Abstract
Objective
To identify features of ablations and trajectories that correlate with optimal seizure control and minimize the risk of neurocognitive deficits in patients undergoing laser interstitial thermal therapy (LiTT) for mesiotemporal epilepsy (mTLE).
Methods
Clinical and radiographic data were reviewed from a prospectively maintained database of all patients undergoing LiTT for the treatment of mTLE at the University of Miami Hospital. Standard pre- and post-operative evaluations, including contrast-enhanced MRI and neuropsychological testing, were performed in all patients. Laser trajectory and ablation volumes were computed both by manual tracing of mesiotemporal structures and by non-rigid registration of ablation cavities to a common reference system based on 7T MRI data.
Results
Among 23 patients with at least one-year follow-up, 15 (65%) were free of disabling seizures since the time of their surgery. Sparing of mesial hippocampal head was significantly correlated with persistent disabling seizures (p = 0.01). A lateral trajectory through the hippocampus showed a trend for poor seizure outcome (p = 0.08). Comparison of baseline and postoperative neurocognitive testing revealed areas of both improvement and worsening, which were not associated with ablation volume or trajectory.
Significance
At one-year follow-up, LiTT appears to be a safe and effective tool for the treatment of mTLE, though a longer follow-up period is necessary to confirm these observations. Better understanding of the impact of ablation volume and location could potentially fine-tune this technique to improve seizure freedom rates and associated neurological and cognitive changes.
Keywords: deformable atlas, volumetric analysis, hippocampus, amygdala, memory
Introduction
Resection of mesial temporal structures, in particular hippocampus and amygdala, have been the bases of the surgical treatment of mesial temporal lobe epilepsy (mTLE).1 Unfortunately, despite long term seizure-freedom rates ranging between 60–80% and an overall 95% rate of improvement in well-selected patients, this treatment has remained vastly underutilized, due to fears of morbidity associated with antero-temporal lobectomies (ATLs), and potential cognitive deficits due to resection of “functional” tissue. Thus, less-invasive treatment strategies have been employed over the years in an effort to reduce the risk of cognitive decline by sparing functional tissue, while at the same time optimizing the likelihood of seizure control.2–6
Laser interstitial thermal therapy (LiTT) was introduced for the treatment of epilepsy in 2012; it offers a less-invasive option for the surgical treatment of mTLE than that of ATL, resulting in shorter hospital stays and lower reported perioperative morbidity.7–10 Several small case series have reported seizure outcomes comparable to those of ATL (ranging between 60% and 70%), but the relatively short post-surgical periods preclude establishment of its definite equivalence and which ablated mesiotemporal areas most-closely associate with seizure freedom has yet to be determined.7,9,10 Additionally, data on the relationship between the ablation of mesiotemporal structures and neurocognitive functions is limited. The purpose of this study was to use non-rigid image registration to investigate the impact of laser fiber trajectory and ablation volume on post-surgical seizure control and neurocognitive changes in patients with mTLE who underwent LiTT at the University of Miami Epilepsy program and had a minimum of 12-months follow-up.
Methods
Patient Selection
Twenty-three consecutive patients suffering from treatment-resistant mTLE who underwent LiTT, and had a minimum of 12 months of post-surgical follow-up, were included in this study. Every patient underwent a presurgical evaluation at our level 4 epilepsy center, which included a video-EEG monitoring study with scalp, basal and antero-temporal electrodes and when necessary intracranial recordings, 3-Tesla high resolution thin-cut MRI scans using epilepsy protocol with volumetric analysis of mesiotemporal structures, interictal positron emission tomography (PET), neuropsychological evaluation and either functional MRI (fMRI) or Wada test as necessary to lateralize language dominance. Surgical eligibility was evaluated in a multidisciplinary conference that included seven epileptologists, two neuropsychologists, and two neurosurgeons. All eligible patients were given the option to undergo an ATL or LiTT. All aspects of this study were approved by the University of Miami Institutional Review Board (IRB).
Operative Procedure
All procedures were performed under general anesthesia. After application of a radionics CRW (Plainsboro, NJ) frame, a stereotactic-protocol CT scan was obtained and fused to the preoperative 3T MRI using a Medtronic (Minneapolis, MN) Stealth Station and Framelink platform. Trajectory planning was completed to direct the laser fiber through the length of the hippocampus and amygdala and was optimized to avoid cerebral vasculature and the ventricular system where possible.
After positioning and confirmation of frame coordinates, a 3 mm diameter electric drill was used to make a craniostomy at the entry point. Durotomy was performed and the rigid bone anchor was affixed to the skull along the trajectory. The laser fiber was then inserted to the prescribed depth. The frame was removed and the patient was brought to the MRI suite.
T1 weighted images were obtained to confirm laser fiber position prior to ablation and axial and sagittal T2 weighted images were used for ablation. Real time fast spoiled gradient-recalled (FSPGR) echo images were obtained at 4 second intervals to generate near real-time thermal (phase) maps. A series of 3–5 thermal ablations was then performed along the fiber trajectory using the Visualase 980 nm 15 Watt laser. Laser power ranging from 70–80% of maximum in the amygdala and head of hippocampus to 50–60% in the body and tail was used for ablation. Test doses at sub-lethal temperatures were performed at all sequential ablation sites prior to lethal ablation. High temperature set points were set at 90° C and low temperature set points at 55° C. Once adequate ablation was done based on the predictive irreversible damage zone (IDZ), a post-ablation MRI using T1-weighted images with gadolinium was performed. The bone anchor, laser applicator and fiber were then removed, a single stitch was placed at the puncture site and patient extubated.
