Abstract
Objective
During verbal communication, humans briefly maintain mental representations of speech sounds conveying verbal information, and constantly scan these representations for comparison to incoming information. We determined the spatio-temporal dynamics of such short-term maintenance and subsequent scanning of verbal information, by intracranially measuring high-gamma activity at 70–110 Hz during a working memory task.
Methods
Patients listened to a stimulus set of two or four spoken letters and were instructed to remember those letters over a two-second interval, following which they were asked to determine if a subsequent target letter had been presented earlier in that trial’s stimulus set.
Results
Auditory presentation of letter stimuli sequentially elicited high-gamma augmentation bilaterally in the superior-temporal and pre-central gyri. During the two-second maintenance period, high-gamma activity was augmented in the left pre-central gyrus, and this effect was larger during the maintenance of stimulus sets consisting of four compared to two letters. During the scanning period following target presentation, high-gamma augmentation involved the left inferior-frontal and supra-marginal gyri.
Conclusions
Short-term maintenance of verbal information is, at least in part, supported by the left pre-central gyrus, whereas scanning by the left inferior-frontal and supra-marginal gyri.
Significance
The cortical structures involved in short-term maintenance and scanning of speech stimuli were segregated with an excellent temporal resolution.
Keywords: High-frequency oscillations (HFOs), Subdural electroencephalography (EEG), Intracranial electrocorticography (ECoG) recording, Pediatric epilepsy surgery, Sternberg paradigm, 4D brain mapping
1. Introduction
Imagine that you are an efficient waiter/waitress and a group of your customers are ordering dishes delivered in a sequence such as ‘turkey’, ‘ham’, ‘chicken’, ‘fish’, ‘chicken’, and so on. You would presumably use verbal working memory operations including maintenance of mental representation of the items and scanning these representations to determine a match among previously encountered items to comprehend what and how many items were ordered (Baddeley, 1986; Hickok and Poeppel, 2007; Rauschecker and Scott, 2009; Sternberg, 1966). Previous studies using functional MRI (fMRI) and positron emission tomography (PET) showed widespread brain activation in the frontal and parietal lobes, which were suggested to support the execution of operations involved in verbal working memory (Awh et al., 1996; Cohen et al., 1997; Huang et al., 2013; Paulesu et al., 1993). Yet, the precise localization of these activations and the specific cortical dynamics supporting verbal working-memory operations of externally-delivered auditory stimuli have remained poorly understood. Previous fMRI studies reported that language tasks tapping verbal working memory function elicited activations in the left inferior-frontal region including Broca’s area, but also in regions outside of typical language areas such as in the pre-central gyrus; thereby, activation outside Broca’s area was presumably attributed to verbal working memory processing (Gaillard et al., 2003; Schapiro et al., 2004; Szaflarski et al., 2006; Wood et al., 2004).
The main aim of this study was to characterize the temporal dynamics of localized cortical activity involved in verbal working memory maintenance and scanning. Patients with focal epilepsy undergoing intracranial electrocorticography (ECoG) were assigned an auditory working-memory task (adapted from Sternberg, 1966), based on which we localized cortical activation taking place specifically during the maintenance and scanning periods. We chose a simple task that would allow us to define the maintenance and scanning periods, while minimizing semantic or syntactic processing and not requiring the operations of long-term memory functions. Participants were asked to listen to either two or four letters and to keep the letters in mind for two seconds (i.e. maintenance period), following which they listened to a target letter and decided whether the target was a new letter or had previously been presented in that trial (i.e. scanning period) (Fig. 1).
Fig. 1. Working memory task.
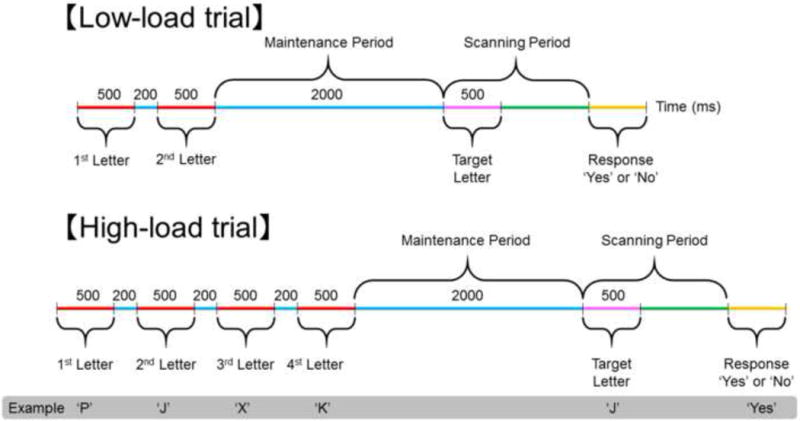
The task consisted of 30 ‘low-load’ and 30 ‘high-load’ trials presented in a pseudorandom order. Auditory stimuli consisted of a set of two alphabet letters for ‘low-load’ trials or four letters for ‘high-load’ trials. After delivery of the final letter in a set, a 2-second delay preceded delivery of a target letter. Patients were instructed to answer ‘yes’ or ‘no’ regarding whether the target letter was in the set for that trial. Thus, the task requires successful short-term maintenance prior to target onset and subsequent scanning of letter stimuli before a relevant response. The probability of each response (‘Yes’ or ‘No’) was 0.5.
In each participant, we measured high-gamma activity on ECoG during the maintenance and scanning periods; thereby, augmentation of high-gamma activity at 70–110 Hz was treated as a biomarker of in-situ cortical activation (Crone et al., 2006; Kojima et al., 2013; Towle et al., 2008). A number of studies, including ours, have reported the spatial concordance between the primary language areas defined by neurostimulation and language task-related augmentation of high-gamma activity including this frequency range (Kojima et al., 2012; Leuthardt et al., 2007; Ruescher et al., 2013; Wang et al., 2016). We assessed whether high-gamma activity would be differentially augmented in the frontal and parietal regions during the two-second maintenance period and during the subsequent scanning period. We also assessed whether there would be an effect of working memory load by comparing the degree of high-gamma augmentation in activated regions during trials presenting four (high-load) compared to two letter (low-load) stimulus sets. The presence of such memory load-dependent pattern of activation would support the key roles of high-gamma activated sites in short-term maintenance and scanning of verbal information, respectively (i.e.: ‘dose-response effect’ in Asano et al., 2013).
2. Methods
2.1. Participants
A consecutive series of 19 patients satisfying the following inclusion and exclusion criteria were studied (age range: 6–44 years; seven females; Table 1). The inclusion criteria consisted of: (i) a history of drug-resistant epilepsy scheduled for chronic subdural ECoG recording as part of presurgical evaluation at Children’s Hospital of Michigan or Harper University Hospital, Detroit, between December 2010 and July 2015, (ii) age of five years or older, (iii) measurement of ECoG amplitude augmentation driven by a letter-based working memory task described in the ‘Working Memory Task’ section below. The exclusion criteria consisted of: (i) presence of massive brain malformations, (ii) right-hemispheric language dominance (Akanuma et al., 2003; Knecht et al., 2000; Kojima et al., 2013), (iii) bilateral seizure foci, (iv) Verbal Intelligence Quotient or Verbal Comprehension Index less than 70, (v) inability to complete the tasks described in the ‘Working Memory Task’ section below due to the lack of adequate vocabulary, comprehension of task instructions, or cooperation, and (vi) history of previous neurological surgery. This study has been approved by the Institutional Review Board at Wayne State University (Protocol number: 048404MP2E), and written informed consent was obtained from all patients, their legal parent, or guardian.
