Abstract
Vascular precursor cells include stem and progenitor cells giving rise to all mature cell types in the wall of blood vessels. Upon tissue injury, local hypoxia and inflammation result in generation of vasculogenic mediators which orchestrate migration of vascular precursor cells from their niche environment to the site of tissue injury. The intricate crosstalk among signaling pathways coordinates vascular precursor cell proliferation and differentiation during neovascularization. Establishment of normal blood perfusion plays an essential role in effective repair of the injured tissue. In recent years, studies on molecular mechanisms underlying the regulation of vascular precursor cell function have achieved substantial progress, which promotes exploration of vascular precursor cell-based approaches to treat chronic wounds and ischemic diseases in vital organ systems. Verification of safety and establishment of specific guidelines for the clinical application of vascular precursor cell-based therapy remain major challenges in the field.
Keywords: vascular precursor cells, stem cells, progenitor cells, tissue injury, repair, vasculogenesis, angiogenesis
Introduction
Reestablishment of blood circulation is essential for repair of tissue injury. Tissue vascularization commonly involves vasculogenesis, angiogenesis, and arteriogenesis. Vasculogenesis is the formation of new blood vessels from vascular precursor cells. Angiogenesis is the process of outgrowing vessels from the existing vasculature. Arteriogenesis involves remodeling of arteries where collateral arterial anastomoses undergo abluminal expansion. Cell composition in blood vessels is highly heterogeneous and dynamic. Endothelial cells assemble in a monolayer lining the inner surface of all blood vessels. Pericytes are cell components of the microvasculature, including capillaries, arterioles, and venules. In addition to these cells, large blood vessels have many other cell types, such as smooth muscle cells (SMCs), fibroblasts, master cells, dendritic cells, and macrophages.
Since the discovery of unique cell subpopulations with the functional similarity to embryonic angioblasts in the peripheral blood of adults1,2, vascular precursor cells have drawn a broad attention for their role in repair of the blood vasculature. At the site of tissue injury, hypoxia and inflammation result in an increase in local production of bioactive mediators which function as chemoattractants and/or activators. These mediators orchestrate migration of vascular precursor cells from their niche environment to the site of tissue injury. Proliferation and differentiation of vascular precursor cells, particularly endothelial progenitor cells (EPCs), contribute to formation of new blood vessel islets. The initially formed vascular cores via vasculogenesis are pruned and extended by angiogenesis. Maturation of the blood vasculature can be subsequently achieved through remodeling processes with the participation of different vascular precursor cell types, such as mesenchymal stem cells (MSCs) and smooth muscle progenitor cells (SMPCs) (Figure 1 sketches the involvement of vascular precursor cells in neovascularization during the process of tissue injury repair). This review discusses the recent progress in characterization of vascular precursor cells and highlights signaling mechanisms underlying the regulation of their function in promoting neovascularization during the process of tissue injury repair. Efforts in exploring the application of vascular precursor cells for the treatment of tissue injury are also addressed.
Figure 1.
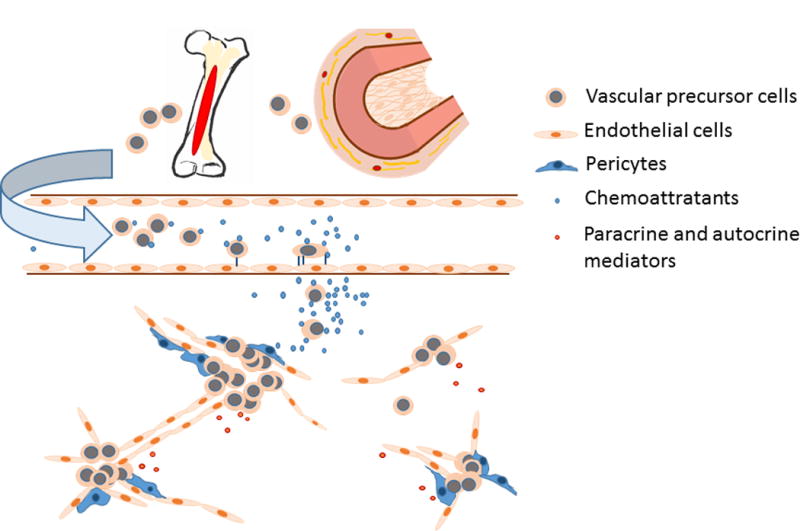
Involvement of vascular precursor cells in neovascularization during tissue injury repair.
Vascular Precursor Cells
Vascular precursor cells denote stem and progenitor cells that give rise to mature cell types in the wall of blood vessels, including endothelial cells, SMCs, and fibroblasts. The existence of circulating EPCs in adults was initially reported by Asahara and colleagues in 19971. In their study, CD34 and Flk1/KDR (Flk1 or fms-like tyrosine kinase-1 is also called vascular endothelial growth factor receptor-2 or VEGFR-2 in mice; KDR or kinase insert domain receptor is the human homolog of VEGFR-2) positive mononuclear cells isolated from human peripheral blood were able to differentiate into endothelial cells in vitro and to participate in neovascularization in vivo. Since then, numerous efforts have been devoted to characterization of adult EPCs. Blood EPCs observed by Asahara and colleagues were in a spindle shape with a limited potential of proliferation for up to 4 weeks in culture1. In 1998, Shi and colleagues isolated an EPC population from human bone marrow and demonstrated that these precursor cells could proliferate to form cobblestone-shaped cell monolayers in the presence of vascular endothelial growth factor (VEGF) in vitro3. These marrow-derived EPCs exhibited a strong potential of proliferation as evidenced by their lifespan of over 30 passages in cell culture. Shortly thereafter, Hur and colleagues reported the presence of two EPC types termed “early” and “late” EPCs, respectively4. Early and late EPCs appeared sequentially in the culture of peripheral blood mononuclear cells (PBMCs). Early EPCs in the spindle shape showed peak growth at 2 to 3 weeks and died at 4 weeks, whereas late EPCs with the cobblestone shape appeared late at 2 to 3 weeks, exhibited exponential growth from 4 to 8 weeks, and lived up to 12 weeks. Early EPCs were proved as a heterogeneous cell population including clones that generated late EPCs. Further investigations on the origin, phenotype, and vasculogenic properties of these cell types have improved understanding about them. Early EPCs are now preferably called “circulating angiogenic cells” (CAC) and late EPC are termed “endothelial colony forming cells” (ECFC)5. The CAC gene signature is highly enriched for markers of M2 macrophages6. These cells do not possess the ability of becoming endothelial cells or directly incorporating into the microvascular network. Instead, they can substantially induce endothelial tube formation in vitro and vascular repair in vivo through generation of vasculogenic mediators. ECFCs are most likely the true EPCs responsible for giving rise to endothelial cells and participating in neovasculization in vivo7.
Ample evidence indicates that EPCs have a close relationship with hematopoietic precursor cells8. In early embryogenesis, endothelial and hematopoietic cells appear to share a common ancestor, i.e. hemangioblasts, in the yolk sac9,10. Shortly later in the embryonic development, hematopoietic stem cells (HSCs) are derived from the hemogenic endothelium in the dorsal aorta9,11. In adults, some surface markers identifying putative EPCs are co-expressed by hematopoietic precursor cells, such as CD34 in humans1,12, and CD117 (stem cell growth factor receptor or c-kit) as well as stem cell antigen-1 (Sca1) in mice13,14. The lineage (lin)−CD34+ cell population or panleukocyte marker CD45−CD34+ cell population in human bone marrow and peripheral blood contains enriched HSCs, while EPCs or ECFCs exhibit a phenotype of CD45−CD34+KDR+15. These facts indicate that human CD45−CD34+ cells are a heterogeneous population of cells containing precursors for both hematopoiesis and vasculogenesis8. Since mature endothelial cells may also express CD3416, CD133 has been introduced for further verification of EPC identity. CD133 antigen (also known as prominin-1) is a marker for hematopoietic stem cells. Mature endothelial cells do not express CD133. CD34+ cells with co-expression of KDR and CD133 has been identified as a unique population of circulating endothelial precursors (CEPs) in humans2. In murine models, transplantation of marrow lin−c-kit+Sca1+ cells (a primitive precursor cell population enriched with HSCs) can not only repopulate blood cells, but also give rise to endothelial cells that are integrated into blood vessels in various organ tissues in the recipients17. A subset of mouse lin−c-kit+Sca1+ cells expressing VEGFR-2/Flk1 is believed to contain enriched EPCs18. In rats, a subset of blood cells bearing lin−Hoechst+CD36+ markers have been characterized to contain putative EPCs19. Blood-derived EPCs in rabbits express CD34+VEGFR-2+Ulex europaeus agglutinin-1(UEA1)+ markers20. These progenitors are also positive for uptake of acetylated low-density lipoprotein.
Numerous efforts have been made for characterizing the vasculogenic/angiogenic activity of cells from the myeloid lineage, particularly of monocytes and macrophages. It is now accepted that monocytes and macrophages do not possess the capacity to further differentiate into fully functional endothelial cells8,21,22. Instead, they promote blood vessel formation through generation of chemoattractants and vasculogenic factors for local vascular precursor cell homing, proliferation, and differentiation. Monocyte/macrophage activation through different pathways can reach two extreme phenotypes: M1 classically activated macrophages that exhibit a pro-inflammatory phenotype, and M2 alternatively activated macrophages that are pro-angiogenic and anti-inflammatory23. Macrophages have a full-range of plasticity allowing them to switch from one phenotype to another under the influence of local environment. Accordingly, either in vitro or in vivo, the distinct milieu is considered to be critical for developing pro-angiogenic activity of monocytes and macrophages21.
Bone marrow houses and produces SMPCs. The existence of SMPCs in the circulation was documented in 200124. In the following year, confirmation for the capacity of human bone marrow-derived mononuclear cells for differentiation into SMCs was reported25. Bone marrow-derived mononuclear cells represent a highly heterogeneous population containing cells from the myeloid origin, such as CD14 positive monocytes and macrophages. Similar to the angiogenic activity described above, accumulated observations suggest that differentiated monocytes/macrophages may primarily generate paracrine effect on remodeling of artery and other large vessels rather than function as the true SMPCs26,27. A CD14 negative SMPC phenotype has been identified recently28. Cells with this phenotype can differentiate into alpha smooth muscle actin (αSMA) expressing cells in the culture system with stimulation of platelet-derived growth factor (PDGF)-BB. Studies using the promoter-sorting method have shown that adhered bone marrow cells transfected with a human smooth muscle (SM)-22α promoter driven green-fluorescent protein (GFP) gene construct express GFP at 5 days after the transfection29. These cells express platelet-derived growth factor receptor (PDGFR)-β but neither mature (calponin) nor immature (embryonic form smooth muscle myosin heavy chain or SMemb) SMC-specific proteins at that time. The cells can eventually grow into individual clones expressing SMC-specific proteins (αSMA, calponin, and SM-1). Culture of murine bone marrow cells with PDGF-BB and monomeric collagen has also been reported to produce SMPCs30. These SMPCs exhibit high proliferative rates prior to their expression of SMC marker αSMA, SM22-α, and smooth muscle-myosin heavy chain (SM-MHC). In vivo studies have revealed that transplantation of bone marrow labelled with enhanced green-fluorescent protein (eGFP) or β-galactosidase (LacZ) in experimental animals and by sex-mismatching in humans result in neointimal SMCs with the respective labeling or gene marker31,32. Furthermore, co-transplantation of adult human peripheral blood-derived EPCs and SMPCs to a nude/SCID mouse model of hindlimb ischemia has been reported to induce a robust neovascularization, improvement of blood perfusion, and enhancement of tissue injury repair33.
At the present time, no marker or makers have been confirmed specific for SMPCs26,27. SMPCs, by definition, can only be specified for their potential of a smooth muscle fate but not expressing differentiated SMC marker proteins27. It has been documented that five surface markers regulating various SMPC functions, including PDGFR-β, carboxypeptidase M (CPM), carbonic anhydrase 12 (CA12), receptor activity-modifying protein 1 (RAMP1), and low-density lipoprotein receptor–related protein (LRP1) can be used for detecting circulating SMPCs in humans34. The reliability of these markers remains to be further validated.
Stem/progenitor cell (SPC) types other than those from hematopoietic tissue may possess the potential to develop into vascular cells. The blood vessel wall is a reservoir for resident precursor cell35,36. The wall of a mature blood vessel typically consists of three layers: the tunica intima, tunica media, and tunica adventitia. All of these three layers contain resident progenitor cells, including EPCs37, SMPCs, and multipotent vascular precursor cells38,39. The initial evidence for the existence of vascular precursors in the vascular wall was obtained from an ex vivo study in which embedding rings of human embryonic aorta in collagen gels led to outgrowth of capillary-like structures with cells expressing markers of endothelial differentiation, such as CD31, CD34, von Willebrand factor (vWF), and Flk1/VEGFR-240. Literatures suggest that precursor cells residing in the vessel wall may divided into two categories, one of which is a typical vascular progenitor cell type giving rise to endothelial cells, SMCs, or both, while the other resembles MSCs37,41.
The intima encompasses a layer of endothelial cells lining the luminal surface of the blood vessel and an elastic lamina of subendothelial connective tissue called the basement membrane. Evidence suggests that the vessel wall-associated endothelial cell pool contains a complete hierarchy of endothelial progenitors42. EPCs isolated from human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAECs) exhibit similar clonogenic potential and endopoietic activity compared to EPCs derived from human umbilical cord blood. EPCs from the blood vessel wall express a profile of endothelial cell-specific antigens including CD31, CD141, CD105, CD146, CD144, vWF, and Flk1, but not the hematopoietic cell surface markers CD45 and CD14. In addition to EPCs associated with the vascular endothelial pool, the intima of the vessel wall contains MSCs that can differentiate into different types of mesenchymal cells43,44. Gene expression by MSCs from the intima shows a strong similarity to that by MSCs from other sources except for two genes related to angiogenesis, interleukin-8 (IL-8) and matrixmetalloproteinase-2 (MMP-2, or gelatinase A), that are expressed more in intima-associated MSCs than in MSCs from other sources, such as bone marrow and umbilical vein44. Furthermore, the expression of both genes is shared with endothelial cells from the umbilical cord vein, suggesting that they may be of particular importance in vascular development and physiology.
Pericytes, also known as Rouget cells or mural cells, predominately reside in the subendothelial space surrounding smaller blood vessels or microvasculature, such as capillaries, precapillary arterioles, and postcapillary venules45,46. These cells are continuous with SMCs of larger-sized arteries and veins46. In addition, pericyte-like cells have been reported to exist in the inner intimal layer, primarily in the subendothelial layer, in large, medium, and small arteries in humans47. Since pericytes are contractile, they apparently play a role in the regulation of vessel tension, vessel permeability, and blood pressure48. Different precursor cell types, including embryonic stem cells (ESCs)49,50, vascular MSCs45,51, bone marrow-derived MSCs52, SMCs53, fibroblasts54, and hematopoietic precursor cells55, have been reported to be able to differentiate into pericytes. Pericytes themselves are multipotent in producing different mature cell types, including SMCs, adipocytes, osteoblasts, chondrocytes, and neurons56–60, which suggests their high degree of plasticity. Pericytes appear to be in a unique status with the capacity to switch phenotypic characteristics in a large range. As capillaries are remodeled into larger vessels to meet increased functional demand, pericytes can further differentiate into true SMCs in order to accommodate the requirement for strengthening the vessel wall56. In addition, the multipotency of pericytes may play a role in the pathogenesis of developing lesions in the vasculature. Pericytes can contribute to plaque formation through differentiation into adipocytes in the lipid core, chondrocytes in the fibrous cap, or osteoblasts in the typically late-stage calcified atherosclerotic plaques37.
Pericytes actively participate in angiogenesis and vasculogenesis. They can stabilize vascular sprouts by migrating along angiogenic sprouts of endothelial cells46. Pericytes can also invade tissues in the absence of endothelial cells to form tubes enabling the subsequent penetration of endothelial cells61,62. These precursors produce a number of mediators, including VEGF63,64, angiopoietin 1 and 2 (Ang-1 and 2)65, and inflammatory cytokines66,67, to regulate endothelial cell migration, survival, and proliferation.
