;10:e0145781. doi: 10.1371/journal.pone.0145781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inzucchi SE. Clinical practice. Diagnosis of diabetes. N Engl J Med. 2012;367:542–550. doi: 10.1056/NEJMcp1103643. [DOI] [PubMed] [Google Scholar]
- 99.Sherr D, Lipman RD. Diabetes educators: skilled professionals for improving prediabetes outcomes. Am J Prev Med. 2013;44:S390–393. doi: 10.1016/j.amepre.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 100.Lappalainen T, Lindstrom J, Paananen J, Eriksson JG, Karhunen L, Tuomilehto J, Uusitupa M. Association of the fat mass and obesity-associated (FTO) gene variant (rs9939609) with dietary intake in the Finnish Diabetes Prevention Study. Br J Nutr. 2012;108:1859–1865. doi: 10.1017/S0007114511007410. [DOI] [PubMed] [Google Scholar]
- 101.Moleres A, Ochoa MC, Rendo-Urteaga T, Martinez-Gonzalez MA, Azcona San Julian MC, Martinez JA, Marti A. Dietary fatty acid distribution modifies obesity risk linked to the rs9939609 polymorphism of the fat mass and obesity-associated gene in a Spanish case-control study of children. Br J Nutr. 2012;107:533–538. doi: 10.1017/S0007114511003424. [DOI] [PubMed] [Google Scholar]
- 102.Razquin C, Martinez JA, Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, Marti A. A 3-year Mediterranean-style dietary intervention may modulate the association between adiponectin gene variants and body weight change. Eur J Nutr. 2010;49:311–319. doi: 10.1007/s00394-009-0090-2. [DOI] [PubMed] [Google Scholar]
- 103.Ahmad T, Lee IM, Pare G, Chasman DI, Rose L, Ridker PM, Mora S. Lifestyle interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes Care. 2011;34:675–680. doi: 10.2337/dc10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814–823. doi: 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pu J, Chewning B. Racial difference in diabetes preventive care. Res Social Adm Pharm. 2013;9:790–796. doi: 10.1016/j.sapharm.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 106.Dailey G. Overall mortality in diabetes mellitus: where do we stand today? Diabetes Technol Ther. 2011;13:S65–74. doi: 10.1089/dia.2011.0019. [DOI] [PubMed] [Google Scholar]
- 107.Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, Sosa JA, Sumner AE, Anton B. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors--an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97:E1579–1639. doi: 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Edelman D, Edwards LJ, Olsen MK, Dudley TK, Harris AC, Blackwell DK, Oddone EZ. Screening for diabetes in an outpatient clinic population. J Gen Intern Med. 2002;17:23–28. doi: 10.1046/j.1525-1497.2002.10420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda O, Garber AJ, Hirsch IB, Horton ES, Ismail-Beigi F, Jellinger PS, Jones KL, Jovanovic L, Lebovitz H, Levy P, Moghissi ES, Orzeck EA, Vinik AI, Wyne KL. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(Suppl 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 110.Nakagami T, Tajima N, Oizumi T, Karasawa S, Wada K, Kameda W, Susa S, Kato T, Daimon M. Hemoglobin A1c in predicting progression to diabetes. Diabetes Res Clin Pract. 2010;87:126–131. doi: 10.1016/j.diabres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 111.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000;16:230–236. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 112.Tuso P. Prediabetes and lifestyle modification: time to prevent a preventable disease. Perm J. 2014;18:88–93. doi: 10.7812/TPP/14-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, Atreja A, Zimmerman RS. Increase in overall mortality risk in patients with type 2 diabetes receiving glipizide, glyburide or glimepiride monotherapy versus metformin: a retrospective analysis. Diabetes Obes Metab. 2012;14:803–809. doi: 10.1111/j.1463-1326.2012.01604.x. [DOI] [PubMed] [Google Scholar]
- 115.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 116.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121:149–157. e142. doi: 10.1016/j.amjmed.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 118.DeFronzo RA, Abdul-Ghani MA. Preservation of beta-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96:2354–2366. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- 119.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rossner S, Savolainen MJ, Van Gaal L. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 121.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 122.Luchsinger JA, Lehtisalo J, Lindstrom J, Ngandu T, Kivipelto M, Ahtiluoto S, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Eriksson JG, Uusitupa M, Tuomilehto J. Cognition in the Finnish diabetes prevention study. Diabetes Res Clin Pract. 2015;108:e63–66. doi: 10.1016/j.diabres.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 123.Boles AN, Khan H, Lenzmeier TA, Molinar-Lopez VA, Ament JC, TeBrink KL, Stonum K, Gonzales RM, Reddy PH. Impact of Exercise and Education in Adults of Lubbock, Texas: Implications for Better Lifestyle. Front Aging Neurosci. 2016;8:85. doi: 10.3389/fnagi.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 125.Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ. 2009;35:641–651. doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- 126.Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Franz MJ, Boucher JL, Evert AB. Evidence-based diabetes nutrition therapy recommendations are effective: the key is individualization. Diabetes Metab Syndr Obes. 2014;7:65–72. doi: 10.2147/DMSO.S45140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P, Yancy WS., Jr Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821–3842. doi: 10.2337/dc13-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sonestedt E, Lyssenko V, Ericson U, Gullberg B, Wirfalt E, Groop L, Orho-Melander M. Genetic variation in the glucose-dependent insulinotropic polypeptide receptor modifies the association between carbohydrate and fat intake and risk of type 2 diabetes in the Malmo Diet and Cancer cohort. J Clin Endocrinol Metab. 2012;97:E810–818. doi: 10.1210/jc.2011-2444. [DOI] [PubMed] [Google Scholar]
- 130.Hojlund K, Beck-Nielsen H, Flyvbjerg A, Frystyk J. Characterisation of adiponectin multimers and the IGF axis in humans with a heterozygote mutation in the tyrosine kinase domain of the insulin receptor gene. Eur J Endocrinol. 2012;166:511–519. doi: 10.1530/EJE-11-0790. [DOI] [PubMed] [Google Scholar]
- 131.Hojlund K, Wojtaszewski JF, Birk J, Hansen BF, Vestergaard H, Beck-Nielsen H. Partial rescue of in vivo insulin signalling in skeletal muscle by impaired insulin clearance in heterozygous carriers of a mutation in the insulin receptor gene. Diabetologia. 2006;49:1827–1837. doi: 10.1007/s00125-006-0312-6. [DOI] [PubMed] [Google Scholar]
- 132.Pantalone KM, Hobbs TM, Wells BJ, Kong SX, Kattan MW, Bouchard J, Yu C, Sakurada B, Milinovich A, Weng W, Bauman JM, Zimmerman RS. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res Care. 2015;3:e000093. doi: 10.1136/bmjdrc-2015-000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Teychenne M, Ball K, Salmon J, Daly RM, Crawford DA, Sethi P, Jorna M, Dunstan DW. Adoption and maintenance of gym-based strength training in the community setting in adults with excess weight or type 2 diabetes: a randomized controlled trial. Int J Behav Nutr Phys Act. 2015;12:105–113. doi: 10.1186/s12966-015-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13–8. doi: 10.1097/01.JAA.0000475460.77476.f6. [DOI] [PubMed] [Google Scholar]
- 135.Narayanaswami V, Dwoskin LP. Obesity: Current and potential pharmacotherapeutics and targets. Pharmacol Ther. 2016;7258:30194. doi: 10.1016/j.pharmthera.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]