Abstract
Obstructive sleep apnea (OSA) is a prevalent sleep-disordered breathing with potential long-term major neurocognitive and cardiovascular sequelae. The pathophysiology of OSA varies between individuals and is composed of different underlying mechanisms. Several components including the upper airway anatomy, effectiveness of the upper airway dilator muscles such as the genioglossus, arousal threshold of the individual, and inherent stability of the respiratory control system determine the pathogenesis of OSA. Their recognition may have implications for the perioperative health care team. For example, OSA patients with a high arousal threshold are likely to be sensitive to sedatives and narcotics with a higher risk of respiratory arrest in the perioperative period. Supplemental oxygen therapy can help to stabilize breathing in OSA patients with inherent respiratory instability. Avoidance of supine position can minimize airway obstruction in patients with a predisposition to upper airway collapse in this posture. In this review, the clinically relevant endotypes and phenotypes of OSA are described. Continuous positive airway pressure (CPAP) therapy is the treatment of choice for most patients with OSA but tolerance and adherence can be a problem. Patient-centered individualized approaches to OSA management will be the focus of future research into developing potential treatment options that will help decrease the disease burden and improve treatment effectiveness.
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder affecting up to 27% of women and 43% of men aged 50 to 70 years and 9% of women and 26% of men in the 30- to 49-years-old category.1,2 OSA is associated with cardiovascular and cerebrovascular diseases, metabolic disorders, and impaired neurocognitive function.3,4
Although surgical patients with OSA have a 2- to 3-fold increased risk of cardiopulmonary adverse events, a majority of patients with OSA are not diagnosed when they present for surgery.5,6 OSA is recognized to be a heterogeneous disorder with both anatomical (upper airway) and nonanatomical traits.7 Several components including the upper airway anatomy, effectiveness of the upper airway dilator muscles like the genioglossus, arousal threshold of the individual, and inherent stability of the respiratory control system determine the pathogenesis of OSA. The heterogeneity in pathophysiology is more evident in patients with mild-to-moderate OSA than in those with severe disease.8 A recent study found that 69% of patients with OSA have one or more predisposing physiological traits.9 Although the apnea hypopnea index (AHI) is the most commonly used metric of OSA severity, it may not be the metric that best correlates with postoperative outcomes.
Although continuous positive airway pressure (CPAP) is the gold standard treatment for symptomatic moderate-to-severe OSA, the acceptance rate is low, approximately 50%.10 The outcomes of CPAP treatment may vary depending on the clinical phenotypes of OSA.11 It may be useful to understand the clinically important endotypes and phenotypes of OSA to target treatment based on the mechanism. This knowledge can also guide the perioperative health care team in the optimal management of surgical patients with OSA.
Pathophysiology of OSA
The important 4 components that determine OSA pathogenesis include the following: (1) upper airway anatomy, (2) the ability of upper airway dilator muscles to respond to pharyngeal collapse during sleep, (3) the propensity to wake up from respiratory stimulus during sleep, and (4) the inherent stability of the respiratory control system. The risk factors and specific pathophysiologic mechanisms predisposing to OSA along with their treatment are described in Figure 1.
Figure 1.
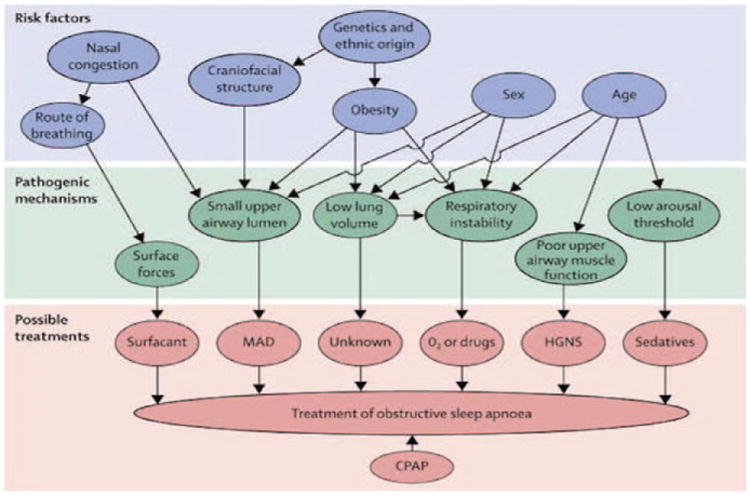
Risk factors, pathogenic mechanisms, and treatments for obstructive sleep apnoea. Specific pathogenetic mechanisms of the various risk factors of OSA have recently been recognized. This paves the way for novel therapeutic approaches targeting individual pathogenic mechanisms, as possible successful alternatives to CPAP, which is the current universal treatment of choice. Reprinted with permission from Jordan et al. Adult obstructive sleep apnoea. Lancet. 2014;383:736–747. CPAP, indicates continuous positive airway pressure; HGNS, hypoglossal nerve stimulation; MAD, mandibular advancement device; O2, oxygen; UPPP, uvulopalatopharyngoplasty.
A narrow upper airway is prone to collapse, but is prevented by a reflex-mediated increase in upper airway dilation when awake. Physiologic studies in rats have shown that sleep reduces serotonergic neural input to motor neurons of upper airway dilator muscles, allowing collapse of the upper airway and contributing to airway obstruction.12 In addition, pharyngeal patency is also dependent on lung volume, which exerts a mechanical traction on the upper airway. Sleep-induced decrements in lung volume lead to reduction in this longitudinal traction yielding a collapsible pharynx.13 The hypercapnic respiratory drive and diaphragmatically generated negative intrapharyngeal pressure during an episode of airway obstruction predisposes the OSA patient to arouse repeatedly from sleep. Each arousal is accompanied by a robust ventilatory response that decreases the carbon dioxide level perpetuating central apnea (cessation of airflow for at least 10 seconds with no respiratory effort), which further destabilizes breathing. Also, ventilatory control is inherently less stable in OSA patients. Additionally, overnight rostral fluid shift from the legs to the neck may narrow the pharyngeal lumen in some patients with edema because of excess extracellular fluid volume.14
Definition of Endotypes and Phenotypes of OSA
Phenotypes have been developed to address the complexities of a disease. A “phenotype” is defined as an observable expression of an individual's characteristics that result from the interaction between the individual's genes (genotype) and the environment, without any implication of a mechanism.15
Phenotype is distinct from an “endotype,” which is the subtype of a disease defined by a unique or distinctive functional or pathophysiologic mechanism. Pathogenic mechanisms of OSA based on craniofacial morphology, obesity, arousal functions, upper airway muscle activity, ventilatory control stability, and nocturnal rostral fluid shift constitute potential endotypes of OSA. A specific phenotype may encompass several endotypes.16
Characterizing the heterogeneity of OSA into various well-defined phenotypes is challenging because there are sparse prospective data and long-term validation. Clinical examination and sleep studies can identify some endotypes; however, recognition of the others is in the experimental stage. High-quality research is underway to help develop clinical tools to identify and target interventions.
Presently, there is no consensus for classifying OSA into various phenotypes. We have suggested a classification of OSA phenotypes with a link to their corresponding predominant endotypes, based on the underlying mechanism (Table 1). The pathophysiologic endotypes are discussed below followed by a description of the various frequently encountered clinical OSA phenotypes. The characteristics of sleep-related breathing disorders similar to OSA and the perioperative management of OSA are also described in this review.
Table 1. OSA Phenotypes and Corresponding Endotypesa.
| OSA Phenotypes | Predominant Endotypes | ||
|---|---|---|---|
| OSA in elderly patients | Low arousal threshold Rostral fluid shift | Hyporesponsive genioglossus | Anatomical (abnormal fat distribution) |
| OSA in males | Anatomical (android obesity) | Rostral fluid shift (age >40 y) | |
| OSA in fluid overloaded states | Rostral fluid shift | ||
| OSA in menopausal women | Anatomical (abnormal fat distribution) | ||
| Ethnic OSA phenotypes | |||
| African Americans | Anatomical (obesity) | ||
| Asians | Anatomical (abnormal craniofacial morphology) | ||
| Caucasians | Anatomical (both obesity and abnormal craniofacial morphology) | ||
| REM-related OSA phenotype | Hyporesponsive | ||
| Genioglossus | |||
| Supine-related OSA phenotype | Anatomical | Hyporesponsive genioglossus | |
Linking underlying pathologic mechanisms with phenotypes of OSA.