Neurocognitive Testing
All patients underwent a comprehensive neuropsychological evaluation during their pre-surgical work-up and six to twelve months following surgery. Given the cultural and linguistic diversity of patients in South Florida, neuropsychological evaluations were completed in both English and Spanish by a team of bilingual clinicians. Parallel measures with empirically validated normative data were utilized for the Spanish neuropsychological battery. For the purpose of this study, visual and verbal memory measures were selected to assess the relationship between hippocampal ablation and change in memory performance. The Wechsler Verbal Memory Scale logical memory subtest (WMS-IV LM) was used to assess verbal memory, and the Brief Visual Memory Test-Revised (BVMT-R) was selected as a measure of visual memory. Additionally, the Boston Naming Test, a measure of confrontational naming, and the Rey Complex Figure Test, which assess visual constructional abilities, were included to assess functions typically associated with neocortical structures. These measures are a part of The National Institute of Neurological Disorders and Stroke (NINDS) epilepsy neuropsychological assessment recommendations (https://www.commondataelements.ninds.nih.gov/).
Image Analysis
Volumetric tracings of the amygdala, hippocampus and ablation zone were performed for all patients using 1 mm thin-cut MPRAGE sequences obtained the week prior to surgery and six-months after surgery. Using Analyze 12.0 Software (Overland Park, KS) coronal images were resliced orthogonal to a line dissecting the hippocampus.12 Amygdala and hippocampus were manually traced on each 1 mm slice using criteria established by the European Alzheimer’s Disease Consortium.13,14 From anterior to posterior, hippocampus was defined as “Head” when it was a three-layered structure, “Body” when it became a one-layer structure and “Tail” posterior to the tectal plate.14 All tracings and analyses of images were performed by a neurosurgeon blinded to neurological and cognitive outcomes. To demonstrate our tracing protocol, we show the tracings performed for a sample patient in Supplemental Figure 1.
Image sets were further processed by leveraging the CranialCloud central repository designed by Vanderbilt University and Neurotargeting LLC and its accompanying CranialSuite clinical software to normalize ablation cavities to a common reference system based on 7T MRIs. This platform performs both a global rigid registration (rotational and translational movements of the entire image), and a non-rigid (local deformations) co-registration. These algorithms have been well validated for typical DBS targets and are currently being used for clinical purposes.12,15 We were therefore able to use this platform to normalize ablation cavities of all patients into a common reference atlas and compare between groups.
Statistical analysis
Binomial and multinomial logistic regression was used to examine the relationship between demographic and epilepsy-related variables and ablation volumes and trajectories and seizure outcome. Patient demographic and epilepsy-related variables were analyzed using ANOVA and chi square tests with an alpha level of P < 0.05.
Neurocognitive deficits prior to surgery were examined by identifying the frequency of patients’ whose standardized scores fell in the lower quartile range. Subgroup analysis of dominant vs. non-dominant patients was completed with repeated measures analysis of variance (ANOVA) followed by pairwise comparisons with Bonferroni correction. Lastly, clinically significant change following surgery was determined by examining the frequency of patients who experienced a > 1 standard deviation (SD) increase or decrease from pre-surgical scores.
Results
Among the 23 patients, 13 were male and their mean age was 40.9 ± 11.9 years (six patients were 50 years or older); the mean duration of their seizure disorder was 28.4 ± 11.2 years. While the minimum post-surgical duration was 12 months their mean follow-up period was 22.4 ± 7.1 months. Twelve patients underwent left and 11 right LiTT. Table 1 summarizes demographic and epilepsy-related data. Fifteen patients (65%) had pre-operative evidence of MTS. Of the eight patients without MTS all but one (patient 23) had hypometabolism on PET. Two patients required invasive video-EEG monitoring with intracranial electrodes. One patient (patient 8) had a normal MRI and had hypometabolism in left temporal lobe regions. He underwent recordings with subdural strips placed bilaterally covering basal temporal and temporal lateral regions. The second patient (patient 11) had MTS and an atrial heterotopia in the left hemisphere. She underwent intracranial recordings with subdural strips covering basal and temporal lateral regions and a depth electrode placed within the heterotopia. This patient was found to have dual pathology, both of which were ablated. These two patients have remained free of disabling seizures. Eighteen patients (78%) were discharged the morning after surgery and in four patients, discharge was delayed by one or two days because of transient headache, nausea and unsteady gait that resolved on post-op day two. Only one patent suffered a post-operative neurological deficit, consisting of a left homonymous hemianopia, which resolved partially (patient 18).12
Table 1. Patient demographics and outcomes.
All 23 patients included in the study are listed in chronological order based on surgery date. Dominance refers to the side the laser was inserted.
| Pt | Sex | Age at onset of epilepsy |
Age at surgery |
Side of surgery |
MTS | Language Dominance |
Follow-up (months) |
Seizure outcome |
Time of first postsurgical sz (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 5 | 50 | Left | No | Dom | 37 | IA | |
| 2 | F | 10 | 59 | Right | Yes | Nondom | 27 | IB | |
| 3 | F | 36 | 50 | Left | No | Dom | 30 | IB | |
| 4 | M | 1 | 39 | Right | Yes | Nondom | 29 | II | 8 |
| 5 | F | 5 | 38 | Left | Yes | Nondom | 25 | IB | |
| 6 | M | 1 | 38 | Left | Yes | Dom | 34 | IA | |
| 7 | F | 0 | 21 | Left | No | Dom | 30 | IA | |
| 8 | M | 14 | 29 | Left | No | Dom | 32 | IC | 12 |
| 9 | F | 0 | 32 | Right | Yes | Nondom | 24 | II | 7 |
| 10 | M | 24 | 59 | Right | Yes | Nondom | 22 | IA | |
| 11 | F | 12 | 30 | Left | No | Dom | 27 | IB | |
| 12 | F | 35 | 50 | Right | Yes | Nondom | 19 | IB | |
| 13 | M | 30 | 60 | Right | Yes | Nondom | 26 | IC | 17 |
| 14 | F | 2 | 44 | Right | Yes | Nondom | 23 | IA | |
| 15 | M | 48 | 59 | Left | Yes | Dom | 20 | II | 12 |
| 16 | M | 4 | 45 | Left | Yes | Dom | 15 | IA | |
| 17 | M | 14 | 36 | Right | No | Nondom | 17 | IV | 1 |
| 18 | M | 7 | 24 | Left | Yes | Dom | 14 | IA | |
| 19 | F | 17 | 47 | Right | Yes | Nondom | 12 | IA | |
| 20 | M | 2 | 38 | Left | Yes | Dom | 14 | IA | |
| 21 | M | 0 | 37 | Left | Yes | Dom | 13 | III | 0 |
| 22 | M | 10 | 25 | Right | No | Nondom | 12 | IV | 1 |
| 23 | F | 0 | 31 | Right | No | Nondom | 12 | III | 7 |
MTS – mesiotemporal sclerosis, pt – patient, sz – seizure.
