Abstract
Introduction and Hypothesis
The paravaginal defect has been a topic of active discussion concerning 1) what it is; 2) how to diagnose it; 3) its role in anterior vaginal wall prolapse; and 4) if and how to repair it. The aim of this article is to review the existing literature on the paravaginal defect and to discuss its role in the anterior vaginal wall support system, with an emphasis on anatomy and imaging.
Methods
Articles related to paravaginal defects were identified through a PUBMED search ending July 1, 2015.
Results
The support of the anterior vaginal wall is a complex system involving the levator ani muscle, the arcus tendineus fascia pelvis (ATFP), the pubocervical fascia, and the uterosacral/cardinal ligaments. Studies conclude that physical examination is inconsistent in detecting paravaginal defects. Ultrasound (US) and magnetic resonance imaging (MRI) have been used to describe patterns in the appearance of the vagina and bladder when a paravaginal defect is suspected. Different terms have been used (e.g. “sagging of bladder base,” “loss of tenting”), which all represent changes in the support of the pelvic floor but which could be due to both paravaginal defects and levator ani defects.
Conclusion
Paravaginal support plays a role in the support of the anterior vaginal wall, but we still do not know the degree to which it contributes to the development of prolapse. Both MRI and US are useful in the diagnosis of paravaginal defects, but further studies are needed to evaluate their use.
Keywords: anatomy, arcus tendineus fascia pelvis, MRI, paravaginal defect, ultrasound, pelvic organ prolapse
INTRODUCTION
Pelvic organ prolapse—especially prolapse of the anterior vaginal wall—is a common condition that increasingly affects women as they advance in age. In 40–60% of parous women, prolapse can be found during gynecologic examination [1] and the lifetime risk of undergoing at least one prolapse surgery is 18.7% [2]. Several factors predispose women to developing prolapse, including pregnancy, vaginal delivery, hormonal status, and aging [3,4].
Despite the fact that prolapse and surgical treatment for the condition is very common, little is known about why surgery (native tissue repair) produces failure rates in the 40% range (taking into account both anatomy and symptoms) [1]. Our understanding of where, how, and why prolapse develops is crucial to efforts to decrease the high recurrence rate after surgery. The “where” has been examined through cadaver, clinical, and imaging studies. For several years, the lateral attachment of the anterior vaginal wall has been a focal point of these investigations. The key issue of how to choose the right operation for each patient remains unresolved. Establishing both the normal and abnormal anatomy in cystourethrocele and how best to determine what factors are most responsible for prolapse in each woman continues to be an area of active investigation.
A. Cullen Richardson coined the term “paravaginal defect” in 1981 [5]. He described it as the detachment of the pubocervical fascia from the arcus tendineus fascia pelvis (ATFP). This defect was associated with descent of the lateral part of the anterior wall, resulting in a cystourethrocele. Richardson proposed the location of the defect in his paper from 1976 [6], highlighting the importance of detecting the exact anatomic site of the defect in the formation of cystocele for the purpose of site-specific repair. He described four failure sites involving anterior wall support: 1) lateral (later called paravaginal); 2) transverse; 3) midline; and 4) pubourethral. In 93 women with cystocele, he found 62 to have a lateral defect. A few years later, an overlooked article from 1909 was discovered, in which George White described the vaginal detachment from the white line (ATFP) as a cause of anterior wall prolapse [7]. White described his observations following a visit to Vienna, where he spent time with the Austrian gynecologist/anatomist duo Halban and Tandler, who conducted landmark studies on the anatomy and etiology of pelvic organ prolapse by performing detailed dissections of cadavers with prolapse [AC Richardson personal communication].
Over the years, the phrase “paravaginal defect” has been subject to extensive discussions throughout the literature. These discussions have addressed a variety of subjects: 1) the anatomical nature of the paravaginal defect; 2) the clinical importance of this defect in the development of cystocele; 3) techniques for diagnosis; and 4) whether or not and how to repair it.
Different authors have used the term “paravaginal defect.” The phrase has been used in different ways to describe: 1) what is seen during retropubic operations, if the retropubic space is opened all the way to the ischial spine where the paravaginal separation can be directly observed [8]; 2) a presumed defect based on indirect inferences made during vaginal dissection in the operating room; 3) the results of tests such as the ring forceps test that elevates the lateral vaginal sulci on examination; and 4) findings in imaging for the abnormal shape of the vagina or bladder base (MRI and US) presumed to be indicative of a paravaginal defect [5,8–13]. As authors in the field use the term for several different types of direct and indirect observations, it is necessary to examine the use of this term and the basis for its use in each circumstance.
The aim of this paper is to review the existing literature on paravaginal defects, with a particular focus on the anatomical support, physiology, and imaging of the anterior wall. In 2001, Nguyen [14] conducted a review of the literature regarding diagnosis and repair of paravaginal defects. Since this review, MRI and US techniques have been further developed and optimized, and paravaginal defects can now be examined using 3D imaging. This development of technology suggests a need for an updated analysis of the anatomy and diagnosis of the paravaginal defect.
MATERIALS AND METHODS
A PubMed literature search in English, Danish, and German languages with the keywords paravaginal defect, cystocele, anatomy, arcus tendineus fascia pelvis, white line, fascial arch, anterior vaginal wall support, ultrasound, and magnetic resonance imaging yielded 138 papers as of July 1, 2015. Additional articles were discovered through review of the references cited in the PubMed-identified articles. In all, 152 articles were reviewed. Only papers regarding anatomy, physical examination, or imaging of paravaginal support/defects were included in this study. Papers regarding only surgical repair of paravaginal defects were excluded.
ANATOMY
The support of the anterior vaginal wall is a complex system involving the levator ani muscle, the ATFP, the pubocervical fascia, and the uterosacral/cardinal ligaments. Understanding the anatomy and physiology of these elements is essential for interpreting both the clinical findings and the images when dealing with anterior wall prolapse due to a paravaginal defect.
Vaginal support
In 1992, DeLancey [15] described that the vagina could be divided into three levels based on cadaver dissection (fig 1). Level I designates the cephalic 2–3 cm of the vagina that is supported by the uterosacral and cardinal ligaments through their attachments to the cervix and upper vagina. At Level II, the pubocervical fascia and the vagina are attached to the ATFP. The vagina is more fixed at this level; imaging studies describe it as having an H-shape in the axial plane [16–17]. Level III is the caudal 2–3 cm above the hymeneal ring, where the vagina is fused with the surrounding structures—namely the urethra, the perineal membrane, and the levator ani.
Fig. 1.
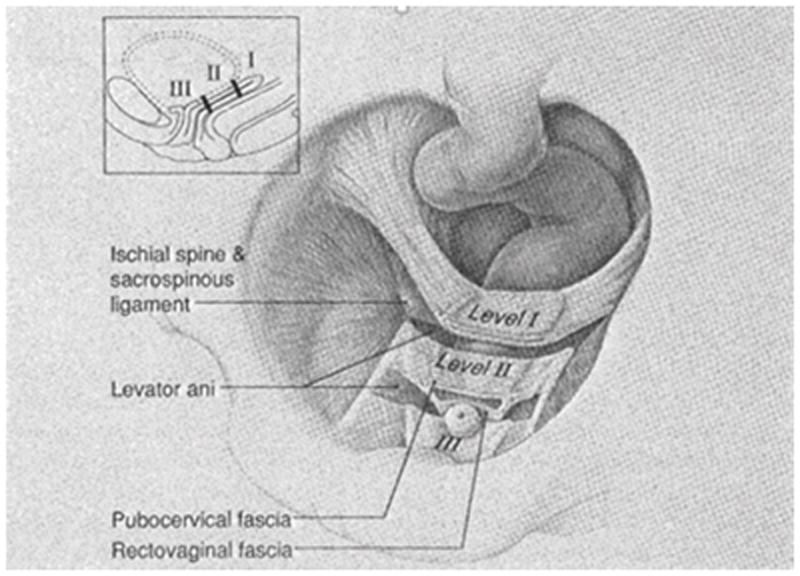
The three levels of vaginal support. Level I: the cephalic 2–3 cm of the vagina supported by the uterosacral and cardinal ligaments. Level II: the middle part of the vagina supported by the attachment between the pubocervical fascia and arcus tendineous fascia pelvis. Level III: the caudal 2–3 cm above the hymeneal ring supported by the surrounding structures - the urethra, perineal membrane and levator ani.
