Abstract
Aims
To review maternal and foetal outcomes in women with mechanical heart valves (MHVs) treated with vitamin-K antagonists (VKAs), first-trimester heparin followed by VKAs (sequential treatment), low molecular weight heparin (LMWH) and unfractionated heparin (UFH) during pregnancy, in order to inform practice.
Methods and results
Medline, Embase and Central were searched from inception until February 2016. Two reviewers independently screened 1786 titles, reviewed 110 full-texts and extracted data and assessed risk-of-bias from 46 articles. Pooled incidence (95% confidence intervals) was calculated for maternal and foetal outcomes. Included studies had a moderate or high risk-of-bias. With VKAs, sequential treatment and LMWH, maternal mortality occurred in 0.9% (0.4–1.4), 2.0% (0.8–3.1) and 2.9% (0.2–5.7), thromboembolic complications in 2.7% (1.4–4.0), 5.8% (3.8–7.7) and 8.7% (3.9–13.4), livebirths in 64.5% (48.8–80.2), 79.9% (74.3–85.6) and 92.0% (86.1–98.0) and anticoagulant-related foetal/neonatal adverse events (embryopathy or foetopathy) in 2.0% (0.3–3.7), 1.4% (0.3–2.5) and 0%, respectively. When UFH is used throughout pregnancy, 11.2% (2.8–19.6) suffered thromboembolic complications. Foetal loss and adverse events occurred with first-trimester warfarin doses ≤ 5 mg/day, although there were more livebirths [83.6% (75.8–91.4) vs. 43.9% (32.8–55.0)] and fewer foetal anomalies [2.3% (0.7–4.0) vs. 12.4% (3.3–21.6)] with lower doses than with warfarin > 5 mg/day.
Conclusions
VKAs are associated with fewest maternal complications but also with fewest livebirths. Sequential treatment does not eliminate anticoagulant-related foetal/neonatal adverse events. LMWH is associated with the highest number of livebirths. The safety of UFH throughout pregnancy and first-trimester warfarin ≤ 5 mg/day remains unconfirmed.
Keywords: Mechanical heart valves , Pregnancy , Anticoagulation , Vitamin-K antagonists , Heparin
Introduction
Patients with mechanical heart valves (MHVs) require life-long anticoagulation to prevent thromboembolic complications (TECs).1 The risk of TECs is increased during pregnancy2,3 and women with MHVs have only a 58% chance of experiencing an uncomplicated pregnancy with a live birth.4 Non-pregnant women with MHVs are treated with vitamin-K antagonists (VKAs), but as VKAs traverse the placenta and are teratogenic, alternative anticoagulation regimens have been used in pregnancy with the aim of reducing foetal risks. High foetal loss and foetal anomaly rates associated with VKAs have been circumvented by replacing VKAs with heparin for either the entire duration of pregnancy or during the period of embryogenesis. Similarly, the increased maternal and foetal bleeding associated with the administration of VKA in the late third trimester has been addressed by changing to heparin in the peripartum period, starting approximately 2 weeks before the anticipated date of delivery. Thus there are three methods of anticoagulation currently used in pregnant women with MHVs – VKAs throughout pregnancy, heparin throughout pregnancy and sequential treatment involving the use of heparin in the first trimester and VKAs in the second and third trimesters. Earlier systematic reviews discussing the risks and benefit of the anticoagulation regimens in pregnant women with MHVs included a large number of women with older style ball-and-cage valves, incorporated studies with less rigorous study designs and did not adequately address outcomes associated with low molecular weight heparin (LMWH).5 An updated assessment of the risks and benefits of anticoagulation in pregnant women with MHVs is therefore needed. The objective of this systematic review was to determine the maternal and foetal risks associated with various anticoagulation regimens in pregnant women with MHVs, to better inform practice.
Methods
The study protocol was registered with PROSPERO6 (CRD42014013286) and conducted and reported according to PRISMA7 and MOOSE8 guidelines (see Supplementary material online, Table S6) respectively.
Data sources and searches
The literature search was conducted using the OvidSP search platform in three bibliographic databases – MEDLINE, EMBASE and CENTRAL, using the indexing terms ‘prosthetic heart valves’, ‘anticoagulation’ and ‘pregnancy’ to include articles indexed as of 10 February 2016 (see Supplementary material online, Figure S1). The search was limited to human data and restricted to the English language. No other restrictions were applied. Additional articles were identified by scanning reference lists, searching the grey literature that included haematology, cardiology and obstetric conference abstracts from the past 5 years and the first 200 hits on Google Scholar after entering variations of the above indexing terms, and contacting authors for full texts where abstracts were identified in conference proceedings.
Study selection
Type of studies and participants
All prospective and retrospective studies that described a minimum of five pregnancies in women with prosthetic heart valves, where at least 80% of the patients comprised women with MHVs, were included. If more than one publication including the same patients was identified, only the most recent study was included. When possible, the type and location of the MHV were recorded.
Types of interventions
Included studies had to describe at least one of the three methods of anticoagulation that comprised VKAs throughout pregnancy, therapeutic heparin throughout pregnancy, or sequential treatment with VKAs in the second and third trimesters and therapeutic heparin in the first trimester. ‘VKAs’ included warfarin, acenocoumarin, phenindione and pelenthan, adjusted to prothrombin time (PT) or international standardized ratio (INR). ‘Heparin’ included unfractionated heparin (UFH) adjusted to the activated partial thromboplastin time (aPTT) of at least 1.5 times normal, or LMWH adjusted to bodyweight or peak/trough anti-Xa levels. Studies that used sub-therapeutic, unadjusted or unclear anticoagulation regimens were excluded.