Seizure Outcome
Among the 23 patients, eight (34.8%) were free of auras and disabling seizures (Engel Class I-A), seven (30.4%) had only auras (Engel Class I-B), but two of these patients experienced a disabling seizure after stopping AEDs on their own. They have continued to have only auras after restarting their AEDs. Four patients (17.4%) had less than three seizures/year (Engel Class II), two (8.7%) had a greater than 90% seizure reduction (Engel Class III) and two had less than 90% seizure reduction (Engel Class IV). Thus, 65.2% of patients have remained free of disabling seizures since the time of surgery.
Having MTS was associated with a higher rate of freedom from disabling seizures (73%) compared to mTLE without radiologic evidence of MTS (62%), but this difference did not reach statistical significance (logistic regression, χ221, p = 0.67). Of note, the two patients with Engel IV outcome did not have MTS.
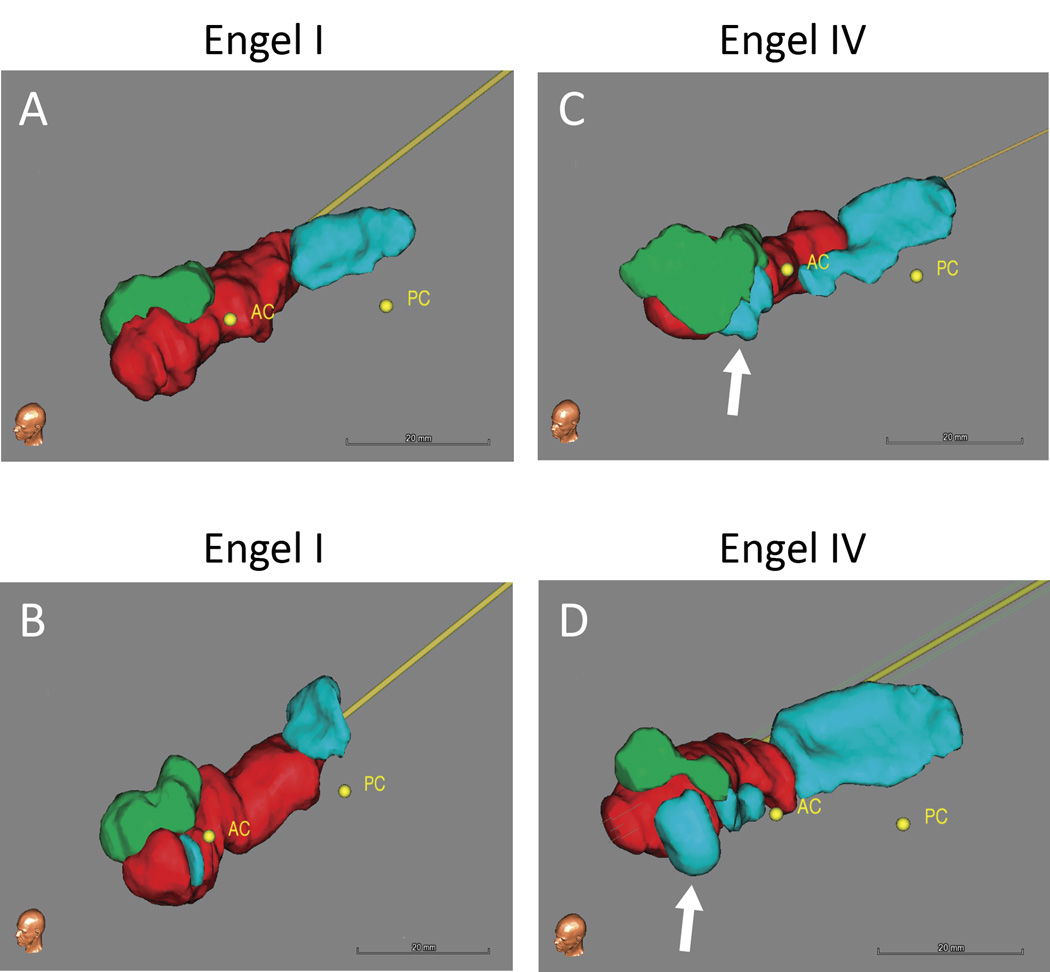
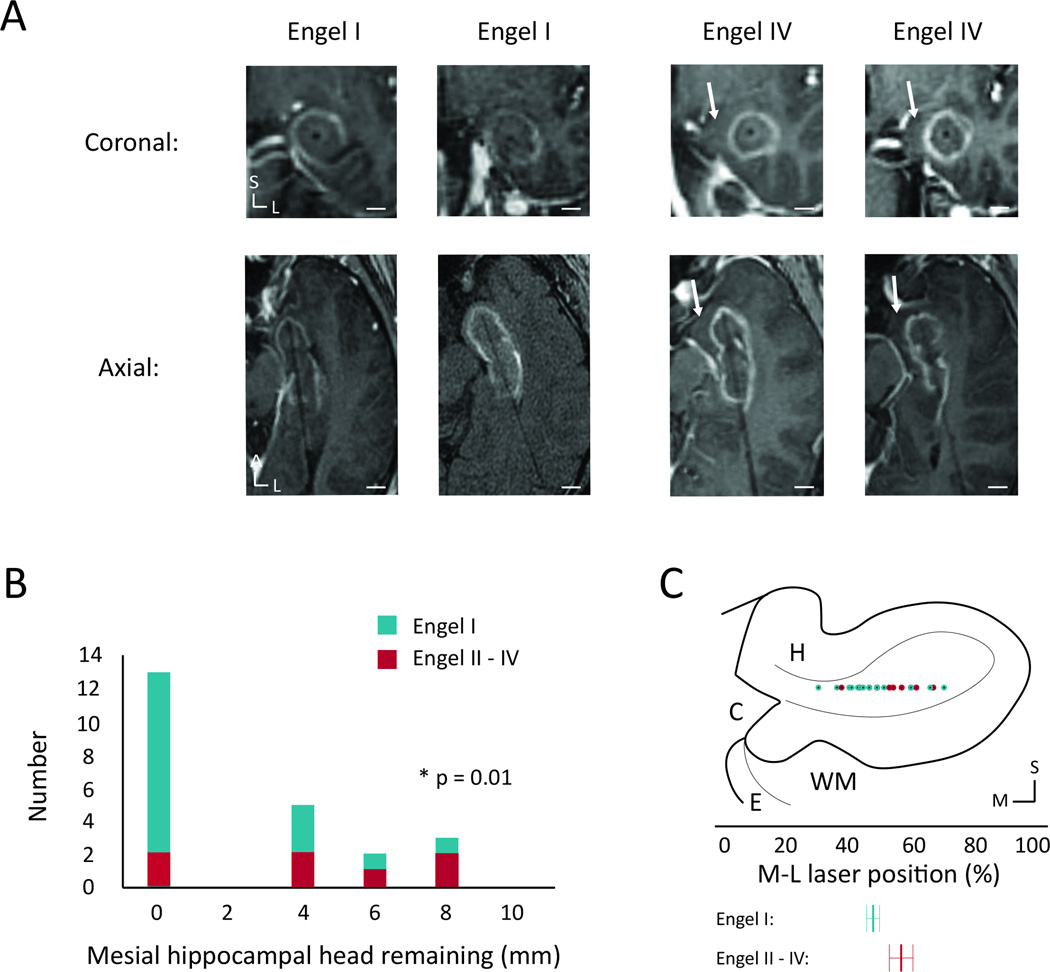
Visual inspection of volumetric renderings derived from postoperative tracings revealed that persistent seizures were associated with greater sparing of mesial hippocampal head tissue (Figure 1). As shown in Table 2, logistic regression analysis (that included TAV, ablation percentages of amygdala and hippocampal head, body and tail, mesial hippocampal head remaining, trajectory angle, laser position in hippocampal head and energy delivered in Joules) demonstrated that sparing of mesial hippocampal head was the only variable significantly associated with persistent seizures (χ221, p = 0.01, Figure 2A, B). A lateral position of the laser along the medial-lateral (M-L) axis of the hippocampal head showed a positive trend toward worse seizure outcomes, but did not reach significance (logistic regression, χ221, p = 0.08, Figure 2C). When multinomial logistic regression was used, no combination of variables together with “remaining mesial hippocampal head” was found that was significantly associated with seizure outcome (i.e. mesial hippocampal head + presence of MTS as independent variables: χ220, p = 0.16).
Figure 1. Postoperative ablation volumes.