DeLancey JO (1992) Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166(6 Pt 1):1717-24
Arcus tendineus fascia pelvis
Several authors describe the ATFP as a key support structure of the anterior wall [8,18–21,27]. From its origin, 2–3 cm lateral to the pubic bone, the ATFP covers the pubococcygeus and iliococcygeus muscles and stretches toward the ischial spine, where it attaches along with the arcus tendineus levator ani. (fig 2).
Fig. 2.
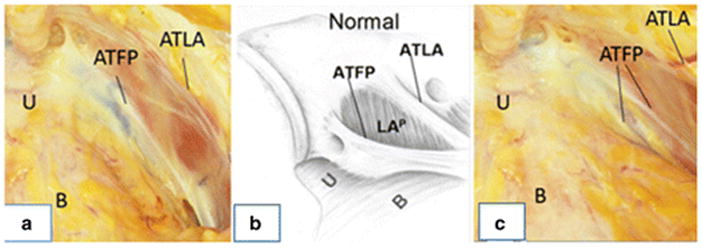
Arcus tendineus fascia pelvis in a cadaver and a drawing at slightly different angles. Image A and B: normal configuration of ATFP. Image C: defect of ATFP from the spine towards the pubic bone. U:urethra, B:bladder, ATFP:arcus tendineus fascia pelvis, ATLA:arcus tendineus levator ani.
Image A and C: private images from DeLancey Image B: Delancey JO (2002) Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol.187(1):93-8. ©DeLancey
Albright et al [22] found the ATFP in a cadaver study to be an average of 8.99 cm long. The anterior section fixed strongly to the surroundings, with a measurement of 5.7–8.2 kg [20,23]. The pubocervical fascia is stretched between the left and right ATFP (fig 1) and serves as a supportive structure underneath the bladder. In this location, it keeps the anterior wall from descending. The pubocervical fascia attaches primarily to the ventral half of the ATFP, with the dorsal connection being much weaker. Therefore, a tear or weakening in the middle of the pubocervical fascia would lead to a midline cystocele, whereas a tear in the lateral attachment of the pubocervical fascia to the ATFP could theoretically lead to a paravaginal cystocele. It is possible to palpate the ATFP during vaginal surgery or to visualize it during abdominal or laparoscopic surgery [8,24–25]. The continuity between the vagina and the ATFP can be seen on MRI, but this connection has not been demonstrated using US.
In 2002, DeLancey conducted a study on 71 women with cystourethrocele and stress urinary incontinence undergoing retropubic operations [8]. Findings observed during surgery were compared with normal findings seen previously during dissection of nulliparous cadavers with normal pelvic organ support. A left paravaginal defect was present in 87% of cases and a right defect in 89% of cases. Moreover, DeLancey described the location of detachment—in more than 95% of the cases, the detachment began at the ischial spine and extended in varying degrees towards the pubic bone, much like a zipper unzipping from just one end (the ischial spine) (fig 2). In the early cadaver and imaging studies, the assumption was that the vagina would be attached to the ATFP in its full length and that the vagina and the ATFP would be located parallel to each other. This led to the hypothesis that detachment of the vagina from the ATFP would be associated with descent of the upper pubocervical fascia and the development of an anterior wall prolapse (fig 3).
Fig. 3.
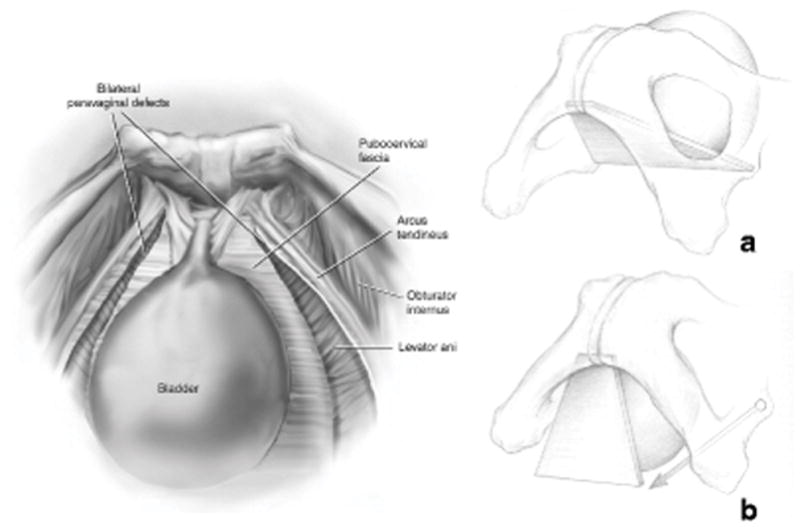
Bilateral paravaginal defect and the development of anterior wall prolapse.
Left: Bilateral paravaginal defect. The pubocervical fascia is no longer attached to ATFP.
Miklos JR, Kohli N (2000) Laparoscopic paravaginal repair plus burch colposuspension: review and descriptive technique Urology Dec 4;56(6 Suppl 1):64-9.[53] © Miklos/Kohli 2000
Right: Image A show the normal attachment of the pubocervical to ATFP. Image B illustrate how a bilateral paravaginal defect theoretically would make the pubocervical fascia drop down and by that create a cystocele.
Delancey JO (2002) Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol.187(1):93-8. ©DeLancey
Objective evaluation of this anatomy without the distortion of opening the space of Retzius has become possible via MRI. In addition, the 3D location of the anterior vaginal wall can be established at maximal Valsalva. Importantly, this non-invasive technique allows the anatomy of women with cystocele to be compared to that of women with normal vaginal wall support who are of similar demographics—something that could not be done in the operating room or dissecting laboratory.
In 2010, Larson et al [26] conducted a MRI study to investigate the relationship between the vagina and the location of the ATFP in women with and without anterior wall prolapse, at rest and at maximal Valsalva. They found that even in women with normal support, the vagina was not parallel to the ATFP (fig 4). In addition, the upper part of the vagina was above the plane of the ATFP, meaning that support systems other than the ATFP (e.g. the apical and the levator ani muscle supports) would need to be responsible for the support of the upper vagina.
Fig. 4.
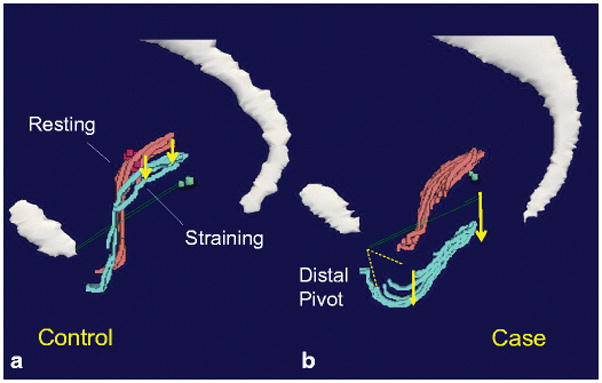
3D model illustrating location of the vagina and ATFP at rest (pink) and at maximum Valsalva (turquoise) in controls (a) and women with anterior wall prolapse (b). ATFP is marked as the green line. P: pubic symphysis. Sa: sacrum. This illustrates that the vagina and the ATFP is not parallel to each other.
