Abstract
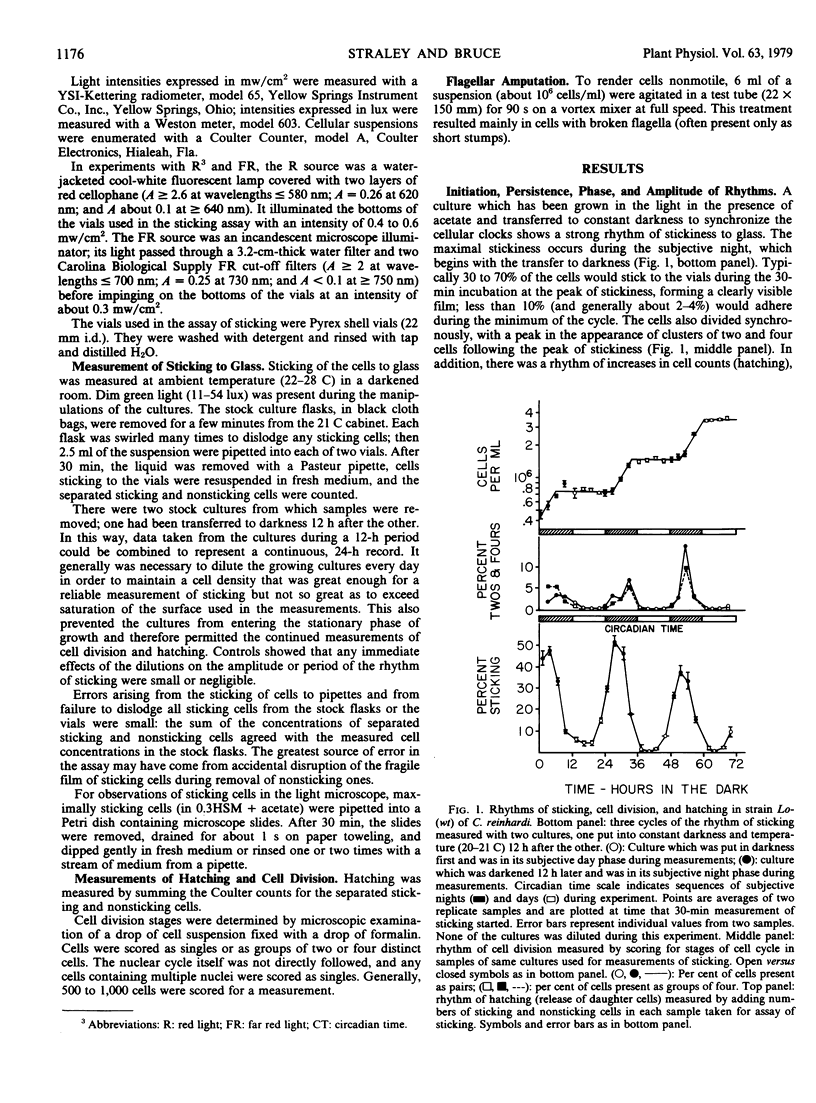
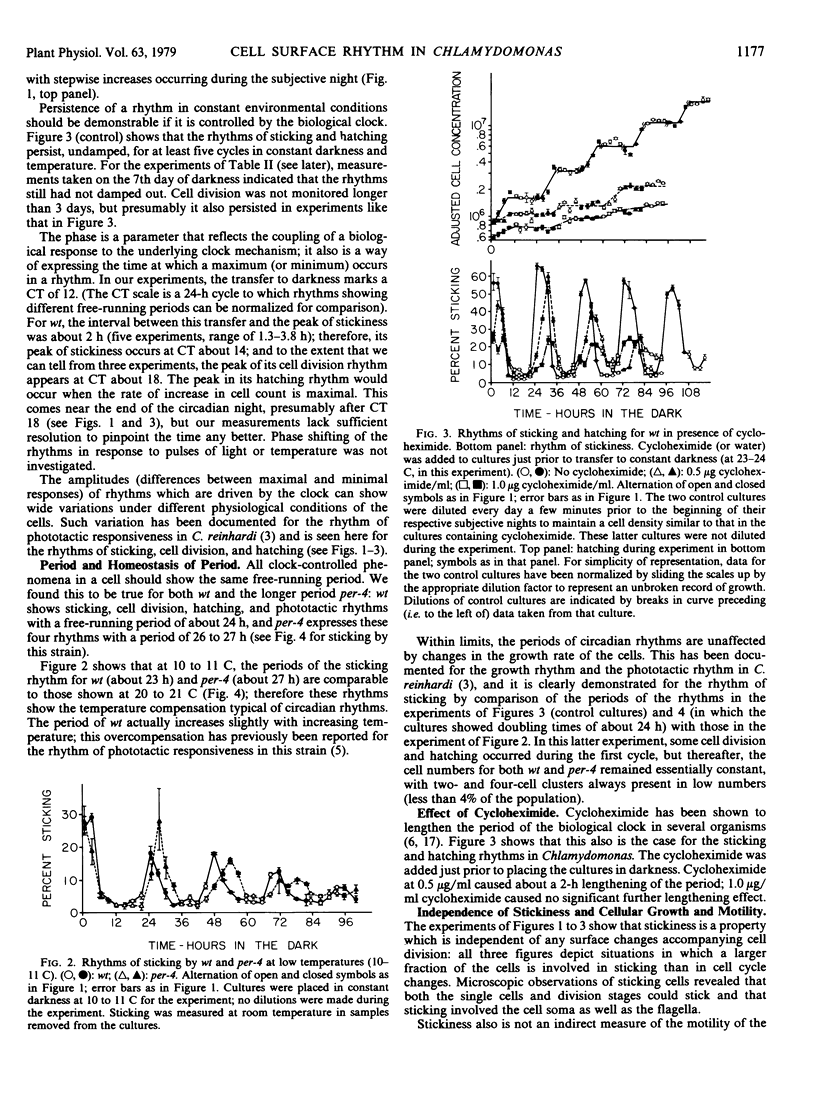
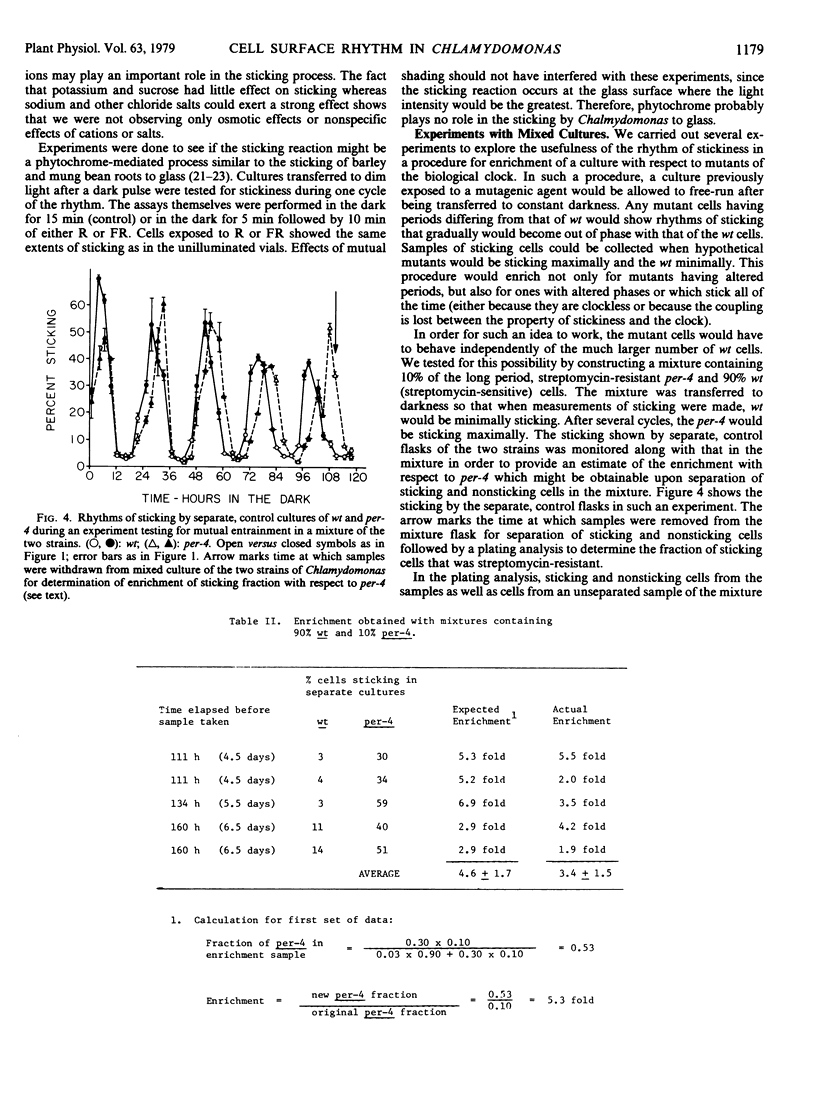
Conditions were found in which Chlamydomonas reinhardi exhibits a circadian alteration of its cell surface, measured as ability to stick to glass. Under these same conditions the cells also show circadian rhythms of cell division and release of daughter cells. The three rhythmic phenomena were shown to have typical properties of rhythms controlled by the biological clock. The rhythm of stickiness was used to demonstrate that in a mixed culture containing two cell populations with natural periods differing by 2 to 3 hours, the cells did not mutally entrain each other and that this rhythm could be successfully applied in an enrichment procedure for mutants of the biological clock. Stickiness was shown to be independent of growth and motility of the cells and unaffected by red or far red illimination. Minimally sticking cells did not affect the sticking of maximally sticking cells in a mixed culture; nor was there a progressive increase in stickiness shown at the minimum from one cycle to the next in a pure culture. These results indicate that sticking probably is not mediated by long lived adhesive material or enzymes excreted into the medium. Several tests of the