Abbreviations: OSA, obstructive sleep apnea; REM, rapid eye movement.
Endotypes and Phenotypes of OSA
Pathophysiologic Endotypes of OSA
These subsets can be classified as anatomical and nonanatomical (physiologic) endotypes.
Based on Anatomy
Obesity and Craniofacial Morphology
Anatomy and upper airway collapsibility are important determinants of the presence or absence of OSA and its severity. Obesity and craniofacial abnormalities account for two-thirds of the variation in OSA severity measured by AHI.17 The relationship between the soft tissues and bony enclosure of the upper airway is depicted in Figure 2.
Figure 2.
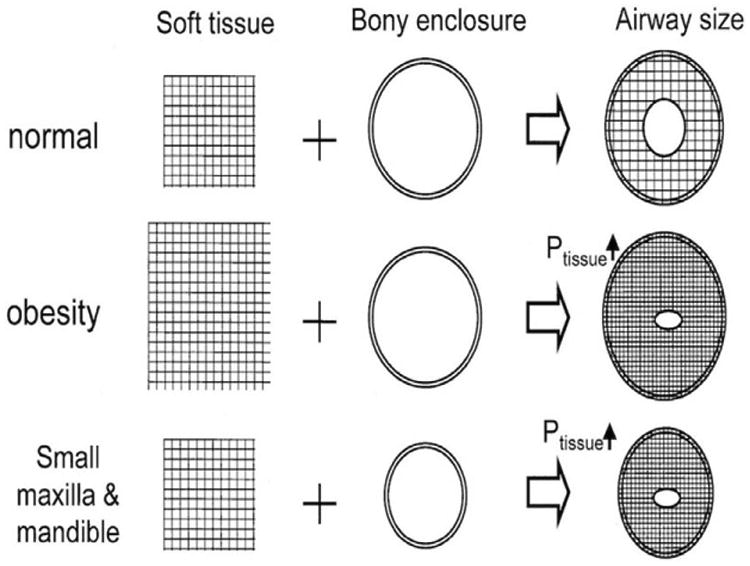
A schematic representation of the interaction between soft tissue and the upper airway bony enclosure and their combined effect on airway size. Reprinted with permission from Watanabe et al; Am J Respir Crit Care Med. 2002; 165:260–265. Ptissue – pressure exerted by soft tissue on upper airway. Ptissue is determined by the balance between the amount of soft material inside the enclosure and the size of the surrounding rigid box. Obesity leads to an excess of soft material inside the rigid box. In contrast, a small bony enclosure reduces the size of the rigid box. Accordingly, an imbalance between body habitus and craniofacial abnormalities may result in increased tissue pressure surrounding the pharyngeal airway, leading to closure of this airway.
Patients with a structurally narrower and more collapsible pharyngeal airway usually manifest severe OSA. They have a high critical closing pressure (Pcrit), which is defined as pressure inside the partial airway at which the airway collapses. Obesity is a prominent feature in OSA patients with high closing pressures exclusively at the retropalatal airway, whereas craniofacial abnormalities such as small maxilla and mandible were predominant in OSA patients with high closing pressures at both retropalatal and retroglossal areas.18
Obesity
Obesity is the most common and well-recognized risk factor for OSA. Moderate-to-severe sleep apnea, defined as an AHI of >30 events/h, is present in 65% of males and 23% of females with severe obesity.19 Although the increased overall body weight is clearly linked to OSA, the particular patterns of fat distribution over the neck and waist underlie the pathophysiologic mechanisms. The neck circumference is a strong predictor of sleep-disordered breathing indicating that fat deposition in the parapharyngeal area is important for the development of sleep apnea.20 The android pattern of fat deposition in the abdomen, seen more commonly in men, reduces the lung volume and thereby the caudal traction on the pharynx, increasing pharyngeal collapsibility.21
Obesity, being a modifiable risk factor, is unique among the other risk factors of OSA. The strong relationship between obesity and OSA is strengthened by the evidence that weight loss measures through diet and bariatric surgery are associated with an improvement in the severity of OSA.22
Craniofacial Morphology
Although obesity is considered a major anatomical risk factor for OSA, craniofacial morphology also plays a role in OSA pathogenesis. The predominant craniofacial characteristics associated with OSA include an inferior positioning of the hyoid bone,23 retropositioning of the mandible,24 a smaller cranial base,23 an increase in the craniocervical extension angle23 as well as abnormal upper airway soft tissue morphology. Craniofacial skeletal enclosure and the amount of soft tissue within is termed as “anatomical balance.”21 Genetic and environmental-mediated imbalances between these anatomical factors are important in OSA pathogenesis.
Craniofacial characteristics are also relevant to target OSA treatment by altering upper airway bony and soft tissue anatomy. Oral appliances such as the mandibular advancement or tongue retaining splints hold the lower jaw or tongue, respectively, in a forward position during sleep, increasing the size of the retropalatal airway.21 They can be considered as alternatives to CPAP for some patients. Likewise, craniofacial morphology can be altered by surgical procedures such as maxillary-mandibular advancement and uvulopalatopharyngoplasty.21
Based on Physiology
OSA Endotype With Ineffective Upper Airway Dilator Muscles (eg, Genioglossus)
The decreased tone of upper airway dilator muscles especially the genioglossus is a key contributor to OSA pathogenesis. The genioglossus is one of the largest extrinsic muscles of the tongue25 and is the main upper airway dilator. The contraction of the genioglossus directly dilates the upper airway by pulling the base of the tongue forward.26 It is highly reactive to chemical drive such as hypoxia and hypercapnia27 and to increased negative intrapharyngeal pressure.28 The electromyographic activity of genioglossus is greater in OSA patients than in healthy individuals during wakefulness,29 but it is reduced at sleep onset in both of them.30 This may be due to inadequate increase in neural drive to the upper airway dilator muscles in response to negative pharyngeal collapsing pressure generated during tidal breathing.
The genioglossal muscle activity is demonstrated to be reduced in both phasic (with eye movements) and tonic (without eye movements) rapid eye movement (REM) sleep compared with nonrapid eye movement (NREM) sleep in OSA patients.31 Thus, obstructive events increase in frequency and duration, and are associated with more pronounced hypoxemia during REM vs NREM sleep.32
Although genioglossus activity can be measured by analyzing the electromyogram obtained by using a surface electrode, it is not routine in the clinical evaluation of OSA patients. A segment of polysomnography (PSG) during an obstructive event is shown in Figure 3. It shows the progressive increase in electromyographic activity of genioglossus muscle throughout the obstructive event, although not sufficient to restore airflow. Hence, it is followed by arousal of the patient.
Figure 3.
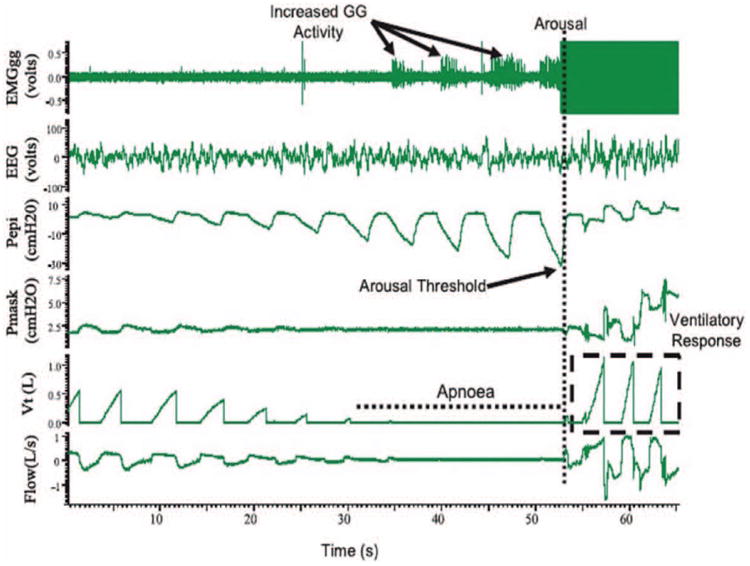
PSG tracings of an obstructive sleep apnea event in a patient with severe OSA. There was increased EMG activity of the genioglossus muscle during the apneic event, although it was not significant enough to restore flow without arousal. The arousal threshold is characterized using Pepi, which is the epiglottic pressure immediately preceding arousal and there is a large ventilatory response following arousal. Reprinted with permission from Campana et al; Indian J Med Res. 2010;131:176–187. EEG indicates electroencephalogram (C3-A2); EMGgg, electromyogram of the genioglossus muscle (intramuscular); Pepi, pressure at the level of the epiglottis; Pmask, pressure measured via nasal mask; Vt, tidal volume.