Most of surface markers expressed by pericytes are not specific. Further, the expression of cell markers by pericytes is dynamic depending on the location and functional status of these cells. The currently accepted identity of human pericytes is the combination of CD146+ PDGFR-β+ phenotype along with negative for hematopoietic, endothelial, and myogenic cell markers (CD34−CD31−CD45−CD56−)45,68–70. Cultured mouse brain vascular pericytes are CD146+PDGFR-β+neural/glial antigen 2 (NG2, also known as chondroitin sulfate proteoglycan 4, or CSPG4)+CD31−71. “Activated” pericytes associated with vascular remodeling and neovascularization express an elevated level of regulator G-protein signaling 5 (RGS5)72. Pericytes in normal capillaries typically express desmin, but not αSMA. However, pericytes in venules are immunoreactive for both46. The expressional pattern of NG2 and αSMA helps to distinguish between three subsets of human pericytes associated with capillaries (NG2+αSMA−), venules (NG2−αSMA+), and arterioles (NG2+αSMA+)69. Particularly, NG2 is considered as an angiogenic marker of pericytes73, which appears to be involved in capillary sprouting74. The function of NG2 during angiogenesis is assumed to facilitate the activity of several angiopoietic mediators, including PDGF-AA, basic fibroblast growth factor (bFGF), and transforming growth factor-β (TGF-β)73.
The media layer of blood vessel primarily consists of concentrically arranged SMCs along with collagen fibers, elastic fibers, elastic lamellae, and proteoglycans. SMCs in media of artery walls can switch between two typical phenotypes: the “contractile/quiescent phenotype” and “synthetic/proliferative phenotype.” The former is for contraction and the later for synthesis of extracellular matrix proteins. In the process of vascular injury repair, the fully differentiated SMCs switch from their contractile phenotype to synthetic phenotype and proliferate at sites of vascular injury. This activation of SMC proliferation is associated with an increase in production of extracellular matrix components and reduction in expression of SMC marker gene products including αSMA, SM-22α, and SM-MHC26,27.
The media layer houses vascular precursor cell types. The majority of these vascular precursor cells are multipotent for differentiation. In the media of adult mouse aorta, a side population (SP) of cells displaying the lin−c-kit−/lowSca1+CD34−/low surface characteristics have been isolated38. These SP cells can differentiate into CD31, VE-cadherin, and vWF expressing endothelial cells when in culture with VEGF, or into αSMA, calponin, and SM-MHC expressing SMCs when in culture with TGF-β1/PDGF-BB. In addition, they can generate vascular-like branching structures, composed of both VE-cadherin+ cells and smooth muscle actin+ cells in Matrigel. SP cells also form myeloid or lymphoid hematopoietic colonies in methylcellulose-based medium. In human thoracic aorta, two distinct cell populations have been observed between the media and adventitia75. One is composed of CD34+ cells and the other is made up with c-kit+ cells. Cells in both of these two populations actively proliferate. In culture systems, these cells co-express mesenchymal stromal cell markers, including CD44, CD90, and CD105, and stem cell gene products, such as octamer-binding transcription factor 4 (OCT4), c-kit, and breakpoint cluster region pseudogene 1 (BCRP1). They can acquire an endothelial cell phenotype in the presence of VEGF, characterized by increase in KDR and vWF expression. These precursor cells are also able to form capillary-like structures in in vitro assessments of angiogenesis.
The adventitia is the outer coating of blood vessels consisting of connective tissue. This layer of the vessel wall has a complex structure containing perivascular nerves, nourishing microvessels, and diverse cell types (including resident vascular progenitor cells) embedded in a collagen-rich extracellular matrix41. Similar to those residing in the media layer, precursor cells in the adventitia are multipotent for differentiation. In adult mice, clusters of cells expressing stem cell markers, such as Sca1+ (21%), c-kit+ (9%), CD34+ (15%), and Flk1+ cells (4%), have been observed in the adventitia of aortic roots76. Sca1+ cells from this region are able to differentiate into SMCs in response to PDGF-BB stimulation in vitro and develop into SMC-like cells in atherosclerotic lesions of the intima following transplantation to the adventitial side of vein grafts in recipient mice. This observation also demonstrates that precursor cells in the adventitia possess the potential to migrate across the vessel wall and differentiate into SMCs. In adult human vascular wall, a “vasculogenic zone” has been described, which is located between the smooth muscle and adventitial layer77. It predominantly contains CD34+CD31− cells that also express VEGFR-2 and Ang 1 receptor (TIE2). In vitro, CD34+ cells from human arterial wall form capillary sprouts. New vessels formed by these precursor cells express markers for angiogenically activated endothelial cells, such as carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), and for mature endothelial cells, such as VE-cadherin or occludin. Vascular precursor cells are found in large and middle-sized arteries and veins from various organs. Studies have shown that Notch homolog 1 (Notch1)+, Stro1 (a MSC marker)+, Sca1+, and Oct4+ cells are distributed along the vasculogenic niche in the arterial wall39. These cells homogeneously express markers of stemness (Stro1+Notch1+Oct4+) and the MSC lineage (CD44+CD90 +CD105 +CD73 +CD29 +CD166+), but are negative for hematopoietic and endothelial markers (CD34−CD45−CD31−vWF−). Vascular wall MSCs exhibit characteristics of stem cells, such as a high efflux capability for Hoechst 33342 dye, the ability to form spheroids when growing in suspension, and to generate colonies when seeded at low density. Furthermore, their multipotency of differentiation along the adipogenic, chondrogenic, and leiomyogenic pathways has been identified by culturing them in the respective induction media. They are also able to differentiate into mature SMCs and pericytes78.
It is now accepted that bone marrow and blood vessel walls of almost all organ tissues in the body provide niche for MSCs70,79–84. In addition to being locally recruited through direct cell migration, tissue resident MSCs may enter the blood stream to travel to tissue of distant organ systems85. MSCs in the peripheral circulation share similarly biological characters with those of bone marrow derived MSCs86,87. Circulating MSCs can also exit the blood stream to home to the blood vessel wall niche environment through transendothelial migration88. It appears that MSCs residing in different organ systems within the body constitute a MSC network, through which they dynamically organize their functional properties, including quiescence, self-renewal, and differentiation. Although sharing similar characteristics, MSCs in different niche environments may vary in phenotype, morphology, capacity for proliferation, and potential for differentiation85. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy has suggested the following fundamental standards to better define characteristics of MSCs89: 1) MSCs must be plastic-adherent when maintained in standard culture conditions; 2) MSCs must express CD105, CD73, and CD90 without expression of CD45, CD34, CD14, CD11b, CD79α, CD19, and human leukocyte antigen-D related (HLA-DR) surface molecules; and 3) MSCs must be able to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro. Further investigation on the signaling regulation of MSC functional states and travel patterns will provide a deeper insight into MSC biology as well as their role in repair of vascular injury. Table 1 lists phenotypic markers and key properties of vascular precursor cell types.
Table 1.
Phenotypic markers and key properties of vascular precursor cells
| Precursor cell | Origin | Phenotypic markers | |
|---|---|---|---|
| EPC | Human | CD45−CD34+CD133+VEGFR-2(KDR)+ | |
| Mouse | Lin−c-kit+Sca1+VEGFR-2(Flk1)+ | ||
| Rat | Lin−Hoechst+CD36+ | ||
| Rabbit | CD34+VEGFR-2+UEA1+ with positive for uptake of acetylated low-density lipoprotein | ||
| MSC | Human | 1) Plastic-adherent when maintained in standard culture conditions; | |
| 2) CD105+CD73+CD90+CD45−CD34−CD14−CD11b−CD79α−CD19−HLA-DR−; | |||
| 3) Differentiation into osteoblasts, adipocytes, and chondroblasts | |||
| Pericyte | Human | CD34−CD31−CD45−CD56−CD146+PDGFRβ+ | |
| Capillary | NG2+aSMA− | ||
| Venule | NG2−aSMA+ | ||
| Arteriole | NG2+aSMA+ | ||
| Mouse | CD31−CD146+PDGFRβ+NG2+ | ||
| SMPC | Human | (PDGFR-β+CPM+CA12+RAMP1+LRP1+)? | |
| Vascular wall SPC | Human | CD31−CD34+VEGFR(KDR)+TIE2+ | |
| C-kit+ | |||
| Mouse | Lin−c-kit−/lowSca1+CD34−/low |
Mediators Regulating the Activity of Vascular Precursor Cells
Vascular precursor cells should appropriately respond to injurious stimuli in order for them to participate in injury repair. Tissue injury caused by various factors, such as trauma, hemorrhage, vascular disease, infection, and inflammation, is commonly accompanied by destruction of nourishing blood vessels and disruption of local blood perfusion. The resulting hypoxic state evokes the adaptive response of local cells. Hypoxia-inducible transcription factors (HIFs) play a critical role in the regulation of cell responses to hypoxic stimulation90,91. In mammals, HIFs are inactivated through ubiquitination-associated degradation of their α subunits (HIF-1α, HIF-2α, and HIF-3α)92. Hypoxia inhibits degradation of HIF-α subunits in the proteasome, allowing translocation of stabilized α subunits to the nucleus to form a complex with constitutively expressed HIF-β and co-activators, which together activate transcription of hypoxia-response element (HRE)-bearing genes90,93. Besides the hypoxia-dependent pathway, activation of HIFs can be induced by various cytokines, growth factors, reactive oxygen/nitrogen species, and microbe-derived components in normoxic conditions94. Mechanisms underlying normoxic activation of HIFs remain to be elucidated, which apparently involve regulations at both transcriptional and post-transcriptional levels. HIFs are master transcription factors that activate expression of over 60 genes crucial for cell survival and metabolism under hostile conditions95.
The promoter region of VEGF gene contains binding sites for HIFs96. Binding of HIFs to the VEGF promoter activates VEGF gene expression97,98. In a rat model of gastric mucosal injury, co-localization of HIF-1α protein with VEGF protein was observed in endothelial cells lining regenerating capillaries99. Along with endothelial cells, many other cell types like macrophages, master cells, and fibroblasts express VEGF under the regulation of the HIF activity90,100. In humans, the VEGF gene contains eight exons occupying a coding region of approximately 14kb in chromosome 6p12101. Due to alternative mRNA slicing, VEGF protein molecules may contain different numbers of amino acid residues. At least six VEGF protein isoforms have been identified, including VEGF121, VEGF145, VEGF165, VEGF183, VEGF189, and VEGF206. Most cell types express multiple variants of VEGF with the predominant expression of VEGF121 and VEGF165102,103. VEGF (or its recent reclassification as VEGF-A) and other members of the VEGF family [VEGF-B, C, D, E and placenta growth factor (PGF)] have been well characterized. The biological features of each VEGF family member and their correspondent receptors have been described in detail in several recently published review articles101,103,104.
VEGF is a secreted protein crucial for vasculogenesis and angiogenesis102. VEGF produced by local cells following tissue injury can diffuse into the blood stream, resulting in an increase in VEGF concentration in the systemic circulation105–107. The active forms of VEGF are mostly homodimers with a molecular weight of 45kDa108. VEGF initiates cell signaling through binding to its receptors, VEGF receptor-1 (VEGFR-1) and VEGFR-2. VEGFR-1 has approximately 10-fold higher affinity for VEGF than that of VEGFR-2, but the kinase activity of VEGFR-1 is about one-tenth that of VEGFR-2104. Furthermore, the VEGFR1 gene produces two products, membrane-bound VEGFR-1 and soluble VEGFR-1. Membrane-bound VEGFR-1 is the full-length, fully functional VEGFR, while soluble VEGFR-1 consists of only the extracellular domain of VEGFR1 without ability to transmit signal across cell membrane. Therefore, VEGFRs may have different roles in vasculogenesis and angiogenesis. VEGFR-2 essentially serves as a positive signal transducer, while VEGFR-1, particularly soluble VEGFR-1, functions as an attenuator109. Both EPCs and differentiated endothelial cells in the blood vasculature express VEGFR-218,110. Engagement of a VEGF homodimer with its receptor causes dimerization of two receptors, triggering their autophosphorylation through the receptor-associated tyrosine kinases. This is followed by activation of several downstream signaling components, including the phospholipase C-gamma (PLC-γ)/protein kinase C (PKC), protein kinase D (PKD), phosphatidylinositol-3 kinase (PI3K), Ras pathway members, and mitogen-activated protein kinase (MAPK), to mediate cell proliferation, differentiation, migration, and contraction103,106,111–117.
The stromal cell–derived factor-1 (SDF-1, also known as CXC motif chemokine 12 or CXCL12)/CXC receptor 4 (CXCR4) axis provides a major driving force for stem/progenitor cell mobilization and homing. EPCs express CXCR4 and respond to SDF-1. The promoter region of SDF-1 contains HIF binding sites118. Engagement of HIF with the SDF-1 promoter activates SDF-1 gene transcription. Cells, particularly endothelial cells, at the site of tissue injury increase their expression and release of SDF-1. In normal circumstance, SDF-1 expression is higher in the bone marrow than in peripheral tissues, but the marrow-periphery gradient of SDF-1 is reversed with the increased release of SDF-1 from sites of injury. Marrow EPCs are then mobilized to the peripheral circulation and recruited to tissue sites with active SDF-1 expression. In addition, SDF-1 causes increases in EPC proliferation and expression of genes for endothelial differentiation, including vWF, TIE2, and VE-cadherin119. SDF-1/CXCR4 signaling has been observed to promote angiogenic activity of EPCs in both in vitro and in vivo experimental models120. SDF-1/CXCR4 signaling is also involved in mediating homing of MSCs and SMPCs121,122.
Macrophage migration inhibitory factor (MIF) is a member of the lately defined ‘chemokine-like function’ (CLF) chemokine family that promotes EPC migration and angiogenesis123. Hypoxia-induced production of MIF by different cell types, including endothelial cells and vascular SMCs, involves the HIF-1α dependent pathway124,125. CD74 is a plasma membrane receptor which binds MIF with a high affinity126. CD74 forms heteromeric complexes with either CXCR2 or CXCR4127,128. These activated complexes may initiate cell signaling through the MAPK, PI3K, and protein kinase B (AKT) cascades126,128,129. MIF potently stimulates EPC chemotaxis124,130. Anti-MIF or anti-CXCR4 antibody is able to block EPC migration in response to supernatants of hypoxia-conditioned HUVECs. Clinical studies have shown that both the serum level of MIF and the number of circulating EPCs in patients receiving flap operations increase markedly following the surgery130. A significant correlation exists between increases in the serum level of MIF and the number of circulating EPCs. Additionally, serum samples from flap patients promotes EPC migration, which can be partially blocked by anti-MIF antibody. MIF also mediates endothelial cell migration and tube formation in vitro as well as to induce angiogenesis in vivo123.
Ang-1 and Ang-2 play important roles in facilitating angiogenesis, especially in EPC adhesion to and migration across established endothelium. HIF regulates the expression of Ang-2 by endothelial cells131. Macrophages can produce Ang-2 in response to different stimuli, including lipopolysaccharide (LPS), interferon-gamma (INFγ), prostaglandin E2 (PGE2), and VEGF132. In human adults, Ang-2 is expressed only at sites of vascular remodeling133. Ang-2 is a ligand for the receptor tyrosine kinase TIE2. An important function of Ang-2 is to destabilize established vasculature allowing formation of new vessels. EPCs express Ang-2 receptor. Ang-2 binding to TIE2 causes a marked stimulation of EPC migration117. Ang-2 and VEGF have an additive effect on EPC migration. Ang-2 also promotes adhesion of EPCs to the endothelial cell monolayer. Such adhesion localizes these progenitor cells to angiogenic endothelial cells in order for them to participate in formation of new blood vessels. Besides Ang-2, Ang-1 has been shown to enhance EPC migration and adhesion to endothelial cells117. Hypoxia and VEGF up-regulate Ang-1 gene transcription by pericytes134. Therefore, Ang-1 appears to participate in the regulation of EPC migration and homing to injured tissue sites during repair and/or the formation of blood vessels.