Table 1.
Patient profile.
| Patient | Age (Year) | Gender | Sampled lobes | Number of electrodes included for analysis | Seizure onset zone | Pathology |
|---|---|---|---|---|---|---|
| 1 | 6 | F | Lt TPOF | 98 | Lt T | Gliosis |
| 2 | 9 | F | Lt TPOF | 91 | Lt T | Tumor |
| 3 | 12 | F | Lt TPOF | 99 | Lt T | Gliosis |
| 4 | 13 | M | Lt TPOF | 93 | Lt T | Gliosis |
| 5 | 14 | M | Lt TPOF | 102 | Lt T | Gliosis |
| 6 | 14 | M | Rt TPOF | 105 | Rt F | Dysplasia |
| 7 | 15 | F | Lt TPOF | 120 | Not captured* | Tumor |
| 8 | 16 | M | Lt TPOF; Rt F | 92 | Lt T | Gliosis |
| 9 | 16 | M | Lt TPOF | 97 | Lt T | Gliosis |
| 10 | 17 | M | Lt TPOF | 79 | Lt T | Gliosis |
| 11 | 17 | F | Lt TPOF | 107 | Lt T | Heterotopia |
| 12 | 18 | M | Rt TPOF | 89 | Rt T | Gliosis |
| 13 | 20 | M | Lt TPOF | 86 | Lt T | Gliosis |
| 14 | 21 | F | Lt TPOF | 84 | Lt T | Gliosis |
| 15 | 27 | F | Lt TPOF | 72 | Lt F | Tumor |
| 16 | 28 | M | Rt TPF | 75 | Rt T | Tumor |
| 17 | 29 | M | Rt TPF; Lt F | 94 | Rt TP | Dysplasia |
| 18 | 31 | M | Rt TPOF; Lt TOF | 118 | Rt T | Tumor |
| 19 | 44 | M | Rt TPOF | 80 | Rt TP | Gliosis |
The location of subdural electrode placement was determined based on clinical necessity, and we did not place electrodes more than clinically indicated (Nonoda et al., 2016).
: MRI showed an area with increased T2 signal in the left parietal lobe, of which radiological diagnosis was ulegyria. Resection of the parietal lesion was performed with sensorimotor, language and visual functions preserved. Pathological examination yielded a diagnosis of ganglioglioma without a surrounding cortical dysplasia. F: Female. M: Male. Lt: Left. Rt: Right. T: Temporal. P: Parietal. O: Occipital. F: Frontal.
2.2. Acquisition of ECoG and three dimensional Magnetic Resonance (3D MR) surface images
Subdural platinum grid electrode (10 mm center-to-center distance; 4 mm diameter) placement was as described previously by our team (Matsuzaki et al., 2015; Nonoda et al., 2016). Extraoperative video–ECoG recordings were obtained for three to five days, using a 192-channel Nihon Kohden Neurofax 1100A Digital System (Nihon Kohden America Inc., Foothill Ranch, CA, USA) at a sampling frequency of 1,000 Hz as previously described. Total electrode contact number ranged from 86 to 138 per patient. The average of ECoG signals derived from the 5th and 6th intracranial electrodes was used as the original reference, and ECoG signals were then re-montaged to an average reference (Fukuda et al., 2008; Nagasawa et al., 2012). Sites classified as seizure onset zone were clinically determined (Asano et al., 2009) and excluded from further analysis (Jacobs et al., 2009; Zijlmans et al., 2012). Likewise, sites showing interictal spikes or artifacts during the task were excluded from analysis. Thus, the number of analyzed electrodes ranged from 72 to 120 per patient. Subsequent ECoG analysis was performed with a common average reference excluding channels classified as seizure onset zone as well as those affected by interictal spikes or artifacts.
Volumetric-T1-weighted spoiled gradient echo MR image was obtained preoperatively using a previously described protocol (Nagasawa et al., 2010). Lateral and anterior–posterior X-rays were acquired following placement of intracranial electrodes; thereby, three metallic fiducial markers were placed at anatomically well-defined locations on the patient’s head for co-registration of the X-ray with the MRI. A 3D MRI brain surface image was created with electrodes delineated (Alkonyi et al., 2009; Matsuzaki et al., 2015; Muzik et al., 2007). Accuracy was confirmed by intraoperative digital photographs of in situ electrodes in each patient (Nonoda et al., 2016; Pieters et al., 2013; Wellmer et al., 2002). Post-implant CT images were reviewed, as needed, to confirm the co-registration accuracy in the medial or inferior surfaces of the cortex. The spatial normalization of each individual electrode site was performed using FreeSurfer scripts (http://surfer.nmr.mgh.harvard.edu). Electrode sites on each individual’s FreeSurfer brain surface were transformed into Talairach coordinates, and finally plotted on the averaged FreeSurfer pial surface image, referred to as “fsaverage” (Chan et al., 2011; Desikan et al., 2006). The surface-based group averaging using FreeSurfer has been validated in children. The mean registration error was reported to be 1.56 mm in those ranging in age from 4 to 11 years old (Ghosh et al., 2010). Automatic parcellation of cortical gyri was performed at both individual and spatially normalized brain surfaces (Desikan et al., 2006; Matsuzaki et al., 2015), and all electrode sites were assigned anatomical labels (Fig. 2A). The regions of interest (ROIs; Fig. 2B) included: (i) superior-temporal, (ii) inferior-frontal, (iii) middle-frontal, (iv) pre-central, (v) post-central, and (vi) supra-marginal regions, bilaterally; neuroimaging studies consistently reported that these regions were involved during auditory working memory tasks (Huang et al., 2013; Paulesu et al., 1993).
Fig. 2. Group coverage map and regions of interest (ROIs).