Hyporesponsiveness of upper airway muscles is amenable to treatment with electrical stimulation of the hypoglossal nerve or the genioglossus muscle directly. This approach has been shown to improve airway patency and reduce the pharyngeal critical closing pressure in several studies.33–35 Serotonergic drugs such as paroxetine and mirtazapine can help in dilating upper airway muscles, but they do not have consistent effects on AHI.36,37
OSA Endotypes of High and Low Respiratory Arousal Threshold
The respiratory arousal threshold is defined as the ease at which a sleeping person can be awakened. Arousals are defined as 3 seconds of high-frequency activity on the electroencephalogram. They are scored in a PSG as respiratory arousal index, defined as the average hourly sleep arousals due to respiratory events. Arousal threshold can be measured using a CPAP drop method via an epiglottic catheter during a PSG.9,38 Figure 3 shows a PSG segment during an event of obstruction. The negative pharyngeal pressure generated during an event of obstruction is seen as negative pressure swings on the epiglottic pressure trace. The nadir epiglottic pressure immediately preceding arousal is quantified as the arousal threshold. To date, these are invasive experimental methods and are not measured during routine PSG.
Some OSA patients are found to have a high threshold for arousal, whereas others with low arousal threshold wake up frequently to minimal stimuli. The notable differences between the 2 categories are described in Table 2.
Table 2. OSA With Low and High Arousal Threshold.
| Low Arousal Threshold | High Arousal Threshold |
|---|---|
| Higher propensity to wake up from sleep | Lower propensity to wake up from sleep |
| More likely to have mild-to-moderate OSA | Predominantly associated with severe OSA |
| Sedatives may be beneficial | Sedatives may evoke a respiratory arrest |
| Associated with less hypoxia due to reduced apnea duration | More prone to hypoxia due to prolonged apneas |
Abbreviation: OSA, obstructive sleep apnea.
OSA and Low Arousal Threshold
Low arousal threshold contributes to OSA, in approximately one-third of patients, by disrupting continuity of sleep and limiting the sufficient accumulation of respiratory stimuli to restore upper airway patency and airflow during sleep.39 The sudden reintroduction of wakefulness that occurs with arousal from sleep may be associated with rapid recruitment of inspiratory upper airway motor neurons, augmenting the pharyngeal dilator muscle activity and reopening the upper airway. The ensuing hyperventilatory response can drive down the carbon dioxide levels below the chemical apnea threshold resulting in a central apnea. This hypocapnia may also reduce the activity of the upper airway dilators by decreasing the neural output to these muscles leading to airway collapse.40 Thus, arousals have the potential to destabilize ventilatory control and perpetuate apnea in these patients.
Patients with a low arousal threshold may be identified from the standard clinically available variables such as AHI, nadir Spo2, and frequency of hypopneas. The criterion for each variable was AHI < 30 events/h, nadir Spo2 > 82.5%, frequency of hypopneas > 58%.41 Individuals with a low arousal threshold would wake up before developing a very low oxygen saturation and are more likely to have a mild-to-moderate OSA rather than a severe OSA. They have an increased frequency of hypopneas rather than apneas due to milder airflow obstruction, possibly because of a favorable anatomy. Although this study describes a noninvasive method to measure the arousal threshold, it requires further validation.
Low respiratory arousal threshold may be a potential therapeutic target. Patients with low respiratory arousal threshold may benefit pharmacologically from certain sedatives to improve sleep quality and reduce OSA severity. Arousal threshold is proven to be increased by 28% and 48%, respectively, with eszopiclone42 and trazodone,43 concomitantly reducing the AHI. Larger clinical trials evaluating long-term clinical outcomes are lacking and need to be done.
OSA and High Arousal Threshold
High arousal threshold means a lower propensity to arouse from sleep. OSA patients may be at a particular risk for developing acquired arousal failure as a function of neural plasticity due to repetitive exposures to brief periods of hypoxemia over many years.44 Respiratory arousal threshold is increased in patients with severe OSA despite regular use of CPAP.9 Although chronic sleep fragmentation (interruption of a sleep stage due to frequent awakenings) and intermittent hypoxia have been suggested as causes for the increased respiratory arousal threshold, the reasons are not clear.45
Patients with a pre-existing high arousal threshold may be at increased risk of adverse respiratory events when opioids are used.46 The opioid-induced reduced respiratory drive to hypoxia and hypercapnia could decrease the neural output to upper airway dilating muscles resulting in upper airway collapsibility. In addition, opioids may impair the arousal mechanisms that occur in response to hypoxia in the perioperative period.47 Thus, sedatives and narcotics can in theory precipitate a respiratory arrest leading to sudden unexpected death in patients with high arousal threshold as they are in a state of “arousal-dependent survival.”
At present, there is no conventional way to identify the patients with low or high arousal threshold preoperatively. Hence, continuous postoperative monitoring has been recommended with high-resolution pulse oximetry to detect early desaturation and initiate treatment.44 Monitoring end-tidal carbon dioxide by using capnography can detect hypoventilation earlier in these patients.48
OSA Endotypes Based on Ventilatory Control Stability
A characteristic feature of OSA patients is the propensity to develop a cyclical breathing pattern whereby the patient oscillates between obstructive breathing events (sleep) and arousal (wakefulness). An increase in ventilatory drive activates the upper airway muscles and promotes patency, whereas a decrease in ventilatory drive relaxes the upper airway muscles and facilitates closure. Thus, instability in ventilatory control is a critical contributor to sleep apnea.
Loop gain is a term used to describe stability or instability in a system controlled by feedback loops that modulate output.49 It is the propensity of the ventilatory control system to develop fluctuations in ventilatory output. It is defined as the ratio of the ventilatory response to a ventilatory disturbance.50 If the magnitude of the response, ie, hyperventilation is greater than or equal to the magnitude of the disturbance, ie, apnea, then the loop gain ratio will be ≥1 and the system is considered to have a high loop gain and is unstable. A system with a loop gain of <1 is stable with little or no fluctuation in breathing.50
High loop gain is characterized by an oversensitive ventilatory control system to hypoxia and hypercapnia. It may have a substantial impact on OSA severity in certain patients, particularly those who do not have a highly collapsible upper airway.51 There are 2 key potential mechanisms that are likely to be important, but neither is definitively proven. First, elevated loop gain would be expected to increase the oscillations from the central ventilatory control in the brainstem, such that the activity of the upper airway dilator muscles which receive the neural output may vary accordingly. Thus, periods of low central respiratory drive may be associated with low upper airway dilator muscle activity, high airway resistance, and increased propensity to airway collapse. Second, elevated loop gain may increase the ventilatory response to arousal. This hyperventilation may culminate in central apnea as a result of hypocapnia and decreased respiratory drive. The central apnea subsequently leads to hypoxia or hypercapnia, perpetuating the cycle of instability leading to periodic breathing. The PSG segment during obstruction in Figure 3 shows ventilatory overshoot following arousal, which may indicate a high loop gain.