sensitivity of stickiness to replacement of the growth medium by distilled water or water containing various compounds suggest that ions might play an important role in the sticking reaction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce V. G. Recombinants between clock mutants of Chlamydomonas reinhardi. Genetics. 1974 Jun;77(2):221–230. doi: 10.1093/genetics/77.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F. Lengthening the period of a biological clock in Euglena by cycloheximide, an inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1080–1087. doi: 10.1073/pnas.57.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacklet J. W. Neuronal circadian rhythm: phase shifting by a protein synthesis inhibitor. Science. 1977 Oct 7;198(4312):69–71. doi: 10.1126/science.897685. [DOI] [PubMed] [Google Scholar]
- Karakashian M. W., Schweiger H. G. Circadian properties of the rhythmic system in individual nucleated and enucleated cells of Acetabularia mediterranea. Exp Cell Res. 1976 Feb;97(2):366–377. doi: 10.1016/0014-4827(76)90628-5. [DOI] [PubMed] [Google Scholar]
- Karakashian M. W., Schweiger H. G. Evidence for a cycloheximide-sensitive component in the biological clock of Acetabularia. Exp Cell Res. 1976 Mar 15;98(2):303–312. doi: 10.1016/0014-4827(76)90442-0. [DOI] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njus D., Sulzman F. M., Hastings J. W. Membrane model for the circadian clock. Nature. 