Abstract
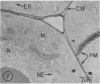
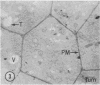
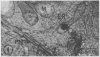
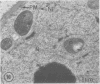
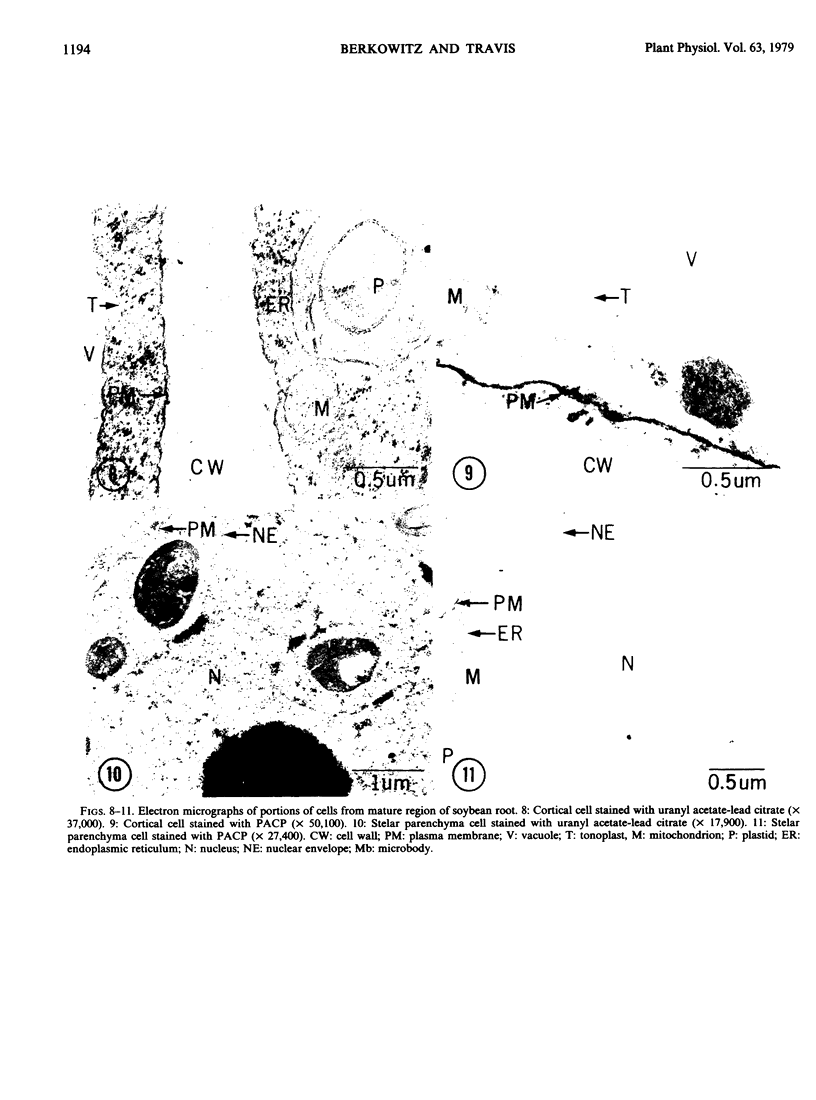
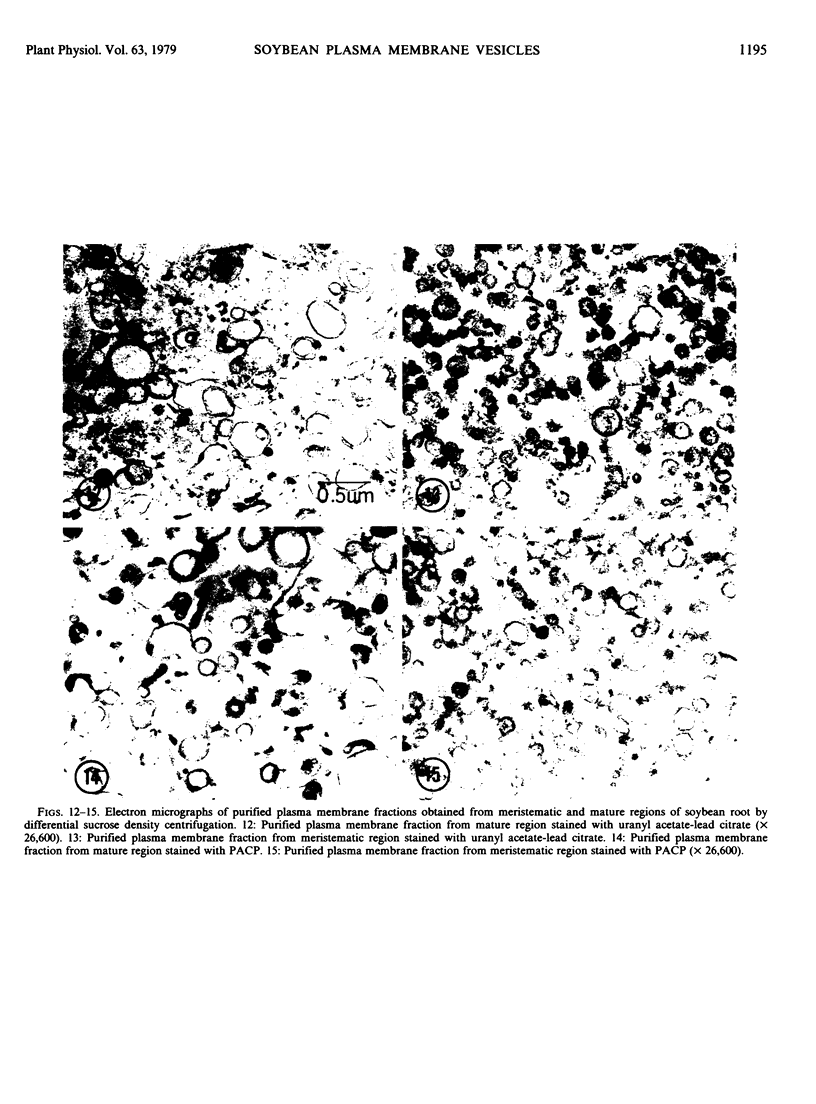
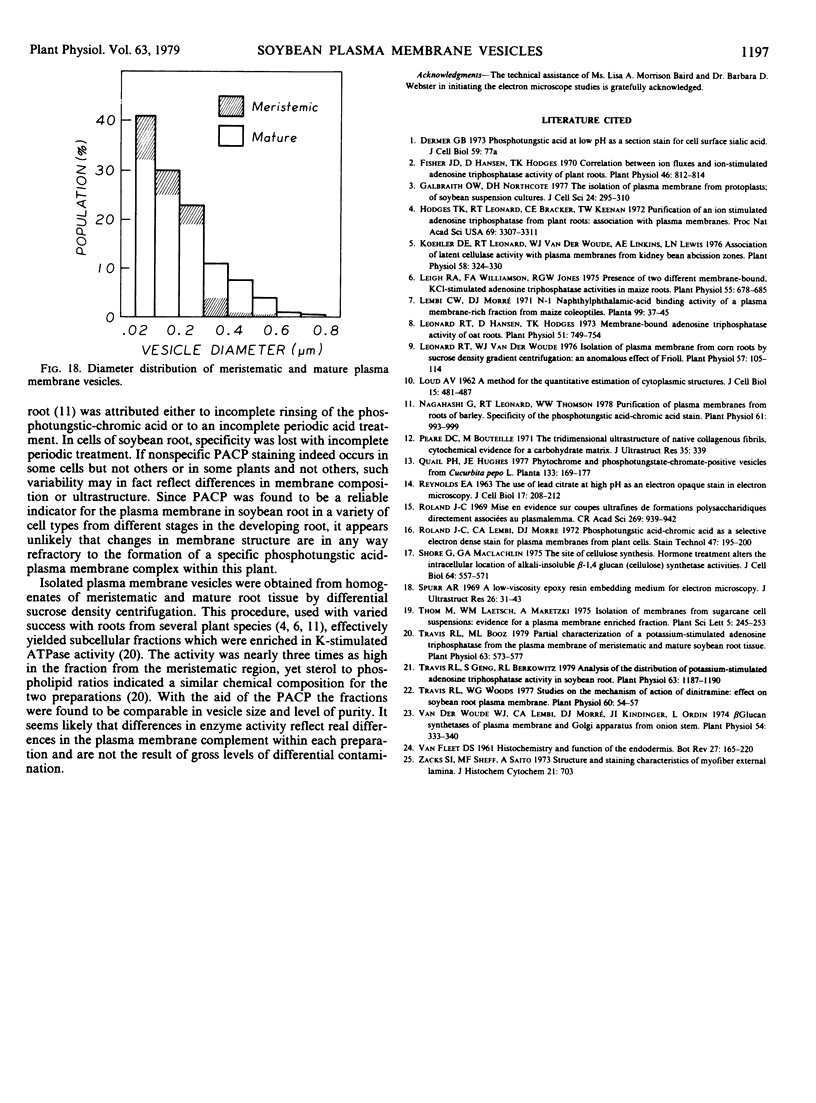
Plasma membrane vesicles were isolated from homogenates of meristematic and mature soybean root tissue by differential sucrose gradient centrifugation. Vesicles were positively identified by the phosphotungstic acid-chromic acid procedure (PACP). The two preparations were comparable in size class distribution, mitochondrial contamination, and per cent plasma membrane vesicles present. Purity levels were estimated to be greater than 75%. The specificity of PACP was observed for a variety of cell types from both regions. Some variability in PACP staining was offset by careful modulation of the stain protocol and was found to be independent of developmental stage in subcellular fractions. Patchy or discontinuous staining, observed in both intact tissue and in subcellular fractions from both regions, was found to be a function of stain time.