Types of outcomes
The primary maternal outcomes were maternal mortality (defined as death of the pregnant woman during pregnancy or in the first 6 postpartum weeks) and TECs (valvular thrombi and extravalvular thrombo-emboli). The primary foetal outcomes were livebirths (defined as the proportion of pregnancies that resulted in live-born infants) and anticoagulant-related foetal adverse events (including ‘embryopathy’ [nasal hypoplasia, stippled epiphyses, or both] resulting from VKA exposure between weeks 6 and 9 and ‘foetopathy’ [central nervous system or ocular abnormalities] resulting from VKA exposure at later gestations).9 Secondary maternal outcomes included major maternal bleeding (defined as blood loss requiring blood transfusion, surgery, readmission to hospital, interruption of anticoagulation or a drop of haemoglobin concentration by greater than 20 g/L), maternal cardiac events (defined as arrhythmias, heart failure and non-thrombotic valvular dysfunction) and adverse effects from anticoagulation including hypersensitivity and heparin-induced thrombocytopenia. Secondary foetal outcomes included foetal intracranial bleeding, small for gestational age infants (birth weight < 10th centile for gestation and sex), preterm birth (before 37 weeks of gestation) and foetal loss including ‘miscarriage’ (foetal loss under 20 weeks), ‘stillbirth’ (foetal loss after 20 weeks), ‘intrauterine foetal loss’ where the gestational age at foetal loss was not reported and neonatal death (deaths occurring after birth and prior to the neonate’s discharge from hospital).
Data extraction and quality assessment
Two reviewers (RD and JO) independently carried out the title and abstract screening, data extraction for included studies and risk-of-bias assessment. Disagreements were resolved by discussion and consensus. When disagreement persisted, a third reviewer (AKM) adjudicated. Authors were contacted with data requests where vital information for inclusion/analysis was unavailable in the original publication. Patients started on a particular treatment regimen were retained in that group for analysis regardless of whether the treatment was changed during the course of pregnancy. The risk for foetal and neonatal outcomes was calculated using the foetuses-at-risk approach,10 where the number of events after a certain gestational age was divided by the number of foetuses/neonates still alive and therefore, at risk for that event. The risk of bias of included studies was assessed using a modified Newcastle-Ottawa Quality Assessment Scale for cohort studies (see Supplementary material online, Figure S2).11 The risk of bias was deemed to be high if a study scored 0–3, moderate if it scored 4–6 and low if it scored 7–9. Publication bias was assessed using visual inspection of funnel plots with 95% and 99.7% control limits, in analyses where more than 10 studies were included.
Data synthesis and analysis
Primary analysis
The estimates of pooled incidence of outcomes were expressed as proportions per 100 pregnancies. Clinical heterogeneity was noted; however, we attempted to minimize it by strict inclusion criteria, the use of standard definitions for maternal and foetal outcomes and the adoption of the foetuses-at-risk approach for foetal/obstetric outcomes. Analysis was performed using OpenMetaAnalyst® software.12 As considerable clinical and methodological heterogeneity between studies was anticipated, it was decided a priori to perform DerSimonian-Laird binary random-effects meta-analyses, presenting pooled proportions with 95% confidence intervals (CIs). To provide a fair comparison between all groups, we used untransformed proportions and estimated CIs around proportions using the modified Wald method that considered Poisson distribution. Where event rates were zero, we used a very small correction factor (1 × 10−15) as recommended and also attempted the Freeman–Tukey Double Arcsine Proportion metric with and without a correction factor, but where this resulted in clinically meaningless results, they were reported as ‘not applicable’. Statistical heterogeneity was assessed using I2 statistics, treating I2 values >75% as having a high degree of heterogeneity.13 Although we considered performing a formal statistical comparison between treatment strategies using Student t tests and non-parametric tests, the significant clinical and methodological heterogeneity between studies meant that the assumptions of these tests were violated. We therefore restricted statistical comparisons only to those studies that reported more than one treatment strategy (see Supplementary material online, Table S4).
Subgroup and sensitivity analyses
Subgroup analysis for VKAs were conducted based on PT or INR targets, the use of VKAs until planned caesarean delivery and the first-trimester dose of VKAs. Studies describing sequential therapy were categorized according to whether the heparin was UFH or LMWH. In addition, subgroup analyses for all categories were conducted including studies that reported outcomes with bileaflet and single-tilting disc valves. Sensitivity analyses were performed according to risk-of-bias scores and the country’s economic status based on the 2015 World Bank report on income level.14
Results
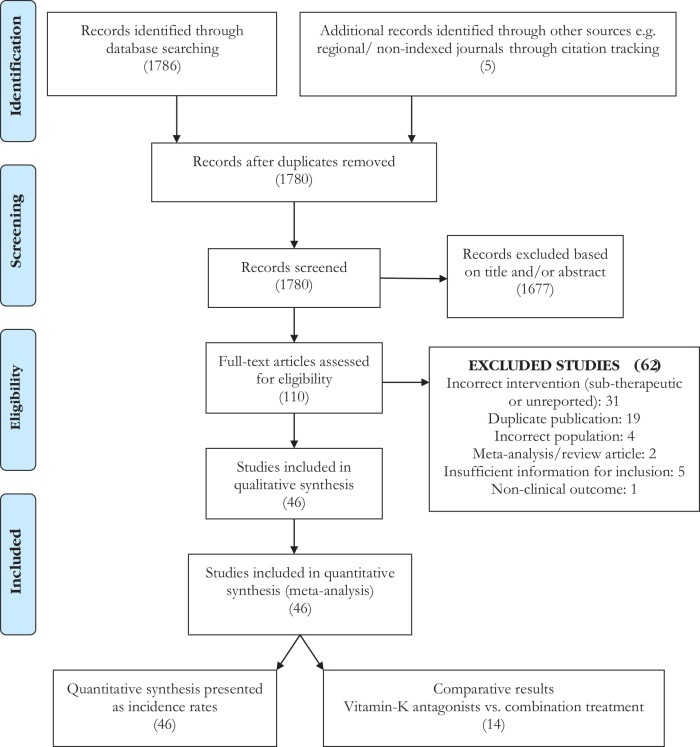
The literature search identified 1786 publications, and five publications were found through citation tracking (Figure 1). After screening titles and abstracts, 110 publications were selected for full-text review, of which 63 were excluded because they did not fulfil the eligibility criteria. The characteristics of the 46 included publications, all of which were non-randomized prospective and retrospective studies involving at least five pregnancies are described in Supplementary material online, Table S1. Excluded studies are described in Supplementary material online, Table S2. The included studies described 2468 pregnancies in at least 1874 women.