Larson KA, Hsu Y, Chen L, Ashton-Miller JA, DeLancey JO (2010) Magnetic resonance imaging-based three-dimensional model of anterior vaginal wall position at rest and maximal strain in women with and without prolapse. Int Urogynecol J. 21(9):1103-9 ©DeLancey 2009
Cadaver studies [15,22] also showed that the pubocervical fascia and the vagina, for the most part, are attached to the anterior part of the ATFP—leading to discussion about whether a detachment in the posterior part of the ATFP (the zipper effect) would lead to a significant prolapse of the anterior wall.
The Larson study [25] also showed that during Valsalva, the movements of the anterior wall primarily occurred along the length of the vagina and that the cystocele formation took place at levels II and III. This supports the theory that the connection between the lateral edges of the vagina and the ATPF in level II is crucial for normal support of the anterior wall.
In 2011, Larson used the “Stress 3D MRI” technique in 10 women with and 10 without anterior wall prolapse to make objective measurements of the change in the relationship between the lateral anterior wall and where the arcus tendineus could be expected to occur, the descent of the apex, and the width of the vagina between rest and Valsalva [28]. By using MRI and 3D models, this group proved that women with prolapse had a significantly larger paravaginal descent than normal women, especially in the lateral part of the midvagina (fig 5). This was the first objective proof that the paravaginal gap between the superior lateral sulcus of the vagina exists. In addition, as hypothesized in earlier studies, it quantified and demonstrated the strong correlation between the apex and the paravaginal gap that was greater in the apical part of the vagina and decreased in segments measured closer to the pubic bone. These findings indicated that apical descent and the paravaginal defect could be “manifestations of the same phenomenon.” Larson’s work was the first time that the paravaginal gap was measured at maximal Valsalva and found to be related to anterior vaginal wall prolapse.
Fig. 5.
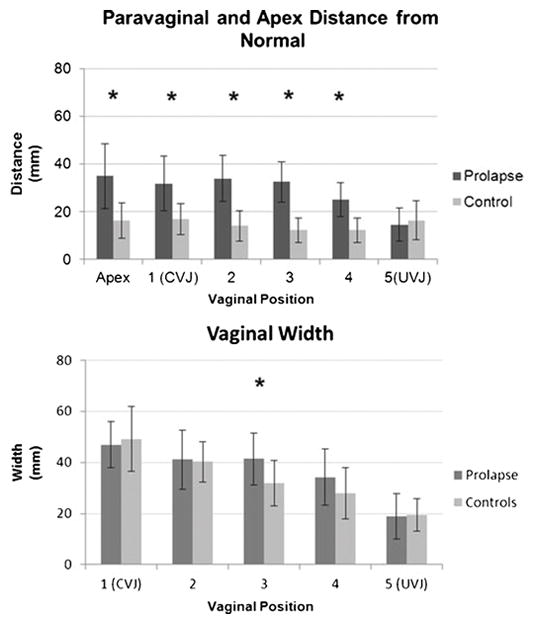
Descent of lateral anterior wall (prolapse vs controls) in six different places along the vagina. “1” is apex at the cervicovaginal junction (CVJ) and “5” is the utherovaginal junction (UVJ). * represents statistically significant differences between cases and controls. This demonstrates significant difference in paravaginal gap in women with prolapse than women without. Only in the mid vagina (“3”) there is a significant difference in vaginal with between cases and controls.
Larson KA, Luo J, Guire KE, Chen L, Ashton-Miller JA, DeLancey JO (2012) 3D analysis of cystoceles using magnetic resonance imaging assessing midline, paravaginal, and apical defects. Int Urogynecol J. 23(3):285-93 (UVJ) ©DeLancey 2010
Anterior vaginal wall prolapse and apical support
The degree of anterior vaginal wall prolapse is strongly associated with Level I prolapse of the cervix [26, 29–31]. In women with normal support, the apex is located above the ATFP [26], suggesting that other structures such as the cardinal and uterosacral ligaments are important for the support of the cervix and the anterior wall. Papers by Summers, Rooney, and Hsu [29–31] showed a strong correlation between apical descent and prolapse of the anterior wall. Their respective studies suggested that between 53 and 77% of anterior wall descent could be explained by the apical descent.
Review of published anatomical descriptions shows that the uterosacral ligament consists of loose connective tissue, smooth muscles, vessels, and nerve fibers [32]. The uterosacral ligament originates from tissue in the region of the os sacrum (the area of S2–S4) and attaches at the lateral and dorsal part of the cervix. Some cadaver and MRI studies suggest a direct attachment at level I of the vagina [33,34]. The uterosacral ligament is strong, and failure is first seen at a weight between 5 and 17 kg [33], depending on where on the ligament the strength is measured. Histologically, the cardinal ligament is not a ligament, but rather a mesentery consisting of vessels and nerves running between the cervix and the origin of the internal iliac artery at the top of the greater sciatic foramen [35].
Levator ani
The levator ani is a muscular diaphragm surrounding a U-shaped central hiatus. It consists of three main parts—the M. Puborectalis, the M. Pubococcygeus, and the M. Iliococcygeus. From its attachment to the pubic bone, the medial part of the pubococcygeal muscle runs laterally to the ATFP. Just caudal to the ATFP, the levator ani muscle and the vagina are attached to each other by collagen and smooth muscle fibers [36]. The urethra, vagina, and rectum pass through the hiatal opening; a contraction of the levator ani pushes the three organs closer to the pubic bone, thereby minimizing the hiatus. The tonic activity of the levator ani muscles creates a high-pressure zone approximately 3 cm long in the lower vagina [37], so that downward forces on the vagina are carried by the levator ani. Pelvic organ prolapse is closely associated with major defects in the pubococcygeal portion of the levator ani [38,39]. In 2014, Berger conducted an MRI study on 284 women with prolapse and 219 without that showed that in 70% of all cases, prolapse could be explained by levator ani defects [41]. Authors have used different terms in the literature for the injury to the levator—most commonly “defect” and “avulsion.” In this review we have chosen to use the term “defect.”
Paravaginal defects and levator ani defects
Paravaginal defects and levator ani defects are separate phenomena; each can occur alone or together. Paravaginal defects seen during surgery in women with AVW prolapse were associated with an abnormal pubococcygeal muscle about half of the time. Approximately half of the women had generalized loss of muscle and the other half had a localized defect [8]. Loss of the levator ani is seen in 15% of women with normal support, so levator defects may also occur in the absence of paravaginal defects 42. Although these problems can occur independently, at least half of the women with a paravaginal defect have an abnormal levator, so the two usually occur together. There is a strong connection (anatomical and physiological) between the ATFP, the levator ani, and the anterior vaginal wall [21], but the exact correlation and their individual impact on the development of cystocele still needs to be demonstrated.
DIAGNOSING A PARAVAGINAL DEFECT
Physical examination for detection of a paravaginal defect
Several authors have used the physical examination technique described by Richardson in 1976 [6] and modified by Shull in 1993 [43]—examining a woman with anterior vaginal wall prolapse to ascertain whether a paravaginal defect is present. With the woman in supine position, both at rest and at maximal Valsalva, the anterior wall is examined with a curved sponge forceps applied to the vaginal wall so that each tip of the forceps is held against the ischial spines in an attempt to imitate the paravaginal support from the ATFP. The patient is asked to perform maximal Valsalva, and if no prolapse is observed, the prolapse is said to be paravaginal. If a prolapse is still observed despite the paravaginal support by the forceps, an element of midline defect is thought to be present. Loss of rugal folds was also thought to be associated with a midline defect, whereas preserved rugal folds are associated with a paravaginal defect. The possibility that the rugal folds are absent due to post-menopausal atrophy was not considered. To evaluate this clinical method, Barber [9] and Segal [44] performed studies on women undergoing vaginal surgery for anterior prolapse and compared the clinical and surgical findings. Overall, there was a surgical prevalence of paravaginal defects in up to 47% of cases, with 50–80% specificity and 24–94% sensitivity. Both studies conclude that physical examination is inconsistent in detecting paravaginal defects. To test the examination technique described earlier, Whiteside [45] did a study in 2004 on inter-rater and intra-rater reliability in diagnosing anterior wall defects as either midline, right lateral, left lateral, or apical, and found both of them to be poor. Inter-rater reliability showed kappa=0.16 (95% CI 0–0.32) and intra-rater reliability showed kappa=0.16 (95% CI 0–0.45). This lack of reproducibility may come from several sources. First, both the degree of elevation and the presence of a paravaginal defect during vaginal surgery are subjective. Second, not only does the placement of ring forceps stretch the vagina laterally, it also elevates it, making it impossible to conclude that reduction of the cystocele is exclusively due to separation from the sidewall, as it could also be caused by apical descent. Third, considering paravaginal defects to be dichotomous is an oversimplification, as there are many different degrees of separation between the vaginal wall and the pelvic sidewall.