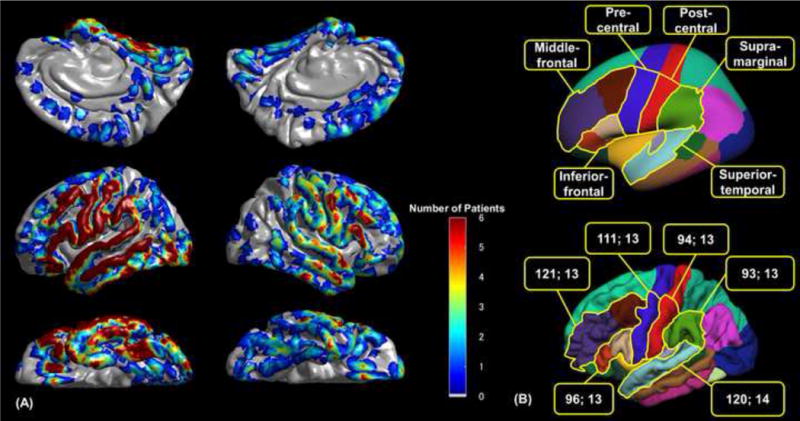
(A) The surface recording zones for a total of 1,781 analyzed electrodes are presented on the FreeSurfer average brain (Dykstra et al., 2012). (B) ROIs are denoted, with different colors, on the inflated (upper) and pial (lower) average brain images (Desikan et al., 2006). In the present study, the upper margin of the pre-central region was defined as the upper limit of the middle-frontal gyrus, whereas the upper margin of the post-central region was defined as the upper limit of the supra-marginal gyrus. The pre- and post-central gyri above the margin are considered to consist of the sensorimotor hand areas. The numbers in each box reflect the number of analyzed electrodes and the number of contributing patients in each ROI, respectively.
2.3. Verbal working memory task
The working memory task employed in this study represents a letter-based, auditory variation on the Sternberg task (Sternberg, 1966), whereas visual stimuli were presented in working memory tasks of other ECoG studies (Axmacher et al., 2010; Meltzer et al., 2008; Noy et al., 2015; Raghavachari et al., 2006; Rodriguez Merzagora et al., 2014; Tertel et al., 2011). None of the patients had a seizure event within two hours prior to or during task performance. While comfortably seated on a bed, patients received 60 question-and-answer trials. All auditory stimuli were delivered via playback of an audio recording of an author’s (E.C.B.) voice using Presentation version 9.81 software (Neurobehavioral Systems Inc., Albany, CA, USA). No warning signal was given before each trial. Each letter was delivered over 500 ms with 200 ms between each letter in a set. None of the letter stimuli were abnormally pronounced or truncated. All consonants of the English alphabet were used, excluding ‘w’ because it is verbalized as a three syllable word. Since the sound of some letters is similar, such as ‘d’ and ‘p’ or ‘f’ and x’, care was taken to ensure that two similar sounding letters were never delivered in succession. As the target letter is a repeat of one of the letters in a set in half of the trials, letters were recorded in three different intonations: rising, falling, and flat. Target letters in such ‘yes’ trials were never an exact replica of the remembered letters in the set since the intonation was always made to be different. The audible session was integrated with ECoG as previously reported (Brown et al., 2008). Subsequently, the onset and offset of stimulus set, target letter, and patient’s response were marked offline for each trial. Response time was defined as the period between target letter onset and response onset. The period between response offset and stimulus onset was jittered across trials and was 6.2 s on average across patients (standard deviation: 2.8 s).
2.4. Evaluation of ECoG amplitude changes
ECoG signals were transformed into the time-frequency domain, and we determined ‘when’ and ‘where’ high-gamma activity70–110 Hz was augmented. The time-frequency analysis used in the present study was previously described and validated (Brown et al., 2012; Kojima et al., 2013; Nishida et al., 2016; Toyoda et al., 2014). The primary measures of interest were the percent change in amplitude (a measure proportional to the square root of power) of high-gamma activity70–110 Hz relative to that during the reference period at 600–200 ms prior to stimulus onset (Supplementary Figure S1). We determined what ROIs were differentially associated with high-gamma augmentation during the maintenance and scanning periods (Fig. 1). We also determined if the degree of such high-gamma augmentation was greater during the high-load compared to low-load trials.
Trials in which the patient failed to provide a correct response were excluded from the time-frequency analysis. ECoG voltage signals during all included trials were transformed into the time-frequency domain using a complex demodulation technique (Brown et al., 2012; Hoechstetter et al., 2004; Papp and Ktonas, 1977). ECoG amplitude was measured at each channel in steps of 5 Hz and 10 ms, and high-gamma amplitude ranging from 70 to 110 Hz was calculated at each 10 ms period. We determined whether the degree of task-related augmentation of high-gamma70–110 Hz activity in each ROI reached significance using studentized bootstrap statistics (Davison and Hinkley, 1997; Terwee et al., 2010) followed by Bonferroni correction (for 275 data points for the maintenance and scanning periods; see the behavioral results below). The level of significance was set at corrected p=0.05 (a critical alpha per test was commonly 0.05/275). This approach is very conservative, and may fail to find a small difference. Furthermore, the percent change in high-gamma amplitude at each 10-ms epoch was presented at each electrode site with a Gaussian half-width at half maximum of 3 mm, and sequentially animated on the average FreeSurfer pial surface image as a function of time throughout the task (Video S1).
3. Results
3.1. Behavioral results
All patients were able to participate in the task until completion. On average, 92.5% (±6.5% standard deviation) of all working memory trials were correctly answered. There was no significant difference in the average percentage of correct responses between the low- and high-load trials (93.9% vs 91.1%, respectively; p = 0.3 on Wilcoxon Signed Rank Test). Compared to low-load trials, high-load trials were associated with a longer response time (mean response time: 1,714 ms and 1,825 ms, respectively; p = 0.02 on Wilcoxon Signed Rank Test). Since the shortest mean response time among all 19 patients was 814 ms, the response motor process may be initiated around 800 ms following target onset in some patients. Therefore, a period of 750 ms immediately following target onset was treated to be the scanning period prior to the motor process, whereas a period of 2,000 ms immediately prior to target onset was the maintenance period (Fig. 1).
3.2. Task-related high-gamma augmentation
Presentation of each letter stimulus elicited high-gamma augmentation in the superior-temporal and then pre-central gyri, bilaterally (Fig. 3A; Video S1). High-gamma augmentation in the pre-central gyri lingered during the maintenance period with left hemispheric dominance, and the degree of such pre-central high-gamma augmentation was larger during the high-load compared to low-load trials (Fig. 3B). Presentation of the target letter again elicited high-gamma augmentation in the superior-temporal and then pre-central gyri, bilaterally. Subsequently, during the scanning period, high-gamma augmentation was observed in the inferior-frontal and supra-marginal gyri (Fig. 3C). Left-hemispheric dominance of high-gamma augmentation during the maintenance period is confirmed by the supplementary analysis (Supplementary Figure S2). Finally, at response onset, high-gamma augmentation involved the pre- and post-central gyri, bilaterally (Fig. 3D). The temporal dynamics of high-gamma amplitude in each ROI are presented in Fig. 4.
Fig. 3. The spatio-temporal dynamics of task-related high-gamma activity.