Figure 1.
PRISMA Diagram: Of the 1786 publications identified through the literature search and five through citation tracking, 110 were selected for full-text review and 46 were included in the analysis .
Of the 46 included studies, 37 studies reported on valve type and 44 on valve position. Of the studies that described valve types, 458/1555 (29%) of the replaced valves were ball-and-cage valves, 1070/1555 (69%) were single-tilting disc or bileaflet valves and no information was available on 27 (2%) of the valves. Of the studies that described valve positions, 1071/1569 (68%) were in the mitral position, 255/1569 (16%) in the aortic position, 238/1569 (15%) were in more than one position (196 mitral + aortic, three mitral + tricuspid and the remainder were not described) and 5/1569 (0.3%) were in the tricuspid position. Only 12 studies reported whether the primary valvular lesion was rheumatic or congenital, of which rheumatic valvular disease was described in 183/236 (78%) and congenital valvular disease in 53/236 (22%). Outcomes were not reported by valve type, position or primary valvular disease.
Of the 30 studies (1373 pregnancies) that reported the use of VKAs throughout pregnancy, 11 (581 pregnancies) used a standard INR target of 2.5–3.5. Twenty studies (530 pregnancies) reported the use of sequential treatment, 10 (132 pregnancies) used LMWH throughout pregnancy either adjusted to the woman’s bodyweight, peak anti-Xa levels, or to both peak and trough anti-Xa levels and four studies (64 pregnancies) used UFH throughout pregnancy adjusted to an aPTT of 1.5–2.5 times normal.
The included studies varied with respect to the risk of bias. As no RCTs were identified, none of the studies were deemed to be at a low risk of bias. Twenty six studies were at high risk of bias and 20 were at moderate risk of bias, from failing to report compliance with the anticoagulation regimen, the absence of the outcome of interest at the start of the study, the method of outcome assessment, adequacy of follow up and/or the lack of adjustment for confounding factors (see Supplementary material online, Figure S3).
Primary outcomes
The use of VKAs with standard (2.5–3.5) INR targets throughout pregnancy was associated with the lowest pooled proportions of maternal mortality and TECs followed by sequential treatment and LMWH respectively (Table 1). In contrast, LMWH throughout pregnancy was associated with the highest number of livebirths followed by sequential treatment and VKAs with standard (2.5–3.5) INR targets throughout pregnancy.
Table 1.
Primary maternal and foetal outcomes
| Anticoagulation regimen | Maternal mortality |
Thromboembolism |
Livebirth rate |
Anticoagulant-related foetal/ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| neonatal adverse events | ||||||||||||||||
| Studies | Events | Estimate (%) | I2 (%) | Studies | Events | Estimate (%) | I2 (%) | Studies | Events | Estimate (%) | I2 (%) | Studies | Events | Estimate (%) | I2 (%) | |
| Vitamin K antagonists (INR target 2.5-3.5) | 11 | 7/581 | 0.9 (0.1, 1.6) | 0 | 11 | 22/581 | 2.7 (1.4, 4.0) | 0 | 10 | 369/531 | 64.5 (48.8, 80.2) | 95 | 11 | 12/407 | 2.0 (0.3, 3.7)a | 24 |
| Sequential treatment | 20 | 11/530 | 2.0 (0.8, 3.1) | 0 | 20 | 44/530 | 5.8 (3.8, 7.7) | 29 | 18 | 381/475 | 79.9 (74.3, 85.6) | 61 | 19 | 5/431 | 1.4 (0.3, 2.5)b | 0 |
| LMWH alone | 10 | 1/132 | 2.9 (0.2, 5.7) | 0 | 9 | 13/127 | 8.7 (3.9, 13.4) | 0 | 7 | 68/74 | 92.0 (86.1, 98.0) | 0 | 8 | 0/103 | NA | 0 |
| UFH alone | 4 | 2/64 | 3.4 (0, 7.7) | 0 | 3 | 7/52 | 11.2 (2.8, 19.6) | 0 | 3 | 33/51 | 69.5 (37.8, 100) | 87 | 4 | 4/44 | 7.6 (0.1, 15.0)b | 0 |
INR, international normalized ratio; LMWH, low molecular weight heparin; NA, not applicable; UFH, unfractionated heparin.