Any intervention that effectively reduces loop gain should possibly benefit OSA.50,52 Oxygen is found to be effective in reducing loop gain by stabilizing ventilation through a reduction in peripheral chemoresponsiveness to hypoxia and hypercapnia.50 Likewise, acetazolamide is a carbonic anhydrase inhibitor that produces metabolic acidosis and increases baseline ventilation. It can increase the respiratory drive to airway obstruction such that the patient can reach stable breathing without arousal.52 Hence, there is a potential for considering alternative treatment modalities to treat OSA patients with a high loop gain and noncompliance to CPAP. It is difficult to measure loop gain in clinical practice53 because the methods are experimental and are not a current routine in sleep laboratories.38
OSA Due to Fluid Retention and Overnight Rostral Fluid Shift
The prevalence of sleep apnea is much higher in patients with fluid-retaining states such as congestive heart failure and end-stage renal disease than in the general population.54,55 Fluid accumulates in the intravascular and interstitial spaces of the legs due to gravity during the day, and upon lying down at night redistributes rostrally, owing to gravity. This hypothesis is called “rostral fluid shift.”56 Some of this fluid may accumulate in the neck, theoretically leading to a narrow upper airway and predisposing to OSA. Spontaneous rostral fluid shift was described in healthy nonobese men, where the leg fluid volume decreased spontaneously overnight, with an associated increase in the neck circumference.57 Nocturnal fluid was associated with an increase in the neck circumference and correlated with the severity of OSA in patients with congestive heart failure.54 and endstage renal disease.55 Similarly, the lack of physical activity in sedentary older individuals increases fluid accumulation in the legs during the day and rostral shift during the night.
The rostral fluid shift could be a potential therapeutic target to treat OSA in some patients. Potential interventions include diuretics, sodium restriction, compression stockings, elevating the head of the bed, exercise interventions, and ultrafiltration. Further work is needed to define the rostral fluid shift in the perioperative period.
Clinical Phenotypes of OSA
Based on Sex, Age, and Ethnicity
Sex Differences in OSA
Males are 2 to 3 times more likely to have OSA than females58 with longer periods of apnea and more significant oxygen desaturations, despite a lower body mass index (BMI).59,60 The male predisposition to OSA appears to be anatomically based with increased fat deposition around the pharyngeal airway.61 The length of the vulnerable pharyngeal airway is greater in males compared with females.62 The android pattern of fat deposition around the abdomen contributes to reduced lung volume in males and increases the susceptibility to upper airway collapsibility as a result of loss of longitudinal caudal traction on the trachea.62
OSA and Age
Elderly patients with OSA are a unique group with a distinct phenotype.63 The frequency of OSA increases with aging with a plateau after 65 years. Reduced airway caliber due to preferential deposition of fat around the pharynx makes the aging population anatomically susceptible to OSA.64 Overnight rostral shift of fluids to the neck,57 higher surface tension of the upper airway,65 and decreases in lung volume tethering effect66 also predispose the elderly population to OSA.
The genioglossal responsiveness to negative intrapharyngeal pressure appears to deteriorate with age.64 Older adults apparently have an increased frequency of spontaneous arousals suggestive of a lower arousal threshold.67 However, the aging process desensitizes the ventilatory control system and lowers the loop gain. Hence, airway anatomy/collapsibility plays a greater role in older adults, whereas a sensitive ventilatory control system is a prominent trait in younger adults with OSA.63 Table 3 illustrates the differences in manifestations of OSA between the young and elderly patients. Nasal CPAP has been shown to improve OSA and increase sleep effectiveness in elderly OSA patients.68
Table 3. OSA Endophenotype in Different Age Groups.
| OSA in Young Patients | OSA in Elderly Patients |
|---|---|
| Sensitive ventilatory control system and high loop gain | Low loop gain and more stabilized breathing |
| Excessive daytime sleepiness: prominent symptom | Excessive daytime sleepiness: rarely reported |
| Overnight rostral fluid shift: rare in nonobese | Overnight rostral shift of fluid: more prevalent in males >40 y, with a higher BMI |
| Airway surface tension: decreased | Airway surface tension: increased |
| Tethering effects of the lung on the upper airway is preserved | Decreased lung volume tethering contributes to airway collapsibility |
Abbreviation: BMI, body mass index; OSA, obstructive sleep apnea.
OSA in Menopausal Women
Menopause, pregnancy, and polycystic ovarian syndrome increase the risk for OSA in women. The odds ratio for OSA was 1.1 in perimenopausal and 3.5 in postmenopausal women.69 After menopause, the worsening severity of OSA predominantly occurs during the NREM sleep versus younger women with a predominantly REM-associated OSA.8 The pharyngeal airway is longer in postmenopausal versus premenopausal women.64 Female sex hormones such as estrogen and progesterone have a protective effect on upper airway patency and ventilatory drive.59 The risk of menopausal OSA can be reduced by hormone replacement therapy.70
OSA in Various Ethnic Populations
The relative importance of the anatomical determinants of OSA varies between ethnicities. The Asian OSA populations are found to primarily display features of craniofacial skeletal restriction, the African Americans display more obesity and enlarged upper airway soft tissues, whereas the Caucasians show evidence of both bony and soft tissue abnormalities21 (Table 4). Craniofacial restriction71 and central fat deposition72 favor a greater predisposition to OSA in Asians, despite a lower overall BMI compared with other populations.73,74 Brachycephaly, which is a disproportionately short and broad head, is associated with a higher AHI in Caucasians but not in African Americans.75
Table 4. Characteristics of Ethnic OSA Endophenotypes.
| African Americans | Caucasians | Asians |
|---|---|---|
| More obese | Both bony and soft tissue abnormalities | Craniofacial skeletal abnormalities |
| Enlarged upper airway | Brachycephaly | Smaller maxilla |
| soft tissues | Retropositioned mandible | |
| Prognathism | Midfacial hypoplasia | |
| Macroglossia |
Abbreviation: OSA, obstructive sleep apnea.
The photographic craniofacial phenotyping, a technique where craniofacial measurements are obtained from computerized photographic analysis, is useful in identifying OSA in various ethnic populations.76 Surgical treatment to alter the craniofacial anatomy carries a higher success rate in treating OSA in certain patients who refuse CPAP.77
OSA in REM Sleep
Hypopneas and apneas are known to be longer in duration and cause an increase in the severity of hypoxemia during REM compared with non-REM sleep in patients with OSA.78 REM-related OSA can be categorized as REM-predominant and REM-isolated OSA. REM-predominant OSA is defined as a doubling of AHI in REM sleep versus the NREM sleep (AHIREM:AHINREM > 2 events/h).8 REM-isolated OSA is characterized by a doubling of AHI in REM sleep in addition to an AHI of less than 5/h in NREM sleep (AHIREM:AHINREM > 2 events/h and AHINREM < 5 events/h).8 The prevalence of REM-related OSA ranges from 10% to 36% of the patient population with OSA undergoing PSG.79 The female preponderance of patients experiencing REM sleep-specific obstruction is well established.59,80 The REM-predominant OSA phenotype comprises older females with more severe OSA versus REM isolated OSA in young females with fewer events of apnea.8
The available data in the literature to identify the reason for worsening of apnea during REM sleep are limited. REM sleep is known to be associated with hypotonia81 and reduced responsiveness of the genioglossus muscle to negative intrapharyngeal pressure.82,83 This is presumably due to withdrawal of excitatory neurochemical inputs to pharyngeal motor neurons, predisposing to upper airway collapse. The critical closing pressure of the pharynx is similar during both REM and NREM sleep, implying that anatomy is not further impaired in REM sleep.
REM sleep is associated with greater sympathetic activity and cardiovascular instability in healthy individuals and OSA patients versus NREM sleep.84,85 REM-related OSA has been found to be associated with a risk of hypertension.86 Treatment measures targeted to improve the genioglossus muscle tone may reduce obstructive events occurring in REM sleep. Transnasal insufflation could also help REM-related OSA as it possibly stabilizes the hypotonic upper airway musculature by increasing the end-expiratory intrapharyngeal pressure.87
Supine Position-Related OSA
Supine position-related OSA is a dominant phenotype of OSA with a prevalence of 20% to 60% in the general population.88 It may be attributable to unfavorable upper airway anatomy, reduced lung volume, and inability of airway dilator muscles to compensate for the airway collapse in the supine position.