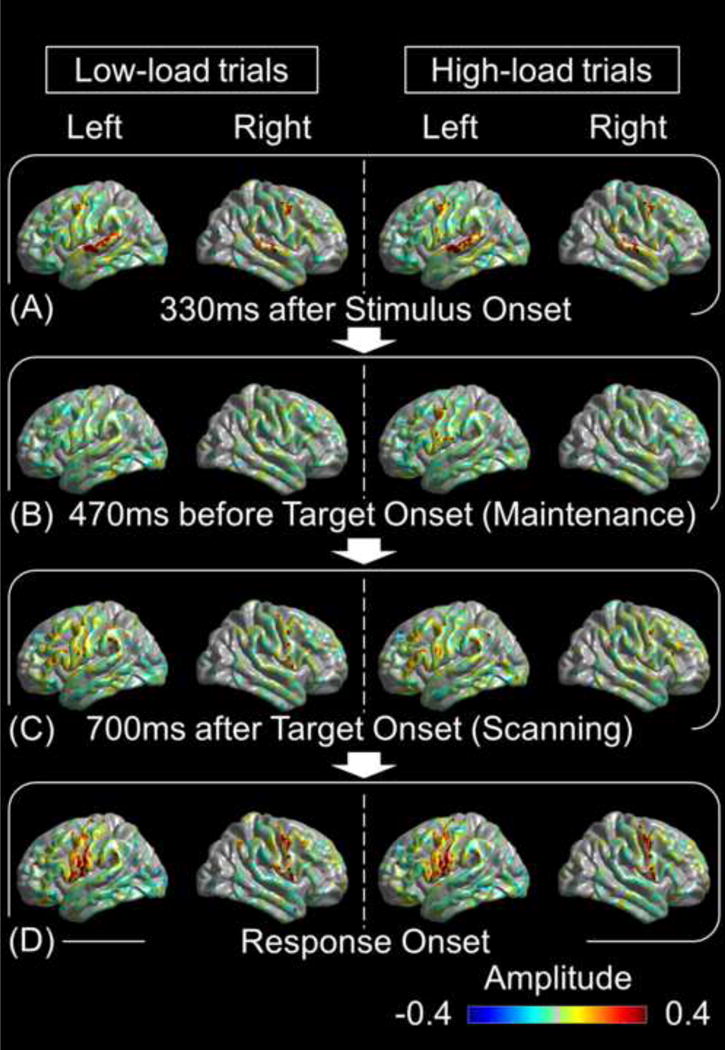
(A) Each letter stimulus elicited high-gamma augmentation in the superior-temporal and pre-central regions, bilaterally. (B) High-load trials (right) elicited high-gamma augmentation in the left pre-central region during the maintenance period. Regardless of the memory load, high-gamma augmentation involved the left inferior-frontal and supra-marginal regions during the scanning period (C), and the bilateral pre- and post-central regions at response onset (D). Amplitude of +0.4 reflects 40% increase in high-gamma amplitude compared to the reference period.
Fig. 4. Task-related high-gamma activity in regions of interest (ROIs) in the left hemisphere.
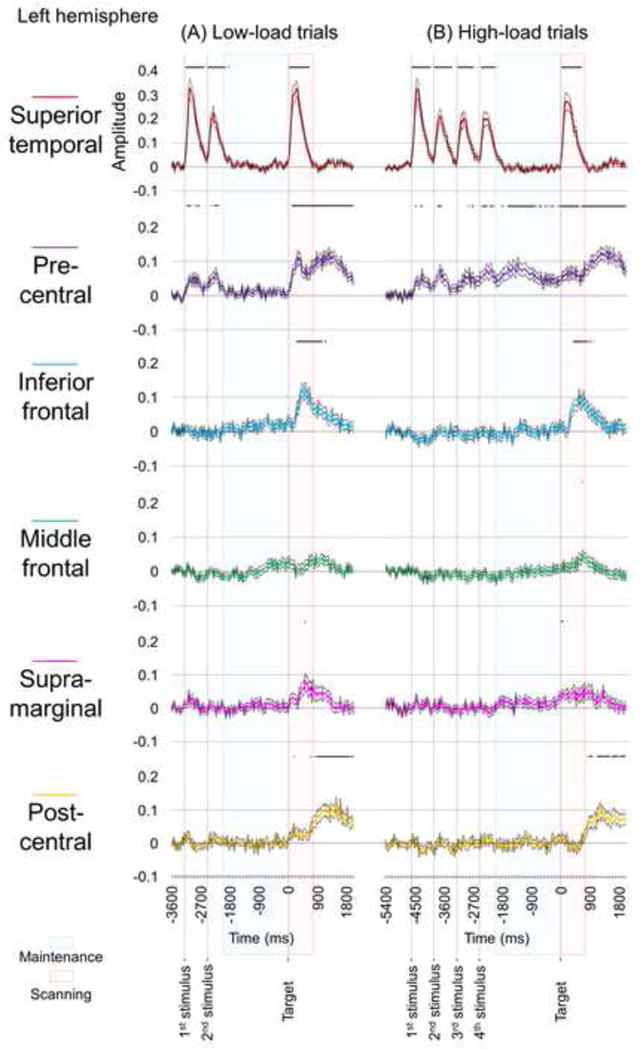
(A) Low-load trials. (B) High-load trials. Bold line: mean amplitude change in high-gamma activity across channels within a given ROI. Thin lines: standard error. Horizontal bars above plots suggest the epochs when high-gamma augmentation reached significance. Left pre-central high-gamma augmentation during the maintenance period reached significance in high-load trials but not in low-load trials (see purple lines). Amplitude of +0.1 reflects 10% increase in high-gamma amplitude compared to the reference period at 600 to 200 ms prior to stimulus onset.
The Wilcoxon Signed Rank test, employed to the six ROIs in the left hemisphere, showed that the degree of pre-central high-gamma augmentation during the 2,000-ms maintenance period was greater in high-load trials compared to low-load trials (Fig. 5A). No other ROIs in the left hemisphere showed a difference in the degree of high-gamma augmentation during the maintenance period between high-load and low-load trials. During the 750-ms scanning period, left superior-temporal and post-central high-gamma amplitudes were rather smaller in high-load trials compared to in low-load ones (Fig. 5B). The spatial-temporal dynamics of high-gamma activity of the right hemisphere is presented in Supplementary Figure S3. In the right hemisphere, no significant difference in the degree of high-gamma augmentation was noted between high-load and low-load trials during the maintenance or scanning period.
Fig. 5. High-gamma modulation in low- and high-memory load trials.