Estimates are presented as proportions per 100 affected pregnancies with 95% confidence intervals.
Of these 7/407 [0.8% (0.0, 1.7)] represent embryopathy and 5/197 [2.1% (0.1, 4.1)] represent foetopathy.
All cases represent foetopathy.
Anticoagulant-related embryopathy and foetopathy was seen in approximately 2% of the foetuses exposed to VKAs throughout pregnancy. Although embryopathy was eliminated in foetuses on sequential treatment, 1.4% of these foetuses encountered foetopathy. Similarly, although embryopathy was eliminated in foetuses exposed to LMWH and UFH, 4/44 neonates on UFH had intraventricular haemorrhage. There were no cases of anticoagulant-related embyropathy or foetopathy in foetuses exposed to LMWH. Maternal and foetal complications were highest when UFH was used throughout pregnancy.
Secondary outcomes
Secondary maternal outcomes – major bleeding, cardiac events and adverse drug events – were lowest with the use of VKAs throughout pregnancy, while foetal and neonatal complications including intrauterine foetal loss, preterm birth and small for gestational age infants were lowest with the use of heparins throughout pregnancy or sequential treatment (see Supplementary material online, Table S3).
Subgroup and sensitivity analyses
Of the studies involving VKAs, nine studies (391 pregnancies) used lower (1.5–2.5) INR targets and six (298 pregnancies) used stratified INR targets based on the type, position and number of MHVs (Table 2). These targets were associated with comparable maternal complications and a higher numbers of livebirths but an increase in foetal anomalies. The use of stratified INR targets reduced TECs without affecting maternal mortality or foetal outcomes. Six studies (240 pregnancies) did not change to peripartum heparin, choosing to discontinue VKAs 24 h prior to a planned caesarean section. Among these studies, there were no maternal deaths and comparable foetal outcomes, but lower TECs were reported than with studies that changed to peripartum heparin [1.6% (1.2–4.3%) vs. 2.7 (1.4–4.0%)]. Ten studies (312 pregnancies) reported foetal outcomes when the daily warfarin dose was ≤ 5 mg, and five studies (121 pregnancies) reported foetal outcomes when the daily warfarin dose was >5 mg (Table 3). The use of ≤5 mg/day warfarin in the first trimester was associated with higher livebirth rates and lower foetal adverse event rates.
Table 2.
Primary outcomes – subgroup and sensitivity analyses
| Anticoagulation regimen | Maternal mortality |
Thromboembolism |
Livebirth rate |
Foetal adverse events |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | Events | Estimate (%) | I2 (%) | Studies | Events | Estimate (%) | I2 (%) | Studies | Events | Estimate (%) | I2 (%) | Studies | Events | Estimate (%) | I2 (%) | |
| VKA (INR target 2.5–3.5) | 11 | 7/581 | 0.9 (0.1, 1.6) | 0 | 11 | 22/581 | 2.7 (1.4, 4.0) | 0 | 10 | 369/531 | 64.5 (48.8, 80.2) | 95 | 11 | 14/410 | 2.6 (0.5, 4.6) | 36 |
| VKA (INR target 1.5–2.5) | 9 | 3/391 | 0.8 (0, 1.7) | 0 | 9 | 13/391 | 2.4 (0.9, 4.0) | 0 | 7 | 242/309 | 84.0 (73.1, 94.8) | 89 | 9 | 19/321 | 4.2 (1.7, 6.6)† | 18 |
| VKA (Stratified INR target) | 6 | 3/298 | 1.2 (0, 2.4) | 0 | 6 | 5/298 | 1.6 (1.2, 4.3) | 0 | 5 | 208/288 | 72.6 (59.3, 85.9) | 87 | 6 | 13/223 | 4.2 (0.5, 7.8) | 47 |
| VKA regardless of INR target | 30 | 14/1373 | 0.9 (0.4, 1.4) | 0 | 30 | 40/1373 | 2.1 (1.3, 2.8) | 0 | 26 | 879/1224 | 71.5 (64.2, 78.8) | 92 | 30 | 49/1014 | 3.5 (2.1, 4.9) | 38 |
| VKA until delivery | 6 | 0/240 | 1.0 (0, 2.3) | 0 | 6 | 3/240 | 1.6 (1.2, 4.3) | 0 | 5 | 141/200 | 70.8 (50.0, 91.5) | 96 | 6 | 9/180 | 4.3 (1.4, 7.3) | 0 |