Volumetric renderings derived from manual tracings of the postoperative ablation zone and remaining non-ablated hippocampal and amygdala tissue, shown for representative Engel I (A and B) and Engel IV (C and D) patients. The white arrows highlight remaining tissue of the mesial hippocampal head in the Engel IV patients. Ablation – red, Amygdala – green, Hippocampaus – blue. AC – anterior commissure, PC – posterior commissure.
Table 2. Amygdalohippocampal ablation and seizure freedom.
All values are presented as mean ± std. error. The bottom row shows p values comparing the variables for Engel I and Engel II–IV patients, computed with logistic regression.
| TAV (mm3) |
Hipp (%) |
Head | Body | Tail | Amyg (%) |
Mesial Head (mm) |
Axial (°) |
Sagittal (°) |
M-L Position (%) |
Energy (J) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Engel I | 3,160 ± 190 | 80 ± 2 | 90 ± 3 | 89 ± 4 | 38 ± 7 | 54 ± 6 | 1.3 ± 0.5 | 11 ± 1 | 16 ± 1 | 46 ± 3 | 7660 ± 480 |
| Engel II–IV | 2,880 ± 150 | 75 + 7 | 90 ± 5 | 76 ± 13 | 34 ± 11 | 66 ± 5 | 3.7 ± 1.1 | 11 ± 2 | 20 ± 3.0 | 55 ± 4 | 8020 ± 1160 |
| Log reg | p=0.24 | p=0.35 | p=0.80 | p=0.22 | p=0.36 | p=0.21 | p=0.01* | p=0.86 | p=0.15 | p=0.08 | p=0.26 |
p < 0.05
Amyg – Amygdala, Hipp – Hippocampus, TAV – total ablation volume.
Figure 2. Ablation of mesial hippocampal head and seizure freedom.
(A) Coronal and axial intraoperative contrasted fast-spin gradient echo images of the laser trajectory through the ablated hippocampal head for two sample Engel I patients and the two Engel IV patients, showing a lateral trajectory with sparing of medial hippocampal tissue for the Engel IV patients. (B) Histogram showing the distribution of thickness of remaining unablated hippocampal head tissue medial to the ablation zone calculated from the same image sequences. (C) Coronal diagram of the hippocampal head showing the M-L distribution of the laser positions, determined from the posterior-most section through the hippocampal head. Below are shown averages for the two Engel groups with std. error shown using the error bars. White scale bars: 0.5 cm. C – cistern, E – entorhinal cortex, H – hippocampus, WM – white matter tracts; L – lateral, M – medial, S – superior.