Ultrasound
Several authors have evaluated US as a diagnostic tool for differentiating between midline and paravaginal defects (table 1). Over time, the quality of US images has improved and advanced 3D and 4D imaging and tomography have become available.
Table 1.
Papers on ultrasound and paravaginal defect. NA: not available AWVP: anterior vaginal wall prolapse. SUI: stress urinary incontinence.
| Author | Ultrasound method | Clinical description | Number of patients | Ultrasound findings | Surgery findings (accura cy compar ed to US) | Levator ani avulsion | Inter-and intra-observer reliability |
|---|---|---|---|---|---|---|---|
| Ostrzenski 1997 [10] | Transabdominal 2D + water filled condom | Sagging of the bladder base (axial) | I. 30 AVWP + SUI II. 3 AVWP no SUI III. 4 nulliparous |
I. 30 PVD (100% - 57% unilateral, 43% bilateral) II. 3 (100% bilateral) III. 0 |
I. 30 (100%) II. 3 (100%) III. NA |
NA | NA |
| Nguyen 2000 [44] | Transabdominal 2D + water filled condom | Sagging of the bladder base (axial) | I. 15 anterior wall II. 15 nulliparous |
I. 15 PVD (100%) II. 15 PVD (100%) |
NA | NA | NA |
| Ostrzenski 1998[11] | Transabdominal 2D | Sagging of the bladder base (axial) | I. 16 + SUI II. 3 parous no POP, no SUI III. 5 nulliparous |
I. 16 PVD (56% unilateral, 44% bilateral) II. 2 PVD (67%) III. 0 |
I. 16 (100%) II. NA III. NA |
NA | NA |
| Martan 2002 [43] | Transabdominal 2D | Sagging of the bladder base (axial) | 20 anterior wall + SUI | 18 (90%) | 18 (100%) | NA | NA |
| Athanasiou 2007[17] | Transvaginal 2D | H-shape of vagina (axial) | I. 43 POP II. 24 no POP |
I. 15 out of 38 (5 unilateral and 10 bilateral) II. 1 (4.2%) |
NA | I. 14 out of 39 (35.9%) II. 0 |
NA |
| Dietz 2005[46] | Translabial 3D | Absence of tenting (axial) | 57 | 32 PVD (57%) (at rest) 21 PVD (37%) (at Valsalva) |
NA | NA | NA |
| Cassadó-Garriga 2014[47] | Translabial 4D | Absence of tenting (axial) | 202 nulliparous before and after childbirth | Before: 65 (32.2%) After: 101 (62%) |
NA | Measured but not outlined as numbers | Inter-rater 88.4%, kappa 0.63 (95% CI 0.52–0.73) |
In 1997 and 1998, Ostrzenski [10,11] published the first papers on US and paravaginal defects. In these studies, the assumption was that the shape of the bladder and vagina would reflect structural defects in the anterior wall supports. Performing transabdominal US on patients with paravaginal defects and nulliparous women, they looked at the appearance of the bladder base in the axial view with the premise that a paravaginal defect would make the bladder base drop on the side of the defect (fig 6). They reported that 100% of the US findings were also found at surgery; however, the evaluators of the US images were not blinded to the clinical findings and the surgeons were not blinded to the US findings. Moreover, the surgical definition of a paravaginal defect was not specified. Martan showed similar results in 2002 [46]. Using US, he observed that 18 out of 20 women with stress urinary incontinence had changes in the bladder base due to a paravaginal defect, results which were 100% accurate when compared to the surgical findings. In this uncontrolled study, each participating woman had a pelvic floor disorder and no available information regarding status of the anterior vaginal wall; therefore, comparisons between women with and without prolapse could not be made. Unlike the other studies, the Ostrzenski study from 1997 [10] was carried out with a water-filled condom in the vagina, which could have caused distortion. In 2000, Nguyen [47] conducted a similar study with asymptomatic nulliparous women that revealed that findings considered diagnostic of paravaginal defects in the earlier studies were present not only in women with prolapse, but also in nulliparous women with normal support, and that the size of the presumptive paravaginal defect was correlated with the amount of water in the condom. Thus, the bladder and the bladder base were very movable and stretchable structures that were dependent on the amount of water in both the bladder and the condom.
Fig. 6.
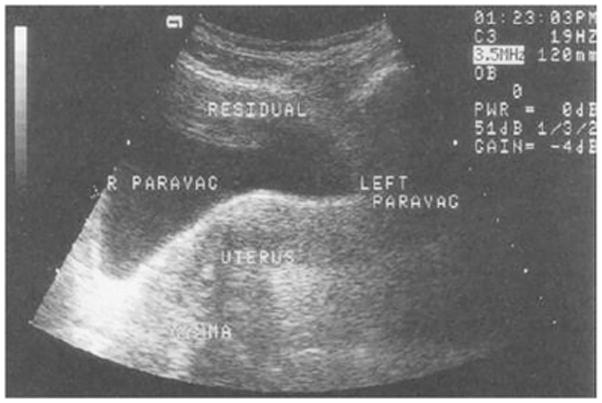
Transabdominal ultrasound image showing a drop of the bladder base on the right.
Ostrzenski A, Osborne NG (1998) Ultrasonography as a screening tool for paravaginal defects in women with stress incontinence: a pilot study. Int Urogynecol J Pelvic Floor Dysfunct 9(4):195-9
The lateral attachments of the vagina at levels II and III makes the vagina appear H-shaped on the axial view [16,17,48,49]. In 2001, Ochsenbein did a study examining 40 women without a vaginal delivery (nulliparous or just after elective caesarean section) [48]. Transanal US was used to describe the location of the lateral edge of the vagina and the fixation of the vaginal corners to the ATFP. The lateral edges were described as above, equal to, or below the suburethral portion of the vagina in the axial plane (the presence or absence of the H-shape). All paravaginal edges were located above the suburethral vagina; the authors hypothesized that this was a sign of no paravaginal defect. No other cases (e.g. women with pelvic floor trauma or known anterior wall prolapse) were observed and the presence/absence of a paravaginal defect was not examined by other methods.
Athanasiou also examined the H-shape of the vagina by transvaginal US in 2007, showing that 40% of women with and 4% of women without prolapse had unilateral or bilateral loss of the H-shape [17]. The data showed that 36% of the women with prolapse had levator ani trauma, but no information was provided in this early study about the correlation between levator ani trauma and changes in the US appearance of the vagina.