| Studies reporting newer valves only | 6 | 5/261 | 2.3 (0.5, 4.1) | 0 | 6 | 8/261 | 2.5 (0.6, 4.4) | 0 | 6 | 156/261 | 65.8 (43.2, 88.4) | 96 | 6 | 8/156 | 3.4 (0.4, 6.5) | 13 |
| Excluding studies with high risk of bias | 9 | 1/350 | 1.1 (0.1, 2.2) | 0 | 9 | 1/350 | 1.2 (0.1, 2.3) | 0 | 9 | 242/333 | 72.9 (59.5, 86.2) | 94 | 9 | 11/249 | 3.3 (1.1, 5.5) | 0 |
| High-income countries | 10 | 0/228 | 2.7 (0.2, 5.2) | 0 | 10 | 3/228 | 1.3 (0, 2.3) | 0 | 9 | 130/217 | 60.6 (41.4, 79.8) | 92 | 10 | 9/142 | 3.9 (0.9, 7.0) | 0 |
| Middle-income countries | 20 | 14/1145 | 0.9 (0.3, 1.4) | 0 | 20 | 37/1145 | 2.4 (1.5, 3.2) | 0 | 17 | 749/1007 | 76.1 (68.3, 83.8) | 91 | 20 | 40/872 | 3.3 (1.8, 4.9) | 47 |
| LMWH alone | 10 | 1/132 | 2.9 (0.2, 5.7) | 0 | 9 | 13/127 | 8.7 (3.9, 13.4) | 0 | 7 | 68/74 | 92.0 (86.1, 98.0) | 0 | 8 | 1/103 | 3.6 (0.1, 7.0) | 0 |
| LMWH studies reporting newer valves | 5 | 1/63 | 4.2 (0, 8.9) | 0 | 4 | 5/58 | 6.9 (0.5, 13.3) | 0 | 4 | 43.48 | 91.4 (83.8, 99.0) | 0 | 4 | 0/43 | NA | 0 |
| UFH alone | 4 | 2/64 | 3.4 (0, 7.7) | 0 | 3 | 7/52 | 11.2 (2.8, 19.6) | 0 | 3 | 33/51 | 69.5 (37.8, 100) | 87 | 3 | 2/35 | 4.3 (0, 100) | 6 |
| UFH studies reporting newer valves | 1 | 0/24 | NA | 0 | 1 | 2/24 | 8.3 (0, 19.4) | 0 | 1 | 9/23 | 39.0 (19.0 – 59.0) | 0 | 0 | – | – | – |
| Excluding studies with high risk of bias | 8 | 1/101 | 3.5 (0, 6.9) | 0 | 7 | 7/96 | 7.1 (2.1, 12.1) | 0 | 7 | 66/85 | 84.1 (70.6, 97.6) | 78 | 6 | 0/57 | NA | 0 |
| High-income countries | 10 | 1/151 | 2.7 (0.2, 5.2) | 0 | 8 | 16/139 | 9.0 (0.4, 13.7) | 0 | 7 | 73/95 | 82.4 (68.9, 95.9) | 77 | 8 | 3/110 | 3.6 (0.3, 7.0) | 0 |
| Middle-income countries | 4 | 2/45 | 5.6 (0, 12.0) | 0 | 3 | 4/40 | 10.3 (1.0, 19.5) | 0 | 3 | 28/30 | 5.6 (0, 12.0) | 0 | 3 | 0/28 | NA | 0 |
| Sequential treatment | 20 | 11/530 | 2.0 (0.8, 3.1) | 0 | 20 | 44/530 | 5.8 (3.8, 7.7) | 29 | 18 | 381/475 | 79.9 (74.3, 85.6) | 61 | 19 | 5/431 | 1.4 (0.3, 2.5) | 0 |
| Sequential with LMWH | 4 | 1/33 | 6.8 (1.4, 15.1) | 0 | 4 | 3/33 | 8.3 (0, 17.5) | 0 | 4 | 28/32 | 89.5 (77.7, 100) | 30 | 4 | 0/28 | NA | 0 |
| Sequential with UFH | 15 | 10/454 | 2.0 (0.7, 3.3) | 0 | 15 | 38/454 | 6.1 (3.6, 8.5) | 0 | 13 | 310/402 | 72.4 (63.6, 81.2) | 80 | 14 | 5/363 | 1.4 (0.2, 2.5) | 0 |
| Studies reporting newer valves only | 4 | 2/86 | 3.4 (0, 7.2) | 0 | 4 | 13/86 | 14.4 (7.0, 21.7) | 0 | 4 | 66/85 | 78.4 (68.3, 88.5) | 14 | 4 | 1/69 | 2.7 (0, 6.3) | 0 |
| Excluding studies with high risk of bias | 7 | 4/133 | 2.4 (0, 4.9) | 0 | 7 | 14/133 | 9.7 (4.7, 14.7) | 0 | 6 | 68/103 | 65.4 (47.6, 83.1) | 74 | 6 | 2/88 | 2.2 (0, 5.1) | 0 |
| High-income countries | 8 | 1/124 | 2.8 (0, 5.6) | 0 | 8 | 8/124 | 4.7 (1.1, 8.4) | 0 | 8 | 76/123 | 65.9 (50.2, 81.7) | 77 | 8 | 3/106 | 3.8 (0.3, 72) | 0 |
| Middle-income countries | 12 | 10/406 | 1.8 (0.5, 3.1) | 0 | 12 | 36/406 | 6.9 (4.1, 9.8) | 22 | 10 | 295/352 | 84.0 (78.2, 89.7) | 54 | 11 | 2/325 | 1.2 (0, 2.3) | 0 |
VKA, Vitamin-K antagonists; INR, international normalized ratio; LMWH, low molecular weight heparin; UFH, unfractionated heparin; NA, not applicable.
Table 3.
Comparison of foetal outcomes based on dose of warfarin
| Warfarin ≤ 5 mg |
Warfarin > 5 mg |
|||||||
|---|---|---|---|---|---|---|---|---|
| Studies | Events | Estimate (%) | I2 (%) | Studies | Events | Estimate (%) | I2 (%) | |
| Livebirths | 10 | 264/312 | 83.6 (75.8, 91.4) | 81 | 5 | 54/121 | 43.9 (32.8, 55.0) | 36 |
| Foetal adverse events | 9 | 11/305 | 2.3 (0.7, 4.0) | 0 | 4 | 9/63 | 12.4 (3.3, 21.6) | 16 |
Estimates are presented as proportions per 100 affected pregnancies with 95% confidence intervals.