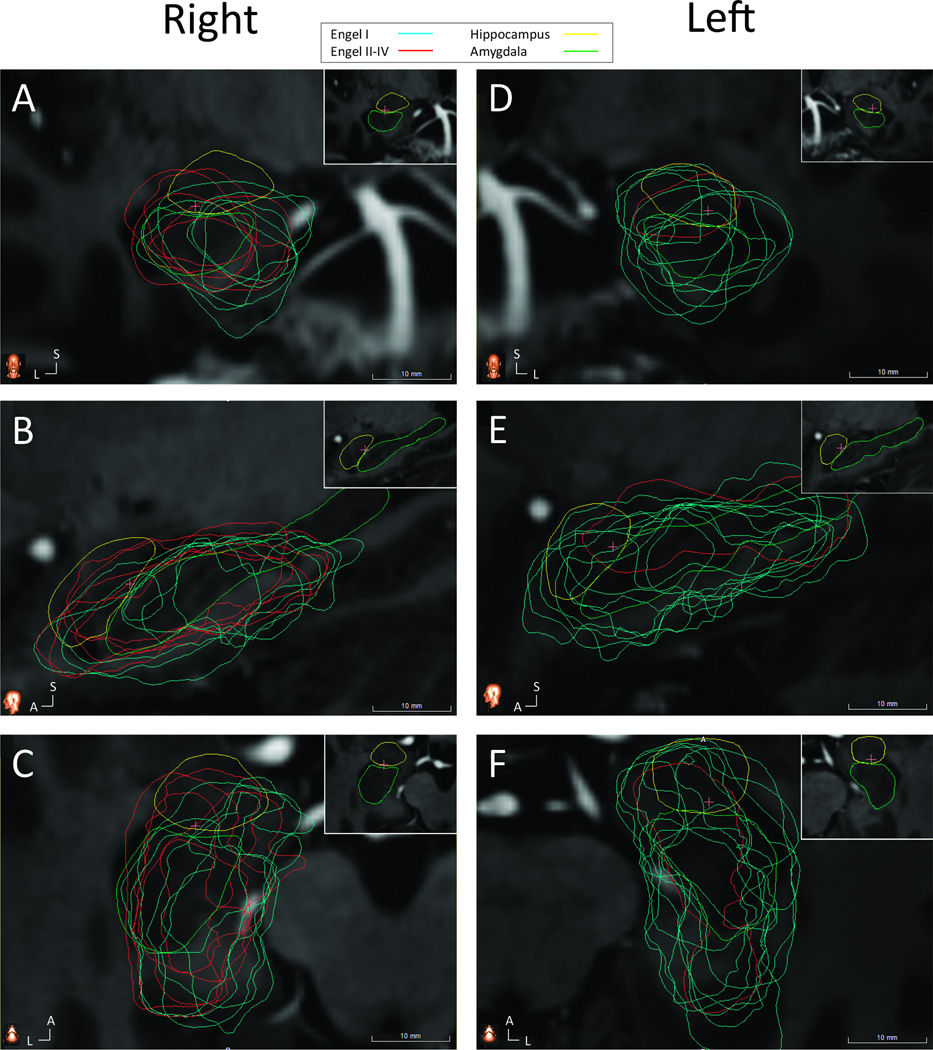
Given the inherent difficulty in comparing imaging data between patients, we next normalized all patient images by registering them to a common reference atlas derived from 7T MRI data. To validate this process, data generated via the atlas platform was compared to manual tracings. Measurements from both methods were strongly correlated (TAV: R2 = 0.7496, F21, p < 0.001; mesial hippocampal head remaining: R2 = 0.9487, F21, p < 0.001; See Supplemental Figure 2). Consistent with the results from manual tracing, the ablations of patients with persistent seizures (Engel II-IV) spared the mesial hippocampal head more frequently than patients free of disabling seizures (Figure 3).
Figure 3. Co-registered ablation cavities and Engel class outcome.
(A) Coronal slice through the right hippocampal head showing greater sparing of medial tissues by the ablations of Engel II–IV patients (red) compared to Engel I patients (blue). Insert in upper right corner demonstrates anatomy, with hippocampus (green) and amygdala (yellow) traced from reference atlas and no ablation cavities shown. (B) Sagittal and (C) axial cuts through right hippocampus and amygdala. The pink crosshairs indicated the intersection of the three imaging planes. (D) Coronal, (E) sagittal and (F) axial slices through left hippocampus with same conventions as (A–C). A – anterior, L – lateral, S – superior.
Neurocognitive Outcomes
Among the 23 patients, 20 had a post-surgical neuropsychological evaluation by the time of this study. Patients were evenly split between side of ablation (left=10; right=10). The mean post-surgical period for testing was 8.4 ± 0.7 months (mean ± std. error). Groups did not show any statistically significant difference in demographic characteristics and epilepsy variables (See Supplemental Figure 3).
Prior to surgery, nine patients with seizures originating in the dominant hemisphere had impairment (scores in the lower quartile range) in at least one measure of verbal memory and/or confrontational naming (Figure 4A). Similarly, seven patients with seizures of non-dominant hemisphere origin had impaired scores on visual spatial and/or memory measures.