Using transvaginal US for examining anatomical changes in the appearance of the vagina has been a topic of discussion because the transducer distends the vagina and might therefore affect the location of the anatomical landmarks. As alternatives, 2D and 3D translabial US techniques have been used. In 2005, Dietz published a paper describing the shape of the vagina in the axial plan using 3D translabial US [49]. These observations were based on the premise that the normal lateral support of the vagina to the ATFP was described as “tenting,” showing that the sulcus was lifted up towards the arcus (fig 7). 59 women with bladder dysfunction (e.g. incontinence and prolapse) were examined. Eleven of them had grade 2 cystocele or greater. Fourteen women had a clinically-suspected paravaginal defect on the left side (24%) and 19 had one on the right side (32%). Upon examination, their US images showed an absence of tenting in 57% of the cases at rest, but this did not correlate with the clinical findings. At Valsalva, 37% showed an absence of tenting, which was weakly associated with the clinical findings. The US findings were not reported and no information regarding levator ani damage was published, but the authors suggest that the loss of tenting could be due to levator ani defects. Cassadó-Garriga examined this concept in 2015 [50]. 4D translabial US was used to examine 163 nulliparous women before and after childbirth. Images were analyzed to measure factors such as the levator ani defect and tenting, among others. Multivariate analysis demonstrated that the absence of tenting was independently associated with the degree of cystocele (p=0.005) even after controlling for levator ani defects. These results suggested that the “loss of tenting” phenomenon could be due to paravaginal defects and not levator ani defects, and as an independent factor, could be involved in the development of anterior wall prolapse.
Fig. 7.
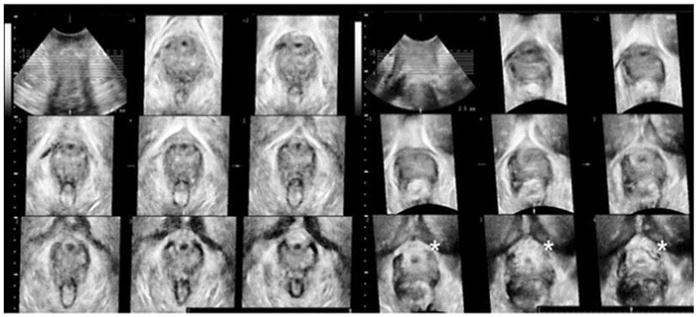
Translabial ultrasound image. The nine images on the left show normal configuration of the vaginal support = no loss of tenting. The nine images on the right, from another woman, show absence of tenting in the last three images (the absence is marked with *)
Cassadó-Garriga J, Wong V, Shek K, Dietz HP (2015) Can we identify changes in fascial paravaginal supports after childbirth? Aust N Z J Obstet Gynaecol.55(1):70-5
Magnetic resonance imaging
MRI has better spatial resolution than US and allows measurements of 3D spatial relationships to be related to the bony pelvis, but it is also considerably more expensive. Its capabilities include dynamic mid-sagittal images that quantify both the direction and magnitude of movement [51]. More recently, 3D stress MRI, in which multi-slice images are captured at the point of maximal Valsalva with the prolapse maximally developed, allows changes in vaginal width, length, and location to be captured and measured relative to bony landmarks. This allows structural hypotheses to be tested, comparing women with normal support to those with anterior vaginal wall prolapse, and the connections and interactions between the different organs to be observed. Several authors have used MRI as a method to describe the attachment of the vagina to the pelvic sidewalls and the relationship between paravaginal defects and prolapse of the anterior vaginal wall.
In 1995, two research groups used static resting MRI to describe an abnormal pattern of vaginal shapes (presumed to be paravaginal defects), but with different classifications. Huddleston performed MRIs on 12 patients with stress urinary incontinence and cystourethrocele, with the aim of describing paravaginal defects in the axial plane in the three levels of the vagina, before and after surgery [18]. The MRIs showed 10 women with bilateral defects at vaginal level I, 12 with bilateral defects at level II, and 9 with bilateral defects at level III. They described the defects subjectively by the terms “chevron sign” (level I), “saddlebag sign” (level II), and “moustache sign” (level III) (fig 8). They evaluated the anatomy of the paravaginal attachment intraoperatively and found 100% agreement with the MRI results. Postoperative MRIs revealed that these signs of a paravaginal defect were gone in all 12 patients. The surgeons not being blinded to pre-operative MRI results and the absence of a control group without prolapse limits the objectivity of these results. In addition, the question of whether or not these abnormal findings might be associated with levator defects was not considered. A similar study by Aronson [52] added a control group. They performed MRI on four continent nulliparous women and four women who had stress urinary incontinence and clinical signs of a paravaginal defect upon physical examination. In the axial plane, they identified the space of Retzius on both sides of the midline and found that in the continent women, the space of Retzius was well-defined and symmetric. In the group of incontinent women, the space of Retzius was enlarged at the side of the paravaginal defect and there was an asymmetric appearance along the midline. Besides the subjective description, they calculated the volume of the space of Retzius, showing that the mean volume of the distal 2.5 cm for the incontinence group was almost double that of the continent group. This suggests that a paravaginal defect led to downward placement of the space of Retzius on the affected side. Correlation coefficients between the two authors were R2=0.97. There was no information regarding the levator ani status.
Figure 8.
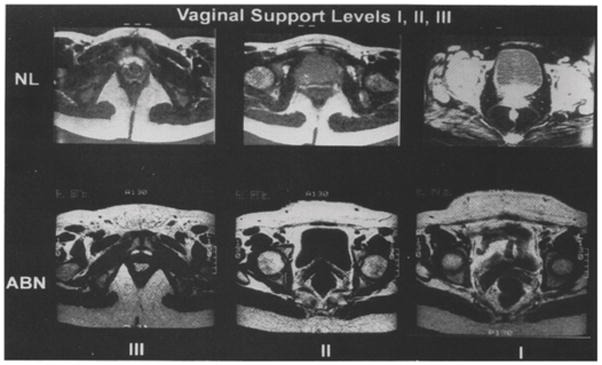
Comparison of the appearance of vagina in the three vaginal levels. NL: normal, ABN: abnormal.
Huddleston HT, Dunnihoo DR, Huddleston PM 3rd, Meyers PC Sr.(1995) Magnetic resonance imaging of defects in DeLancey’s vaginal support levels I, II, and III. Am J Obstet Gynecol.172(6):1778-82
The abnormal appearance of pelvic sidewall tissues at the level of the vesical neck was called “architectural distortion” by Huebner [53] in 2009. This was defined as lateral “spill” of the vagina in the axial plane beyond the normal location (fig 9). MRI scans from 144 women with and 126 women without prolapse were categorized into one of the following groups: 1) no levator ani defect/no architectural distortion; 2) levator ani defect/no architectural distortion; or 3) levator ani defect/architectural distortion. Inter-rater agreement was 87% and kappa=0.64 (95% CI 0.54–0.75). No women had architectural distortion without levator ani defect. 31% (33 out of 108) of women in group 1, 62% (60 out of 97) in group 2, and 78% (51 out of 65) in group 3 showed significantly higher risk of prolapse if both MRI signs were present, compared to those with only levator ani defects. They concluded that architectural distortion was associated, but not always seen together with levator ani defects, and because levator ani defects could be seen without the distortion, the distortion was a result of connective tissue damage (paravaginal defect) rather than muscle defect.
Fig. 9.
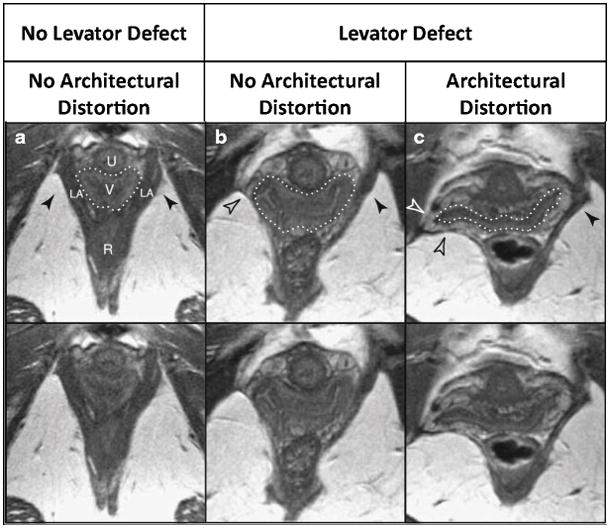
MRI scans from women with and without architectural distortion and with and without levator ani defect.