For studies using sequential treatment, maternal and foetal adverse outcomes were lower with first-trimester UFH (15 studies, 454 pregnancies) than with LMWH (four studies, 33 pregnancies). Although 18 studies reported data on two or more anticoagulation regimens, direct comparisons between VKAs and heparins were not possible due to insufficient numbers. Of the 14 studies reporting VKAs and sequential treatment where direct comparison was possible (see Supplementary material online, Table S4), VKAs were associated with fewer TECs [OR 0.38 (95%CI 0.22, 0.67)] but more miscarriages [OR 2.43 (1.20, 4.93)] and preterm births [OR 1.65 (95%CI 1.01, 2.70)]. There were no differences in other primary or secondary outcomes.
Only 13 studies (470 pregnancies) reported the exclusive use of single-tilting discs and bileaflet valves. Of these six (261 pregnancies) used VKAs throughout pregnancy, four (86 pregnancies) used sequential treatment, five (63 pregnancies) used LMWH and one (24 pregnancies) used UFH throughout pregnancy. Due to the small number of included studies, pooled incidences for most outcomes with sequential treatment, LMWH and UFH were not estimable, but those for maternal mortality, TECs, livebirths and foetal adverse events with VKAs throughout pregnancy were 2.3% (0.5–4.1%), 2.5% (0.6–4.4%), 65.8% (43.2–88.4%) and 3.4% (0.4–6.5%) respectively.
Analysis based on the countries’ economic status showed a higher rate of maternal TECs and livebirths in middle-income countries when compared with high-income countries, regardless of treatment regimen.
Discussion
In this systematic review of cohort studies, mostly including pregnancies with single-tilting discs and bileaflet valves, maternal mortality and TECs were lower with VKAs (with the recommended INR targets of 2.5–3.5 throughout pregnancy) when compared to sequential treatment and LMWH. However, livebirths were also lowest with this regimen. For women taking VKA throughout pregnancy, there is a 2% risk of anticoagulant-related foetal/neonatal adverse events. Although sequential treatment eliminates the risk of anticoagulant-related embryopathy, there remains a risk of anticoagulant-related foetopathy. There are no drug-related foetal adverse events in women taking LMWH, as heparins do not cross the placenta.
VKAs have been recommended for women with MHVs in the second and third trimesters and their use in the first trimester is to be considered if the daily dose required to achieve a therapeutic INR is ≤5mg.2,15 While this approach is reasonable, there are a few issues to consider. First, livebirths and foetal adverse events stratified according to warfarin doses >5 mg/day vs. ≤5 mg/day, must be interpreted cautiously. Although livebirths are twice as high and foetal adverse events five-times lower with warfarin doses ≤5 mg/day, these data are based on very small numbers [10 studies (312 pregnancies) with warfarin ≤5 mg/day, and five studies (121 pregnancies) with warfarin >5 mg/day]. Indeed recent data from the Registry of Pregnancy and Cardiac disease (ROPAC) registry did not demonstrate reduced foetal loss with the use of low first-trimester doses of VKAs [warfarin ≤5 mg/day, acenocoumarol ≤2 mg/day or phenprocoumon ≤3 mg/day)].4 Also, most studies do not make a distinction between anticoagulant-related and -unrelated adverse events, and do not mention foetopathy, thereby underestimating the numbers of anticoagulant-related foetal adverse events. In addition, these studies only describe the first-trimester dose of warfarin, which may or may not reflect the dose used later in pregnancy, when warfarin fetopathy can continue to occur. Finally, women with stable INRs in the three months prior to pregnancy may require higher doses of VKAs during pregnancy.16 Thus, while it appears that foetal adverse events increase when the dose of warfarin is >5 mg/day, foetal loss and adverse events can occur in women with doses of warfarin ≤5 mg/day. The safety of first-trimester VKA doses ≤5 mg/day still needs to be confirmed, and requires discussion with the patient.
VKA use in pregnancy was variable. While most centres used standard (2.5–3.5) INR targets for VKAs, some stratified INR targets based on valve type, position and number, and others used lower INR targets (1.5–2.5) regardless of these parameters. The use of stratified INR targets was associated with lower TECs, unchanged maternal mortality and more foetal adverse events, and the use of lower INR targets was associated with comparable TECs, higher number of livebirths and higher foetal adverse events. The safety of the use of lower or stratified INR targets therefore has not yet been determined. Additionally, few centres reported the use of VKAs until 24 hours prior to a planned caesarean delivery without changing to heparin in the peripartum period. Although these studies did not report any maternal deaths, fewer TECs and comparable foetal outcomes, there are limited data on maternal and foetal bleeding risks to safely support this practice.
Most (15) studies that reported the use of sequential treatment (454 pregnancies) used UFH and four (33 pregnancies) used LMWH. Where the timing of TECs was reported, eight occurred in the first trimester, five in the second trimester around the time of bridging, four in the third trimester while on VKAs and five in the postpartum period (see Supplementary material online, Table S5). Maternal mortality, TECs and livebirths were higher with sequential treatment than with VKAs and lower than with LMWH. Sequential treatment is also associated with lowest numbers of small-for-gestational age babies, miscarriages and preterm births (see Supplementary material online, Tables S3 and S4). It is therefore an attractive option, especially in middle-income countries where the use of UFH and LMWH may be cost-prohibitive and continued anticoagulant monitoring may be difficult due to geographical, educational and financial constraints. However, these data are self-reported and it is unclear whether birth weight was adjusted to gestational age, or whether the preterm birth was spontaneous or iatrogenic. Also, although sequential treatment eliminates the risk of anticoagulant-related embryopathy, the risk of anticoagulant-related foetopathy from second- and third-trimester exposure is 1.4%.