1974 Mar 8;248(5444):116–120. doi: 10.1038/248116a0. [DOI] [PubMed] [Google Scholar]
- Rothman B. S., Strumwasser F. Phase shifting the circadian rhythm of neuronal activity in the isolated Aplysia eye with puromycin and cycloheximide. Electrophysiological and biochemical studies. J Gen Physiol. 1976 Oct;68(4):359–384. doi: 10.1085/jgp.68.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger H. G., Schweiger M. Circadian rhythms in unicellular organisms: an endeavor to explain the molecular mechanism. Int Rev Cytol. 1977;51:315–342. doi: 10.1016/s0074-7696(08)60230-2. [DOI] [PubMed] [Google Scholar]
- Sweeney B. M. A physiological model for circadian rhythms derived from the acetabularia rhythm paradoxes. Int J Chronobiol. 1974;2(1):25–33. [PubMed] [Google Scholar]
- Tanada T. A rapid photoreversible response of barley root tips in the presence of 3-indoleacetic Acid. Proc Natl Acad Sci U S A. 1968 Feb;59(2):376–380. doi: 10.1073/pnas.59.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson T. R., Corpe W. A. Enhancement of adhesion of the marine Chlorella vulgaris to glass. Can J Microbiol. 1975 Jul;21(7):1025–1031. doi: 10.1139/m75-152. [DOI] [PubMed] [Google Scholar]