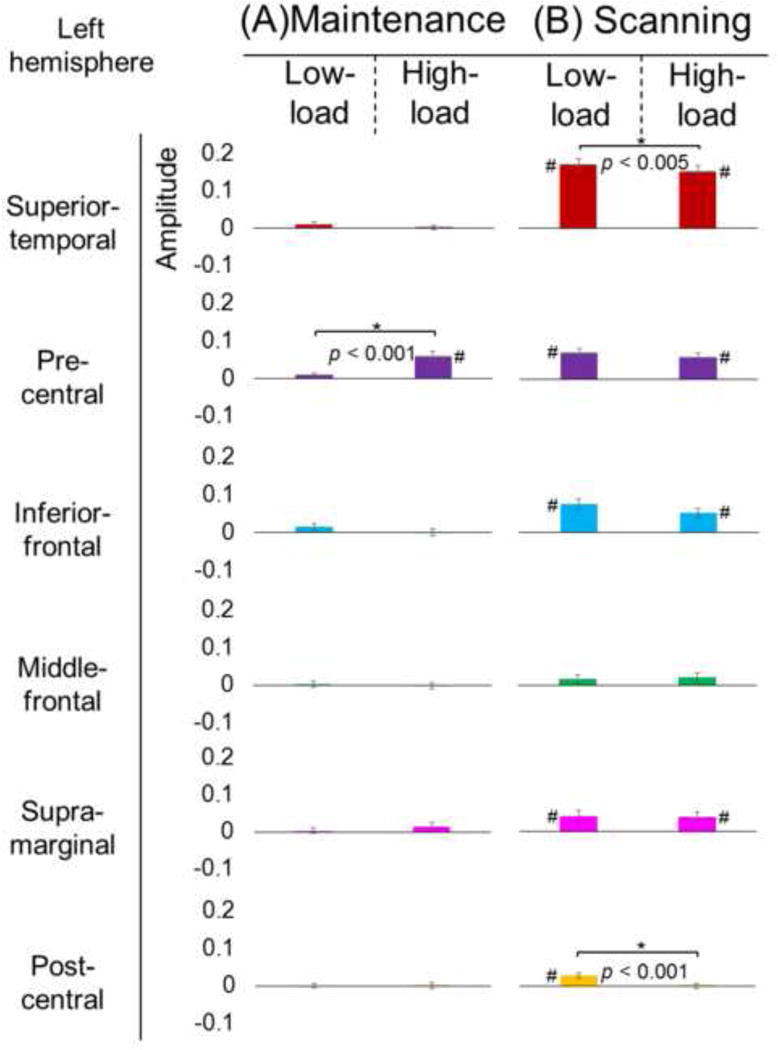
(A) During the maintenance period (a period of 2,000 ms prior to target onset), high-gamma augmentation in the left pre-central region was greater in high-load trials compared to low-load trials (mean percent changes in high-gamma amplitude: 0.9% vs 5.8%; standard error bars presented). (B) During the scanning period (a period of 750 ms following target onset), high-gamma augmentations in the left superior-temporal (16.9% vs 15.0%) and post-central regions (3.0% vs 0.0%) were rather smaller in high-load trials compared to low-load trials. *: uncorrected p-values, which survived the Bonferroni correction for six ROIs, are presented. #: gyrus showing significant augmentation of high-gamma activity70–110Hz averaged within the maintenance and scanning periods, respectively, compared to that during the reference period (studentized bootstrap test).
As a post-hoc analysis to explore the significance of high-gamma modulations during the scanning period, we compared the amplitude of high-gamma activity between the trials with the target letter included in the preceding stimulus set (‘Yes’ trials) and those without (‘No’ trials) (Wilcoxon-Signed Rank test). Within the left inferior-frontal, supra-marginal, and post-central gyri, high-gamma amplitude during the scanning period was greater during ‘Yes’ trials compared to during ‘No’ (Fig. 6).
Fig. 6. Differential high-gamma modulation during the scanning period.
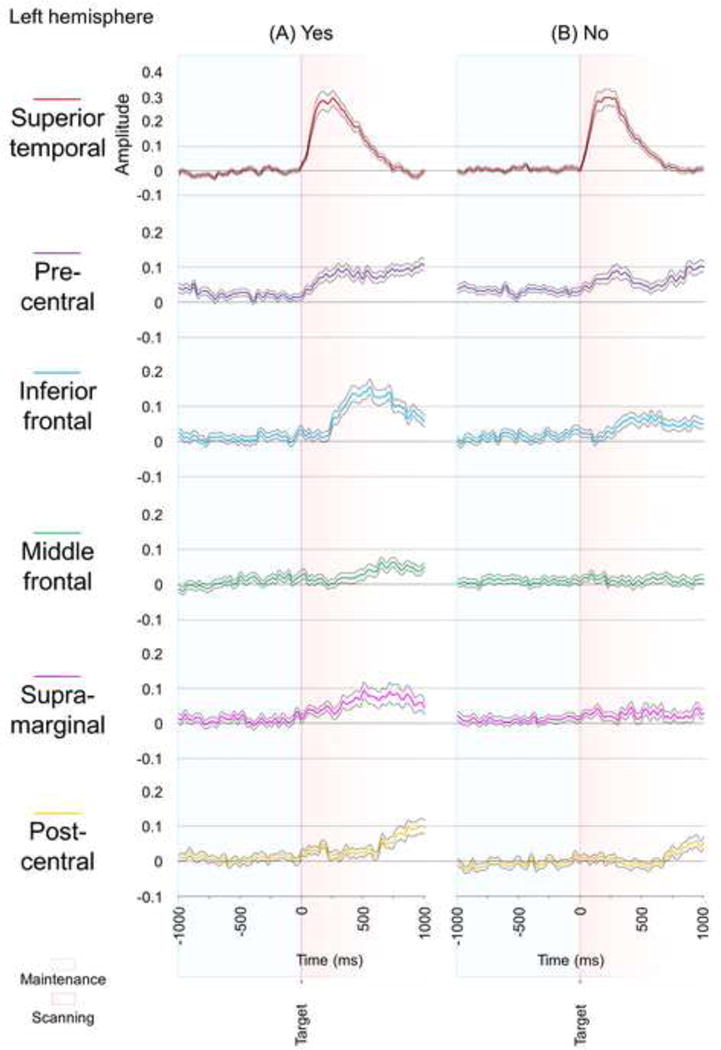
(A) ‘Yes’ trials when the target letter was included in the stimulus set for that trial. (B) ‘No’ trials. (C) ‘Yes’ trials, compared to ‘No’, were associated with larger high-gamma augmentation during the scanning period in the left inferior-frontal, supra-marginal, and post-central gyri. #: gyrus showing significant augmentation of high-gamma activity70–110Hz in ‘Yes’ and ‘No’ trials, respectively, compared to that during the reference period (studentized bootstrap test).
4. Discussion
4.1. Role of the left pre-central gyrus in verbal working memory maintenance
In this study, we successfully identified key cortical structures involved in working memory maintenance and scanning of speech stimuli. Furthermore, we provided novel evidence that short-term maintenance of auditory-verbal information is supported by the pre-central gyrus with left hemispheric dominance (Video S1). During the maintenance period, high-gamma augmentation specifically involved the left pre-central gyrus; furthermore, high-gamma augmentation differed by working memory loads, with the high-load condition associated with a significantly longer response time and greater pre-central high-gamma augmentation, compared to the low-load condition (Fig. 5). Thus, left-hemisphere dominant pre-central high-gamma augmentation sustaining during the maintenance period is strongly related to working memory maintenance of verbal stimuli. Alternatively, it is possible that bilaterally-symmetric pre-central high-gamma augmentation immediately following stimulus presentation, as best presented in Fig. 3A, could reflect sensory-motor transformations for speech sounds (Cogan et al., 2014) or automatic/short-term buffer of perceived acoustic representations (Baddeley, 1986; 2000); i.e. covert rehearsal. Further studies using electrical stimulation of the left pre-central gyrus would be useful to determine if this region plays a critical role in verbal working memory maintenance. The left pre-central ROI is a part of the dorsal and ventral attention network (Macaluso, 2010; Vossel et al., 2014), and increase in working memory load might modulate attention to stimuli.
