Abstract
A new model of diabetic nephropathy in T1D emerged from our studies of Joslin Clinic patients. The dominant feature is progressive renal decline, not albuminuria. This decline is a unidirectional process commencing while patients have normal renal function and, in the majority, progresses steadily (linearly) to ESRD. While an individual’s rate of renal decline is constant, the eGFR slope varies widely among individuals from −72 to −3.0 ml/min/year. KDIGO guidelines define rapid progression as rate of eGFR decline <−5 ml/min/year, a value exceeded by 80% of patients in Joslin’s T1D ESRD cohort. The extraordinary range of slopes within the rapid progression category prompted us to partition it into “very fast”, “fast” and “moderate” decline. We showed for the first time that very fast and fast decline from normal eGFR to ESRD within 2–10 years constitutes 50% of the Joslin cohort. In this review we present data about frequency of fast decliners in both diabetes types, survey some mechanisms underlying fast renal decline, discuss methods of identifying patients at risk and comment on the need for effective therapeutic interventions.
Whether the initiating mechanism of fast renal decline affects glomerulus, tubule, interstitium or vasculature is unknown. Since no animal model mimics progressive renal decline, studies in humans are needed. Prospective studies searching for markers predictive of the rate of renal decline yield findings that may make detection of fast decliners feasible. Recognizing such patients will be the foundation for developing effective personalized methods to prevent or delay onset of ESRD in diabetes.
Keywords: chronic kidney disease, diabetic nephropathy
1. Risk of ESRD in Type 1 Diabetes Remains High
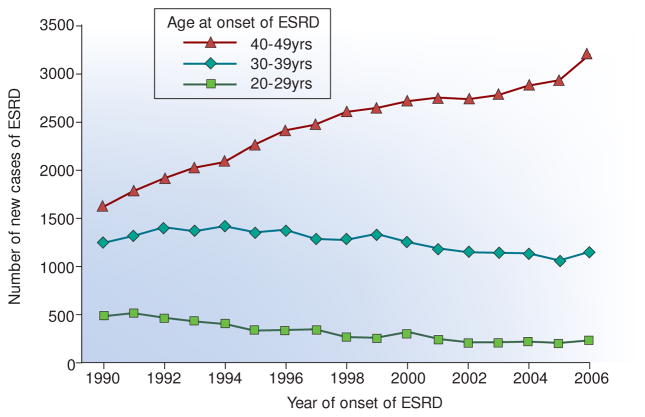
For Type 1 diabetes (T1D) patients in the USA, ESRD remains the major cause of premature morbidity and mortality. Lifetime risk of ESRD for these patients is 10–15%1–3. Typically, it develops decades after the onset of T1D and results from a period of progressive renal decline.1, 3–6 Despite almost universal implementation of reno-protective therapies over the last 20 years and attempts to improve glycemic control, the risk ESRD in T1D is not decreasing but increasing.4 Based on data from the US Renal Data System (http://www.usrds.org/), the number of new cases of ESRD attributed to T1D in the US Caucasian population increased steadily from 3400 in 1990 to 4600 in 2006, an average increase of about 9% per year. The age distribution of cases, however, changed as shown In Figure 1. While the number of cases aged 20–39 years declined slightly, the number of cases aged 40–49 years almost doubled. This pattern suggests the risk of ESRD increased at the same time that improved care of T1D during the last 20 years, perhaps, postponed onset of ESRD to a longer duration of T1D and older age (see data in Table 1).
Figure 1.
Number of new cases of ESRD in the white US population attributed to diabetes between 1990–2006 according to age of onset of ESRD. In these patients, T1DM is the predominant diabetes and the almost exclusive type in those with sufficient diabetes duration to develop diabetes-associated ESRD.
The data were obtained from the US Renal Data System (http://www.usrds.org/). Figure adapted from Rosolowsky et al. JASN 2012.4
Using data presented in Table 1, one may interpret that patients with ESRD onset between aged 20–39 years were enriched for fast decliners and those 40–49 years for moderate and slow decliners.