Huebner M, Margulies RU, DeLancey JO (2008) Pelvic architectural distortion is associated with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct.19(6):863-7
Larson examined the anatomical basis for “architectural distortion” in 2011 [54]. She included a group of 14 women who had unilateral architectural distortion that could be compared with normal anatomy on the other side using MRI and 3D models. The position of the ATFP and the arcus tendineus levator ani at rest was evaluated, and the distorted side was compared with the normal side (fig 10). The fascial and the levator arches are too small to be seen directly on MRI, but their positions can be determined by their relation to other anatomical structures. The 3D model showed that both arches had moved downward (caudally) on the side with distortion compared to the side with normal configuration. On the distorted side, only the anterior half (the region closest to the pubic bone) of the arches were lower (more caudal) than the normal side. Both anterior parts of the arches were placed more medial and inferior on the distorted side. Surprisingly, the section of the ATFP closest to the ischial spine on the affected side did not change position in any of the directions compared to the unaffected side. Previous studies have shown that paravaginal defects involving the ATFP almost always only occur in the part closest to the ischial spine [8], indicating that the ATFP in this region would descend as well. This is probably because the ATFP was not directly visible on the MRI scans and the scans were made at rest, when the separation was not provoked. As mentioned earlier, the study by Larson 2011 [28] measured the paravaginal gap (the distance between the position of the lateral margin of the anterior wall) in women with and without prolapse. She found the greatest difference in the apex and the midvagina, where the vagina was closest to the ATFP. This suggests that paravaginal defects could play a significant role in the development of cystocele. The same paper showed a strong correlation between the paravaginal gap and apical descent.
Figure 10.
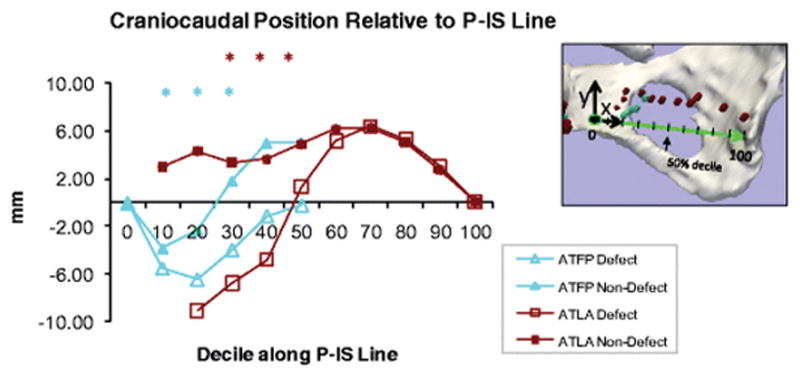
Comparison of the craniocaudal position of arcus tendineus fascia pelvis and arcus tendineus levator ani along a line between os pubis and ischial spine(P-IS Line) on the side with architectural distortion (defect) and the side with no distortion (non-defect) “0” is the pubic bone and “100” is the ischial spine.
Larson KA, Luo J, Yousuf A, Ashton-Miller JA, Delancey JO (2012) Measurement of the 3D geometry of the fascial arches in women with a unilateral levator defect and “architectural distortion”. Int Urogynecol J. 23(1):57–63
In a recent study from 2015 [55], two authors sought to determine the prevalence of architectural distortion by examining MRI images from 32 women referred to Radiology with “pelvic floor weakness” and from 44 women without weakness. The meaning of pelvic floor weakness was not specified. In the “weakness” group, one observer found distortion in 84% (27) of women, while the other one found it in only 41% (13) of women. In the “no weakness” group, the two observers reported distortion in 32% (14) and 9% (4) of women respectively. The correlation between pelvic floor weakness and architectural distortion was significant for both authors; however, there was a significant lack of agreement between examiners. They hypothesized that architectural distortion was a sign of pelvic floor relaxation in general and not linked to the anterior wall or paravaginal defects. The authors were blinded in regard to knowing whether the MRI scans were from women in the “weakness” or “no weakness” groups, but the paper lacked information regarding the degree of “pelvic floor weakness” and levator ani status.
DISCUSSION
As demonstrated above, the anterior vaginal wall support system involves a complex interaction between muscular and connective tissue that keeps the vagina and the surrounding organs from descending. Expanding anatomical knowledge is crucial to understanding the mechanism behind the development of prolapse, which is multi-factorial. The studies show that the paravaginal defect is just one aspect of this support system, and the term itself has been used in many different ways. Anatomically, it represents a separation between the anterior vaginal wall and the ATFP that can only be seen directly by observing it through the space of Retzius. Indirect ways of establishing whether or not a paravaginal defect is present have been evaluated, since direct observation is not always possible. These include imaging, physical examination, and observations made during vaginal surgery where a view from the space of Retzius is not always available.
MRI and US have been used in an attempt to find patterns that might indicate whether or not a paravaginal defect was present in women with anterior wall prolapse. These studies have, for the most part, used the changes in the shape of the vagina and bladder in the axial plane and suggested that these indicated whether or not a paravaginal defect was present. Stress 3D MRI that shows the location of the lateral margin of the vagina relative to the normal location of the ATFP and allows this distance to be directly measured is, at present, the most objective way to evaluate paravaginal defects. These evaluations can be conducted in women with and without anterior prolapse so that the differences can be compared. It is expected that similar techniques will become available with US.
Current studies of resting anatomy, however, are incomplete. No studies have fully demonstrated the relationship between changes in vaginal shape and the observation of paravaginal defects through the space of Retzius. Early studies lacked the blinding of investigators (cases vs. controls, imaging results before surgery, etc.). Because the appearance of levator ani muscle defects was not fully appreciated at the time, most of these studies lacked information on levator ani status. In retrospect, many of the images from these papers clearly demonstrated levator defects as a cause of prolapse. Levator ani defects are strongly correlated with the development of cystocele, but the fact that prolapse of the anterior wall can develop without levator ani damage leads to the thought that other defects in the support system could be important.
Areas of future research
There is no doubt that changes in the shape of the vagina, to some degree, are associated with levator ani defects, but we still have no answers as to whether an isolated paravaginal defect alone could cause changes in the shape of the vagina/bladder base. Future MRI studies examining the lateral edges of the vagina in women with anterior wall prolapse but no levator ani defect would be of interest. Moreover, a blinded prospective study determining the status of the levator ani and the shape of the vagina by MRI or US before surgery for an assumed paravaginal defect, where it would be possible to determine intraoperatively whether or not a paravaginal defect was in fact present, would provide further information about the relationship between the different anterior wall support structures. We also lack information on whether women without anterior wall prolapse could have a paravaginal defect, since this term represents a separation between the anterior vaginal wall and the ATFP that can only be seen directly by observing it through the space of Retzius when the space is opened to its full extent all the way to the ischial spine, and there have been no studies exploring the space of Retzius during surgery for conditions other than prolapse/stress urinary incontinence. Opening the space of Retzius for no reason other than urogynecologic surgery would be unethical due to the surgical risks. In the future, imaging could prove helpful in answering this question.
The paravaginal defect is an anatomical observation, and its clinical implication in the development of anterior wall prolapse is yet to be fully clarified. The recent 3D MRI studies performed at rest and at maximal Valsalva allow the location of the lateral edge of the anterior vaginal wall to be evaluated relative to the normal location of the anterior vaginal wall and show that at rest in normal women, the lower and middle part of the vagina is in close proximity to the ATFP. Paravaginal descent is greater in the middle part of the vagina, suggesting that the connection between the vagina and the ATFP is important for vaginal support.