Supine position-related OSA can be categorized as supine-predominant and supine-isolated OSA.8 On the one hand, supine-isolated OSA is characterized by a doubling of AHI in a supine position in addition to an AHI of <5 events/h in a nonsupine position (AHISupine:AHINSupine > 2 events/h and AHINSupine < 5 events/h). On the other hand, supine-predominant OSA presents as a doubling of AHI in the supine position versus the nonsupine position, where the nonsupine AHI may remain higher than 5 events/h (AHISupine:AHINSupine > 2 events/h and AHINSupine ≥ 5 events/h).8 The comparison between supine-related OSA and REM-related OSA is shown in Table 5. In the supine-isolated OSA, the patients tend to be younger males (48 vs 51 years, P < .05) with a lower BMI (28.6 vs 30 kg/m2, P < .05) versus supine predominant OSA.8 Patients with the supine position-related OSA were subjectively more sleepy versus other patients with OSA indicating that respiratory events occurring in the supine position may increase subjective sleepiness.8
Table 5. Differences Between REM-Related and Supine-Related OSA.
| REM-Related OSA | Supine-Related OSA |
|---|---|
| Females | Males |
| Older in age | Younger in age |
| Higher mean BMI | Lower mean BMI |
| Genioglossal hyporesponsiveness | Unfavorable airway geometry due to smaller craniofacial volume (midline obstruction such as thyroid, retroglossal thyroid, or tonsillar enlargement needs to be excluded) |
| Reduced lung volume |
Abbreviations: BMI, body mass index; OSA, obstructive sleep apnea; REM, rapid eye movement.
Recognition of the supine position-related OSA may be therapeutically useful because these patients respond to oral appliances better than other types of nonpostural OSA.89 The avoidance of supine sleep with a positional device should improve AHI in these patients.90 Upper body elevation to 30°, and to a lesser extent lateral positioning, significantly improved upper airway stability during sleep.91 Hence, oral appliances and positional devices can be considered as alternate treatment modalities in these patients if they are noncompliant to CPAP.
Other Sleep-Disordered Breathing Similar to OSA
Upper Airway Resistance Syndrome
The upper airway resistance syndrome is a recently described form of sleep-disordered breathing in which repetitive increases in resistance to airflow within the upper airway lead to brief arousals from 2 to 14 seconds termed as respiratory effort-related arousal (RERA) and daytime somnolence, followed immediately by decreased airway resistance.92 These events are brief and typically last for 1 to 3 breaths, without meeting the criteria for hypopnea, in the absence of frank apnea or oxygen desaturation unlike OSA.
Contrary to OSA patients, patients with upper airway resistance syndrome are typically nonobese and younger, with a mean BMI of ≤25 kg/m2.93 Craniofacial abnormalities include low soft palate, long uvula, increased overbites, and a high and narrow hard palate. Although there is controversy whether upper airway resistance syndrome constitutes a distinct phenotype versus a condition on the spectrum of sleep-disordered breathing, upper airway resistance syndrome is underrecognized in sleep centers and many patients remained untreated. Twenty-six percent of patients without preoperative sleep apnea develop postoperative sleep apnea.94 This may be attributable to upper airway resistance syndrome. RERAs may have been converted to apneas and hypopneas postoperatively because of increased upper airway collapsibility.94
Obesity Hypoventilation Syndrome
Obesity hypoventilation syndrome (OHS) patients manifest with obesity (BMI ≥ 30 kg/m2), daytime hypoventilation (Paco2 > 40 mm Hg), and sleep-disordered breathing in the absence of other causes of hypoventilation.95 The prevalence of OHS is estimated to be 0.15% to 0.3% in the general population95 and 8% in patients undergoing bariatric surgery.96 In 90% of patients with OHS, the sleep-disordered breathing is OSA and the remaining 10% have nonobstructive sleep hypoventilation. Patients with OHS present with severe upper airway obstruction, restrictive pulmonary physiology, blunted central respiratory drive, and pulmonary hypertension.97,98 Serum bicarbonate is considered to be a surrogate marker for daytime hypercapnia and levels ≥27 mmol/L may be indicative of OHS.99 The combination of serum HCO3− ≥ 28 mmol/L and a STOP-Bang score ≥3 may help to distinguish patients with moderate-to-severe OSA or OHS.98
OHS can pose a higher risk of postoperative complications and is often unrecognized at the time of surgery, requiring better emphasis on preoperative recognition of hypercapnia among patients with OSA.100 Therapeutic interventions for OHS therapy include CPAP therapy, bilevel positive airway pressure therapy, supplemental oxygen and weight reduction surgery.101
OSA in Surgical Patients
What Can We Learn from PSG of Surgical Patients?
Information from PSG may help in the perioperative risk stratification of OSA patients. PSG quantifies the number of obstructive events, the resultant hypoxemia and arousals related to the respiratory events.102 A recent cohort study found that patients with a higher preoperative AHI had a higher postoperative AHI, and slow wave (NREM) sleep percentage was inversely associated with postoperative AHI.103 Although AHI is the most commonly used metric of OSA severity, it might not be the best metric to correlate with postoperative outcomes. The same AHI may have a different connotation for the severity of OSA depending on the severity of oxygen desaturation during each episode of apnea/hypopnea, the cumulated time of overnight oxygen desaturation and the respiratory arousal threshold.104 Supine respiratory disturbance had been proposed to be one of the measure of OSA severity.105 Other parameters such as oxygen desaturation index, cumulated duration of oxygen desaturation <90%, the lowest Spo2 and/or mean Spo2 may help in the prediction of postoperative complications.106
Perioperative Management of OSA
There has been a growing concern regarding the increased risk of postoperative complications in surgical patients with OSA.107 Preoperative preparation is key in patients with OSA.108 The guidelines by the Society of Anesthesia and Sleep Medicine recommended additional preoperative evaluation for suspected OSA patients with certain conditions such as hypoventilation syndromes, severe pulmonary hypertension, and resting hypoxemia not attributable to other cardiopulmonary disease.109 Surgical patients with OSA adherent to CPAP therapy should continue CPAP therapy at their previously prescribed setting perioperatively.110 A recent meta-analysis indicated that the use of CPAP may have beneficial effects of reduction of AHI in the postoperative period.111 Two large retrospective database studies provide incremental evidence that confirms the benefits of establishing the diagnosis of OSA preoperatively and provide a preliminary rationale for treating OSA with CPAP during the perioperative period.112,113 Consideration should be given to using CPAP or an oral appliance during sedation to patients previously treated with these modalities.108 Some patients may be nonadherent to CPAP therapy. Simple maneuvers such as refitting a mask, the addition of heated humidification, or the control of nasal congestion with nasal corticosteroid sprays may help patients to adhere to therapy.114,115 Automatically titrated positive airway pressure (APAP) may be suitable for postoperative patients with moderate-to-severe OSA, especially those with a diagnosis of OSA awaiting CPAP titration in a sleep laboratory.116 Adaptive servoventilation, which delivers servocontrolled inspiratory pressure support on top of expiratory positive airway pressure, may be more effective to treat opioid-induced respiratory depression and central sleep apnea that are not responsive to CPAP.117 Similarly, overlap syndrome, which is a combination of OSA and chronic obstructive pulmonary disease, and OHS are effectively treated with BPAP because they require different levels of positive pressure during inspiration and expiration and a lower expiratory positive pressure.97,118
It may be useful for the perioperative team to have knowledge of the various endotypes and phenotypes of OSA to provide optimal perioperative management (Table 6). Obesity and abnormal craniofacial morphology can be associated with poor glottic visualization and unexpected difficult intubation in OSA patients. This is compounded by the use of sedatives and anesthetics, which worsen upper airway collapsibility. The sniffing and ramped up positions can facilitate intubation. Preparation for a difficult intubation should be done by ensuring the availability of airway adjuncts and rescue equipment.108
Table 6. Perioperative Management of OSA.