The introduction of LMWH with its ease of administration and lower bleeding risks compared to UFH has successfully replaced UFH for treatment of thromboembolism in many centres. The data in women with MHVs is promising with one reported maternal death, no anticoagulant-related foetal/neonatal anomalies and 92% live-born babies. Except for the one fatal antepartum case where peak anti-Xa level was 1U/ml (authors’ reference range 1-1.2U/ml),17 and one postpartum case where anti-Xa levels were not reported,18 all other cases of TECs were associated with non-compliance and/or sub-therapeutic LMWH doses.17–22 These data are limited, however, to 10 studies (132 pregnancies) resulting in wide confidence intervals around the estimates. Moreover, LMWH is more expensive, potentially requires close monitoring and there is a precaution in the label regarding its use in pregnancy for MHVs.
Although very few studies reported the use of UFH throughout pregnancy, its use even with therapeutic doses, is associated with high rates of maternal and foetal complications. In the published literature, four cases of intraventricular haemorrhage in premature infants were reported. Although UFH does not cross the placenta and the foetal bleeding complications were more likely to be secondary to prematurity, the use of UFH throughout pregnancy is not supported by the systematic review.
Although recent guidelines2,15 conducted semi-quantitative reviews of recent studies and contemporaneous reviews on the topic continue to be published, an updated systematic review of all published studies using accepted methods of anticoagulation is needed to inform clinical practice. The main strengths of this review are the comprehensive nature of the literature search from the first published series in 1969, the strict inclusion criteria, the large number of included studies involving women from all continents, the use of the foetuses-at-risk approach for describing pregnancy outcomes, the extensive subgroup- and sensitivity analyses and the lack of significant publication bias among included studies. Given the large number of maternal and foetal outcomes and the competing interests of mother and foetus, we chose to focus on outcomes vital to women for making informed decisions – a healthy mother without TECs and a live baby without embryopathy or foetopathy. We chose ‘livebirths’ because not all studies reported the gestational age at which foetal loss occurred and when reported, miscarriage and stillbirths were inconsistently defined, with gestational age criteria between the two varying from 20 weeks in the US, Australia, Canada and New Zealand, to 24 weeks in the United Kingdom and the Middle East and 28 weeks in India, Pakistan and North Africa.
While this study provides important up-to-date risk estimates for this complex high-risk pregnancy population, it has a number of limitations, the most important being the moderate-to-high risk of bias of included studies. As RCTs were not identified, and cohort studies carry an inherent risk of bias, it was not surprising that all included studies fell either into the ‘moderate’ or ‘high’ risk-of-bias categories. Reassuringly, sensitivity analysis showed that results were not altered significantly by excluding studies at high risk of bias. Another limitation is that almost 30% of the MHVs in the included studies were older style and highly thrombogenic ball-and-cage MHVs. Although these MHVs have largely been replaced by single-tilting discs and bileaflet MHVs, many centres including those in high-income countries continue to report significant proportions (13–98%) of older style MHVs. Our attempt to stratify outcomes by valve type and position, important determinants of the risk of valve thrombosis, was unsuccessful. Only 13 of the 46 included studies reported the exclusive use of newer MHVs and outcomes were not reported by valve type and position. Yet, almost 70% of the MHVs included in this review were of single-tilting disc and bileaflet variety, which seems representative of contemporary practice. Another limitation was the small number of studies involving the use of LMWH and UFH during pregnancy. This led to higher pooled incidences of maternal mortality and TECs, several non-estimable results and wide confidence intervals, so that definitive conclusions for the use UFH and LMWH could not be drawn. Further, we used a strategy that analysed pregnancies based on the initial anticoagulation regimen, akin to an intent-to-treat analysis used in RCTs. Although this strategy has the drawback of failing to identify the true regimen responsible for the adverse event, we were able to identify only two cases where treatments were changed (see Supplementary material online, Table S5) and do not believe that this would alter the results. Recently published data from the ROPAC Registry4 was not included in the analysis because we could not ensure that these data were not duplicated in studies published by other European centres and also because our strict inclusion criteria excluded studies that did not specify the dose of therapeutic anticoagulation. Our review was not able to address the effect of antiplatelet agents. Of the 46 included studies, antiplatelet agents were used in 14, not used in 14 and not reported in 18. Of the 14 studies that used antiplatelet agents, two early publications used higher doses than currently recommended; otherwise, the use of antiplatelet agents ranged widely from 100% in two studies to 1.2% in one. As outcomes were not reported based on the use of antiplatelet agents and sample sizes were too small, a meaningful analysis could not be performed to elucidate the role of antiplatelet agents in this population. Finally, although we restricted our search strategy by language, we believe that the large number of included studies representing all continents adequately compensate for this, and that the discrepant results of a higher maternal TECs and livebirths in middle-income countries compared with high-income countries are likely due to the variability in reporting these outcomes.