In level I, the upper third of the vagina is connected to the apical supports that also attach to the cervix, where there is still a lateral attachment between the vagina, the ATFP, and the cardinal uterosacral ligament complex. Apical descent is closely associated with paravaginal defect, and it is unknown whether apical descent found in conjunction with a paravaginal defect causes a lateral detachment of the vagina or vice versa. In conclusion, it is possible that a paravaginal defect and descent of the lateral part of the vagina might drag down the apical part of the vagina. When the lateral part of the vagina descends, the apical part will be dragged down as well. Future studies are needed to evaluate this connection.
Acknowledgments
Dr. DeLancey gratefully acknowledges NIH grants P50 HD044406 and R01 HD038665 that provide support for his effort.
Abbreviations
- ATFP
arcus tendineus fascia pelvis
- US
ultrasound
- MRI
magnetic resonance imaging
Footnotes
Conflict of interest:
LTS Arenholt has accepted travel grant from Astellas and has consulted Astellas on topics not relevant to this manuscript. M Glavind-Kristensen and K Glavind have accepted travel grants from Astellas.
Each author’s contribution to the manuscript: Louise T.S. Arenholt: literature search, literature reading, manuscript writing
Bodil Ginnerup Pedersen: literature reading, manuscript writing/editing
Karin Glavind: literature reading, manuscript writing/editing
Marianne Glavind-Kristensen: literature reading, manuscript writing/editing
John O.L. DeLancey: literature reading, manuscript writing/editing
Contributor Information
Louise T.S. Arenholt, Centre for Clinical Research, Department of Obstetrics and Gynecology, North Denmark Regional Hospital, Bispensgade 67, 9800 Hjorring, Denmark
Bodil Ginnerup Pedersen, Department of Radiology, Aarhus University Hospital, Skejby, Denmark.
Karin Glavind, Department of Obstetrics and Gynecology, Aalborg University Hospital, Denmark.
Marianne Glavind-Kristensen, Department of Obstetrics and Gynecology, Aarhus University Hospital, Skejby, Denmark.
John O.L. DeLancey, Pelvic Floor Research Group, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA
References
- 1.Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013 Apr;30:4. doi: 10.1002/14651858.CD004014.pub5. [DOI] [PubMed] [Google Scholar]
- 2.Løwenstein E, Ottesen B, Gimbel H. Incidence and lifetime risk of pelvic organ prolapse surgery in Denmark from 1977 to 2009. Int Urogynecol J. 2015;26(1):49–55. doi: 10.1007/s00192-014-2413-y. [DOI] [PubMed] [Google Scholar]
- 3.Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG. 1013;120(2):152–60. doi: 10.1111/1471-0528.12020. [DOI] [PubMed] [Google Scholar]
- 4.Tegerstedt G, Miedel A, Maehle-Schmidt M, Nyrén O, Hammarström M. Obstetric risk factors for symptomatic prolapse: a population-based approach. Am J Obstet Gynecol. 2006;194(1):75–81. doi: 10.1016/j.ajog.2005.06.086. [DOI] [PubMed] [Google Scholar]
- 5.Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57(3):357–62. [PubMed] [Google Scholar]
- 6.Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126(5):568–73. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- 7.White GR. Cystocele--a radical cure by suturing lateral sulci of the vagina to the white line of pelvic fascia. 1909. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(5):288–92. doi: 10.1007/BF02765486. [DOI] [PubMed] [Google Scholar]
- 8.Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187(1):93–8. doi: 10.1067/mob.2002.125733. [DOI] [PubMed] [Google Scholar]
- 9.Barber MD, Cundiff GW, Weidner AC, Coates KW, Bump RC, Addison WA. Accuracy of clinical assessment of paravaginal defects in women with anterior vaginal wall prolapse. Am J Obstet Gynecol. 1999;181(1):87–90. doi: 10.1016/s0002-9378(99)70440-0. [DOI] [PubMed] [Google Scholar]
- 10.Ostrzenski A, Osborne NG, Ostrzenska K. Method for diagnosing paravaginal defects using contrast ultrasonographic technique. J Ultrasound Med. 1997;16(10):673–7. doi: 10.7863/jum.1997.16.10.673. [DOI] [PubMed] [Google Scholar]
- 11.Ostrzenski A, Osborne NG. Ultrasonography as a screening tool for paravaginal defects in women with stress incontinence: a pilot study. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9(4):195–9. doi: 10.1007/BF01901603. [DOI] [PubMed] [Google Scholar]
- 12.Hosni MM, El-Feky AE, Agur WI, Khater EM. Evaluation of three different surgical approaches in repairing paravaginal support defects: a comparative trial. Arch Gynecol Obstet. 2013;288(6):1341–8. doi: 10.1007/s00404-013-2927-4. [DOI] [PubMed] [Google Scholar]
- 13.Behnia-Willison F, Seman EI, Cook JR, O’Shea RT, Keirse MJ. Laparoscopic paravaginal repair of anterior compartment prolapse. J Minim Invasive Gynecol. 2007;14(4):475–80. doi: 10.1016/j.jmig.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen JK. Current concepts in the diagnosis and surgical repair of anterior vaginal prolapse due to paravaginal defects. Obstet Gynecol Surv. 2001;56(4):239–46. doi: 10.1097/00006254-200104000-00025. [DOI] [PubMed] [Google Scholar]
- 15.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 Pt 1):1717–24. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 16.Macura KJ. Magnetic resonance imaging of pelvic floor defects in women. Top Magn Reson Imaging. 2006;17(6):417–26. doi: 10.1097/RMR.0b013e3180417dc8. [DOI] [PubMed] [Google Scholar]
- 17.Athanasiou S, Chaliha C, Toozs-Hobson P, Salvatore S, Khullar V, Cardozo L. Direct imaging of the pelvic floor muscles using two-dimensional ultrasound: a comparison of women with urogenital prolapse versus controls. BJOG. 2007;114(7):882–8. doi: 10.1111/j.1471-0528.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- 18.Huddleston HT, Dunnihoo DR, Huddleston PM, 3rd, Meyers PC., Sr Magnetic resonance imaging of defects in DeLancey’s vaginal support levels I, II, and III. Am J Obstet Gynecol. 1995;172(6):1778–82. doi: 10.1016/0002-9378(95)91411-0. [DOI] [PubMed] [Google Scholar]
- 19.Mauroy B, Goullet E, Stefaniak X, Bonnal JL, Amara N. Tendinous arch of the pelvic fascia: application to the technique of paravaginal colposuspension. Surg Radiol Anat. 2000;22(2):73–9. doi: 10.1007/s00276-000-0073-8. [DOI] [PubMed] [Google Scholar]
- 20.Pit MJ, De Ruiter MC, Lycklama A, Nijeholt AA, Marani E, Zwartendijk J. Anatomy of the arcus tendineus fasciae pelvis in females. Clin Anat. 2003;16(2):131–7. doi: 10.1002/ca.10102. [DOI] [PubMed] [Google Scholar]
- 21.Fritsch H, Zwierzina M, Riss P. Accuracy of concepts in female pelvic floor anatomy: facts and myths! World J Urol. 2012;30:429–435. doi: 10.1007/s00345-011-0777-x. [DOI] [PubMed] [Google Scholar]
- 22.Albright TS, Gehrich AP, Davis GD, Sabi FL, Buller JL. Arcus tendineus fascia pelvis: a further understanding. Am J Obstet Gynecol. 2005;193(3 Pt 1):677–81. doi: 10.1016/j.ajog.2005.02.129. [DOI] [PubMed] [Google Scholar]
- 23.Cosson M, Boukerrou M, Lacaze S, Lambaudie E, Fasel J, Mesdagh H, Lobry P, Ego A. A study of pelvic ligament strength. Eur J Obstet Gynecol Reprod Biol. 2003;109(1):80–7. doi: 10.1016/s0301-2115(02)00487-6. [DOI] [PubMed] [Google Scholar]
- 24.Shull BL, Baden WF. A six-year experience with paravaginal defect repair for stress urinary incontinence. Am J Obstet Gynecol. 1989;160(6):1432–9. doi: 10.1016/0002-9378(89)90867-3. [DOI] [PubMed] [Google Scholar]