Several members of the interleukin family regulate vascular precursor cell function during the process of new vessel formation. Interleukin-1beta (IL-1β) is a cytokine that promotes vasculogenesis and angiogenesis either though directly exerting its effect on EPCs or indirectly via the HIF-VEGF pathway135. Cultures of murine EPCs with IL-1β display increased numbers of cells and colonies. IL-1β also significantly increases the number of vessel-like structures in a Matrigel assay of EPCs, demonstrating its ability to augment EPC vasculogenic function. IL-1β significantly stimulates human EPC proliferation, migration, and adhesion through activation of the PI3K-AKT signal pathway and extracellular-signal-regulated kinases 1/2 (ERK1/2) signaling136. EPCs express IL-1 receptor-I (IL-1R). In in vivo Matrigel plug experiments, it has been observed that IL-1 produced from infiltrated marrow myeloid cells stimulates VEGF production by recruited endothelial cells from the neighboring tissues, which in turn promotes angiogenesis137. Subcutaneous administration of interleukin-1alpha (IL-1α) to mice can cause a strong angiogenic response locally, which is accompanied by infiltration of VEGF-expressing cells. Treatment with VEGFR-2 neutralizing antibodies blocks this IL-1α-induced angiogenic response138. Interleukin-6 (IL-6) has also been found to stimulate EPC proliferation, migration, and Matrigel tube formation. EPCs express IL-6 receptor (IL-6R)139. Binding of IL-6 to its receptor activates the gp80/gp130 signaling pathway, leading to activation of the downstream ERK1/2 and signal transducer and activator of transcription 3 (STAT3) cascades.
Genes encoding PDGFs are targeted by hypoxia and/or HIF140–143. Certain inflammatory cytokines and growth factors can also stimulate PDGF expression by different cell types144,145. Mammalian PDGFs are divided into two classes (class I and II), depending on the presence of basic retention motifs (PDGF-A and B) or CUB domains (PDGF-C and D)145. Ligand PDGFs predominantly form homodimers except for one heterodimer (PDGF-AB) that has been identified in the culture of human platelets146. PDGFs bind to two similar protein tyrosine-kinase receptors: PDGFR-α and β. PDGF-AA and PDGF-CC function via binding to PDGFR-α, while PDGF-BB acts through engagement with PDGFR-β. Cell types from mesenchymal, hematopoietic, endothelial, and epithelial origins, including MSCs, pericytes, vascular SMCs, blood mononuclear cells, and EPCs, express PDGFRs. The expression of PDGFRs by cells can be substantially upregulated by inflammatory cytokines, growth factors, and microbe-derived substances145,147. Binding of PDGFs to their receptors initiates dimerization of receptors, allowing for receptor autophosphorylation on tyrosine residues in the intracellular domain. Downstream signaling involves activation of several major pathways, including the Ras/MAPK, PI3K, PLC-γ, and signal transducer and activator of transcription 5 (STAT5) cascades, during the process of modulating cell proliferation, differentiation, migration, and secretion144. Endothelial cells in the developing vasculature, particularly at the tip of angiogenic sprouts and in the growing arteries, strongly express PDGF-BB145. Locally produced PDGF-BB by endothelial cells plays a crucial role in recruiting pericytes in order for both cell types to function coordinately during the development of new vessels and maturation of existing vessels. PDGF signaling regulates EPC survival, proliferation, and migration148,149. In addition, PDGFs are major mitogens for a number of vascular cell types, including pericytes, fibroblasts, SMPCs, and SMCs150.
TGF-β is another important mediator for formation and remodeling of blood vessels. In mammals, three isomeric forms (TGF-β1-3) have been identified in the TGF-β family151. In humans, TGF-β1 is the predominant isoform that commonly generates homodimers with a mass around 25kDa. Almost all types of cells produce TGF-β1. Members of the TGF-β family exert their effects by binding to specific receptors152. In mammals, seven type I [TβRI, also known as activin receptor-like kinase (ALK) 1 to 7] and five type II (TβRII) TGF-β receptors have been identified. TGF-β binds to the type II receptor with a high affinity. Upon binding, a type I receptor is recruited to form a ligand-induced heteromeric receptor complex within which the constitutively active type II receptor phosphorylates the type I receptor on specific serine and threonine residues to activate the type I receptor153. ALK5 and ALK1 are major type I receptors for TGF-β signaling in vasculogenic cells154. Interaction of ligands with TGF-β receptors can be affected by soluble ligand binding proteins and accessory type III receptors, such as endoglin and betaglycan155,156. Signaling molecules positioned immediately downstream of the type I receptor activation are receptor Smads (R-Smads). With the activation of the receptor complex, R-Smads are recruited to and phosphorylated by type I receptors. Typically, activation of ALK5 will induce activation of the Smad 2/3 pathway, whereas activation of ALK1 will lead to activation of the Smad 1/5/8 pathway157. A complex interplay exists between TGF-β/ALK5 and TGF-β/ALK1 signals, which adjust the overall effect of TGF-β on the downstream signaling activities158. Activated R-Smads will interact with the common Smad4 (C-Smad) to form heteromeric complexes that will then translocate to the nucleus to regulate the transcription of target genes with the assistance of other partner proteins.
Studies on genetic TGF-β deficiencies have demonstrated that TGF-β1 and TβRII are critical for both vasculogenesis and angiogenesis152,153,158. TGF-β1 promotes EPC vasculogenic activity159. TGF-β signaling also regulates differentiation of EPCs from human pluripotent stem cells160. TGF-β1 alone or with PDGF-BB promotes proliferation and SMC differentiation of bone marrow-derived multipotent progenitor cells161. TGF-β1 also promotes SMC differentiation from MSCs162 and enhances contractile function of vascular constructs generated from hair follicle mesenchymal stem cells163. Except for the direct effect on vascular precursor cells, TGF-β acts on HIF-1 vasculogenic activity through enhancing HIF-1α gene expression, increasing HIF-1α protein stability, and inducing HIF-1 DNA binding activity164–166.
In contrast to mediators stimulating the activity of vascular precursor cells as discussed above, certain bioactive molecules exert inhibitory effects on vascular precursor cell function in various physiological and pathological conditions. Vascular endothelial growth inhibitor (VEGI), also termed as tumor necrosis factor superfamily 15 (TNFSF15), is a cytokine produced predominantly by endothelial cells in established blood vessels. Three isoforms of VEGI including VEGI-174, VEGI-192, and VEGI-251 have been identified167. Since VEGI inhibits endothelial cell proliferation and induces apoptosis in these cells, VEGI appears functioning as an autocrine cytokine to inhibit angiogenesis and stabilize the established vasculature168. VEGI also exerts a strong inhibitory effect on EPC activities, including their differentiation into endothelial cells, adherence, migration, and vasculogenesis169,170. In addition, VEGI induces apoptosis of differentiated EPCs, but not early-stage EPCs. VEGI exerts its effect on EPCs at least partially through engagement with the death domaincontaining receptor 3 (DR3), a member of the TNF receptor superfamily, that is expressed by differentiated EPCs, endothelial cells, and other mature cell types169,171. One possible mechanism underlying VEGI-mediated inhibition of EPC function is to stimulate soluble VEGFR-1 expression by activating the PKC, Src, and Erk1/2 signaling pathway170. VEGI promotes alternative splicing of the VEGFR-1 gene in favor of soluble VEGFR-1 production by down-regulating nuclear protein Jumonji domain-containing protein 6 (Jmjd6), thus alleviating Jmjd6-inhibited soluble VEGFR-1 expression. Since soluble VEGFR-1 essentially functions as a VEGF trapper/inhibitor104, the biological activity of VEGF is subsequently inhibited by VEGI. Immunohistochemical staining has shown that VEGI expression in tissues of acute wounds is down-regulated172. The underlying mechanisms remain to be elucidated. It is apparent that this reduction of VEGI expression is in favor of revascularization during the process of tissue injury repair.
Pigment epithelium–derived factor (PEDF) is a 50-kDa secreted glycoprotein in the serine proteinase inhibitor (Serpins) family173. PEDF is a potent anti-angiogenic factor174. The anti-angiogenic activity of PEDF is selective in that it is effective against newly forming vessels but spares existing ones. PEDF functions on the target cells likely through interaction with its receptor(s). A putative PEDF receptor of 80–85-kDa has been isolated175. There is a reciprocal interaction between PEDF and VEGF176,177. In vitro studies have observed that PEDF decrease VEGF expression by cultured cells176. Silencing of the PEDF gene upregulates VEGF expression at both the RNA and protein levels. PEDF inhibits VEGF expression likely through inhibiting hypoxia-induced increase in VEGF promoter activity, HIF-1 nuclear translocation, and mitogen activated protein kinase phosphorylation. In diabetic patients with foot ulcer, the plasma level of PEDF is elevated178. In animal experiments, it has been observed that the increase in plasma level of PEDF is correlated with the reduction of EPC counts in the peripheral circulation. PEDF also inhibits EPC functional activities, including adhesion, migration, and tube formation, in in vitro culture systems. In addition to function on EPCs, PEDF stimulates the surface expression of Fas ligand by endothelial cells174. Activated endothelial cells that are migrating out from the established vasculature to form new vessels in response to inducers of angiogenesis (such as VEGF) display Fas receptor. The interaction of Fas ligand with Fas receptor induces apoptosis in these activated endothelial cells. Since regular endothelial cells in existing vessels do not express Fas receptor, they appear to be protected from PEDF-induced apoptosis.
Interleukin-10 (IL-10) is generally considered as an anti-inflammatory cytokine. In vitro culture of human EPCs with IL-10 has shown that IL-10 causes a dose-dependent inhibition of EPC differentiation into mature endothelial cells179. The presence of IL-10 in serum is inversely correlated with EPC function in patients with chronic inflammatory diseases, such as systemic lupus erythematosus (SLE). Observations have also shown that activation of the inflammasome pathway in patients with SLE leads to elevation of serum interleukin-18 (IL-18) level, which is correlated with EPC dysfunction180. IL-18 can cause a dose-dependent inhibition of EPC differentiation into mature endothelial cells in the in vitro culture system. In addition, culturing EPCs with angiotensin II (Ang II) has been shown to accelerate the rate of senescence as well as reduction of proliferation in EPCs181,182. Inhibition of telomerase activity appears playing an important role in Ang II-induced impairment of EPC function. Table 2 lists major activators and inhibitors for vascular precursor cell activities.
Table 2.
Major factors regulating the activity of vascular precursor cells
| Mediators | Receptors | Function |
|---|---|---|
| Activators | ||
| Ang | TIE2 | EPC adhesion, migration, and homing |
| HIF | Master transcription factor for expression of over 60 genes related to vasculogenesis, angiogenesis, and inflammation | |
| IL-1 | IL-1R | EPC proliferation, adhesion, migration, and differentiation |
| IL-6 | IL-6R | EPC proliferation, migration, and differentiation |
| MIF | CD74 | EPC migration and chemotaxis |
| PDGF | PDGFR | Survive, proliferation, migration, differentiation, and secretion of EPCs, MSCs, and pericytes |
| SDF-1/CXCL12 | CXCR4 | Retention of SPCs in their niche; recruitment and homing of EPCs, MSCs, and SMPs; EPC proliferation and differentiation |
| TGF-β | TβR | Proliferation and differentiation of EPCs and MSCs; HIF expression, stability, and activity in cells |
| VEGF | VEGFR-1, VEGFR-2 | EPC proliferation, migration, and differentiation |
| Inhibitors | ||
| VEGI/TNFSF15 | ??, DR3 | EPC survive, adhesion, migration, and differentiation |
| PEDF | PEDF receptor? | EPC adhesion, migration, and differentiation; expression of VEGF |
| IL-10 | IL-10 receptor (IL-10R) | EPC differentiation |
| IL-18 | IL-18 receptor (IL-18R) | EPC differentiation |
| Ang II | Ang II receptor (Ang IIR) | EPC proliferation, induction of senescence in EPCs |
Vascular Precursor Cells in Vascularization during Tissue Injury Repair
A typical process of wound healing encompasses distinct, yet overlapping, phases of hemostasis, inflammation, proliferation, and maturation. Effective vascularization is essential for delivering oxygen and nutrients to cells in organ tissues. Upon traumatic injury, the initial effort of the host defense is to establish hemostasis, where damaged blood vessels constrict along with local formation of platelet plugs and blood clots. Tissue injury caused by pathological changes of the vascular wall is also accompanied by either bleeding from the rupture of blood vessel or ischemia due to occlusion of blood vessel. Activation of the HIF pathway caused by interruption of tissue oxygen supply ignites chain reactions of cytokine production90. Lipid and peptide mediators derived from aggregated platelets participate in initiation of the inflammatory reaction183. In the meanwhile, activation of pattern recognition receptors (PRRs) in cells through engagement with damage-associated molecular pattern ligands (DAMPs) and pathogen-associated molecular pattern ligands (PAMPs, in the presence of infection) evokes the local and/or systemic inflammatory response184. Recruitment of leukocytes to the site of tissue injury reinforces generation of cytokines, chemokines, and growth factors during the inflammatory phase. These soluble mediators work in concert to recruit vascular precursor cells from their niche environment, such as the bone marrow and vascular wall, to the site of tissue injury. Clinical investigations have shown that EPCs in the peripheral circulation can increase by 50-fold within the first 6 to 12 hours in patients with burn injuries or coronary artery bypass grafting185.
The bone marrow hematopoietic environment contains an endosteal niche for retention of quiescent stem cells and a permissive sinusoidal site for precursor cell proliferation and their release into the systemic circulation186–188. NG2+ pericytes around small arterioles in the endosteum appear to be the major player for maintaining quiescence in marrow stem cells, whereas leptin receptor+ cells in the perisinusoidal niche facilitate stem/progenitor cell cycling and proliferation189. Mobilization of stem and progenitor cells from bone marrow involves cell detachment from the stromal niche and egress into the circulation. During this process, cells are activated for proliferation to expend the pool of these precursors. Programing of precursor cells to improve their potential for differentiation into vascular cells also takes place.
Bone marrow contains diverse types of stromal cells187. The retention of stem and progenitor cells in their niche relies on ligand binding to the cell membrane. Several ligand/receptor pairs, including kit ligand (kitL)/c-kit, SDF-1/CXCR4, and vascular cell adhesion molecule-1 (VCAM-1)/integrins (α4β1, α4β7, and α9β1), play important roles in the retention of these cells187,188,190. Many cytokines, such as SDF-1, VEGF, stem cell growth factor (SCF), granulocyte colony-stimulating factor (G-CSF), macrophage colony stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, interleukin-3 (IL-3), IL-6, IL-8, and pattern recognition receptor ligands generated from the site of tissue injury stimulate marrow stromal cells and hematopoietic cells to secret matrixmetalloproteinase-9 (MMP-9)188,191,193. MMP-9 converts kitL from a membrane-bound adhesion- and survival-promoting molecule to a soluble survival/mitogenic factor through proteolytic cleavage. Increase in soluble kitL drives transfer of stem cells from the quiescent niche to the proliferative niche188. In addition, MMP-9 can cleave c-kit from cell surfaces to facilitate their mobilization194.