In summary, VKAs are associated with fewest maternal complications but also with fewest livebirths, sequential treatment is associated with higher maternal complications when compared with VKAs and does not eliminate anticoagulant-related foetopathy and LMWH is associated with the most livebirths but data are limited to draw definitive conclusions. While the findings of this systematic review might seem to support current guidelines, it must be mentioned that the safety of UFH throughout pregnancy and first-trimester warfarin ≤5 mg/day remains unproven. There are insufficient data to support the use of lower or stratified INR targets and VKAs until planned caesarean delivery. This review, in addition to providing up-to-date data for counselling women with MHVs, highlights the fact that when compared with the earlier systematic review,5 the increased use of MHVs with lower thrombogenic potential has not necessarily resulted in a lower risk of adverse outcomes, and that the optimal method of anticoagulation in pregnant women with MHVs remains undetermined. As absolute equipoise of maternal vs. foetal well-being is unlikely, patient-preferences for maternal and foetal health states resulting from the use anticoagulation in pregnancy should be considered when determining the optimal method of anticoagulation in these women.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors acknowledge Daphne Horn for her assistance with development and execution of the search strategy and Josie Chundamala for her editorial assistance.
Funding
NS is a recipient of a Canadian Institute of Health Research/Canadian Blood Services New Investigator Award.
Conflict of interest: NS has received educational funding from Sanofi-Aventis (Canada), honoraria for educational sessions and support from a Canadian Institute of Health Research (CIHR)/Canadian Blood Services New Investigator Award. PS is supported by an Applied Research Chair in Reproductive and Child Health Services and Policy Research from CIHR. Other authors report no conflicts of interest.
References
- 1. Pibarot P, Dumesnil JG.. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation 2009;119:1034–1048. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD, ACC/AHA Task Force Members 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American Heart Association task force on practice guidelines. Circulation 2014;129:e521–643. [DOI] [PubMed] [Google Scholar]
- 3. Lawley CM, Lain SJ, Algert CS, Ford JB, Figtree GA, Roberts CL.. Prosthetic heart valves in pregnancy, outcomes for women and their babies: a systematic review and meta-analysis. BJOG 2015;122:1446–1455. [DOI] [PubMed] [Google Scholar]
- 4. van Hagen IM, Roos-Hesselink JW, Ruys TP, et al. Pregnancy in women with a mechanical heart valve: data of the European society of cardiology registry of pregnancy and cardiac disease (ROPAC). Circulation 2015;132:132–142. [DOI] [PubMed] [Google Scholar]
- 5. Chan WS, Anand S, Ginsberg JS.. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Int Med 2000;160:191–196. [DOI] [PubMed] [Google Scholar]
- 6. PROSPERO. Contemporary Methods of Anticoagulation in Pregnant Women with Mechanical Heart Valves. York, UK: University of York: Centre for Reviews and Dissemination; 2015. [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stroup DF, Berlin JA, Morton SC, Olkin I,Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 9. McLintock C. Thromboembolism in pregnancy: challenges and controversies in the prevention of pregnancy-associated venous thromboembolism and management of anticoagulation in women with mechanical prosthetic heart valves. Best Practice Res Clinl Obst Gynaecol 2014;28:519–536. [DOI] [PubMed] [Google Scholar]
- 10. Joseph KS. The fetuses-at-risk approach: clarification of semantic and conceptual misapprehension. BMC Pregnancy Childbirth 2008;8:11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newcastle-Ottawa Quality Assessment Scale for Cohort Studies. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (12 July 2016).
- 12. Wallace B, Dahabreh I, Trikalinos T, Lau J, Trow P, Schmid C.. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 2012; 49, https://www.jstatsoft.org/article/view/v049i05 (14 February 2017). [Google Scholar]
- 13. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Bank Data: Income-level. 2015. http://data.worldbank.org/income-level/MIC (25 April 2015).
- 15. Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L.. ESC Guidelines on the management of cardiovascular diseases during pregnancy. European Heart J 2011;32:3147–3197. [DOI] [PubMed] [Google Scholar]
- 16. Hassouna A, Ammar A, Elnahas Y, Toema A, Allam H.. Limited dose warfarin throughout pregnancy in high-risk patients with mechanical valves: a randomized clinical trial. Egyptian Heart J 2015;67:115–122. [Google Scholar]
- 17. Yinon Y, Siu SC, Warshafsky C, Maxwell C, McLeod A, Colman JM, Sermer M, Silversides CK.. Use of low molecular weight heparin in pregnant women with mechanical heart valves. Am J Cardiol 2009;104:1259–1263. [DOI] [PubMed] [Google Scholar]
- 18. McLintock C, McCowan LM, North RA.. Maternal complications and pregnancy outcome in women with mechanical prosthetic heart valves treated with enoxaparin. BJOG 2009;116:1585–1592. [DOI] [PubMed] [Google Scholar]
- 19. Saeed CR, Frank JB, Pravin M, Aziz RH, Serasheini M, Dominique TG.. A prospective trial showing the safety of adjusted-dose enoxaparin for thromboprophylaxis of pregnant women with mechanical prosthetic heart valves. Clin Appl Thromb/Hemostasis 2011;17:313–319. [DOI] [PubMed] [Google Scholar]
- 20. Quinn J, Von Klemperer K, Brooks R, Peebles D, Walker F, Cohen H.. Use of high intensity adjusted dose low molecular weight heparin in women with mechanical heart valves during pregnancy: a single-center experience. Haematologica 2009;94:1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abildgaard U, Sandset PM, Hammerstrom J, Gjestvang FT, Tveit A.. Management of pregnant women with mechanical heart valve prosthesis: thromboprophylaxis with low molecular weight heparin. Thromb Res 2009;124:262–267. [DOI] [PubMed] [Google Scholar]
- 22. Nelson-Piercy C, Greer IA.. Anticoagulation with Tinzaparin for women with mechanical valves in pregnancy: a retrospective case series. Thromb Res 2013;131:185–186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.