Table 1.
Characteristics of the Joslin ESRD Cohort according to category of progressive renal decline
| CATEGORY OF PROGRESSIVE RENAL DECLINE | |||||
|---|---|---|---|---|---|
| Characteristics | Very Fast | Fast | Moderate | Slow | |
| N=89 | N=79 | N=123 | N=73 | P | |
| Men | 40.5% | 58.2% | 56.1% | 48.0% | 0.28 |
| Age at T1D Dx (y) | 13 (9, 18) | 12 (7, 16) | 13 (8, 17) | 10 (6, 16) | 0.08 |
| At entry to follow-up (first serum creatinine counted): | |||||
| Duration of T1D (y) | 17 (14, 22) | 19 (15, 28) | 18 (15, 25) | 23 (18, 28) | 0.13* |
| Age (y) | 32 (27, 38) | 34 (28, 40) | 33 (25, 40) | 33 (29, 42) | 0.22* |
| HbA1c (%) | 10.6 (8.7, 12.4) | 9.8 (8.7, 11.0) | 9.2 (8.2, 10.0) | 8.9 (7.9, 10.3) | <0.001 |
| ACR g albumin/g creatinine | 1.8 (0.5, 3.3) | 1.9 (0.5, 2.6) | 1.1 (0.5, 2.3) | 0.8 (0.5, 1.7) | 0.015 |
| eGFR ml/min | 84 (67, 108) | 78 (63, 103) | 77 (63, 97) | 49 (38, 69) | <0.001 |
| During follow-up | |||||
| eGFR slopes (ml/min/year) | −22 (−31, −18) | −12 (−13, −11) | −7.5 (−8.7, −6.4) | −3.7 (−4.2, −2.7) | By design |
| Time to ESRD (y) | 3.2 (2.2, 4.0) | 5.7 (4.4, 7.1) | 8.9 (6.7, 11.7) | 10.7 (7.6, 14.5) | By design |
| At onset of ESRD | |||||
| Duration of T1D at ESRD onset(y) | 20 (17, 26) | 25 (22, 33) | 28 (24, 34) | 34 (31, 40) | <0.001* |
| Age at ESRD onset (y) | 36 (30, 41) | 40 (34, 46) | 42 (36, 47) | 46 (41, 53) | <0.001* |
Data are median (1st, 3rd quartiles)
Re-analyzed data from Skupien et al. Diabete Care 2016.6
Limited success in reducing the risk of ESRD in T1D, as reflected by the numbers in Figure 1, provides motivation to seek new strategies for developing more effective therapeutic programs to prevent ESRD or postpone its onset by many years or even decades, i.e. into the fifties and sixties. In this review, we focus on the subset of diabetic patients whose renal function loss is so fast that only an interval of 2 to less than 10 years separates normal function from ESRD.6 We call them “fast decliners”, and they account for the majority of ESRD cases in T1D. Our aim is to spotlight this subgroup of diabetic patients that deserves special attention and is in urgent need of more effective interventions. As the underlying pathophysiology may differ from patient to patient, the treatment may need to be individualized, i.e. a need for precision medicine. As literature relevant to fast decliners is lacking, our review predominantly uses published, reanalyzed and unpublished results for T1D and T2D obtained from the Joslin Kidney Studies. As the concept of “progressive renal decline as a new paradigm of diabetic nephropathy” was previously reviewed by us, only some aspects of it will be discussed briefly.7
2. Joslin Kidney Studies
Established more than a century ago to provide long term diabetes management for patients of all ages, Joslin Clinic currently follows about 4000 T1D and 16,000 T2D patients. The majority resides in Eastern Massachusetts and 85% are Caucasian. A large proportion remains under the Clinic care for a long time. Over the last 20 years we have enrolled these patients into our Joslin Kidney Studies. The studies include the Joslin Proteinuria4,5 and ESRD Cohorts,6 cross-sectional groups of patients for studies of the genetics of diabetic nephropathy,7–10 and multiple cohorts of patients assembled for prospective follow-up studies that focused on determinants of albuminuria11,12 and early and late renal function decline in T1D4,13–18 and T2D.19–20 Altogether about 5000 Joslin patients (half with T1D) have been enrolled.