The spatial characteristics of high-gamma modulation in our ECoG study were consistent with those reported in previous fMRI studies of working memory which employed Sternberg-type paradigms (Sternberg, 1966) in both visual and auditory domains. For example, an fMRI study of visual working memory reported that hemodynamic activation involved the occipital lobe initially during a 2–3 second encoding period, whereas subsequent activation involved the left inferior-frontal, supra-marginal, and pre-central gyri most intensely both during 3–6 second maintenance and 2 second scanning periods (Michels et al., 2010; Narayanan et al., 2005). Memory-load effects in the left pre-central gyrus were reported in some fMRI studies (Chang et al., 2007; Habeck et al., 2005; Huang et al., 2013; Kirschen et al., 2010) but not in another (Narayanan et al., 2005).
Several of the pre-central high-gamma sites associated with verbal working memory maintenance function were located over or proximal to the frontal eye field (Kirchner et al., 2009). In five patients (#4, 5, 6, 10, and 12; Table 1 and Supplementary Figure S4), we found the instance of eye deviation upon electrical brain stimulation of an electrode pair exactly overlying or immediately adjacent to the electrode associated with verbal working memory maintenance. The frontal eye fields, identified by lateral eye deviation toward the contralateral side upon electrical brain stimulation, have been shown to be involved in both fast auditory and visual processing (Brown et al., 2012, Brown et al., 2014b; Kirchner et al., 2009).
Our ECoG study was unique in that we assessed verbal working memory using an auditory Sternberg task, whereas previous ECoG studies employed a visually-based task (Axmacher et al., 2010; Howard et al., 2003; Meltzer et al., 2008; Noy et al., 2015; Raghavachari et al., 2006; Rodriguez Merzagora et al., 2014; Tertel et al., 2011). Those studies with sufficient spatial coverage reported significant augmentation of gamma to high-gamma activity at 30–150 Hz in the left pre-central gyrus during the maintenance period (Howard et al., 2003; Meltzer et al., 2008; Noy et al., 2015). Unlike in our study, few ECoG studies have employed a load manipulation and therefore satisfactorily assessed a pattern of load-dependence in pre-central high-gamma responses during the maintenance period (Roux and Uhlhaas, 2014).
The onset of left pre-central high-gamma augmentation during the scanning period preceded that in the left inferior-frontal region. Though not significant with the entire scanning period taken into account (Fig. 5B), left pre-central high-gamma augmentation in high-load trials (Fig. 4B) was somewhat smaller than that in low-load ones (Fig. 4A). The functional role of left pre-central high-gamma augmentation during the scanning period is not completely understood, and may not be limited to sensory-motor transformation of speech sounds alone (Cogan et al., 2014). A smaller degree of amplitude augmentation in high-load trials, compared to that in low-load ones, can be explained by the hypothesis that much of the neural resources in this gyrus could have been allocated to the working memory maintenance of a larger number of letter stimuli. The temporal proximity of high-gamma augmentation in the left precentral, inferior-frontal, and supra-marginal gyri raises the possibility of neural interaction exerted across these cortical structures during the scanning period. Many of the neurobiological models of language propose that the left inferior-frontal region receives mental representations of speech sounds primarily from the temporal neocortex rather than the pre-central gyrus (Rauschecker and Scott, 2009; Skeide and Friederici, 2016). Further studies using network analysis (Flinker et al., 2015) may better determine how the superior-temporal, pre-central, inferior-frontal, and supra-marginal gyri interact with each other when the human brain determines a match among previously encountered speech items.
4.2. Role of the left inferior-frontal and supra-marginal gyri in working memory scanning
In this ECoG study, we identified high-gamma augmentation in the left inferior-frontal and supra-marginal gyri during scanning to determine a match for an auditory stimulus. This effect was particularly large when participants indeed found a match (Fig. 6). This is consistent with the results of previous neuroimaging (Chen and Desmond, 2005; Paulesu et al., 1993) and ECoG studies (Howard et al., 2003; Mainy et al., 2007) showing hemodynamic or electrophysiological activation during working memory tasks. A plausible interpretation of these activations is that left inferior-frontal high-gamma augmentations reflects the attentive judgement of a match, whereas left supra-marginal augmentations reflects the retrieval of phonological information. This interpretation is in part derived from the report that left inferior-frontal hemodynamic activation can be induced by non-verbal visual working memory, whereas working memory tasks using verbal stimuli are often associated with left supra-marginal gyrus activation (Cohen et al., 1997; Courtney et al., 1997; Crottaz-Herbette et al., 2004).
In this ECoG study, we failed to find load-dependent high-gamma modulations in left inferior-frontal and supra-marginal regions, unlike some but not all fMRI studies of visual verbal working memory (Braver et al., 1997; Cairo et al., 2004; Chen and Desmond, 2005). This null effect in our ECoG study could be attributed to the experimental design; each patient was assigned only 2- or 4-letter trials. Conversely, participants in fMRI studies were often given 6-letter trials. Since our patients underwent a working memory task under an invasive presurgical evaluation, we designed a relatively easy task. Nonetheless, our behavioral results indicated that 4-letter (high-load) trials, compared to 2-letter (low-load) trials, took a longer time to execute, and were associated with a load-effect in the left pre-central gyrus as discussed above.
4.3. Significance of high-gamma attenuation in the left superior-temporal and post-central gyri
Left superior-temporal high-gamma activity was minimally augmented during the maintenance period and significantly augmented at the beginning of the scanning period (Fig. 4). Trials with a match (‘Yes’ trials) and those without (‘No’ trials) were associated with a similar degree of left superior-temporal high-gamma augmentation during the scanning period (Fig. 6AB), whereas ‘Yes’ trials, compared to ‘No’, showed larger high-gamma augmentation later seen in the left inferior-frontal and supra-marginal regions (Fig. 6C). If match-preferential high-gamma augmentation in the left inferior-frontal or supra-marginal regions is interpreted to reflect the output of the scanning process, a plausible candidate for the structure exerting true scanning activity may be the cortical network across the left superior-temporal and the aforementioned frontal-parietal regions.