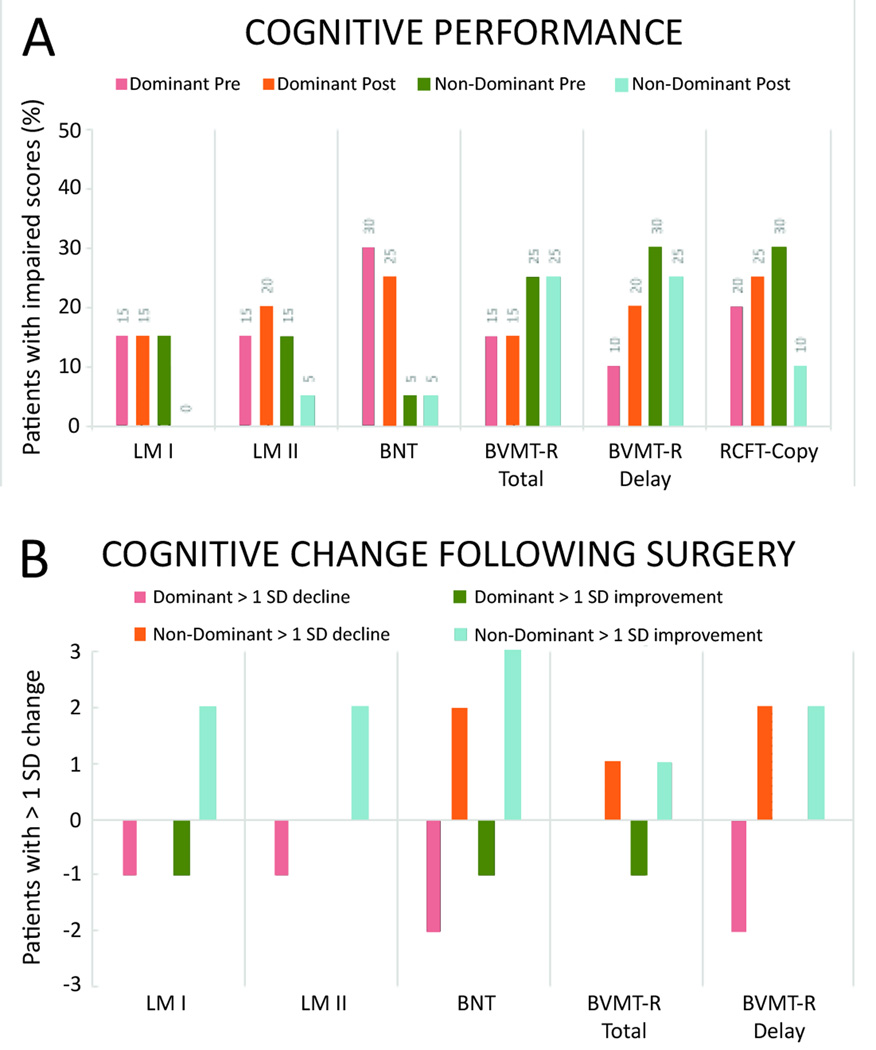
Figure 4. Incidence of clinically-significant neurocognitive changes.
(A) Percent of dominant and non-dominant ablation patients showing neurocognitive deficits (>1 SD) prior to surgery. (B) Number of patients displaying greater than 1 SD change in performance for each of the neurocognitive measures. Both groups contain ten patients. BVMT- brief visual memory test, BNT- measure of confrontational naming, LM- logical memory subtest, RCFT- measure of visual constructional praxis.
A one-way repeated measures ANOVA was conducted to determine whether there were statistically significant differences in memory performance following surgery. There were no outliers and the data was normally distributed, as assessed by boxplot and Shapiro-Wilk test (p > .05), respectively. There was a statistically significant decline in delayed verbal memory performance for dominant hemisphere ablation patients only (F (1,8) = 6.2, p < .05, partial η2 = .40). Verbal memory score changes were not significantly different for seizure-free patients (0.1 ± 6.9) vs. patients with persistent disabling seizures (Engel II–IV) (6.0 ± 8.5, mean ± SD, p < 0.05, logistic regression, χ217, p > 0.05). Performance on the WMS-IV Logical Memory subtest decreased from pre-surgical scores of 41.6 ± 16.1 to 38.0 ± 17.9 (mean ± SD) following LiTT. While change in performance was also observed in the non-dominant ablation group, it was not statistically significant. For full summary of scores, please refer to Table 3.
Table 3. Changes in group memory performance following surgery.
T-scores are shown for verbal and visual memory measures for all patients (10 dominant, 10 non-dominant).
| Dominant | Non-Dominant | ||||
|---|---|---|---|---|---|
| Pre T- Score |
Post T- Score |
Pre T- Score |
Post T- Score |
Statistic | |
| WMS-IV Logical Memory I | 39.8 ± 4.6 | 40.2 ± 4.1 | 42.0 ± 4.3 | 48.3 ± 3.8 | p= 0.11 |
| WMS-IV Logical Memory II | 41.6 ± 5.9 | 38.0 ± 5.8 | 41.2 ± 5.4 | 46.2 ± 5.3 | p= 0.03* |
| BMVT-R Total Learning | 35.6 ± 5.9 | 38.2 ± 6.0 | 35.2 ± 5.3 | 34.5 ± 5.4 | p= 0.43 |
| BMVT-R Delayed Recall | 37.2 ± 6.9 | 35.0 ± 7.4 | 34.0 ± 6.4 | 35.8 ± 6.7 | p= 0.47 |
p < 0.05
Each value shows mean ± SD. Significance was measured with ANOVA followed by post-hoc analysis.
Clinically significant changes were observed in both the dominant and non-dominant hemisphere ablation groups, with the incidence of a significant cognitive decline (>1 SD drop in t-scores) occurring with greater frequency in the dominant hemisphere group (5 vs 3, Figure 4B). In this group, of the five patients who experienced a significant decline, one had deficits in naming only, one in visual memory, two in verbal memory, and one in both naming and visual memory. In the non-dominant hemisphere group, of the three patients who experienced a decline, one had naming deficits only, one verbal memory decline, and one visual memory change.
In contrast, the likelihood of cognitive improvement (>1 SD increase in t-scores) following surgery was greater for non-dominant compared to dominant hemisphere ablations (7 vs 2). Of the seven non-dominant hemisphere patients with post-surgical cognitive improvement, one had better visual and verbal memory scores, one improved in visual memory performance, two demonstrated better verbal memory, and three improved in confrontational naming. Of the two dominant hemisphere patients with improvement following surgery, one had better visual memory performance, and the other showed improvements in both visual memory and naming scores.
To evaluate the relationship between ablation volume and trajectory with neurocognitive outcome, patients were separated into those who had ablations in dominant and non-dominant hemispheres. There was no relation between ablation volume and trajectory and neurocognitive outcome. The normalized ablations of patients that suffered a >1 SD decrease in verbal or visual memory are compared to all other patients in Supplemental Figure 4).
Discussion
Our results suggest that LiTT may be a safe and effective treatment for mTLE. Patients had a rapid recovery with very short hospital stay and low incidence of neurologic and cognitive complications. Seventy-five percent of our patients were able to return home the morning after surgery, with the remaining patients discharged 2–3 days post-procedure. However, while our post-surgical follow-up period of at least 12 months appears to demonstrate seizure-free rates comparable to those reported with ATL, the efficacy of LiTT can only be established with longer post-surgical follow-up periods (at least 24 months and then of 5 and 10 year-duration).1,2,7–10 In our case series, 14 patients had a minimum of 24-months follow up, 12 of whom were free of disabling seizures.