Full text
PDF

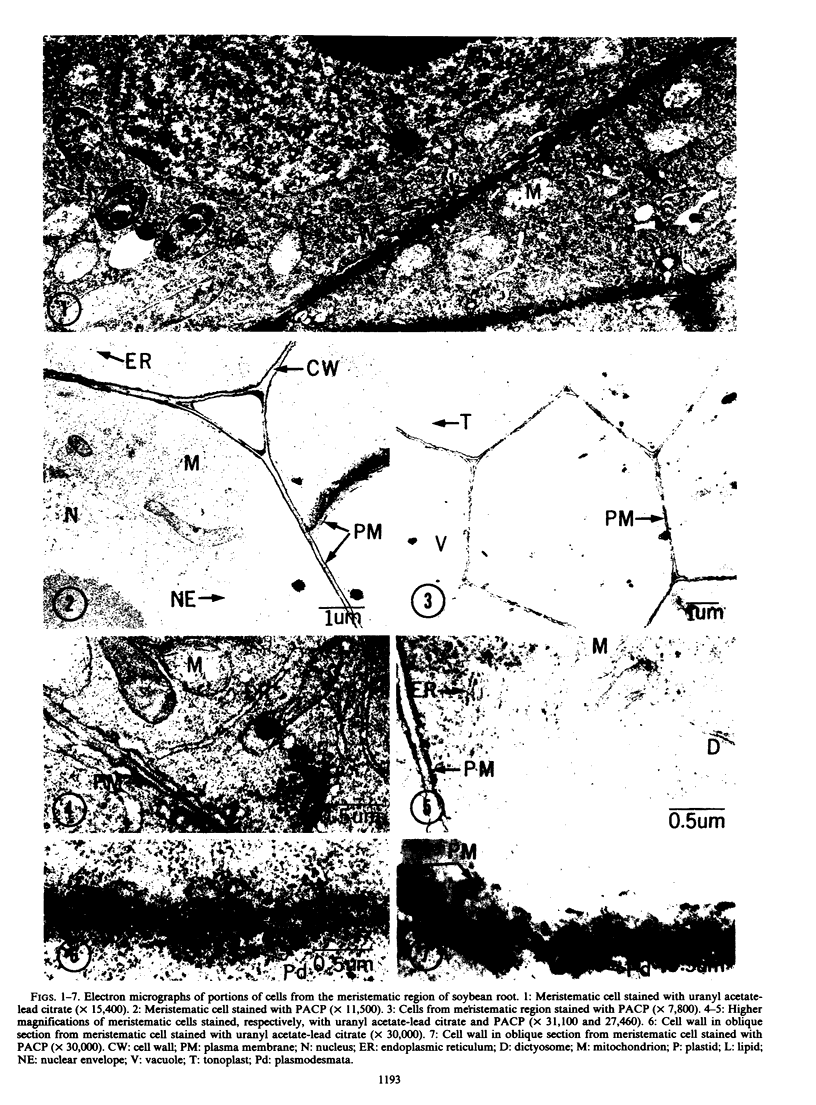

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith D. W., Northcote D. H. The isolation of plasma membrane from protoplasts of soybean suspension cultures. J Cell Sci. 1977 Apr;24:295–310. doi: 10.1242/jcs.24.1.295. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler D. E., Leonard R. T., Vanderwoude W. J., Linkins A. E., Lewis L. N. Association of latent cellulase activity with plasma membranes from kidney bean abscission zones. Plant Physiol. 1976 Sep;58(3):324–330. doi: 10.1104/pp.58.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. A., Williamson F. A., Jones R. G. Presence of Two Different Membrane-bound, KCl-stimulated Adenosine Triphosphatase Activities in Maize Roots. Plant Physiol. 1975 Apr;55(4):678–685. doi: 10.1104/pp.55.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi G., Leonard R. T., Thomson W. W. Purification of plasma membranes from roots of barley: specificity of the phosphotungstic Acid-chromic Acid stain. Plant Physiol. 1978 Jun;61(6):993–999. doi: 10.1104/pp.61.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease D. C., Bouteille M. The tridimensional ultrastructure of native collagenous fibrils, cytochemical evidence for a carbohydrate matrix. J Ultrastruct Res. 1971 May;35(3):339–358. doi: 10.1016/s0022-5320(71)80162-4. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble beta-1,4-glucan (cellulose) synthetase activities. J Cell Biol. 1975 Mar;64(3):557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Booz M. L. Partial Characterization of a Potassium-stimulated Adenosine Triphosphatase from the Plasma Membrane of Meristematic and Mature Soybean Root Tissue. Plant Physiol. 1979 Mar;63(3):573–577. doi: 10.1104/pp.63.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Geng S., Berkowitz R. L. Analysis of the Distribution of Potassium-stimulated Adenosine Triphosphatase Activity in Soybean Root. Plant Physiol. 1979 Jun;63(6):1187–1190. doi: 10.1104/pp.63.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Woods W. G. Studies on the mechanism of action of dinitramine: effect on soybean root plasma membrane. Plant Physiol. 1977 Jul;60(1):54–57. doi: 10.1104/pp.60.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks S. I., Sheff M. F., Saito A. Structure and staining characteristics of myofiber external lamina. J Histochem Cytochem. 1973 Aug;21(8):703–714. doi: 10.1177/21.8.703. [DOI] [PubMed] [Google Scholar]