High-load trials were associated with reduced high-gamma augmentation in the left superior-temporal gyrus during the scanning period (Fig. 5B). Such reduced high-gamma activity during high-load trials may be, in part, attributed to the phenomenon of ‘repetition suppression/neural habituation’ (Engell and McCarthy, 2014; Matsuzaki et al., 2012), since the high-load trials were intrinsically associated with a larger number of voice stimuli preceding the target onset. Alternatively, reduced high-gamma augmentation in high-load trials may be explained by the notion that greater attention to high-load trials resulted in greater suppression (or reduced activation) of primary sensory functions (i.e.: reduced attention to external sounds or sensations, increased selectivity of attention with increased working memory load) which may be obtrusive at the moment in which the scanning process is executed. Reduction of high-gamma activation in the left superior-temporal cortex may have been induced by increased working memory maintenance load in the left pre-central gyrus via the arcuate fasciculus (Brown et al., 2014a); likewise, suppression of the post-central gyrus may have been mediated via U-fibers between pre- and post-central gyri. Such transient suppression of the sensory cortex has been reported in the primary auditory cortex during retrieval of a relevant answer for a question (Towle et al., 2008), in the primary somatosensory cortex during articulation of phonemes (Toyoda et al., 2014), and in the primary visual cortex for the peripheral vision during attention directed to central vision (Uematsu et al., 2013).
5. Conclusion
In this study we have generated rich and unique data identifying working memory processing associated with verbal auditory stimuli. The left pre-central gyrus was identified here as a seat of verbal working memory maintenance for auditory stimuli with a ‘dose-response’ correlating with working memory load. Working memory scanning function for auditory stimuli was localized primarily in the inferior-frontal gyrus, within Broca’s area, and the supra-marginal gyrus of the left hemisphere, and this observation highlights the role of traditional language cortex in auditory verbal working memory function.
A particular region of cortex, midway up the lateral surface of the left pre-central gyrus, appears to be rich with various functions. While this study was not designed to explore this region specifically, we believe that further study of this gyrus proximal to the frontal eye fields may yield new insights into the cortical function of language, working memory, and beyond. The clinical significance of sites in this particular region of the pre-central gyrus also requires further investigation.
Supplementary Material
Red: High-gamma augmentation. Blue: High-gamma attenuation. Amplitude of +0.1 reflects 10% increase in high-gamma amplitude compared to the reference period at 600 to 200 ms prior to stimulus onset.
Highlights.
-
-
Intracranial electrocorticography recordings revealed high-gamma augmentation during working memory maintenance in left pre-central gyrus.
-
-
Such pre-central high-gamma augmentation was larger when memory load was increased.
-
-
Left inferior-frontal and supra-marginal gyri showed high-gamma augmentation during scanning.
Acknowledgments
This work was supported by NIH grants NS64033 (to E. Asano) and MH107512 (to N. Ofen) as well as the intramural grant from Children’s Hospital of Michigan Foundation (to E. Asano). We are grateful to Sandeep Sood, MD, Aashit Shah, MD, Sandeep Mittal, MD, Robert Rothermel, PhD, Alanna Carlson, MS, LLP, Carol Pawlak, REEG/EPT at Detroit Medical Center, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None of the authors have potential conflicts of interest to be disclosed.
References
- Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanuma N, Alarcón G, Lum F, Kissani N, Koutroumanidis M, Adachi N, et al. Lateralising value of neuropsychological protocols for presurgical assessment of temporal lobe epilepsy. Epilepsia. 2003;44:408–18. doi: 10.1046/j.1528-1157.2003.24502.x. [DOI] [PubMed] [Google Scholar]
- Asano E, Brown EC, Juhász C. How to establish causality in epilepsy surgery. Brain Dev. 2013;35:706–20. doi: 10.1016/j.braindev.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132:1038–47. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci USA. 2010;107:3228–33. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychol Sci. 1996;7:25–31. [Google Scholar]
- Baddeley A. Working Memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brown EC, Jeong JW, Muzik O, Rothermel R, Matsuzaki N, Juhász C, et al. Evaluating the arcuate fasciculus with combined diffusion-weighted MRI tractography and electrocorticography. Hum Brain Mapp. 2014a;35:2333–47. doi: 10.1002/hbm.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Muzik O, Rothermel R, Juhász C, Shah AK, Fuerst D, et al. Evaluating signal-correlated noise as a control task with language-related gamma activity on electrocorticography. Clin Neurophysiol. 2014b;125:1312–23. doi: 10.1016/j.clinph.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Muzik O, Rothermel R, Matsuzaki N, Juhász C, Shah AK, et al. Evaluating reverse speech as a control task with language-related gamma activity on electrocorticography. Neuroimage. 2012;60:2335–45. doi: 10.1016/j.neuroimage.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, et al. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–31. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo TA, Liddle PF, Woodward TS, Ngan ET. The influence of working memory load on phase specific patterns of cortical activity. Brain Res Cogn Brain Res. 2004;21:377–87. doi: 10.1016/j.cogbrainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Chan AM, Baker JM, Eskandar E, Schomer D, Ulbert I, Marinkovic K, et al. First-pass selectivity for semantic categories in human anteroventral temporal lobe. J Neurosci. 2011;31:18119–29. doi: 10.1523/JNEUROSCI.3122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–69. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005;24:332–8. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Cogan GB, Thesen T, Carlson C, Doyle W, Devinsky O, Pesaran B. Sensory-motor transformations for speech occur bilaterally. Nature. 2014;507:94–8. doi: 10.1038/nature12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–8. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–11. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–95. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Anagnoson RT, Menon V. Modality effects in verbal working memory: differential prefrontal and parietal responses to auditory and visual stimuli. Neuroimage. 2004;21:340–51. doi: 10.1016/j.neuroimage.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge University Press; 1997. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dykstra AR, Chan AM, Quinn BT, Zepeda R, Keller CJ, Cormier J, et al. Individualized localization and cortical surface-based registration of intracranial electrodes. Neuroimage. 2012;59:3563–70. doi: 10.1016/j.neuroimage.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell AD, McCarthy G. Repetition suppression of face-selective evoked and induced EEG recorded from human cortex. Hum Brain Mapp. 2014;35:4155–62. doi: 10.1002/hbm.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, Crone NE. Redefining the role of Broca’s area in speech. Proc Natl Acad Sci USA. 2015;112:2871–5. doi: 10.1073/pnas.1414491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, Asano E. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131:1793–805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SS, Kakunoori S, Augustinack J, Nieto-Castanon A, Kovelman I, Gaab N, et al. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. Neuroimage. 2010;53:85–93. doi: 10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, et al. An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2005;23:207–20. doi: 10.1016/j.cogbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–8. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–74. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Huang S, Seidman LJ, Rossi S, Ahveninen J. Distinct cortical networks activated by auditory attention and working memory load. Neuroimage. 2013;83:1098–108. doi: 10.1016/j.neuroimage.2013.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Châtillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–37. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H, Barbeau EJ, Thorpe SJ, Regis J, Liegeois-Chauvel C. Ultra-rapid sensory responses in the human frontal eye field region. J Neurosci. 2009;29:7599–7606. doi: 10.1523/JNEUROSCI.1233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Desmond JE. Modality specific cerebro-cerebellar activations in verbal working memory: an fMRI study. Behav Neurol. 2010;23:51–63. doi: 10.3233/BEN-2010-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–8. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Fuerst D, Matsuzaki N, et al. Clinical significance and developmental changes of auditory-language-related gamma activity. Clin Neurophysiol. 2013;124:857–69. doi: 10.1016/j.clinph.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Matsuzaki N, Shah A, Atkinson M, Mittal S, Fuerst D, Sood S, Asano E. Multimodality language mapping in patients with left-hemispheric language dominance on Wada test. Clin Neurophysiol. 2012;123:1917–24. doi: 10.1016/j.clinph.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthardt EC, Miller K, Anderson NR, Schalk G, Dowling J, Miller J, Moran DW, Ojemann JG. Electrocorticographic frequency alteration mapping: a clinical technique for mapping the motor cortex. Neurosurgery. 2007;60:260–70. doi: 10.1227/01.NEU.0000255413.70807.6E. [DOI] [PubMed] [Google Scholar]
- Macaluso E. Orienting of spatial attention and the interplay between the senses. Cortex. 2010;46:282–97. doi: 10.1016/j.cortex.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Neural correlates of consolidation in working memory. Hum Brain Mapp. 2007;28:183–93. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki N, Nagasawa T, Juhász C, Sood S, Asano E. Independent predictors of neuronal adaptation in human primary visual cortex measured with high-gamma activity. Neuroimage. 2012;59:1639–46. doi: 10.1016/j.neuroimage.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki N, Schwarzlose RF, Nishida M, Ofen N, Asano E. Upright face-preferential high-gamma responses in lower-order visual areas: evidence from intracranial recordings in children. Neuroimage. 2015;109:249–59. doi: 10.1016/j.neuroimage.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, et al. Effects of working memory load on oscillatory power in human intracranial EEG. Cereb Cortex. 2008;18:1843–55. doi: 10.1093/cercor/bhm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L, Bucher K, Lüchinger R, Klaver P, Martin E, Jeanmonod D, et al. Simultaneous EEG-fMRI during a working memory task: modulations in low and high frequency bands. PLoS One. 2010;5:e10298. doi: 10.1371/journal.pone.0010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Zou G, Hua J, Lu Y, Lu S, et al. Multimodality data integration in epilepsy. Int J Biomed Imaging. 2007;2007:13963. doi: 10.1155/2007/13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Juhász C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: intracranial recording in epileptic patients. Hum Brain Mapp. 2012;33:569–83. doi: 10.1002/hbm.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Rothermel R, Juhász C, Fukuda M, Nishida M, Akiyama T, et al. Cortical gamma-oscillations modulated by auditory-motor tasks-intracranial recording in patients with epilepsy. Hum Brain Mapp. 2010;31:1627–42. doi: 10.1002/hbm.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JD. The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related FMRI analysis. Neuropsychology. 2005;19:223–32. doi: 10.1037/0894-4105.19.2.223. [DOI] [PubMed] [Google Scholar]
- Nishida M, Zestos MM, Asano E. Spatial-temporal patterns of electrocorticographic spectral changes during midazolam sedation. Clin Neurophysiol. 2016;127:1223–32. doi: 10.1016/j.clinph.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoda Y, Miyakoshi M, Ojeda A, Makeig S, Juhász C, Sood S, et al. Interictal high-frequency oscillations generated by seizure onset and eloquent areas may be differentially coupled with different slow waves. Clin Neurophysiol. 2016;127:2489–99. doi: 10.1016/j.clinph.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy N, Bickel S, Zion-Golumbic E, Harel M, Golan T, Davidesco I, et al. Intracranial recordings reveal transient response dynamics during information maintenance in human cerebral cortex. Hum Brain Mapp. 2015;36:3988–4003. doi: 10.1002/hbm.22892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–45. [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–5. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Pieters TA, Conner CR, Tandon N. Recursive grid partitioning on a cortical surface model: an optimized technique for the localization of implanted subdural electrodes. J Neurosurg. 2013;118:1086–97. doi: 10.3171/2013.2.JNS121450. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95:1630–8. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–24. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Merzagora A, Coffey TJ, Sperling MR, Sharan A, Litt B, Baltuch G, et al. Repeated stimuli elicit diminished high-gamma electrocorticographic responses. Neuroimage. 2014;85:844–52. doi: 10.1016/j.neuroimage.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ. Working memory and neural oscillations: α-γ versus θ-γ codes for distinct WM information? Trends Cogn Sci. 2014;18:16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Ruescher J, Iljina O, Altenmüller DM, Aertsen A, Schulze-Bonhage A, Ball T. Somatotopic mapping of natural upper- and lower-extremity movements and speech production with high gamma electrocorticography. Neuroimage. 2013;81:164–77. doi: 10.1016/j.neuroimage.2013.04.102. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, Holland SK. BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15:2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeide MA, Friederici AD. The ontogeny of the cortical language network. Nat Rev Neurosci. 2016;17:323–32. doi: 10.1038/nrn.2016.23. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–4. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertel K, Tandon N, Ellmore TM. Probing brain connectivity by combined analysis of diffusion MRI tractography and electrocorticography. Comput Biol Med. 2011;41:1092–9. doi: 10.1016/j.compbiomed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwee CB, Roorda LD, Dekker J, Bierma-Zeinstra SM, Peat G, Jordan KP, Croft P, de Vet HC. Mind the MIC: large variation among populations and methods. J Clin Epidemiol. 2010;63:524–34. doi: 10.1016/j.jclinepi.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, et al. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–27. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda G, Brown EC, Matsuzaki N, Kojima K, Nishida M, Asano E. Electrocorticographic correlates of overt articulation of 44 English phonemes: intracranial recording in children with focal epilepsy. Clin Neurophysiol. 2014;125:1129–37. doi: 10.1016/j.clinph.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu M, Matsuzaki N, Brown EC, Kojima K, Asano E. Human occipital cortices differentially exert saccadic suppression: Intracranial recording in children. Neuroimage. 2013;83:224–36. doi: 10.1016/j.neuroimage.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–9. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fifer MS, Flinker A, Korzeniewska A, Cervenka MC, Anderson WS, Boatman-Reich DF, Crone NE. Spatial-temporal functional mapping of language at the bedside with electrocorticography. Neurology. 2016;86:1181–9. doi: 10.1212/WNL.0000000000002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer J, von Oertzen J, Schaller C, Urbach H, König R, Widman G, et al. Digital photography and 3D MRI-based multimodal imaging for individualized planning of resective neocortical epilepsy surgery. Epilepsia. 2002;43:1543–50. doi: 10.1046/j.1528-1157.2002.30002.x. [DOI] [PubMed] [Google Scholar]
- Wood AG, Harvey AS, Wellard RM, Abbott DF, Anderson V, Kean M, et al. Language cortex activation in normal children. Neurology. 2004;63:1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jiruska P, Zelmann R, Leijten FS, Jefferys JG, Gotman J. High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71:169–78. doi: 10.1002/ana.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Red: High-gamma augmentation. Blue: High-gamma attenuation. Amplitude of +0.1 reflects 10% increase in high-gamma amplitude compared to the reference period at 600 to 200 ms prior to stimulus onset.