Identifying the optimal LiTT trajectory and ablation is made difficult not only by our limited understanding of the pathophysiology of mTLE, but also by the large variability in human anatomy.15 Here, in addition to performing manual tracings for volumetric analyses, we are the first to apply a non-rigid, deformable brain atlas to the study of a neuroablative procedure. Spatial normalization of images has a long history in deep brain stimulation (DBS), with automated targeted methods based on non-rigid image registration recently demonstrating accuracy comparable to popular manual methods of DBS targeting.15,16 This study further demonstrates the potential of this technology for normalizing and comparing imaging data between groups of patients. Also, because ablation cavities were normalized to the 7T MRI reference map, manual tracing of hippocampus or amygdala was not required, which saved significant time spent on volumetric analyses when computed with the deformable atlas vs. the standard method based on manual tracings. Moving forward, we expect deformable atlases to have a large future impact on the study of neurological diseases.
Several important findings emerged from our analyses: 1) Two-thirds of our patients achieved freedom from disabling seizures (Engel I), which compares favorably to reported seizure outcomes in previous series of MR-guided LiTT.1,2,7,9 2) Inadequate ablation of the mesial hippocampal head, possibly from too lateral a trajectory through the hippocampus, was most-closely associated with poor seizure outcome. 3) There was a small decrease in verbal memory performance in patients with dominant hemisphere ablations.
Consistent with our findings, prior studies of LiTT for mTLE have also reported treatment failures with sparing of mesial hippocampal head structures noted on postoperative imaging.7,9 Treatment of this residual tissue with re-ablation has yielded seizure freedom, though follow-up was inadequate.7 Thus, while the size of an ablation is important, ultimately what matters is that the correct area is ablated. This is illustrated by Patients 17 and 22 (both Engel IV) in our series. While the latter had a well-positioned laser through the hippocampal head but too small of an ablation, the former had an above-average ablation volume but the laser was positioned too lateral.
Although our analyses do suggest anatomical differences between the ablations of Engel I vs. Engel II-IV patients, other factors may have led to treatment failure, such as bilateral atrophy or no evidence of MTS. As in other case series, the presence of MTS in our study was associated with a good seizure outcome after LiTT.7,9 Of the four Engel Class III+IV patients, three did not show preoperative evidence of MTS, suggesting this may have played a role in treatment failure.
Data on the relationship between the ablation of mesiotemporal structures and neurocognitive functions has been historically limited. One of the larger case series that compared 19 patients who had undergone LiTT with 39 patients that had ATL, found no significant decline in cognitive outcome for the LiTT group, compared to deficits identified for the ATL group.8 However, cognitive information was provided on one standardized measure of verbal naming (Boston Naming Tests) and one experimental visual naming measure (Famous Faces). These measures examine functions associated with extra-mesial temporal lobe structures and thus, do not provide information of hippocampal performance with respect to learning and retaining new memories. A similar study by Kang et al. that examined memory performance looked at test re-test change in six patients using a reliable change index (RCI) for the WMS-IV Logical Memory subtest.9 In this study, there was no significant change in performance for all six patients, of which five had undergone dominant hemisphere surgeries. Memory decline was seen in a list of learning tasks, but no information was available on visual memory performance.
The findings in this study suggest that patients who undergo dominant hemisphere LiTT may experience a decline in verbal memory performance. Decline in visual memory performance was also seen in this group, but may be related to imperfect construct validity of commonly used visual memory measures, or a dual encoding process. In contrast, patients who undergo non-dominant hemisphere ablations may be more likely to experience improvement on both visual and verbal memory measures. Similar to previous studies, preservation of functions typically associated with neocortical temporal lobe structures was observed in this series.
Although we have yet to realize the full potential of this therapy, our results are encouraging when viewed in the context of the appeal of MR-guided LiTT as minimally invasive, safe, and offering a faster recovery when compared to standard open resection techniques. Previous observations that LiTT treatment failures often respond well to subsequent ATL, may be explained in part by our data which suggest specific subregions of the amygdalohippocampal complex may be better-suited for targeting in MR-guided LiTT to achieve optimal seizure control.7,9
Thanks to advances in stereotactic targeting the important question is no longer whether the laser is in the hippocampus, but where in the hippocampus? If the probe is positioned too lateral within the hippocampal head, mesial hippocampal head tissue may be spared and seizures unaffected. Thus we recommend a strategy of careful preoperative planning to optimize coverage of the entire hippocampal head, particularly the medial portion.
As we continue to elucidate the optimal procedure for treatment of mTLE using thermal ablation, we can reasonably expect the efficacy and consistency of this treatment to further improve. This will be greatly aided by the continuous evolution of spatial normalization techniques for images and big data sharing. Similarly, as we improve our understanding of the adverse cognitive effects of mesial temporal thermal ablation, we can better define the risks to our patients and accurately predict outcomes. It is our sincere hope that these endeavors continue to eliminate the many barriers obstructing our ability to offer robust treatment to patients suffering from this debilitating disease.
Supplementary Material
Sample manual tracings of hippocampus (red) and amygdala (green). Every other 1 mm thin-cut image is shown, from anterior (A) to posterior (M). White scale bar: 0.5 mm. L – lateral, S – superior.
(A) Comparison of TAV determined using with methods of volumetric analysis. (B) Correlation of mesial hippocampal determined using with methods of volumetric analysis. Each observation was measured from the coronal hippocampal head slice with the most residual mesial tissue.
Data are shown for the 20 patients used for the neurocognitive portion of the paper. FSIQ – Full-scale intelligence quotient. SD – standard deviation.