- 25.Claydon CS, Maccarone JL, Grody MH, Steinberg A, Oyama I, Holzberg AS, Caraballo R. The distance between the perceived and the actual arcus tendineus fascia pelvis during vaginal paravaginal repair. Am J Obstet Gynecol. 2005;192(5):1707–11. doi: 10.1016/j.ajog.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 26.Larson KA, Hsu Y, Chen L, Ashton-Miller JA, DeLancey JO. Magnetic resonance imaging-based three-dimensional model of anterior vaginal wall position at rest and maximal strain in women with and without prolapse. Int Urogynecol J. 2010;21(9):1103–9. doi: 10.1007/s00192-010-1161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ersoy M, Sagsoz N, Bozkurt MC, Apaydin N, Elhan A, Tekdemir I. Important anatomical structures used in paravaginal defect repair: cadaveric study. Eur J Obstet Gynecol Reprod Biol. 2004;112(2):206–13. doi: 10.1016/j.ejogrb.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Larson KA, Luo J, Guire KE, Chen L, Ashton-Miller JA, DeLancey JO. 3D analysis of cystoceles using magnetic resonance imaging assessing midline, paravaginal, and apical defects. Int Urogynecol J. 2012;23(3):285–93. doi: 10.1007/s00192-011-1586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438–43. doi: 10.1016/j.ajog.2006.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol. 2006;195(6):1837–40. doi: 10.1016/j.ajog.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 31.Hsu Y, Chen L, Summers A, Ashton-Miller JA, DeLancey JO. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):137–42. doi: 10.1007/s00192-007-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramanah R, Berger MB, Parratte BM, DeLancey JO. Anatomy and histology of apical support: a literature review concerning cardinal and uterosacral ligaments. Int Urogynecol J. 2012;23(11):1483–94. doi: 10.1007/s00192-012-1819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buller JL, Thompson JR, Cundiff GW, Krueger Sullivan L, Schön Ybarra MA, Bent AE. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97(6):873–9. doi: 10.1016/s0029-7844(01)01346-1. [DOI] [PubMed] [Google Scholar]
- 34.Umek WH1, Morgan DM, Ashton-Miller JA, DeLancey JO. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;103(3):447–51. doi: 10.1097/01.AOG.0000113104.22887.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Range RL, Woodburne RT. The gross and microscopic anatomy of the transverse cervical ligaments. Am J Obstet Gynecol. 1964;90:460. doi: 10.1016/0002-9378(64)90802-6. [DOI] [PubMed] [Google Scholar]
- 36.DeLancey JO, Starr RA. Histology of the connection between the vagina and levator ani muscles. Implications for urinary tract function. J Reprod Med. 1990;35(8):765–71. [PubMed] [Google Scholar]
- 37.Jung SA, Pretorius DH, Padda BS, Weinstein MM, Nager CW, den Boer DJ, Mittal RK. Vaginal high-pressure zone assessed by dynamic 3-dimensional ultrasound images of the pelvic floor. Am J Obstet Gynecol. 2007;197(1):52.e1–7. doi: 10.1016/j.ajog.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 39.Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG. 2008;115(8):979–84. doi: 10.1111/j.1471-0528.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 40.Lammers K, Fütterer JJ, Prokop M, Vierhout ME, Kluivers KB. Diagnosing pubovisceral avulsions: a systematic review of the clinical relevance of a prevalent anatomical defect. Int Urogynecol J. 2012;23(12):1653–1664. doi: 10.1007/s00192-012-1805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger MB, Morgan DM, DeLancey JO. Levator ani defect scores and pelvic organ prolapse: is there a threshold effect? Int Urogynecol J. 2014;25(10):1375–9. doi: 10.1007/s00192-014-2388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007 Feb;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 43.Shull BL. Clinical evaluation of women with pelvic support defects. Clin Obstet Gynecol. 1993;36(4):939–51. doi: 10.1097/00003081-199312000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Segal JL, Vassallo BJ, Kleeman SD, Silva WA, Karram MM. Paravaginal defects: prevalence and accuracy of preoperative detection. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(6):378–83. doi: 10.1007/s00192-004-1196-y. [DOI] [PubMed] [Google Scholar]
- 45.Whiteside JL, Barber MD, Paraiso MF, Hugney CM, Walters MD. Clinical evaluation of anterior vaginal wall support defects: interexaminer and intraexaminer reliability. Am J Obstet Gynecol. 2004;191(1):100–4. doi: 10.1016/j.ajog.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 46.Martan A, Masata J, Halaska M, Otcenásek M, Svabik K. Ultrasound imaging of paravaginal defects in women with stress incontinence before and after paravaginal defect repair. Ultrasound Obstet Gynecol. 2002;19:496–500. doi: 10.1046/j.1469-0705.2002.00686.x. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen JK, Hall CD, Taber E, Bhatia NN. Sonographic diagnosis of paravaginal defects: a standardization of technique. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11(6):341–5. doi: 10.1007/s001920070003. [DOI] [PubMed] [Google Scholar]
- 48.Ochsenbein N, Kurmanavicius J, Huch R, Huch A, Wisser J. Volume sonography of the pelvic floor in nulliparous women and after elective cesarean section. Acta Obstet Gynecol Scand. 2001;80(7):611–5. [PubMed] [Google Scholar]
- 49.Dietz HP, Pang S, Korda A, Benness C. Paravaginal defects: a comparison of clinical examination and 2D/3D ultrasound imaging. Aust N Z J Obstet Gynaecol. 2005;45(3):187–90. doi: 10.1111/j.1479-828X.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 50.Cassadó-Garriga J, Wong V, Shek K, Dietz HP. Can we identify changes in fascial paravaginal supports after childbirth? Aust N Z J Obstet Gynaecol. 2015;55(1):70–5. doi: 10.1111/ajo.12261. [DOI] [PubMed] [Google Scholar]
- 51.Spahlinger DM, Newcomb L, Ashton-Miller JA, DeLancey JO, Chen L. Relationship between intra-abdominal pressure and vaginal wall movements during Valsalva in women with and without pelvic organ prolapse: technique development and early observations. Int Urogynecol J. 2014;25(7):873–8.1. doi: 10.1007/s00192-013-2298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aronson MP, Bates SM, Jacoby AF, Chelmow D, Sant GR. Periurethral and paravaginal anatomy: an endovaginal magnetic resonance imaging study. Am J Obstet Gynecol. 1995;173(6):1702–8. doi: 10.1016/0002-9378(95)90413-1. [DOI] [PubMed] [Google Scholar]
- 53.Huebner M, Margulies RU, DeLancey JO. Pelvic architectural distortion is associated with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):863–7. doi: 10.1007/s00192-007-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larson KA, Luo J, Yousuf A, Ashton-Miller JA, Delancey JO. Measurement of the 3D geometry of the fascial arches in women with a unilateral levator defect and “architectural distortion”. Int Urogynecol J. 2012;23(1):57–63. doi: 10.1007/s00192-011-1528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tillack AA, Joe BN, Yeh BM, Jun SL, Kornak J, Zhao S, Deng D. Vaginal shape at resting pelvic MRI: predictor of pelvic floor weakness? Clin Imaging. 2015;39(2):285–8. doi: 10.1016/j.clinimag.2014.10.007. [DOI] [PubMed] [Google Scholar]