3. How do patients with diabetes progress to ESRD?
The Joslin ESRD Cohort
To generate empirical data about trajectories of the decline of renal function to ESRD, we assembled the Joslin T1D ESRD Cohort6. This cohort comprises 364 Caucasian residents of Eastern Massachusetts with T1D who were under the care of the Joslin Clinic for many years and developed ESRD between 1991 and 2011. These patients could be considered as a representation of the patients depicted in Figure 1. Characteristics of our cohort are typical of T1D patients (see Table 1). From the archives of the Joslin Clinical Laboratory, we were able to retrieve many years of serum creatinine determinations before onset of ESRD for the majority of them and used the determinations to trace eGFR trajectories preceding its onset. The median interval spanned by the determinations was 6.6 years (1st and 3rd quartiles 4.2 and 9.9 years, respectively).
For most, renal replacement therapy began with dialysis. A few (4.7%) received a preemptive transplant, and the proportion of such early interventions has recently doubled.4 Renal replacement therapy was not registered in the USRDS for 17.3% of the cohort because they died at diagnosis of ESRD or soon after.
Renal function declines linearly to ESRD
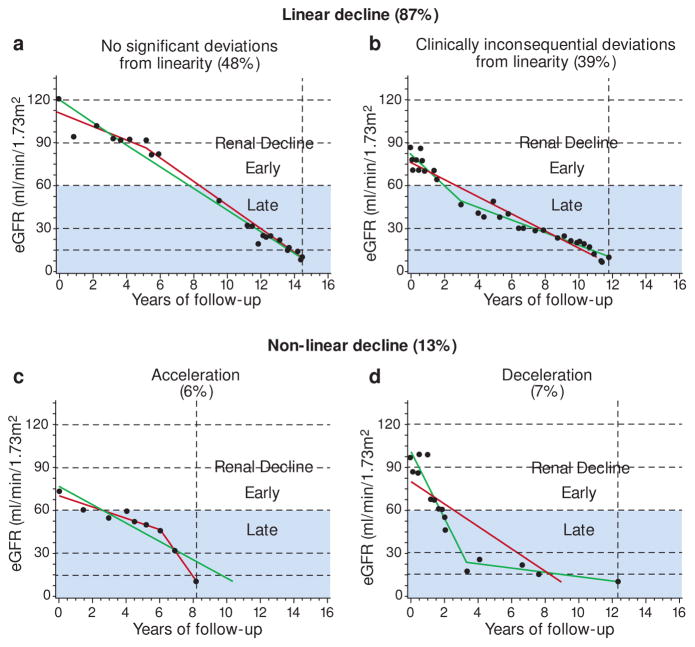
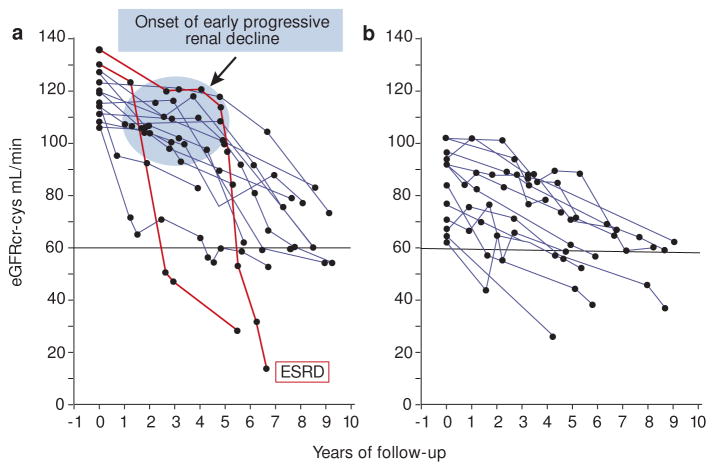
While most eGFR trajectories appeared linear by inspection, we sought to validate this impression statistically by fitting both linear and spline models to each patient’s data.6 For 48% of patients, the linear model was not rejected statistically and their trajectories were classified as linear (Figure 2A). For another 39% of the patients the data rejected the linear model but the non-linearity had a clinically inconsequential effect on the predicted time of ESRD (Figure 2B). Thus, linear renal decline was seen in the vast majority of patients (i.e. 87%). The remaining 13% of trajectories were sufficiently non-linear to have clinically consequential impacts on the anticipated time to ESRD: the trajectories of 6% of the patients accelerated (Figure 2C) and 7% decelerated (Figure 2D). In summary, a linear inexorable progression to ESRD was the predominate pattern of eGFR decline in this large cohort of T1D – the least biased study group reported so far. This is in agreement with our previous findings in the Joslin Proteinuria Cohort.5
Figure 2.