| General recommendations | Preoperative CPAP |
| Preoperative mandibular advancement/oral appliances | |
| Preoperative weight loss | |
| Sniffing and ramped up positions for intubation | |
| Preparation for a difficult intubation | |
| Minimizing sedatives and opioids, plan for multimodal analgesia | |
| Considering regional anesthesia techniques whenever possible | |
| Recovery in the lateral, semiupright or other nonsupine positions | |
| Postoperative use of CPAP therapy | |
| Supplemental oxygen as required | |
| Continuous monitoring with pulse oximetry and capnography | |
| Incentive spirometry and early ambulation | |
| Specific considerations for various OSA endophenotypes Morbidly obese | Preoperative weight loss |
| Preparation for a difficult mask ventilation and intubation | |
| Ramped up position for intubation | |
| PAP therapy postextubation | |
| Screen for OHS, and continued use of special PAP therapy such as CPAP, BPAP, or ASV in preoperative and postoperative period | |
| Craniofacial abnormalities involving maxilla and mandible | Preparation for a difficult mask ventilation and intubation |
| Airway adjuncts such as videolaryngoscopes or fiber optic bronchoscopes | |
| Awake intubation may be considered | |
| Possible use of dental devices (not tested in perioperative testing) | |
| Craniofacial surgeries as a long-term therapy | |
| High arousal threshold | Proven in research studies. Feasible method of identification required in future |
| Regional anesthesia whenever possible | |
| Multimodal analgesia | |
| Short-acting anesthetic agents | |
| Judicious use of opioids/sedatives | |
| Continuous postoperative monitoring with high-resolution pulse oximetry | |
| High loop gain | Proven in research studies. Feasible method of identification required in future |
| Oxygen therapy beneficial in stabilizing breathing | |
| Supine-related OSA phenotype |
Avoidance of supine position |
| Semiupright/lateral position for recovery | |
| Fluid overloaded conditions and rostral fluid shift | Potential interventions that may be of benefit: |
| Elevated body position | |
| Diuretics | |
| Avoidance of excessive fluid administration | |
| Use of compression stockings to decrease leg fluid volume |
Abbreviations: ASV, adaptive servo ventilation; BPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; OHS, obesity hypoventilation syndrome; PAP, positive airway pressure.
Although, in theory, postoperative use of oxygen therapy in OSA patients may mask hypoxia that accompanies obstruction with a risk of significant carbon dioxide retention,119 it is often required. Oxygen therapy can be justified in OSA patients with hypoxemia in the early postoperative period until basal preoperative oxygen saturation level is reached.120 In addition, oxygen therapy has proven benefits in OSA patients with a high loop gain.50 When patient is on supplemental oxygen, pulse oximetry may not be reliable to detect a respiratory compromise. Respiratory rate and capnography may have to be monitored.44
Certain surgical patients with OSA have a high arousal threshold and may be more sensitive to opioids and sedatives with a higher risk of respiratory arrest.46 Regional anesthesia, by an opioid-sparing effect, decreases airway collapsibility and respiratory depression and is beneficial in these patients.121 These patients may require prolonged continuous postoperative monitoring with high-resolution pulse oximetry44 and capnography.48
The supine position can worsen symptoms in patients with OSA. Anesthetizing and recovering patients without OSA in the head position elevated up to 6 cm from horizontal increases the stability of the airway.122 It is useful to have patients with supine-related OSA in lateral or semiupright positions throughout the perioperative period. Although the role of fluid shift in worsening of OSA is not studied in the perioperative period, it may be prudent to restrict perioperative fluid administration in the elderly OSA patients and those with fluid retention states. Aggressive incentive spirometry and early ambulation are found to optimize the pulmonary status in OSA patients undergoing laparoscopic Roux-en-Y gastric bypass.123 Breathing disturbances during sleep were found to be highest on the third postoperative night, while the disturbances in sleep architecture were greatest on first postoperative night with a significant decrease in sleep efficiency, slow-wave sleep, and REM sleep in both OSA and non-OSA patients.124 The cause of the breathing disturbances may be partly due to recovery of REM sleep by the third postoperative night.124 Transnasal insufflation with a nasal cannula has been shown to relieve obstruction associated with REM-related OSA, by stabilizing the hypotonic upper airway dilators.87
Alternative therapies used to treat OSA, such as oral appliances, oral negative pressure devices, hypoglossal nerve stimulation, body positioners, nasal resistive valves, and other treatments, although proven to be effective, have not been systematically studied in the perioperative setting. Patients using the alternative therapies as their primary treatment or due to noncompliance to CPAP should be encouraged to continue them in the perioperative period.
Conclusion
In conclusion, OSA has recently been recognized as a complex multifactorial disease with distinct endotypes and phenotypes. This knowledge is of particular importance in providing the optimal perioperative care to OSA patients. In addition to empirical CPAP therapy, supplemental oxygen can help to stabilize breathing in OSA patients with high loop gain. Avoidance of supine position can minimize airway obstruction in patients with a supine-related OSA. In the future, OSA patients with a high arousal threshold should be recognized because they are sensitive to sedatives and narcotics with a risk of respiratory arrest in the perioperative period. Hence, understanding the pathophysiologic mechanisms of OSA is critical to the success of individualized therapeutic approaches.
Acknowledgments
Supported by Department of Anesthesiology and Pain Medicine, Toronto Western Hospital, University Health Network, University of Toronto, Toronto, Ontario, Canada.
Footnotes
Contribution: Yamini Subramani, MD. This author helped to design the review, review the literature, and write the manuscript.
Mandeep Singh, MBBS, FRCPC. This author helped to write the manuscript.
Jean Wong, MD, FRCPC. This author helped to write the manuscript.
Clete A. Kushida, MD, PhD. This author helped to write the manuscript.
Atul Malhotra, MD. This author helped to write the manuscript.
Frances Chung, MBBS, FRCPC. This author helped to design the review, review the literature, and write the manuscript.
Disclosures: Conflicts of Interest: Yamini Subramani declares no Conflicts of interest.
Mandeep Singh declares no Conflicts of interest.
Jean Wong declares no Conflicts of interest.
Clete A. Kushida declares no Conflicts of interest.
Atul Malhotra declares no Conflicts of interest.
Frances Chung, MBBS, FRCPC. STOP Bang tool is proprietary to University Health Network. Royalties from UpToDate. Research grants from ResMed Foundation and Acadia Pharma.
Attestation: Yamini Subramani attests to the integrity of the manuscript and has approved the final manuscript.
Mandeep Singh approved the final manuscript.
Jean Wong approved the final manuscript.
Clete A. Kushida attests to the integrity of the manuscript and has approved the final manuscript.
Atul Malhotra attests to the integrity of the manuscript and has approved the final manuscript.