Stromal cells in perivascular regions express high levels of SDF-1 that binds to CXCR4 expressed by stem cells to retain them in the marrow niche187,195,196. These perivascular stromal cells, including SDF-1-abundant reticular (CAR) cells, nestin+ stromal cells, and leptin receptor+ stromal cells, are essentially mesenchymal precursor cells in a considerable overlap with each other. Uncoupling of SDF-1 from CXCR4 in the marrow facilitates the release of these stem cells197. Certain soluble mediators, such as G-CSF, Flt3 ligand (Flt3L), and SCF induce down-regulation of SDF-1 expression by marrow niche cells198,199. Membrane associated SDF-1 is a substrate for MMP-9 and neutrophil elastase. Degradation of SDF-1 by MMP-9 and elastase in the marrow niche promotes stem cell mobilization200,201. In vitro studies have shown that soluble SDF-1 induces transendothelial migration of hematopoietic stem and progenitor cells across marrow endothelium202. Marrow endothelium constitutively expresses endothelial VCAM-1. Engagement of VCAM-1 with its receptors (including integrins α4β1, α4β7, and α9β1) expressed by stem/progenitor cells helps retain these precursors in the bone marrow203. G-CSF stimulates marrow neutrophil release of elastase and cathepsin G, which cleave VCAM-1 expressed by marrow stromal cells to further facilitate stem cell mobilization204. In addition to the regulation of stem/progenitor cell mobilization mediated by ligand/receptor pairs described above, actions of other cellular and molecular factors contribute as well. These include changes in cell membrane lipid rafts, activation of the complement cascade and fibrinolytic system, interaction of heat-resistant bioactive lipids [such as sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P)] with their receptors, and regulation through the sympathetic nervous system205,206.
Due to the close relationship between EPCs and HSCs, these two types of precursors may share similar mechanisms during their mobilization from the bone marrow. Comparable pathways appear to be used for mobilization of other vascular precursor cells. HIF signaling through VEGF and SDF-1 has been shown to promote mobilization of MSCs from the bone marrow207, and SDF-1 induces marrow mobilization of vascular SMPCs208. Nevertheless, evidence suggests that mechanisms do exist for differentially mobilizing subsets of stem/progenitor cells from the bone marrow209. Administration of VEGF, followed by treatment with CXCR4 antagonist AMD3100 in mice has been reported to mobilize both EPCs and stromal progenitor cells, while suppressing hematopoietic stem and progenitor cell activation. Further investigation on mechanisms for optimal mobilization of vascular precursor cells appears to be helpful for developing an effective approach to enhance vascularization during the process of injury repair.
Mobilized marrow precursors gain access to tissue primarily through the systemic circulation. Homing of vascular precursors, requires a series of coordinated efforts, including chemotaxis, adhesion, migration, and vascular integration. Chemoattractants generated at the site of tissue injury build up a gradient of chemoattractive force to guide the migration of vascular precursor cells. Locally produced HIF stimulates surface membrane expression of SDF-1 by endothelial cells, injured SMCs, and activated platelets210, which mediates the homing of CXCR4+ progenitor cells211. Additionally, IL-8, human growth-regulated oncogene (GRO), keratinocyte chemoattractant (KC), and neutrophil-activating peptide 2 (NAP-2) expressed by cells in the injured tissue facilitate homing of EPCs through interaction with CXCR2212. The coupling of CC chemokines and their receptors, including monocyte chemoattractant protein-1 (MCP-1)/CC receptor 2 (CCR2) and RANTES/CCR5, play a significant role in promoting EPC homing to sites of new vessel formation213–215. Interactions between selectins (E-, L-, and P-selectins) with their ligands helps EPC tethering and recruitment to the site of tissue injury216–218. Functional activities of integrin adhesion molecules are critical in various steps of EPC homing219. Specifically, integrins α5β1, α6β1, αvβ3 and αvβ5 are major contributors for EPC homing, invasion, differentiation, and paracrine factor production. β2 integrins mediate EPC attachment and transendothelial migration. Both VEGF and SDF-1 stimulate expression of intercellular adhesion molecule-1 (ICAM-1, a ligand of β2 integrins) by vascular endothelial cells220. The upregulated expression of ICAM-1 mediates EPC homing by interacting with their surface β2 integrins. In addition to EPCs, other marrow-derived vascular precursors, such as MSCs and SMPCs, are able to home to sites of new vessel formation through diverse mechanisms including the interactions between their expressed integrins and endothelial adhesion molecules, the SDF-1/CXCR4 axis, and PDGF-BB/PDGFR-β ligand-receptor signaling46,121,122,221–223. Currently, mechanisms for recruitment of vascular precursor cells from the blood vessel wall niche are less well defined. Recruitment of cells from remote locations requires transportation through the blood circulation, while cell mobilization from nearby sites might be achieved via direct migration of cells.
Vascular precursor cells contribute to neovascularization during tissue injury repair through two major functions: structural incorporation by converting to functional vascular wall cells and signaling regulation via generation of paracrine and autocrine mediators. Recruited EPCs actively proliferate and differentiate into endothelial cells during vascularization. One estimate suggests that EPCs contribute anywhere between 2-25% of endothelial cells in newly formed vessels224. In a murine model of new corneal vessel formation, 53% of pericytes were derived from the bone marrow225. Other in vivo studies of injury repair reported that 11-50% of regenerated vascular SMCs were from the bone marrow origin226. Besides differentiation into mature vascular cells along the same lineage, translineage commitment of vascular precursor cells may also contribute to new vessel formation. With appropriate stimulation, EPCs can give rise to vascular SMCs through endothelial-to-mesenchymal transdifferentiation (EnMT)227,228. MSCs can differentiate into multiple types of vascular cells, including SMCs, pericytes, and endothelial cells229–231. Figure 2 illustrates differentiation, transdifferentiation, and interchange of vascular precursor cells and vascular cells. Currently, the relative contributions of recruited and resident cells to new vessel formation during the process of injury repair remains uncertain225,232. It may vary depending on the type and extent of injury as well as the tissue type involved in injury.
Figure 2.
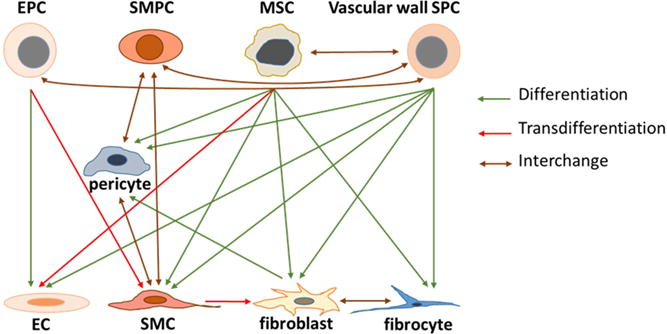
Differentiation, transdiffertiation, and interchange of vascular precursor cells and vascular cells.
Vascular precursor cells are potent producers for bioactive mediators233. Mediators expressed by EPCs include HIF-1α, VEGF, PDGF, TGF-β1, SDF-1, insulin-like growth factor-1 (IGF-1), and IL-8234–238. Attachment of EPCs to extracellular matrices upregulates expression of vasculogenic mediators by these precursors235. EPCs also release extracellular vesicles, including microvesicles (50-1000 nm) and exosomes (30-120 nm), containing cell-associated protein, RNA or microRNA, and DNA components239,240. These extracellular vesicles activate the angiogenic activity of vascular cells, particularly mature endothelial cells, through horizontal transfer of signaling molecules. Exposure to EPC-derived exosomes enhances proliferation, migration, and tube formation of endothelial cells in in vitro culture models241,242. Endothelial cells stimulated with these exosomes have been shown to increase the expression of angiogenesis-related molecules, including FGF, VEGF, VEGFR-2, Ang, E-selectin, CXC motif chemokine 16 (CXCL16), nitric oxide synthase (eNOS) and IL-8. Encountering extracellular vesicles, pericytes increase expression of VEGF243, which further promotes the survival and stabilization of endothelial cells. In vivo treatment with EPC-derived exosomes has been reported to accelerate re-endothelialization in the early phase post endothelial damage in the carotid artery in rats241 and to enhance cutaneous wound healing in diabetic rats242. MSCs are known to produce many paracrine factors for neovascularization, including VEGF, IGF-1, TGF-β1, MCP-1, and IL-8244–247. Activation of MSCs following exposure to inflammatory cytokines (such as TNF-α, IL-6, and TGF-α) and PAMPs (such as LPS) enhances their production of vasculogenic factors248–251. Like EPCs, MSCs produce extracellular vesicles to promote migration and angiogenic activity of endothelial cells and pericytes252,253. Activities of MSC-derived extracellular vesicles in promoting neovascularization as well as the potential of their application for clinical treatment have been comprehensively discussed in a recent review article254.
At the present time, recognition about the relative contribution of direct cell integration and generating paracrine/autocrine mediators by vascular precursors to new vessel formation during tissue injury repair remains incomplete. Discrepancy also exists in reports regarding the significance of vascular precursor cells in revascularization during injury repair255. Improvement of techniques for tracing vascular precursor cells and further investigation on activities of vasculogenic mediators produced by vascular precursor cells will be helpful for understanding the integrated efforts in the regulation of new vessel formation.
Application of Vascular Precursor Cells in the treatment of Tissue Injury
Recently, efforts in exploring the potential of using vascular precursor cells in the treatment of chronic wounds (such as diabetic ulcers and delayed healing bone fractures), traumatic injury (such as bone fracture repair), and ischemic damage in vital organ systems (such as myocardial infarction and stroke) has been substantially expanded. Non-healing ulcers in lower extremities are frequent complications of diabetes. Insufficiency in the number and function of vascular precursor cells is one of the common causes for the development of these wounds256–258. Accordingly, stimulating release of vascular precursor cells from their niche environment has been investigated as a therapeutic approach for the treatment of these chronic ulcers. G-CSF stimulates bone marrow release of granulocytes, HSCs, and EPCs. In a randomized placebo-controlled study of 40 patients with diabetic foot infection, administration of G-CSF as adjunctive therapy was beneficial for earlier eradication of pathogens from the infected ulcer, quicker resolution of cellulitis, and avoiding surgical interventions259. An analysis of five clinical trials including a total of 167 patients with diabetic foot infection has also revealed that G-CSF administration significantly reduces likelihood of lower extremity surgical interventions, such as amputation, and shortens the duration of hospital stay260. In these cases, the beneficial effect of G-CSF appears to be linked to the improvement of neutrophil-mediated immune defense. It remains unclear if mobilization of EPCs plays a role. Ang-1 is another potent factor stimulating EPC mobilization and activation. Overexpression of Ang-1 at the site of injury through adenoviral vector mediated Ang-1 gene transduction has been reported to result in improvement of EPC recruitment, neovascularization, and re-epithelialization in diabetic mice261. Other strategies have also been found to improve diabetic wound closure and neovascularization in mice, such as systemic administration of agent AMD3100 promoting stem and progenitor cell mobilization along with local application of PDGF-BB262.
Cell based-therapies aiming at increasing local vascular precursor cells and/or enhancing functional activities of these cells have been shown to improve healing outcomes of chronic wounds in diabetes. One prospective clinical trial phase I/IIa study reported that patients with non-healing diabetic foot ulcers achieved complete wound closure with increased vascular perfusion at an average of 18 weeks following local transplantation of autologous blood CD34+ cells263. Local implantation of EPCs or topical application of vascular progenitors (EPCs and CD133+ progenitor cells) has been documented to promote neovascularization and accelerated wound healing in diabetic murine models236,237,264. These precursors actively produce vasculogenic mediators (such as VEGF, IL-8, and PDGF) and other growth factors [such as bFGF, keratinocyte growth factor (KGF)] to exert paracrine stimulation of vascularization and injury repair. Local proangiogenic priming by injecting a mixture of VEGF, bFGF, and PDGF prior to implantation of EPCs has been observed to further improve the effectiveness of neovascularization and wound healing in diabetic animals265. Enhancing growth of marrow-derived EPCs in PolyCaprolactone-Gelatin (PCG) nano-fiber matrix in culture and then applying this PCG-EPCs matrix to the wound of diabetic mice can achieve sustained delivery of EPCs onto the diabetic wounds and enhance fibrosis-free wound healing266. In addition to EPCs, topical application of MSCs can also achieve inducing early granulation tissue formation and stabilizing neovasculature at wound sites267. Implantation of collagen scaffolds containing MSCs in murine skin wounds has been reported to enhance vascularization during the dermal regeneration process268. Since paracrine interactions among vascular precursor cells, inflammatory cells, and tissue cells at the site of tissue injury play a pivotal role in the regulation of wound repair, developing scaffold matrices containing optimal components of EPCs, MSCs, and vasculogenic mediators highlights an area of current interest for improving the treatment of chronic diabetic wounds.
Vascularization is also critical for healing of bone injury. Strategies for utilizing vascular precursor cells to enhance vascularization during bone injury repair include stimulation of native vascular precursor cell homing to the site of injury, local delivery of vascular precursor cells, and the combination of both techniques. The advantages of stimulating vascular precursor cell homing are the simplicity in application, inexpensive cost, and utilization of self-cell resources. VEGF, PDGF, SDF-1, and FGF have been tested in this application269. The direct absorption of vasculogenic mediators can achieve an immediate burst of stimulation, while continuing release from carrier materials entrapping vasculogenic factors, such as hydrogels, microspheres, and nanoparticles, allows generation of sustained and/or dynamic stimulations. Success has also been reported in enhancing local production of vasculogenic factors by tissue cells with genetic-engineering techniques270,271. Local delivery of marrow-derived EPCs to the site of bone fractures has been reported to enhance VEGF expression and to promote bone healing in experimental animals272,273. Development of bioengineered scaffolds modified with vasculogenic mediators and containing stem and progenitor cells may enhance vascularization in large bone grafts242. Along this path, a variety of precursor cells, including EPCs, MSCs, HSCs, pericytes, and ESCs, have been tested. Since interplays among cells in the bone and marrow regulate bone regeneration and its vascularization, including elements of bone marrow in bioengineered scaffolds deserves more attention in the future investigations.
Ischemia-induced myocardial infarction is a life threatening injury. Myocardial ischemia causes a rapid mobilization of EPCs into the systemic circulation275 and increase in homing of these vascular precursor cells to the injured heart276,277. These observations identify that EPCs actively participate in the host response to ischemic injury in the heart. EPCs infused intravenously can actively incorporate into foci of myocardial neovascularization276. Administration of G-CSF to promote mobilization of stem and progenitor cells from the bone marrow has been shown to improve vascular precursor cell homing, infarcted tissue repair, and left ventricular function in animal models of myocardial ischemia278–280. However, results of G-CSF treatment in patients with ischemic heart diseases remain controversial281–285. In experimental studies, implantation of EPCs and other vascular precursor cells around the ischemic areas appears having promising effects. Direct transplantation of human EPCs or MSCs into the border regions of ischemic heart tissue is able to improve cardiac function and enhance vascularization in rats233,286. Infusion of autologous EPCs in the circumflex artery following myocardial infarction can substantially reduce the size of infarction with an enhancement of vascularization in pigs287. Sequential transplantation of EPCs followed by implantation of fetal cardiomyocytes or EPCs again to marginal zones of infarction has been reported to additively improve myocardial function and vascularization in rats288. In addition, local implantation of genetically engineered EPCs and MSCs overexpressing vasculogenic mediators (IGF-1, FGF-1, and SDF-1) has been demonstrated to further enhance the beneficial effects of these precursor cells on neovascularization and myocardial function in animal models of myocardial ischemia289–291. Treatment of mouse lin−Sca1+CD31+ EPCs and human CD34+ cells with inhibitors of DNA methyltransferases (5-Azacytidine), histone deacetylases (valproic acid), and G9a histone dimethyltransferase (BIX-01294) can globally increase the activity of the transcriptome, including reactivation of pluripotency-associated genes and up-regulation of cardiomyocyte- and endothelial cell-specific gene expression292. Intramyocardial transplantation of these reprogrammed mouse and human EPCs into mice with acute myocardial infarction has been shown to significantly improve ventricular function, enhance de novo cardiomyocyte differentiation of transplanted cells, and increase capillary density. Several clinical trials have reported that intracoronary transfer of autologous bone marrow cells or bone marrow-derived stem cells result in a better recovery of regional systolic function and reduction in infarct size in patients with myocardial infarction293,294. Recent meta-analyses of several clinical studies have confirmed that stem/progenitor cell therapy improves left ventricular contractility in patients with myocardial infarction294–298. Moreover, intracoronary cell therapy has been reported to be accompanied with a decrease in incidence of death, recurrent of acute myocardial infarction, and readmission for heart failure294,297. At the present time, however, clinical reports regarding the effect of intracoronary stem/progenitor cell treatment on the infarct volume and remodeling remain inconsistent294,295,297,299. A phase 3, randomized, double-blinded, active-controlled, unblinded standard of care study (the RENEW study) is underway to assess the efficacy and safety of intramyocardial administration of autologous CD34+ cells in patients with refractory angina300.