(A) Coronal image of right hippocampal head with ablation cavities of patients with >1 SD decline in neurocognitive function (red) shown with the ablation cavities of patients that did not suffer a significant decline. Insert in upper right corner demonstrates anatomy without the ablation cavities. Hippocampus (green) and amygdala (yellow) are traced from reference atlas. (B) Sagittal and (C) axial cuts through right hippocampus and amygdala. Pink crosshairs indicated the intersection of the three images. (D) Coronal, (E) sagittal and (F) axial slices through left hippocampus with same conventions as (A-C). A – anterior, L – lateral, S – superior.
Key Points.
Non-rigid image registration using a deformable brain atlas allows for the normalization and comparison of patient images.
In our study 65 % of patients had Engel I outcome one year after surgery; longer follow-up is needed to confirm durability of findings.
Sparing of mesial hippocampal head is correlated with failure to treat seizures.
Some LiTT patients suffer a decline in memory performance, which is not correlated with ablation volume or trajectory.
Acknowledgments
We thank the NIH for their support of Waypoint Navigator and the deformable 7T atlas developed at Vanderbilt University (R01-EB006136 and R01-NS095291). We express our gratitude to Dr. Wayne Winston at The University of Houston for consultation and guidance with statistical analyses.
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflicts of interest to disclose.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;3455:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 2.Josephson CB, Dykeman J, Fiest KM, et al. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013;80:1669–1676. doi: 10.1212/WNL.0b013e3182904f82. [DOI] [PubMed] [Google Scholar]
- 3.Lutz MT, Clusmann H, Elger CE, et al. Neuropsychological outcome after selective amygdalohippocampectomy with transsylvian versus transcortical approach: a randomized prospective clinical trial of surgery for temporal lobe epilepsy. Epilepsia. 2004;45:809–816. doi: 10.1111/j.0013-9580.2004.54003.x. [DOI] [PubMed] [Google Scholar]
- 4.von Rhein B, Nelles M, Urbach H, et al. Neuropsychological outcome after selective amygdalohippocampectomy: subtemporal versus transsylvian approach. J Neurol Neurosurg Psychiatry. 2012;83:887–893. doi: 10.1136/jnnp-2011-302025. [DOI] [PubMed] [Google Scholar]
- 5.Henz BD, Friedman PA, Bruce CJ, et al. Advances in radiofrequency ablation of the cerebral cortex in primates using the venous system: Improvements for treating epilepsy with catheter ablation technology. Epilepsy Res. 2014;108:1026–1031. doi: 10.1016/j.eplepsyres.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medvid R, Ruiz A, Komotar RJ, et al. Current Applications of MRI-Guided Laser Interstitial Thermal Therapy in the Treatment of Brain Neoplasms and Epilepsy: A Radiologic and Neurosurgical Overview. AJNR Am J Neuroradiol. 2015;36:1998–2006. doi: 10.3174/ajnr.A4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willie JT, Laxpati NG, Drane DL, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74:569–584. doi: 10.1227/NEU.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56:101–113. doi: 10.1111/epi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2016;57:325–334. doi: 10.1111/epi.13284. [DOI] [PubMed] [Google Scholar]
- 10.Waseem H, Osborn KE, Schoenberg MR, et al. Laser ablation therapy: An alternative treatment for medically resistant mesial temporal lobe epilepsy after age 50. Epilepsy Behav. 2015;51:152–157. doi: 10.1016/j.yebeh.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Bouman Z, Elhorst D, Hendriks M, et al. Clinical utility of the Wechsler Memory Scale — Fourth Edition in patients with intractable temporal lobe epilepsy. Epilepsy and Behavior. 2016;55:178–182. doi: 10.1016/j.yebeh.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Jermakowicz WJ, Ivan ME, Cajigas I, et al. Visual deficit from laser interstitial thermal therapy for temporal lobe epilepsy: Anatomical considerations. Operative Neurosurg. 2017 doi: 10.1093/ons/opx029. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR. MRI-based hippocampal volume measurements in epilepsy. Epilepsia. 2015;35(6):S21–S29. doi: 10.1111/j.1528-1157.1994.tb05986.x. [DOI] [PubMed] [Google Scholar]
- 14.Frisoni GB, Jack CR, Bocchetta M, et al. The EADC-ADNI Harmonized Protocol for manual hippocampal segmentation on magnetic resonance: evidence of validity. Alzheimers Dement. 2015;11:111–125. doi: 10.1016/j.jalz.2014.05.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Haese PF, Pallavaram S, Li R, Remple MS, et al. CranialVault and its CRAVE tools: a clinical computer assistance system for deep brain stimulation (DBS) therapy. Med Image Anal. 2012;16:744–753. doi: 10.1016/j.media.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallavaram S, D’Haese PF, Lake W, et al. Fully automated targeting using non-rigid image registration matches accuracy and exceeds precision of best manual approaches to subthalamic deep brain stimulation targeting in Parkinson’s disease. Neurosurgery. 2015;76:756–765. doi: 10.1227/NEU.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample manual tracings of hippocampus (red) and amygdala (green). Every other 1 mm thin-cut image is shown, from anterior (A) to posterior (M). White scale bar: 0.5 mm. L – lateral, S – superior.
(A) Comparison of TAV determined using with methods of volumetric analysis. (B) Correlation of mesial hippocampal determined using with methods of volumetric analysis. Each observation was measured from the coronal hippocampal head slice with the most residual mesial tissue.
Data are shown for the 20 patients used for the neurocognitive portion of the paper. FSIQ – Full-scale intelligence quotient. SD – standard deviation.
(A) Coronal image of right hippocampal head with ablation cavities of patients with >1 SD decline in neurocognitive function (red) shown with the ablation cavities of patients that did not suffer a significant decline. Insert in upper right corner demonstrates anatomy without the ablation cavities. Hippocampus (green) and amygdala (yellow) are traced from reference atlas. (B) Sagittal and (C) axial cuts through right hippocampus and amygdala. Pink crosshairs indicated the intersection of the three images. (D) Coronal, (E) sagittal and (F) axial slices through left hippocampus with same conventions as (A-C). A – anterior, L – lateral, S – superior.