Frances Chung attests to the integrity of the manuscript and has approved the final manuscript.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 5.Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109:897–906. doi: 10.1093/bja/aes308. [DOI] [PubMed] [Google Scholar]
- 6.Singh M, Liao P, Kobah S, Wijeysundera DN, Shapiro C, Chung F. Proportion of surgical patients with undiagnosed obstructive sleep apnoea. Br J Anaesth. 2013;110:629–636. doi: 10.1093/bja/aes465. [DOI] [PubMed] [Google Scholar]
- 7.Owens RL, Eckert DJ, Yeh SY, Malhotra A. Upper airway function in the pathogenesis of obstructive sleep apnea: a review of the current literature. Curr Opin Pulm Med. 2008;14:519–524. doi: 10.1097/MCP.0b013e3283130f66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joosten SA, Hamza K, Sands S, Turton A, Berger P, Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17:99–107. doi: 10.1111/j.1440-1843.2011.02037.x. [DOI] [PubMed] [Google Scholar]
- 9.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 11.Gagnadoux F, Le Vaillant M, Paris A, et al. Institut de Recherche en Santé Respiratoire des Pays de la Loire Sleep Cohort Group. Relationship between OSA clinical phenotypes and CPAP treatment outcomes. Chest. 2016;149:288–290. doi: 10.1016/j.chest.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- 13.Phillipson EA, Sullivan CE. Arousal: the forgotten response to respiratory stimuli. Am Rev Respir Dis. 1978;118:807–809. doi: 10.1164/arrd.1978.118.5.807. [DOI] [PubMed] [Google Scholar]
- 14.Lam T, Singh M, Yadollahi A, Chung F. Is perioperative fluid and salt balance a contributing factor in postoperative worsening of obstructive sleep apnea? Anesth Analg. 2016;122:1335–1339. doi: 10.1213/ANE.0000000000001169. [DOI] [PubMed] [Google Scholar]
- 15.Campo P, Rodríguez F, Sánchez-García S, et al. Severe Asthma Workgroup. SEAIC Asthma Committee. Phenotypes and endotypes of uncontrolled severe asthma: new treatments. J Investig Allergol Clin Immunol. 2013;23:76–88. [PubMed] [Google Scholar]
- 16.Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42:650–658. doi: 10.1111/j.1365-2222.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 17.Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122:840–851. doi: 10.1378/chest.122.3.840. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:260–265. doi: 10.1164/ajrccm.165.2.2009032. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5:185–192. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland K, Lee RW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology. 2012;17:213–222. doi: 10.1111/j.1440-1843.2011.02082.x. [DOI] [PubMed] [Google Scholar]
- 22.Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. 2013;23:414–423. doi: 10.1007/s11695-012-0862-2. [DOI] [PubMed] [Google Scholar]
- 23.Sakakibara H, Tong M, Matsushita K, Hirata M, Konishi Y, Suetsugu S. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur Respir J. 1999;13:403–410. doi: 10.1183/09031936.99.13240399. [DOI] [PubMed] [Google Scholar]
- 24.Hui DS, Ko FW, Chu AS, et al. Cephalometric assessment of craniofacial morphology in Chinese patients with obstructive sleep apnoea. Respir Med. 2003;97:640–646. doi: 10.1053/rmed.2003.1494. [DOI] [PubMed] [Google Scholar]
- 25.Takemoto H. Morphological analyses of the human tongue musculature for three-dimensional modeling. J Speech Lang Hear Res. 2001;44:95–107. doi: 10.1044/1092-4388(2001/009). [DOI] [PubMed] [Google Scholar]
- 26.Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:772–779. doi: 10.1152/jappl.1979.46.4.772. [DOI] [PubMed] [Google Scholar]
- 27.Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ, Younes M. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep. 2011;34:1061–1073. doi: 10.5665/SLEEP.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol (1985) 2008;105:1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 29.Fogel RB, Malhotra A, Pillar G, et al. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–2030. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- 30.Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol (1985) 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 31.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The infuence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–964. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985;87:432–436. doi: 10.1378/chest.87.4.432. [DOI] [PubMed] [Google Scholar]
- 33.Malhotra A. Hypoglossal-nerve stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:170–171. doi: 10.1056/NEJMe1314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kezirian EJ, Goding GS, Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23:77–83. doi: 10.1111/jsr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White DP. New therapies for obstructive sleep apnea. Semin Respir Crit Care Med. 2014;35:621–628. doi: 10.1055/s-0034-1390074. [DOI] [PubMed] [Google Scholar]
- 36.Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea: therapeutic potential. Am J Respir Med. 2003;2:21–29. doi: 10.1007/BF03256636. [DOI] [PubMed] [Google Scholar]
- 37.Berry RB, Yamaura EM, Gill K, Reist C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep. 1999;22:1087–1092. doi: 10.1093/sleep/22.8.1087. [DOI] [PubMed] [Google Scholar]
- 38.Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) 2013;114:911–922. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 40.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190:1293–1300. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–1312. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynn LA, Curry JP. Patterns of unexpected in-hospital deaths: a root cause analysis. Patient Saf Surg. 2011;5:3. doi: 10.1186/1754-9493-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zavodny J, Roth C, Bassetti CL, Mathis J, Douglas NJ, Gugger M. Effects of sleep fragmentation on the arousability to resistive loading in NREM and REM sleep in normal men. Sleep. 2006;29:525–532. doi: 10.1093/sleep/29.4.525. [DOI] [PubMed] [Google Scholar]
- 46.Lam KK, Kunder S, Wong J, Doufas AG, Chung F. Obstructive sleep apnea, pain, and opioids: is the riddle solved? Curr Opin Anaesthesiol. 2016;29:134–140. doi: 10.1097/ACO.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273–1285. doi: 10.1213/ANE.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 48.Weinger MB, Lee LA. No patient shall be harmed by opioidinduced respiratory depression. APSF Newsletter Fall. 2011 [Google Scholar]
- 49.Khoo MC. Using loop gain to assess ventilatory control in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1044–1045. doi: 10.1164/ajrccm.163.5.ed1101c. [DOI] [PubMed] [Google Scholar]
- 50.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naughton MT. Loop gain in apnea: gaining control or controlling the gain? Am J Respir Crit Care Med. 2010;181:103–105. doi: 10.1164/rccm.200909-1449ED. [DOI] [PubMed] [Google Scholar]
- 54.Yumino D, Redolfi S, Ruttanaumpawan P, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121:1598–1605. doi: 10.1161/CIRCULATIONAHA.109.902452. [DOI] [PubMed] [Google Scholar]
- 55.Elias RM, Bradley TD, Kasai T, Motwani SS, Chan CT. Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transplant. 2012;27:1569–1573. doi: 10.1093/ndt/gfr605. [DOI] [PubMed] [Google Scholar]
- 56.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591:1179–1193. doi: 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–246. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 58.Redline S, Strohl KP. Recognition and consequences of obstructive sleep apnea hypopnea syndrome. Clin Chest Med. 1998;19:1–19. doi: 10.1016/s0272-5231(05)70428-7. [DOI] [PubMed] [Google Scholar]
- 59.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12:481–496. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subramanian S, Jayaraman G, Majid H, Aguilar R, Surani S. Influence of gender and anthropometric measures on severity of obstructive sleep apnea. Sleep Breath. 2012;16:1091–1095. doi: 10.1007/s11325-011-0607-9. [DOI] [PubMed] [Google Scholar]
- 61.Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54:323–328. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 63.Edwards BA, Wellman A, Sands SA, et al. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37:1227–1236. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119:72.e9–e14. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Relationship between surface tension of upper airway lining liquid and upper airway collapsibility during sleep in obstructive sleep apnea hypopnea syndrome. J Appl Physiol (1985) 2003;95:1761–1766. doi: 10.1152/japplphysiol.00488.2003. [DOI] [PubMed] [Google Scholar]
- 66.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 68.McMillan A, Bratton DJ, Faria R, et al. PREDICT Investigators. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804–812. doi: 10.1016/S2213-2600(14)70172-9. [DOI] [PubMed] [Google Scholar]
- 69.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 70.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 71.Lee RW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33:1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 73.Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest. 2004;125:127–134. doi: 10.1378/chest.125.1.127. [DOI] [PubMed] [Google Scholar]
- 74.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol (1985) 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 75.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–950. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 76.Lee RW, Chan AS, Grunstein RR, Cistulli PA. Craniofacial phenotyping in obstructive sleep apnea–a novel quantitative photographic approach. Sleep. 2009;32:37–45. [PMC free article] [PubMed] [Google Scholar]