Stroke is a devastating insult to the brain. Optimal repair of brain injury relies on effective revascularization to support neurogenesis and synaptic plasticity. Like what has been observed in ischemic-induced myocardial infarction, stroke causes mobilization of vascular precursor cells into the systemic circulation301–304. In a rat model of stroke, endogenously mobilized EPCs were observed to participate in neovascularization in the boundary zone of ischemia, which was beneficial for maintaining neurobehavioral functions of animals305. Active homing of nanoprobe-labeled EPCs to the peri-infarct area in the brain of mice has been detected by non-invasive imaging after the ischemic attack. Transplanted EPCs are able to incorporate into vessels around areas of infarction through neovascularization306. Studies on patients suffering from strokes have revealed that an increase in EPC concentration in the circulation is a positive sign for better outcomes of recovery302,303,307, while failure to elevate blood EPCs often implies a poorer prognosis301,308. In animal models of brain ischemia, systemic or intra-carotid arterial administration of EPCs has been shown to enhance neovascularization and neurogenesis, reduce the severity of brain injuries, and improve long-term neurobehavioral outcomes309–311. EPC-derived paracrine mediators also play an important role in promoting neovascularization and neurogenesis following the ischemic attack312,313. Treatment with G-CSF in combination with transplantation of human umbilical cord blood cells in animal models of stoke has been reported to be beneficial in coordinating efforts of both transplanted precursor cells and endogenously mobilized stem/progenitors in integration of graft-host cells, production of growth factors, and promotion of neurogenesis314. In patients with stroke, treatment with G-CSF has been shown to substantially increase CD34+ cells in the circulation315,316, which is associated with a tendency toward reduction in ischemic lesion in the brain316. Several phase I clinical trials have been completed involving intra-arterial or intravenous transplantation of autologous marrow mononuclear cells, including CD34+ precursor cells, in patients with stroke317,318. The results suggest that this therapy is feasible and seems to be safe. A phase II, randomized, dose-finding, controlled multicenter trial on intra-arterial bone marrow cells transplantation in patients with acute ischemic stroke (IBIS trial) has been launched recently319.
Perspective Remarks
Vascular precursor cells in adults represent a highly heterogeneous population of tissue stem/progenitor cells residing in different niche environments. A complex signaling network regulates their self-renewal, proliferation, differentiation, transdifferentiation, mobilization, and homing. These cells actively participate in tissue injury repair through structural integration into the vasculature and generation of soluble mediators that promote the coordinated efforts among various types of cells in new vessel formation and tissue regeneration. Improved understanding of underlying mechanisms will enhance the development of vascular precursor cell-based therapies for effective treatment of wounds and ischemic attacks in vital organ systems. Current attention is primarily focused on exploring the maximal efficacy of treatment using optimized combinations of vascular precursor cells and vasculogenic agents. Genetic engineering of vascular precursor cells to boost their function in promoting vascularization represents another new direction of research. However, identifying the risk and safety of vascular cell-based therapies are vital for their clinical application in the future.
Background.
Tissue injury is commonly associated with damage to blood vasculature. Restoring blood supply is essential for wound healing. Crosstalk among signaling pathways coordinates recruitment of vascular precursor cells to the site of tissue injury and regulates their activity during neovascularization. In recent years, substantial progress has been achieved in basic studies on molecular mechanisms underlying the regulation of vascular precursor cell function during their participation in tissue injury repair.
Translational Significance.
Novel information obtained from these basic investigations drives exploration of vascular precursor cell-based approaches to treat chronic wounds and ischemic diseases in vital organ systems.
Acknowledgments
Supported by NIH grants R01AA019676 (PZ) and R01AA022816 (PZ); National Science Foundation of China grant U1404814 (WZ).
Funding institutes had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Definition of abbreviations
- 7AAD
7-Aminoactinomycin D
- αSMA
alpha smooth muscle actin
- AKT
protein kinase B
- ALK
activin receptor-like kinase
- Ang
angiopoietin
- Ang II
angiotensin II
- BCRP1
breakpoint cluster region pseudogene
- bFGF
basic fibroblast growth factor
- C1P
ceramide-1-phosphate
- CA12
carbonic anhydrase 12
- CAC
circulating angiogenic cell
- CAR
SDF-1-abundant reticular
- CCR
CC receptor
- CD
clusters of differentiation
- CEACAM1
carcinoembryonic antigen-related cell adhesion molecule 1
- CEP
circulating endothelial precursor
- c-kit
stem cell growth factor receptor
- CLF
chemokine-like function
- CPM
carboxypeptidase M
- CSPG4
chondroitin sulfate proteoglycan 4
- CXCL12
CXC motif chemokine 12
- CXCL16
CXC motif chemokine 16
- CXCR
CXC receptor
- DAMP
damage-associated molecular pattern ligand
- DR3
death domaincontaining receptor 3
- ECFC
endothelial colony forming cell
- eGFP
enhanced green-fluorescent protein
- EnMT
endothelial-to-mesenchymal transdifferentiation
- eNOS
nitric oxide synthase
- EPC
endothelial progenitor cell
- ERK1/2
extracellular-signal-regulated kinases 1/2
- ESC
embryonic stem cell
- FGF
fibroblast growth factor
- Flk1
fms-like tyrosine kinase-1
- Flt3L
Flt3 ligand
- G-CSF
granulocyte colony-stimulating factor
- GFP
green-fluorescent protein
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GRO
human growth-regulated oncogene
- HAEC
human aortic endothelial cell
- HIF
hypoxia-inducible transcription factor
- HLA-DR
human leukocyte antigen - antigen D related
- HRE
hypoxia-response element
- HSC
hematopoietic stem cell
- HUVEC
human umbilical vein endothelial cell
- ICAM-1
intercellular adhesion molecule-1
- IFNγ
interferon-gamma
- IGF-1
insulin-like growth factor-1
- IL-1α
interleukin-1 alpha
- IL-1β
interleukin-1 beta
- IL-1R
interleukin-1 receptor
- IL-3
interleukin-3
- IL-6
interleukin-6
- IL-6R
interleukin-6 receptor
- IL-8
interleukin-8
- IL-10
interleukin-10
- IL-18
interleukin-18
- Jmjd6
Jumonji domain-containing protein 6
- KC
keratinocyte chemoattractant
- KDR
kinase insert domain receptor
- KGF
keratinocyte growth factor
- kitL
kit ligand
- LacZ
β-galactosidase
- Lin
lineage
- LRP1
low-density lipoprotein receptor–related protein
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- M-CSF
macrophage colony-stimulating factor
- MIF
macrophage migration inhibitory factor
- MMP-2
matrixmetalloproteinase-2
- MMP-9
matrixmetalloproteinase-9
- MSC
mesenchymal stem cell
- NAP-2
neutrophil-activating peptide 2
- NG2
neural/glial antigen 2
- Notch1
Notch homolog 1
- OCT4
octamer-binding transcription factor 4
- PAMP
pathogen-associated molecular pattern ligand
- PBMC
peripheral blood mononuclear cell
- PCG
PolyCaprolactone-Gelatin
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PEDF
pigment epithelium–derived factor
- PGE2
prostaglandin E2
- PGF
placenta growth factor
- PI3K
phosphatidylinositol-3 kinase
- PKC
protein kinase C
- PKD
protein kinase D
- PLC-γ
phospholipase C-gamma
- PRR
pattern recognition receptors
- RAMP1
receptor activity-modifying protein 1
- RGS5
regulator G-protein signaling 5
- S1P
sphingosine-1-phosphate
- Sca1
stem cell antigen-1
- SCF
stem cell growth factor
- SDF-1
stromal cell-derived factor-1
- SLE
systemic lupus erythematosus
- SM-22α
smooth muscle-22α
- SMC
smooth muscle cell
- SMemb
embryonic form smooth muscle myosin heavy chain
- SMPC
smooth muscle progenitor cell
- SP
side population
- SPC
stem/progenitor cell
- STAT
signal transducer and activator of transcription
- TβR
transforming growth factor beta receptor
- TGF-β
transforming growth factor beta
- TIE2
receptor for angiopoietin
- TNF
tumor necrosis factor
- TNFSF15
Tumor necrosis factor superfamily 15
- UEA1
Ulex europaeus agglutinin-1
- VCAM-1
vascular cell adhesion molecule-1
- VEGF
vascular endothelial growth factor
- VEGFR-1
vascular endothelial growth factor receptor-1
- VEGFR-2
vascular endothelial growth factor receptor-2
- VEGI
vascular endothelial growth inhibitor
- vWF
von Willebrand factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest and have none to declare. The manuscript has been reviewed by and approved by all named authors
References
- 1.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 3.Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- 4.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 5.Van Craenenbroeck EM, Van Craenenbroeck AH, van Ierssel S, et al. Quantification of circulating CD34+/KDR+/CD45dim endothelial progenitor cells: analytical considerations. Int J Cardiol. 2013;167:1688–95. doi: 10.1016/j.ijcard.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 6.Medina RJ, O’Neill CL, O’Doherty TM, et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med. 2011;17:1045–55. doi: 10.2119/molmed.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–7. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoder MC. Endothelial progenitor cell: a blood cell by many other names may serve similar functions. J Mol Med (Berl) 2013;91:285–95. doi: 10.1007/s00109-013-1002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollerot K, Pouget C, Jaffredo T. The embryonic origins of hematopoietic stem cells: a tale of hemangioblast and hemogenic endothelium. APMIS. 2005;113:790–803. doi: 10.1111/j.1600-0463.2005.apm_317.x. [DOI] [PubMed] [Google Scholar]
- 10.Schatteman GC, Awad O. Hemangioblasts, angioblasts, and adult endothelial cell progenitors. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:13–21. doi: 10.1002/ar.a.10131. [DOI] [PubMed] [Google Scholar]
- 11.Park C, Ma YD, Choi K. Evidence for the hemangioblast. Exp Hematol. 2005;33:965–70. doi: 10.1016/j.exphem.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler BL, Valtieri M, Porada GA, et al. KDR receptor: a key marker defining hematopoietic stem cells. Science. 1999;285:1553–8. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- 13.Russell JS, Brown JM. Circulating mouse Flk1+/c-Kit+/CD45- cells function as endothelial progenitors cells (EPCs) and stimulate the growth of human tumor xenografts. Mol Cancer. 2014;13:177. doi: 10.1186/1476-4598-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–84. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 15.Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb and Vasc Biol. 2007;27:1572–9. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 16.Fina L, Molgaard HV, Robertson D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–26. [PubMed] [Google Scholar]
- 17.Bailey AS, Jiang S, Afentoulis M, et al. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood. 2004;103:13–9. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 18.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 19.Thomas RA, Pietrzak DC, Scicchitano MS, Thomas HC, McFarland DC, Frazier KS. Detection and characterization of circulating endothelial progenitor cells in normal rat blood. J Pharmacol Toxicol Methods. 2009;60:263–74. doi: 10.1016/j.vascn.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Chen W, Liang Z, Chen L. Autotransplantation of circulating endothelial progenitor cells protects against lipopolysaccharide-induced acute lung injury in rabbit. Int immunopharmacol. 2011;11:1584–90. doi: 10.1016/j.intimp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Favre J, Terborg N, Horrevoets AJ. The diverse identity of angiogenic monocytes. Eur J Clin Invest. 2013;43:100–7. doi: 10.1111/eci.12009. [DOI] [PubMed] [Google Scholar]
- 22.Shantsila E, Wrigley BJ, Shantsila A, Tapp LD, Gill PS, Lip GY. Monocyte-derived and CD34+/KDR+ endothelial progenitor cells in heart failure. J Throm Haemost. 2012;10:1252–61. doi: 10.1111/j.1538-7836.2012.04753.x. [DOI] [PubMed] [Google Scholar]
- 23.Chambers SE, O’Neill CL, O’Doherty TM, Medina RJ, Stitt AW. The role of immune-related myeloid cells in angiogenesis. Immunobiology. 2013;218:1370–5. doi: 10.1016/j.imbio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Saiura A, Sata M, Hirata Y, Nagai R, Makuuchi M. Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nat Med. 2001;7:382–3. doi: 10.1038/86394. [DOI] [PubMed] [Google Scholar]
- 25.Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation. 2002;106:1199–204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- 26.Daniel JM, Sedding DG. Circulating smooth muscle progenitor cells in arterial remodeling. J Mol Cell Cardiol. 2011;50:273–9. doi: 10.1016/j.yjmcc.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108:365–77. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simper D, Mayr U, Urbich C, et al. Comparative proteomics profiling reveals role of smooth muscle progenitors in extracellular matrix production. Arterioscler Thromb Vasc Biol. 2010;30:1325–32. doi: 10.1161/ATVBAHA.110.204651. [DOI] [PubMed] [Google Scholar]
- 29.Kashiwakura Y, Katoh Y, Tamayose K, et al. Isolation of bone marrow stromal cell-derived smooth muscle cells by a human SM22alpha promoter: in vitro differentiation of putative smooth muscle progenitor cells of bone marrow. Circulation. 2003;107:2078–81. doi: 10.1161/01.CIR.0000070082.64414.B5. [DOI] [PubMed] [Google Scholar]
- 30.Lin C, Yuan Y, Courtman DW. Differentiation of Murine Bone Marrow-Derived Smooth Muscle Progenitor Cells Is Regulated by PDGF-BB and Collagen. PLoS One. 2016;11:e0156935. doi: 10.1371/journal.pone.0156935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–9. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 32.Caplice NM, Bunch TJ, Stalboerger PG, et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A. 2003;100:4754–9. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joo HJ, Seo HR, Jeong HE, et al. Smooth muscle progenitor cells from peripheral blood promote the neovascularization of endothelial colony-forming cells. Biochem Biophys Res Commun. 2014;449:405–11. doi: 10.1016/j.bbrc.2014.05.061. [DOI] [PubMed] [Google Scholar]
- 34.Wang CH, Lee YS, Lin SJ, et al. Surface markers of heterogeneous peripheral blood-derived smooth muscle progenitor cells. Arterioscler Thromb Vasc Biol. 2012;32:1875–83. doi: 10.1161/ATVBAHA.112.245852. [DOI] [PubMed] [Google Scholar]
- 35.Ergun S, Tilki D, Klein D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid Redox Signal. 2011;15:981–95. doi: 10.1089/ars.2010.3507. [DOI] [PubMed] [Google Scholar]
- 36.Chen CW, Corselli M, Peault B, Huard J. Human blood-vessel-derived stem cells for tissue rep. air and regeneration. J Biomed Biotechnol. 2012;2012:597439. doi: 10.1155/2012/597439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50:304–11. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Sainz J, Al Haj Zen A, Caligiuri G, et al. Isolation of “side population” progenitor cells from healthy arteries of adult mice. Arterioscler Thromb Vasc Biol. 2006;26:281–6. doi: 10.1161/01.ATV.0000197793.83391.91. [DOI] [PubMed] [Google Scholar]
- 39.Pasquinelli G, Pacilli A, Alviano F, et al. Multidistrict human mesenchymal vascular cells: pluripotency and stemness characteristics. Cytotherapy. 2010;12:275–87. doi: 10.3109/14653241003596679. [DOI] [PubMed] [Google Scholar]
- 40.Alessandri G, Girelli M, Taccagni G, et al. Human vasculogenesis ex vivo: embryonal aorta as a tool for isolation of endothelial cell progenitors. Lab Invest. 2001;81:875–85. doi: 10.1038/labinvest.3780296. [DOI] [PubMed] [Google Scholar]
- 41.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs. 2012;195:73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–6. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 43.Covas DT, Siufi JL, Silva AR, Orellana MD. Isolation and culture of umbilical vein mesenchymal stem cells. Braz J Med Biol Res. 2003;36:1179–83. doi: 10.1590/s0100-879x2003000900006. [DOI] [PubMed] [Google Scholar]
- 44.Covas DT, Piccinato CE, Orellana MD, et al. Mesenchymal stem cells can be obtained from the human saphena vein. Exp Cell Res. 2005;309:340–4. doi: 10.1016/j.yexcr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Wanjare M, Kusuma S, Gerecht S. Perivascular cells in blood vessel regeneration. Biotechnol J. 2013;8:434–47. doi: 10.1002/biot.201200199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261–8. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 47.Andreeva ER, Pugach IM, Gordon D, Orekhov AN. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell. 1998;30:127–35. doi: 10.1016/s0040-8166(98)80014-1. [DOI] [PubMed] [Google Scholar]