- 77.Islam S, Uwadiae N, Ormiston IW. Orthognathic surgery in the management of obstructive sleep apnoea: experience from maxillofacial surgery unit in the United Kingdom. Br J Oral Maxillofac Surg. 2014;52:496–500. doi: 10.1016/j.bjoms.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Mokhlesi B, Punjabi NM. “REM-related” obstructive sleep apnea: an epiphenomenon or a clinically important entity?”. Sleep. 2012;35:5–7. doi: 10.5665/sleep.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conwell W, Patel B, Doeing D, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16:519–526. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 80.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–1472. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 81.Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol (1985) 1991;71:488–497. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]
- 82.Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol. 1999;520(pt 3):897–908. doi: 10.1111/j.1469-7793.1999.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–264. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 86.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190:1158–67. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nilius G, Wessendorf T, Maurer J, et al. Predictors for treating obstructive sleep apnea with an open nasal cannula system (transnasal insufflation) Chest. 2010;137:521–528. doi: 10.1378/chest.09-0357. [DOI] [PubMed] [Google Scholar]
- 88.Dieltjens M, Braem MJ, Van de Heyning PH, Wouters K, Vanderveken OM. Prevalence and clinical significance of supine-dependent obstructive sleep apnea in patients using oral appliance therapy. J Clin Sleep Med. 2014;10:959–964. doi: 10.5664/jcsm.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 1998;114:1630–1635. doi: 10.1378/chest.114.6.1630. [DOI] [PubMed] [Google Scholar]
- 90.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115:771–781. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 91.Neill AM, Angus SM, Sajkov D, McEvoy RD. Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1997;155:199–204. doi: 10.1164/ajrccm.155.1.9001312. [DOI] [PubMed] [Google Scholar]
- 92.Exar EN, Collop NA. The upper airway resistance syndrome. Chest. 1999;115:1127–1139. doi: 10.1378/chest.115.4.1127. [DOI] [PubMed] [Google Scholar]
- 93.Guilleminault C, Stoohs R, Clerk A, Simmons J, Labanowski M. From obstructive sleep apnea syndrome to upper airway resistance syndrome: consistency of daytime sleepiness. Sleep. 1992;15:S13–S16. [PubMed] [Google Scholar]
- 94.Chung F, Liao P, Yang Y, et al. Postoperative sleep-disordered breathing in patients without preoperative sleep apnea. Anesth Analg. 2015;120:1214–1224. doi: 10.1213/ANE.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 95.Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care. 2010;55:1347–1362. discussion 1363–1365. [PubMed] [Google Scholar]
- 96.Domínguez-Cherit G, Gonzalez R, Borunda D, Pedroza J, Gonzalez-Barranco J, Herrera MF. Anesthesia for morbidly obese patients. World J Surg. 1998;22:969–973. doi: 10.1007/s002689900501. [DOI] [PubMed] [Google Scholar]
- 97.Chau EH, Mokhlesi B, Chung F. Obesity hypoventilation syndrome and anesthesia. Sleep Med Clin. 2013;8:135–147. doi: 10.1016/j.jsmc.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung F, Chau E, Yang Y, Liao P, Hall R, Mokhlesi B. Serum bicarbonate level improves specificity of STOP-Bang screening for obstructive sleep apnea. Chest. 2013;143:1284–1293. doi: 10.1378/chest.12-1132. [DOI] [PubMed] [Google Scholar]
- 99.Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11:117–124. doi: 10.1007/s11325-006-0092-8. [DOI] [PubMed] [Google Scholar]
- 100.Kaw R, Bhateja P, Paz Y, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest. 2016;149:84–91. doi: 10.1378/chest.14-3216. [DOI] [PubMed] [Google Scholar]
- 101.Chau EH, Lam D, Wong J, Mokhlesi B, Chung F. Obesity hypoventilation syndrome: a review of epidemiology, pathophysiology, and perioperative considerations. Anesthesiology. 2012;117:188–205. doi: 10.1097/ALN.0b013e31825add60. [DOI] [PubMed] [Google Scholar]
- 102.Bloch KE. Polysomnography: a systematic review. Technol Health Care. 1997;5:285–305. [PubMed] [Google Scholar]
- 103.Chung F, Liao P, Elsaid H, Shapiro CM, Kang W. Factors associated with postoperative exacerbation of sleep-disordered breathing. Anesthesiology. 2014;120:299–311. doi: 10.1097/ALN.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 104.Cooksey J, Mokhlesi B. Postoperative complications in obesity hypoventilation syndrome and hypercapnic OSA: CO2 levels matter! Chest. 2016;149:11–13. doi: 10.1016/j.chest.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 105.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 106.Chung F, Zhou L, Liao P. Parameters from preoperative overnight oximetry predict postoperative adverse events. Minerva Anestesiol. 2014;80:1084–1095. [PubMed] [Google Scholar]
- 107.Opperer M, Cozowicz C, Bugada D, et al. Does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the society of anesthesia and sleep medicine task force on preoperative preparation of patients with sleep-disordered breathing. Anesth Analg. 2016;122:1321–1334. doi: 10.1213/ANE.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 108.Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea an updated report by the American society of anesthesiologists task force on perioperative management of patients with obstructive sleep apnea. Anesthesiology. 2014;120:268–286. doi: 10.1097/ALN.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 109.Chung F, Memtsoudis S, Krishna Ramachandran S, et al. Society of anesthesia and sleep medicine guideline on preoperative screening and assessment of patients with obstructive sleep apnea. Anesth Analg. 2016 [Google Scholar]
- 110.Chung F, Nagappa M, Singh M, Mokhlesi B. CPAP in the perioperative setting: evidence of support. Chest. 2016;149:586–597. doi: 10.1378/chest.15-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nagappa M, Mokhlesi B, Wong J, Wong DT, Kaw R, Chung F. The effects of continuous positive airway pressure on postoperative outcomes in obstructive sleep apnea patients undergoing surgery: a systematic review and meta-analysis. Anesth Analg. 2015;120:1013–1023. doi: 10.1213/ANE.0000000000000634. Available at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medl&NEWS=N&AN=25899270. [DOI] [PubMed] [Google Scholar]
- 112.Mutter TC, Chateau D, Moffatt M, Ramsey C, Roos LL, Kryger M. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121:707–718. doi: 10.1097/ALN.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 113.Abdelsattar ZM, Hendren S, Wong SL, Campbell DA, Jr, Ramachandran SK. The impact of untreated obstructive sleep apnea on cardiopulmonary complications in general and vascular surgery: a cohort study. Sleep. 2015;38:1205–1210. doi: 10.5665/sleep.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Russell T. Enhancing adherence to positive airway pressure therapy for sleep disordered breathing. Semin Respir Crit Care Med. 2014;35:604–612. doi: 10.1055/s-0034-1390070. [DOI] [PubMed] [Google Scholar]
- 115.Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med. 2007;3:706–712. [PMC free article] [PubMed] [Google Scholar]
- 116.Liao P, Luo Q, Elsaid H, Kang W, Shapiro CM, Chung F. Perioperative auto-titrated continuous positive airway pressure treatment in surgical patients with obstructive sleep apnea: a randomized controlled trial. Anesthesiology. 2013;119:837–847. doi: 10.1097/ALN.0b013e318297d89a. [DOI] [PubMed] [Google Scholar]
- 117.Kasai T, Kasagi S, Maeno K, et al. Adaptive servo-ventilation in cardiac function and neurohormonal status in patients with heart failure and central sleep apnea nonresponsive to continuous positive airway pressure. JACC Heart Fail. 2013;1:58–63. doi: 10.1016/j.jchf.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 118.McNicholas WT, Verbraecken J, Marin JM. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev. 2013;22:365–375. doi: 10.1183/09059180.00003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fu ES, Downs JB, Schweiger JW, Miguel RV, Smith RA. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 2004;126:1552–1558. doi: 10.1378/chest.126.5.1552. [DOI] [PubMed] [Google Scholar]
- 120.Kaw R, Gali B, Collop NA. Perioperative care of patients with obstructive sleep apnea. Curr Treat Options Neurol. 2011;13:496–507. doi: 10.1007/s11940-011-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Memtsoudis SG, Stundner O, Rasul R, et al. Sleep apnea and total joint arthroplasty under various types of anesthesia: a population-based study of perioperative outcomes. Reg Anesth Pain Med. 2013;38:274–281. doi: 10.1097/AAP.0b013e31828d0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kobayashi M, Ayuse T, Hoshino Y, et al. Effect of head elevation on passive upper airway collapsibility in normal subjects during propofol anesthesia. Anesthesiology. 2011;115:273–281. doi: 10.1097/ALN.0b013e318223ba6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jensen C, Tejirian T, Lewis C, Yadegar J, Dutson E, Mehran A. Postoperative CPAP and BiPAP use can be safely omitted after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008:512–514. doi: 10.1016/j.soard.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 124.Chung F, Liao P, Yegneswaran B, Shapiro CM, Kang W. Postoperative changes in sleep-disordered breathing and sleep architecture in patients with obstructive sleep apnea. Anesthesiology. 2014;120:287–298. doi: 10.1097/ALN.0000000000000040. [DOI] [PubMed] [Google Scholar]