- 48.Kutcher ME, Kolyada AY, Surks HK, Herman IM. Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am J Pathol. 2007;171:693–701. doi: 10.2353/ajpath.2007.070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 50.Marchand M, Anderson EK, Phadnis SM, et al. Concurrent generation of functional smooth muscle and endothelial cells via a vascular progenitor. Stem Cells Transl Med. 2014;3:91–7. doi: 10.5966/sctm.2013-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol. 2005;289:C1396–407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]
- 52.Loibl M, Binder A, Herrmann M, et al. Direct cell–cell contact between mesenchymal stem cells and endothelial progenitor cells induces a pericyte–like phenotype in vitro. Biomed Res Int. 2014;2014:395781. doi: 10.1155/2014/395781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicosia RF, Villaschi S. Rat aortic smooth muscle cells become pericytes during angiogenesis in vitro. Lab Invest. 1995;73:658–66. [PubMed] [Google Scholar]
- 54.Rhodin JA, Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J Submicrosc Cytol Pathol. 1989;21:1–34. [PubMed] [Google Scholar]
- 55.Diaz-Flores L, Gutierrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–69. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 56.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–98. [PubMed] [Google Scholar]
- 57.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–32. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 58.Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–38. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 59.Diaz-Flores L, Gutierrez R, Lopez-Alonso A, Gonzalez R, Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992:280–6. [PubMed] [Google Scholar]
- 60.Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–24. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 61.Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270:469–74. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- 62.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–9. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Redmer DA, Doraiswamy V, Bortnem BJ, et al. Evidence for a role of capillary pericytes in vascular growth of the developing ovine corpus luteum. Biol Reprod. 2001;65:879–89. doi: 10.1095/biolreprod65.3.879. [DOI] [PubMed] [Google Scholar]
- 64.Wu Q, Jing Y, Yuan X, et al. The distinct abilities of tube-formation and migration between brain and spinal cord microvascular pericytes in rats. Clin Hemorheol Microcirc. 2015;60:231–40. doi: 10.3233/CH-141856. [DOI] [PubMed] [Google Scholar]
- 65.Wakui S, Yokoo K, Muto T, et al. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest. 2006;86:1172–84. doi: 10.1038/labinvest.3700476. [DOI] [PubMed] [Google Scholar]
- 66.Guijarro-Munoz I, Compte M, Alvarez-Cienfuegos A, Alvarez-Vallina L, Sanz L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. J Biol Chem. 2014;289:2457–68. doi: 10.1074/jbc.M113.521161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hurtado-Alvarado G, Cabanas-Morales AM, Gomez-Gonzalez B. Pericytes: brain-immune interface modulators. Front Integr Neurosci. 2014;7:80. doi: 10.3389/fnint.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Crisan M, Corselli M, Chen WC, Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851–60. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen WC, Park TS, Murray IR, et al. Cellular kinetics of perivascular MSC precursors. Stem Cell Int. 2013;2013:983059. doi: 10.1155/2013/983059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boroujerdi A, Tigges U, Welser-Alves JV, Milner R. Isolation and culture of primary pericytes from mouse brain. Methods Mol Biol. 2014;1135:383–92. doi: 10.1007/978-1-4939-0320-7_31. [DOI] [PubMed] [Google Scholar]
- 72.Berger M, Bergers G, Arnold B, Hammerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105:1094–101. doi: 10.1182/blood-2004-06-2315. [DOI] [PubMed] [Google Scholar]
- 73.Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL. Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res. 2014;51:163–74. doi: 10.1159/000362276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murfee WL, Rehorn MR, Peirce SM, Skalak TC. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation. 2006;13:261–73. doi: 10.1080/10739680600559153. [DOI] [PubMed] [Google Scholar]
- 75.Pasquinelli G, Tazzari PL, Vaselli C, et al. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–34. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- 76.Hu Y, Zhang Z, Torsney E, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–65. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zengin E, Chalajour F, Gehling UM, et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–51. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 78.Klein D, Benchellal M, Kleff V, Jakob HG, Ergun S. Hox genes are involved in vascular wall-resident multipotent stem cell differentiation into smooth muscle cells. Sci Rep. 2013;3:2178. doi: 10.1038/srep02178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cordeiro-Spinetti E, de Mello W, Trindade LS, Taub DD, Taichman RS, Balduino A. Human bone marrow mesenchymal progenitors: perspectives on an optimized in vitro manipulation. Front Cell Dev Biol. 2014;2:7. doi: 10.3389/fcell.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47:126–31. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 81.Mosna F, Sensebe L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19:1449–70. doi: 10.1089/scd.2010.0140. [DOI] [PubMed] [Google Scholar]
- 82.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watt SM, Gullo F, van der Garde M, et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108:25–53. doi: 10.1093/bmb/ldt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sousa BR, Parreira RC, Fonseca EA, et al. Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications. Cytometry A. 2014;85:43–77. doi: 10.1002/cyto.a.22402. [DOI] [PubMed] [Google Scholar]
- 85.Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–91. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 86.Chong PP, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30:634–42. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 87.Fu WL, Zhang JY, Fu X, et al. Comparative study of the biological characteristics of mesenchymal stem cells from bone marrow and peripheral blood of rats. Tissue Eng Part A. 2012;18:1793–803. doi: 10.1089/ten.TEA.2011.0530. [DOI] [PubMed] [Google Scholar]
- 88.Nassiri SM, Rahbarghazi R. Interactions of mesenchymal stem cells with endothelial cells. Stem Cells Dev. 2014;23:319–32. doi: 10.1089/scd.2013.0419. [DOI] [PubMed] [Google Scholar]
- 89.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 90.Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol. 2010;345:105–20. doi: 10.1007/82_2010_74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cribbs SK, Martin GS, Rojas M. Monitoring of endothelial dysfunction in critically ill patients: the role of endothelial progenitor cells. Curr Opin Crit Care. 2008;14:354–60. doi: 10.1097/MCC.0b013e3282fc216d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elks PM, Renshaw SA, Meijer AH, Walmsley SR, van Eeden FJ. Exploring the HIFs, buts and maybes of hypoxia signalling in disease: lessons from zebrafish models. Dis Model Mech. 2015;8:1349–60. doi: 10.1242/dmm.021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mole DR, Maxwell PH, Pugh CW, Ratcliffe PJ. Regulation of HIF by the von Hippel-Lindau tumour suppressor: implications for cellular oxygen sensing. IUBMB life. 2001;52:43–7. doi: 10.1080/15216540252774757. [DOI] [PubMed] [Google Scholar]
- 94.Gorlach A, Bonello S. The cross-talk between NF-kappaB and HIF-1: further evidence for a significant liaison. Biochem J. 2008;412:e17–9. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- 95.Semenza GL. Targeting HIF-1 for cancer therapy. Nature reviews Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 96.Gerig JT, Reinheimer JD, Robinson RH. Modification of human serum albumin with N-(2,5-dinitro-4-fluorophenyl)-4-amino-2,2,6,6-tetramethyl-piperidinooxy radical. Biochim Biophys Acta. 1979;579:409–20. doi: 10.1016/0005-2795(79)90068-0. [DOI] [PubMed] [Google Scholar]
- 97.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahluwalia A, Narula J, Jones MK, Deng X, Tarnawski AS. Impaired angiogenesis in aging myocardial microvascular endothelial cells is associated with reduced importin alpha and decreased nuclear transport of HIF1 alpha: mechanistic implications. J Physiol Pharmacol. 2010;61:133–9. [PubMed] [Google Scholar]
- 99.Szabo IL, Kawanaka H, Jones MK, et al. Activation of hypoxia inducible factor-1alpha in gastric mucosa in response to ethanol injury: a trigger for angiogenesis? Life Sci. 2001;69:3035–44. doi: 10.1016/s0024-3205(01)01410-2. [DOI] [PubMed] [Google Scholar]
- 100.Aranha AM, Zhang Z, Neiva KG, Costa CA, Hebling J, Nor JE. Hypoxia enhances the angiogenic potential of human dental pulp cells. J Endod. 2010;36:1633–7. doi: 10.1016/j.joen.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 101.Xie K, Wei D, Shi Q, Huang S. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev. 2004;15:297–324. doi: 10.1016/j.cytogfr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 102.Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789–91. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 103.Ramakrishnan S, Anand V, Roy S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol. 2014;9:142–60. doi: 10.1007/s11481-014-9531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–9. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fox A, Smythe J, Fisher N, et al. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br J Surg. 2008;95:244–51. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 106.Svensen CH. Vascular endothelial growth factor (VEGF) in plasma increases after hip surgery. J Clin Anestha. 2004;16:435–9. doi: 10.1016/j.jclinane.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 107.Futami R, Miyashita M, Nomura T, et al. Increased serum vascular endothelial growth factor following major surgical injury. J Nippon Med Sch. 2007;74:223–9. doi: 10.1272/jnms.74.223. [DOI] [PubMed] [Google Scholar]
- 108.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–8. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 109.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 110.Van Craenenbroeck EM, Van Craenenbroeck AH, Van Ierssel S, et al. Quantification of circulating CD34+/KDR+/CD45dim endothelial progenitor cells: analytical considerations. Int J Cardiol. 2013 Sep 1;167(5):1688–95. doi: 10.1016/j.ijcard.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 111.Rahimi N. Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials. Exp Eye Res. 2006;83:1005–16. doi: 10.1016/j.exer.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ha CH, Jin ZG. Protein kinase D1, a new molecular player in VEGF signaling and angiogenesis. Mol Cells. 2009;28:1–5. doi: 10.1007/s10059-009-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sandra F, Oktaviono YH, Widodo MA, Dirgantara Y, Chouw A, Sargowo D. Endothelial progenitor cells proliferated via MEK-dependent p42 MAPK signaling pathway. Mol Cell Biochem. 2015;400:201–6. doi: 10.1007/s11010-014-2276-z. [DOI] [PubMed] [Google Scholar]
- 114.Yu D, Chen W, Ren J, et al. VEGF-PKD1-HDAC7 signaling promotes endothelial progenitor cell migration and tube formation. Microvasc Res. 2014;91:66–72. doi: 10.1016/j.mvr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 115.Zhang X, Mao H, Chen JY, et al. Increased expression of microRNA-221 inhibits PAK1 in endothelial progenitor cells and impairs its function via c-Raf/MEK/ERK pathway. Biochem Biophys Res Commun. 2013;431:404–8. doi: 10.1016/j.bbrc.2012.12.157. [DOI] [PubMed] [Google Scholar]
- 116.Kim JW, Jung SY, Kwon YH, et al. Ginsenoside Rg3 inhibits endothelial progenitor cell differentiation through attenuation of VEGF-dependent Akt/eNOS signaling. Phytother Res. 2012;26:1286–93. doi: 10.1002/ptr.3722. [DOI] [PubMed] [Google Scholar]
- 117.Gill KA, Brindle NP. Angiopoietin-2 stimulates migration of endothelial progenitors and their interaction with endothelium. Biochem Biophys Res Commun. 2005;336:392–6. doi: 10.1016/j.bbrc.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 118.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 119.Bai Li B, Sun W, Zhou P, Hu B, Ying BJ. The effect of CXCL12 on endothelial progenitor cells: potential target for angiogenesis in intracerebral hemorrhage. J Interferon Cytokine Res. 2015;35:23–31. doi: 10.1089/jir.2014.0004. [DOI] [PubMed] [Google Scholar]
- 120.Kanzler I, Tuchscheerer N, Steffens G, et al. Differential roles of angiogenic chemokines in endothelial progenitor cell-induced angiogenesis. Basic Res Cardiol. 2013;108:310. doi: 10.1007/s00395-012-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 122.Hill WD, Hess DC, Martin-Studdard A, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 123.Asare Y, Schmitt M, Bernhagen J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thromb Haemost. 2013;109:391–8. doi: 10.1160/TH12-11-0831. [DOI] [PubMed] [Google Scholar]
- 124.Simons D, Grieb G, Hristov M, et al. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J Cell Mol Med. 2011;15:668–78. doi: 10.1111/j.1582-4934.2010.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fu H, Luo F, Yang L, Wu W, Liu X. Hypoxia stimulates the expression of macrophage migration inhibitory factor in human vascular smooth muscle cells via HIF-1alpha dependent pathway. BMC Cell Biol. 2010;11:66. doi: 10.1186/1471-2121-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leng L, Metz CN, Fang Y, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 128.Schwartz V, Lue H, Kraemer S, et al. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lrtt. 2009;583:2749–57. doi: 10.1016/j.febslet.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93:321–9. doi: 10.1161/01.RES.0000087641.56024.DA. [DOI] [PubMed] [Google Scholar]
- 130.Grieb G, Piatkowski A, Simons D, et al. Macrophage migration inhibitory factor is a potential inducer of endothelial progenitor cell mobilization after flap operation. Surgery. 2012;151:268–77 e1. doi: 10.1016/j.surg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 131.Skuli N, Liu L, Runge A, et al. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–77. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hubbard NE, Lim D, Mukutmoni M, Cai A, Erickson KL. Expression and regulation of murine macrophage angiopoietin-2. Cell Immunol. 2005;234:102–9. doi: 10.1016/j.cellimm.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 133.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 134.Park YS, Kim NH, Jo I. Hypoxia and vascular endothelial growth factor acutely up-regulate angiopoietin-1 and Tie2 mRNA in bovine retinal pericytes. Microvasc Res. 2003;65:125–31. doi: 10.1016/s0026-2862(02)00035-3. [DOI] [PubMed] [Google Scholar]
- 135.Rosell A, Arai K, Lok J, et al. Interleukin-1beta augments angiogenic responses of murine endothelial progenitor cells in vitro. J Cereb Blood Flow Metab. 2009;29:933–43. doi: 10.1038/jcbfm.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang L, Guo XG, Du CQ, et al. Interleukin-1 beta increases activity of human endothelial progenitor cells: involvement of PI3K-Akt signaling pathway. Inflammation. 2012;35:1242–50. doi: 10.1007/s10753-012-9434-9. [DOI] [PubMed] [Google Scholar]
- 137.Carmi Y, Voronov E, Dotan S, et al. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol. 2009;183:4705–14. doi: 10.4049/jimmunol.0901511. [DOI] [PubMed] [Google Scholar]
- 138.Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–3. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- 139.Fan Y, Ye J, Shen F, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood flow Metab. 2008;28:90–8. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gleadle JM, Ebert BL, Firth JD, Ratcliffe PJ. Regulation of angiogenic growth factor expression by hypoxia, transition metals, and chelating agents. Am J Physiol. 1995;268:C1362–8. doi: 10.1152/ajpcell.1995.268.6.C1362. [DOI] [PubMed] [Google Scholar]
- 141.Kuwabara K, Ogawa S, Matsumoto M, et al. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci U S A. 1995;92:4606–10. doi: 10.1073/pnas.92.10.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoshida D, Kim K, Noha M, Teramoto A. Hypoxia inducible factor 1-alpha regulates of platelet derived growth factor-B in human glioblastoma cells. J Neurooncol. 2006;76:13–21. doi: 10.1007/s11060-005-3279-0. [DOI] [PubMed] [Google Scholar]
- 143.Schito L, Rey S, Tafani M, et al. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc Natl Acad Sci U S A. 2012;109:E2707–16. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 145.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heldin CH, Johnsson A, Ek B, et al. Purification of human platelet-derived growth factor. Methods Enzymol. 1987;147:3–13. doi: 10.1016/0076-6879(87)47094-8. [DOI] [PubMed] [Google Scholar]
- 147.Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clinic Proc. 2006;81:1241–57. doi: 10.4065/81.9.1241. [DOI] [PubMed] [Google Scholar]
- 148.Raz O, Lev DL, Battler A, Lev EI. Pathways mediating the interaction between endothelial progenitor cells (EPCs) and platelets. PloS One. 2014;9:e95156. doi: 10.1371/journal.pone.0095156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sufen G, Xianghong Y, Yongxia C, Qian P. bFGF and PDGF-BB have a synergistic effect on the proliferation, migration and VEGF release of endothelial progenitor cells. Cell Biol Int. 2011;35:545–51. doi: 10.1042/CBI20100401. [DOI] [PubMed] [Google Scholar]
- 150.Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103–14. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- 151.Kajdaniuk D, Marek B, Borgiel-Marek H, Kos-Kudla B. Transforming growth factor beta1 (TGFbeta1) in physiology and pathology. Endokrynol Pol. 2013;64:384–96. doi: 10.5603/EP.2013.0022. [DOI] [PubMed] [Google Scholar]
- 152.Bertolino P, Deckers M, Lebrin F, ten Dijke P. Transforming growth factor-beta signal transduction in angiogenesis and vascular disorders. Chest. 2005;128:585S–90S. doi: 10.1378/chest.128.6_suppl.585S. [DOI] [PubMed] [Google Scholar]
- 153.Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–27. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 154.Orlova VV, Liu Z, Goumans MJ, ten Dijke P. Controlling angiogenesis by two unique TGF-beta type I receptor signaling pathways. Histol Histopathol. 2011;26:1219–30. doi: 10.14670/HH-26.1219. [DOI] [PubMed] [Google Scholar]
- 155.ten Dijke P, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 156.Shimmi O, Newfeld SJ. New insights into extracellular and post-translational regulation of TGF-beta family signalling pathways. J Biochem. 2013;154:11–9. doi: 10.1093/jb/mvt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–73. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 158.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–67. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 159.Evrard SM, d’Audigier C, Mauge L, et al. The profibrotic cytokine transforming growth factor-beta1 increases endothelial progenitor cell angiogenic properties. J Thromb Haemost. 2012;10:670–9. doi: 10.1111/j.1538-7836.2012.04644.x. [DOI] [PubMed] [Google Scholar]
- 160.Bai H, Xie YL, Gao YX, Cheng T, Wang ZZ. The balance of positive and negative effects of TGF-beta signaling regulates the development of hematopoietic and endothelial progenitors in human pluripotent stem cells. Stem Cells Dev. 2013;22:2765–76. doi: 10.1089/scd.2013.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ross JJ, Hong Z, Willenbring B, et al. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116:3139–49. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Narita Y, Yamawaki A, Kagami H, Ueda M, Ueda Y. Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 2008;333:449–59. doi: 10.1007/s00441-008-0654-0. [DOI] [PubMed] [Google Scholar]
- 163.Liang MS, Andreadis ST. Engineering fibrin-binding TGF-beta1 for sustained signaling and contractile function of MSC based vascular constructs. Biomaterials. 2011;32:8684–93. doi: 10.1016/j.biomaterials.2011.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rozen-Zvi B, Hayashida T, Hubchak SC, Hanna C, Platanias LC, Schnaper HW. TGF-beta/Smad3 activates mammalian target of rapamycin complex-1 to promote collagen production by increasing HIF-1alpha expression. Am J Physiol Renal Physiol. 2013;305:F485–94. doi: 10.1152/ajprenal.00215.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281:24171–81. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 166.Shih SC, Claffey KP. Role of AP-1 and HIF-1 transcription factors in TGF-beta activation of VEGF expression. Growth Factors. 2001;19:19–34. doi: 10.3109/08977190109001073. [DOI] [PubMed] [Google Scholar]
- 167.Duan L, Yang G, Zhang R, Feng L, Xu C. Advancement in the research on vascular endothelial growth inhibitor (VEGI) Target Oncol. 2012;7:87–90. doi: 10.1007/s11523-012-0206-0. [DOI] [PubMed] [Google Scholar]
- 168.Metheny-Barlow LJ, Li LY. Vascular endothelial growth inhibitor (VEGI), an endogenous negative regulator of angiogenesis. Semin Ophthalmol. 2006;21:49–58. doi: 10.1080/08820530500511446. [DOI] [PubMed] [Google Scholar]
- 169.Tian F, Liang PH, Li LY. Inhibition of endothelial progenitor cell differentiation by VEGI. Blood. 2009;113:5352–60. doi: 10.1182/blood-2008-08-173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qi JW, Qin TT, Xu LX, et al. TNFSF15 inhibits vasculogenesis by regulating relative levels of membrane-bound and soluble isoforms of VEGF receptor 1. Proc Natl Acad Sci U S A. 2013;110:13863–8. doi: 10.1073/pnas.1304529110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xu LX, Grimaldo S, Qi JW, et al. Death receptor 3 mediates TNFSF15- and TNFα-induced endothelial cell apoptosis. Int J Biochem Cell Biol. 2014;55:109–18. doi: 10.1016/j.biocel.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 172.Conway KP, Price P, Harding KG, Jiang WG. The role of vascular endothelial growth inhibitor in wound healing. Int Wound J. 2007;4:55–64. doi: 10.1111/j.1742-481X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 174.Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends Mol Med. 2002;8:330–4. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 175.Alberdi E, Aymerich MS, Becerra SP. Binding of pigment epithelium-derived factor (PEDF) to retinoblastoma cells and cerebellar granule neurons. Evidence for a PEDF receptor. J Biol Chem. 1999;274:31605–12. doi: 10.1074/jbc.274.44.31605. [DOI] [PubMed] [Google Scholar]
- 176.Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 177.Yafai Y, Lange J, Wiedemann P, Reichenbach A, Eichler W. Pigment epithelium-derived factor acts as an opponent of growth-stimulatory factors in retinal glial-endothelial cell interactions. Glia. 2007;55:642–51. doi: 10.1002/glia.20495. [DOI] [PubMed] [Google Scholar]
- 178.Qi W, Yang C, Dai Z, et al. High levels of pigment epithelium-derived factor in diabetes impair wound healing through suppression of Wnt signaling. Diabetes. 2015;64:1407–19. doi: 10.2337/db14-1111. [DOI] [PubMed] [Google Scholar]
- 179.Cates AM, Holden VI, Myers EM, Smith CK, Kaplan MJ, Kahlenberg JM. Interleukin 10 hampers endothelial cell differentiation and enhances the effects of interferon α on lupus endothelial cell progenitors. Rheumatology (Oxford) 2015;54:1114–23. doi: 10.1093/rheumatology/keu431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol. 2011;187:6143–56. doi: 10.4049/jimmunol.1101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Imanishi T, Hano T, Nishio I. Estrogen reduces angiotensin II-induced acceleration of senescence in endothelial progenitor cells. Hypertens Res. 2005;28:263–71. doi: 10.1291/hypres.28.263. [DOI] [PubMed] [Google Scholar]
- 182.Imanishi T, Hano T, Nishio I. Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens. 2005;23:97–104. doi: 10.1097/00004872-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 183.Valluru M, Staton CA, Reed MW, Brown NJ. Transforming Growth Factor-beta and Endoglin Signaling Orchestrate Wound Healing. Front Physiol. 2011;2:89. doi: 10.3389/fphys.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 185.Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–74. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 186.Silberstein LE, Lin CP. A new image of the hematopoietic stem cell vascular niche. Cell Stem Cell. 2013;13:514–6. doi: 10.1016/j.stem.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014;35:32–7. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–43. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ratajczak MZ. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia. 2015;29:776–82. doi: 10.1038/leu.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Janowska-Wieczorek A, Marquez LA, Nabholtz JM, et al. Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34(+) cells and their transmigration through reconstituted basement membrane. Blood. 1999;93:3379–90. [PubMed] [Google Scholar]
- 192.Tanaka A, Yamane Y, Matsuda H. Mast cell MMP-9 production enhanced by bacterial lipopolysaccharide. J Vet Med Sci. 2001;63:811–3. doi: 10.1292/jvms.63.811. [DOI] [PubMed] [Google Scholar]
- 193.Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma. 2003;44:575–82. doi: 10.1080/1042819021000037985. [DOI] [PubMed] [Google Scholar]
- 194.Levesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31:109–17. doi: 10.1016/s0301-472x(02)01028-7. [DOI] [PubMed] [Google Scholar]
- 195.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Van Overstraeten-Schlogel N, Beguin Y, Gothot A. Role of stromal-derived factor-1 in the hematopoietic-supporting activity of human mesenchymal stem cells. Eur J Haematol. 2006;76:488–93. doi: 10.1111/j.1600-0609.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 197.Mohty M, Ho AD. In and out of the niche: perspectives in mobilization of hematopoietic stem cells. Exp Hematol. 2011;39:723–9. doi: 10.1016/j.exphem.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 198.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–7. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–9. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jin F, Zhai Q, Qiu L, et al. Degradation of BM SDF-1 by MMP-9: the role in G-CSF-induced hematopoietic stem/progenitor cell mobilization. Bone Marrow Transplant. 2008;42:581–8. doi: 10.1038/bmt.2008.222. [DOI] [PubMed] [Google Scholar]
- 201.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 202.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–30. [PubMed] [Google Scholar]
- 203.Levesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–92. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 204.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–97. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 205.Lapid K, Glait-Santar C, Gur-Cohen S, Canaani J, Kollet O, Lapidot T. StemBook [Internet] Cambridge (MA): Harvard Stem Cell Institute; 2008–2012. Egress and Mobilization of Hematopoietic Stem and Progenitor Cells: A Dynamic Multi-facet Process. [PubMed] [Google Scholar]
- 206.Alvarez P, Carrillo E, Velez C, et al. Regulatory systems in bone marrow for hematopoietic stem/progenitor cells mobilization and homing. Biomed Res Int. 2013;2013:312656. doi: 10.1155/2013/312656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu L, Yu Q, Lin J, et al. Hypoxia-inducible factor-1alpha is essential for hypoxia-induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells Dev. 2011;20:1961–71. doi: 10.1089/scd.2010.0453. [DOI] [PubMed] [Google Scholar]
- 208.Yokoi H, Yamada H, Tsubakimoto Y, et al. Bone marrow AT1 augments neointima formation by promoting mobilization of smooth muscle progenitors via platelet-derived SDF-1|alpha} Arterioscler Thromb Vasc Biol. 2010;30:60–7. doi: 10.1161/ATVBAHA.109.192161. [DOI] [PubMed] [Google Scholar]
- 209.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 210.Hristov M, Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res. 2008;58:148–51. doi: 10.1016/j.phrs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 211.Cheng M, Huang K, Zhou J, et al. A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J Mol Cell Cardiol. 2015;81:49–53. doi: 10.1016/j.yjmcc.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hristov M, Zernecke A, Bidzhekov K, et al. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res. 2007;100:590–7. doi: 10.1161/01.RES.0000259043.42571.68. [DOI] [PubMed] [Google Scholar]
- 213.Fujiyama S, Amano K, Uehira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93:980–9. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 214.Hristov M, Zernecke A, Liehn EA, Weber C. Regulation of endothelial progenitor cell homing after arterial injury. Thromb Haemost. 2007;98:274–7. [PubMed] [Google Scholar]
- 215.Ishida Y, Kimura A, Kuninaka Y, et al. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest. 2012;122:711–21. doi: 10.1172/JCI43027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Oh IY, Yoon CH, Hur J, et al. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood. 2007;110:3891–9. doi: 10.1182/blood-2006-10-048991. [DOI] [PubMed] [Google Scholar]
- 217.Biancone L, Cantaluppi V, Duo D, Deregibus MC, Torre C, Camussi G. Role of L-selectin in the vascular homing of peripheral blood-derived endothelial progenitor cells. J Immunol. 2004;173:5268–74. doi: 10.4049/jimmunol.173.8.5268. [DOI] [PubMed] [Google Scholar]
- 218.Lev EI, Estrov Z, Aboulfatova K, et al. Potential role of activated platelets in homing of human endothelial progenitor cells to subendothelial matrix. Thromb Haemost. 2006;96:498–504. [PubMed] [Google Scholar]
- 219.Caiado F, Dias S. Endothelial progenitor cells and integrins: adhesive needs. Fibrogenesis Tissue Repair. 2012;5:4. doi: 10.1186/1755-1536-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hur J, Yoon CH, Lee CS, et al. Akt is a key modulator of endothelial progenitor cell trafficking in ischemic muscle. Stem Cells. 2007;25:1769–78. doi: 10.1634/stemcells.2006-0385. [DOI] [PubMed] [Google Scholar]
- 221.Langer HF, Stellos K, Steingen C, et al. Platelet derived bFGF mediates vascular integrative mechanisms of mesenchymal stem cells in vitro. J Mol Cell Cardiol. 2009;47:315–25. doi: 10.1016/j.yjmcc.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 222.Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–12. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 223.Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–45. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 224.Balaji S, King A, Crombleholme TM, Keswani SG. The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing. Adv Wound Care (New Rochelle) 2013;2:283–95. doi: 10.1089/wound.2012.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ozerdem U, Alitalo K, Salven P, Li A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Invest Ophthalmol Vis Sci. 2005;46:3502–6. doi: 10.1167/iovs.05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hirschi KK, Majesky MW. Smooth muscle stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:22–33. doi: 10.1002/ar.a.10128. [DOI] [PubMed] [Google Scholar]
- 227.Diez M, Musri MM, Ferrer E, Barbera JA, Peinado VI. Endothelial progenitor cells undergo an endothelial-to-mesenchymal transition-like process mediated by TGFbetaRI. Cardiovasc Res. 2010;88:502–11. doi: 10.1093/cvr/cvq236. [DOI] [PubMed] [Google Scholar]
- 228.Yoshida M, Okubo N, Chosa N, et al. TGF-beta-operated growth inhibition and translineage commitment into smooth muscle cells of periodontal ligament-derived endothelial progenitor cells through Smad- and p38 MAPK-dependent signals. Int J Biol Sci. 2012;8:1062–74. doi: 10.7150/ijbs.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–7. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 230.Zaniboni A, Bernardini C, Bertocchi M, et al. In vitro differentiation of porcine aortic vascular precursor cells to endothelial and vascular smooth muscle cells. Am J Physiol Cell Physiol. 2015;309:C320–31. doi: 10.1152/ajpcell.00049.2015. [DOI] [PubMed] [Google Scholar]
- 231.Hegner B, Lange M, Kusch A, et al. mTOR regulates vascular smooth muscle cell differentiation from human bone marrow-derived mesenchymal progenitors. Arterioscler Thromb Vasc Biol. 2009;29:232–8. doi: 10.1161/ATVBAHA.108.179457. [DOI] [PubMed] [Google Scholar]
- 232.Zhang M, Malik AB, Rehman J. Endothelial progenitor cells and vascular repair. Curr Opin Hematol. 2014;21:224–8. doi: 10.1097/MOH.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kim SW, Jin HL, Kang SM, et al. Therapeutic effects of late outgrowth endothelial progenitor cells or mesenchymal stem cells derived from human umbilical cord blood on infarct repair. Int J Cardiol. 2016;203:498–507. doi: 10.1016/j.ijcard.2015.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shen WC, Liang CJ, Wu VC, et al. Endothelial progenitor cells derived from Wharton’s jelly of the umbilical cord reduces ischemia-induced hind limb injury in diabetic mice by inducing HIF-1alpha/IL-8 expression. Stem Cells Dev. 2013;22:1408–18. doi: 10.1089/scd.2012.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Caiado F, Carvalho T, Silva F, et al. The role of fibrin E on the modulation of endothelial progenitors adhesion, differentiation and angiogenic growth factor production and the promotion of wound healing. Biomaterials. 2011;32:7096–105. doi: 10.1016/j.biomaterials.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 236.Kim JY, Song SH, Kim KL, et al. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010;19:1635–44. doi: 10.3727/096368910X516637. [DOI] [PubMed] [Google Scholar]
- 237.Barcelos LS, Duplaa C, Krankel N, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095–102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 239.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–8. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 240.Ranghino A, Cantaluppi V, Grange C, et al. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol. 2012;25:75–85. doi: 10.1177/039463201202500110. [DOI] [PubMed] [Google Scholar]
- 241.Li X, Chen C, Wei L, et al. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy. 2016;18:253–62. doi: 10.1016/j.jcyt.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 242.Li X, Jiang C, Zhao J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications. 2016;30:986–92. doi: 10.1016/j.jdiacomp.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 243.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 244.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kawai T, Katagiri W, Osugi M, Sugimura Y, Hibi H, Ueda M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy. 2015;17:369–81. doi: 10.1016/j.jcyt.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 246.Yang Y, Chen QH, Liu AR, Xu XP, Han JB, Qiu HB. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther. 2015;6:250. doi: 10.1186/s13287-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lin X, Robinson M, Petrie T, Spandler V, Boyd WD, Sondergaard CS. Small intestinal submucosa-derived extracellular matrix bioscaffold significantly enhances angiogenic factor secretion from human mesenchymal stromal cells. Stem Cell Res Ther. 2015;6:164. doi: 10.1186/s13287-015-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hou Y, Ryu CH, Jun JA, Kim SM, Jeong CH, Jeun SS. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int. 2014;38:1050–9. doi: 10.1002/cbin.10294. [DOI] [PubMed] [Google Scholar]
- 249.Herrmann JL, Weil BR, Abarbanell AM, et al. IL-6 and TGF-alpha costimulate mesenchymal stem cell vascular endothelial growth factor production by ERK-, JNK-, and PI3K-mediated mechanisms. Shock. 2011;35:512–6. doi: 10.1097/SHK.0b013e31820b2fb9. [DOI] [PubMed] [Google Scholar]
- 250.Wang Y, Crisostomo PR, Wang M, Markel TA, Novotny NM, Meldrum DR. TGF-alpha increases human mesenchymal stem cell-secreted VEGF by MEK- and PI3-K- but not JNK- or ERK-dependent mechanisms. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1115–23. doi: 10.1152/ajpregu.90383.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–82. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 252.Beltramo E, Lopatina T, Berrone E, et al. Extracellular vesicles derived from mesenchymal stem cells induce features of diabetic retinopathy in vitro. Acta Diabetol. 2014;51:1055–64. doi: 10.1007/s00592-014-0672-1. [DOI] [PubMed] [Google Scholar]
- 253.Burlacu A, Grigorescu G, Rosca AM, Preda MB, Simionescu M. Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem Cells Dev. 2013;22:643–53. doi: 10.1089/scd.2012.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Merino-González C, Zuñiga FA, Escudero C, et al. Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: Potencial Clinical Application. Front Physiol. 2016;7:24. doi: 10.3389/fphys.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rüder C, Haase T, Krost A, et al. Combinatorial G-CSF/AMD3100 treatment in cardiac repair after myocardial infarction. PLoS One. 2014;9:e104644. doi: 10.1371/journal.pone.0104644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869–82. doi: 10.1089/ars.2008.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Drela E, Stankowska K, Kulwas A, Rosc D. Endothelial progenitor cells in diabetic foot syndrome. Adv Clin Exp Med. 2012;21:249–54. [PubMed] [Google Scholar]
- 258.Kim KA, Shin YJ, Kim JH, et al. Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Arch Pharm Res. 2012;35:223–34. doi: 10.1007/s12272-012-0203-y. [DOI] [PubMed] [Google Scholar]
- 259.Gough A, Clapperton M, Rolando N, Foster AV, Philpott-Howard J, Edmonds ME. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet. 1997;350:855–9. doi: 10.1016/S0140-6736(97)04495-4. [DOI] [PubMed] [Google Scholar]
- 260.Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst Rev. 2009:CD006810. doi: 10.1002/14651858.CD006810.pub2. [DOI] [PubMed] [Google Scholar]
- 261.Balaji S, Han N, Moles C, et al. Angiopoietin-1 improves endothelial progenitor cell-dependent neovascularization in diabetic wounds. Surgery. 2015;158:846–56. doi: 10.1016/j.surg.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Allen RJ, Jr, Soares MA, Haberman ID, et al. Combination therapy accelerates diabetic wound closure. PLoS One. 2014;9:e92667. doi: 10.1371/journal.pone.0092667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tanaka R, Masuda H, Kato S, et al. Autologous G-CSF-mobilized peripheral blood CD34+ cell therapy for diabetic patients with chronic nonhealing ulcer. Cell Transplant. 2014;23:167–79. doi: 10.3727/096368912X658007. [DOI] [PubMed] [Google Scholar]
- 264.Asai J, Takenaka H, Ii M, et al. Topical application of ex vivo expanded endothelial progenitor cells promotes vascularisation and wound healing in diabetic mice. Int Wound J. 2013;10:527–33. doi: 10.1111/j.1742-481X.2012.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ackermann M, Pabst AM, Houdek JP, Ziebart T, Konerding MA. Priming with proangiogenic growth factors and endothelial progenitor cells improves revascularization in linear diabetic wounds. Int J Mol Med. 2014;33:833–9. doi: 10.3892/ijmm.2014.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kanitkar M, Jaiswal A, Deshpande R, Bellare J, Kale VP. Enhanced growth of endothelial precursor cells on PCG-matrix facilitates accelerated, fibrosis-free, wound healing: a diabetic mouse model. PLoS One. 2013;8:e69960. doi: 10.1371/journal.pone.0069960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sorrell JM, Caplan AI. Topical delivery of mesenchymal stem cells and their function in wounds. Stem Cell Res Ther. 2010;1:30. doi: 10.1186/scrt30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Egana JT, Fierro FA, Kruger S, et al. Use of human mesenchymal cells to improve vascularization in a mouse model for scaffold-based dermal regeneration. Tissue Eng Part A. 2009;15:1191–200. doi: 10.1089/ten.tea.2008.0097. [DOI] [PubMed] [Google Scholar]
- 269.Herrmann M, Verrier S, Alini M. Strategies to Stimulate Mobilization and Homing of Endogenous Stem and Progenitor Cells for Bone Tissue Repair. Front Bioeng Biotechnol. 2015;3:79. doi: 10.3389/fbioe.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tarkka T, Sipola A, Jamsa T, et al. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med. 2003;5:560–6. doi: 10.1002/jgm.392. [DOI] [PubMed] [Google Scholar]
- 271.Li R, Stewart DJ, von Schroeder HP, Mackinnon ES, Schemitsch EH. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J Orthop Res. 2009;27:8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 272.Li R, Nauth A, Li C, Qamirani E, Atesok K, Schemitsch EH. Expression of VEGF gene isoforms in a rat segmental bone defect model treated with EPCs. J Orthop Trauma. 2012;26:689–92. doi: 10.1097/BOT.0b013e318266eb7e. [DOI] [PubMed] [Google Scholar]
- 273.Li R, Atesok K, Nauth A, et al. Endothelial progenitor cells for fracture healing: a microcomputed tomography and biomechanical analysis. J Orthop Trauma. 2011;25:467–71. doi: 10.1097/BOT.0b013e31821ad4ec. [DOI] [PubMed] [Google Scholar]
- 274.Mercado-Pagan AE, Stahl AM, Shanjani Y, Yang Y. Vascularization in bone tissue engineering constructs. Ann Biomed Eng. 2015;43:718–29. doi: 10.1007/s10439-015-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Massa M, Rosti V, Ferrario M, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 276.Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–7. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 277.Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–9. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 278.Misao Y, Takemura G, Arai M, et al. Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 2006;71:455–65. doi: 10.1016/j.cardiores.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 279.Zhao Q, Sun C, Xu X, et al. Early use of granulocyte colony stimulating factor improves survival in a rabbit model of chronic myocardial ischemia. J Cardiol. 2013;61:87–94. doi: 10.1016/j.jjcc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 280.Sato T, Suzuki H, Kusuyama T, et al. G-CSF after myocardial infarction accelerates angiogenesis and reduces fibrosis in swine. Int J Cardiol. 2008;127:166–73. doi: 10.1016/j.ijcard.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 281.Toyama T, Hoshizaki H, Kasama S, et al. Low-dose and long-term G-CSF treatment can improve severe myocardial ischemia in patients with severe coronary artery disease. J Nucl Cardiol. 2011;18:463–71. doi: 10.1007/s12350-011-9350-7. [DOI] [PubMed] [Google Scholar]
- 282.Kang S, Yang Y, Li CJ, Gao R. Effectiveness and tolerability of administration of granulocyte colony-stimulating factor on left ventricular function in patients with myocardial infarction: a meta-analysis of randomized controlled trials. Clin Ther. 2007;29:2406–18. doi: 10.1016/j.clinthera.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 283.Zohlnhofer D, Ott I, Mehilli J, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003–10. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 284.Zohlnhofer D, Kastrati A, Schomig A. Stem cell mobilization by granulocyte-colony-stimulating factor in acute myocardial infarction: lessons from the REVIVAL-2 trial. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S106–9. doi: 10.1038/ncpcardio0745. [DOI] [PubMed] [Google Scholar]
- 285.Zohlnhofer D, Dibra A, Koppara T, et al. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51:1429–37. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 286.Schuh A, Liehn EA, Sasse A, et al. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol. 2008;103:69–77. doi: 10.1007/s00395-007-0685-9. [DOI] [PubMed] [Google Scholar]
- 287.Dubois C, Liu X, Claus P, et al. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol. 2010;55:2232–43. doi: 10.1016/j.jacc.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 288.Alexander S, Sasse A, Konschalla S, et al. Repetitive transplantation of different cell types sequentially improves heart function after infarction. J Cell Mol Med. 2012;16:1640–7. doi: 10.1111/j.1582-4934.2011.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chen SY, Wang F, Yan XY, et al. Autologous transplantation of EPCs encoding FGF1 gene promotes neovascularization in a porcine model of chronic myocardial ischemia. Int J Cardiol. 2009;135:223–32. doi: 10.1016/j.ijcard.2008.12.193. [DOI] [PubMed] [Google Scholar]
- 290.Sen S, Merchan J, Dean J, et al. Autologous transplantation of endothelial progenitor cells genetically modified by adeno-associated viral vector delivering insulin-like growth factor-1 gene after myocardial infarction. Hum Gene Ther. 2010;21:1327–34. doi: 10.1089/hum.2010.006. [DOI] [PubMed] [Google Scholar]
- 291.Tang J, Wang J, Yang J, et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36:644–50. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 292.Thal MA, Krishnamurthy P, Mackie AR, et al. Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair. Circ Res. 2012;111:180–90. doi: 10.1161/CIRCRESAHA.112.270462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 294.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–68. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sun L, Zhang T, Lan X, Du G. Effects of stem cell therapy on left ventricular remodeling after acute myocardial infarction: a meta-analysis. Clin Cardiol. 2010;33:296–302. doi: 10.1002/clc.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Delewi R, Hirsch A, Tijssen JG, et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J. 2014;35:989–98. doi: 10.1093/eurheartj/eht372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Delewi R, Andriessen A, Tijssen JG, Zijlstra F, Piek JJ, Hirsch A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a meta-analysis of randomised controlled clinical trials. Heart. 2013;99:225–32. doi: 10.1136/heartjnl-2012-302230. [DOI] [PubMed] [Google Scholar]
- 298.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–5. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 299.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 300.Povsic TJ, Junge C, Nada A, et al. A phase 3, randomized, double-blinded, active-controlled, unblinded standard of care study assessing the efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina: design of the RENEW study. Am Heart J. 2013;165:854–61 e2. doi: 10.1016/j.ahj.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 301.Yip HK, Chang LT, Chang WN, et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 302.Marti-Fabregas J, Crespo J, Delgado-Mederos R, et al. Endothelial progenitor cells in acute ischemic stroke. Brain Behav. 2013;3:649–55. doi: 10.1002/brb3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Sobrino T, Hurtado O, Moro MA, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–64. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 304.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, et al. Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res. 2010;80:317–23. doi: 10.1016/j.mvr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 305.Mao L, Huang M, Chen SC, et al. Endogenous endothelial progenitor cells participate in neovascularization via CXCR4/SDF-1 axis and improve outcome after stroke. CNS Neurosci Ther. 2014;20:460–8. doi: 10.1111/cns.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bai YY, Wang L, Peng XG, et al. Non-invasive monitoring of transplanted endothelial progenitor cells in diabetic ischemic stroke models. Biomaterials. 2015;40:43–50. doi: 10.1016/j.biomaterials.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 307.Bogoslovsky T, Chaudhry A, Latour L, et al. Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology. 2010;75:2059–62. doi: 10.1212/WNL.0b013e318200d741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tsai NW, Hung SH, Huang CR, et al. The association between circulating endothelial progenitor cells and outcome in different subtypes of acute ischemic stroke. Clin Chim Acta. 2014;427:6–10. doi: 10.1016/j.cca.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 309.Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–97. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Bai YY, Peng XG, Wang LS, et al. Bone Marrow Endothelial Progenitor Cell Transplantation After Ischemic Stroke: An Investigation Into Its Possible Mechanism. CNS Neurosci Ther. 2015;21:877–86. doi: 10.1111/cns.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Chen YL, Tsai TH, Wallace CG, et al. Intra-carotid arterial administration of autologous peripheral blood-derived endothelial progenitor cells improves acute ischemic stroke neurological outcomes in rats. Int J Cardiol. 2015;201:668–83. doi: 10.1016/j.ijcard.2015.03.137. [DOI] [PubMed] [Google Scholar]
- 312.Navarro-Sobrino M, Hernandez-Guillamon M, Fernandez-Cadenas I, et al. The angiogenic gene profile of circulating endothelial progenitor cells from ischemic stroke patients. Vasc Cell. 2013;5:3. doi: 10.1186/2045-824X-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Rosell A, Morancho A, Navarro-Sobrino M, et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS One. 2013;8:e73244. doi: 10.1371/journal.pone.0073244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Pena I, Borlongan CV. Translating G-CSF as an Adjunct Therapy to Stem Cell Transplantation for Stroke. Transl Stroke Res. 2015;6:421–9. doi: 10.1007/s12975-015-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sprigg N, Bath PM, Zhao L, et al. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery EnhanceMent after Stroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092) Stroke. 2006;37:2979–83. doi: 10.1161/01.STR.0000248763.49831.c3. [DOI] [PubMed] [Google Scholar]
- 316.England TJ, Abaei M, Auer DP, et al. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke. 2012;43:405–11. doi: 10.1161/STROKEAHA.111.636449. [DOI] [PubMed] [Google Scholar]
- 317.Moniche F, Montaner J, Gonzalez-Marcos JR, et al. Intra-arterial bone marrow mononuclear cell transplantation correlates with GM-CSF, PDGF-BB, and MMP-2 serum levels in stroke patients: results from a clinical trial. Cell Transplant. 2014;23(Suppl 1):S57–64. doi: 10.3727/096368914X684934. [DOI] [PubMed] [Google Scholar]
- 318.Prasad K, Sharma A, Garg A, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45:3618–24. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- 319.Moniche F, Escudero I, Zapata-Arriaza E, et al. Intra-arterial bone marrow mononuclear cells (BM-MNCs) transplantation in acute ischemic stroke (IBIS trial): protocol of a phase II, randomized, dose-finding, controlled multicenter trial. Int J Stroke. 2015;10:1149–52. doi: 10.1111/ijs.12520. [DOI] [PubMed] [Google Scholar]