Examples and frequencies of trajectories of eGFR decline in the Joslin ESRD cohort, classified as linear or non-linear by a linear spline approach.
Trajectories were classified by comparing linear (solid gray line) and spline (solid black line) regression models. Dots represent eGFR observations, horizontal gridlines are boundaries of CKD stages, and vertical interrupted line indicates onset of ESRD. Grey area distinguishes late from early progressive renal decline.
Figure adapted from Skupien et al. Diabetes Care 2016.6
Linearity of renal decline was postulated four decades ago for various kidney conditions.21 However, those studies were unpersuasive due to various shortcomings. The reciprocal of serum creatinine concentration, used to approximate GFR, has been plotted to predict ESRD onset.21–23 Subsequent studies using other creatinine-based eGFRs to trace trajectories included only a few patients reaching ESRD. Some studies concluded that the trajectories were linear 22, 24–26 while others found significant variation and non-linearity.27–29 All these studies were small, included many patients with advanced renal failure, and often had short follow-up time. Our study in a large cohort of T1D patients who progressed to ESRD overcomes these shortcomings.
Renal decline to ESRD in the majority of the cohort was fast
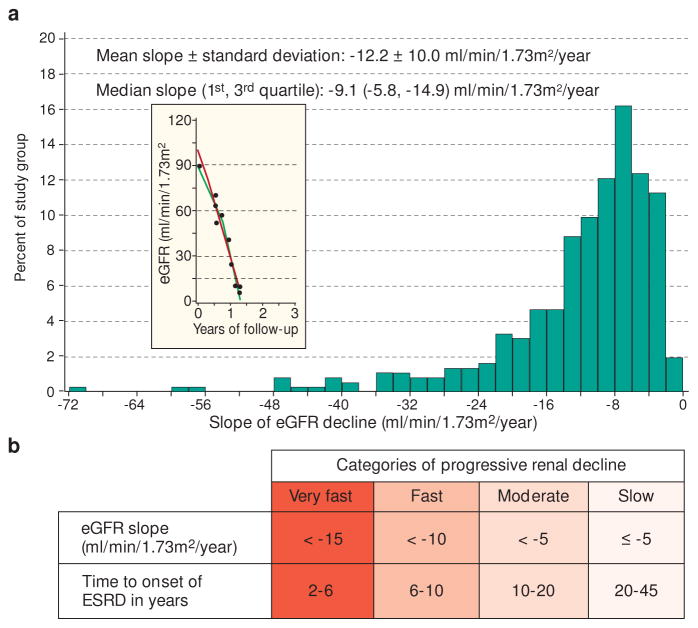
To characterize the distribution of rates of eGFR decline in the Joslin ESRD Cohort, we extracted the linear component of each trajectory as a simple slope. The distribution of eGFR slopes was highly skewed with the tail to the left – toward steeply negative slopes (Figure 3, Panel A). The first quartile was a slope of −14.9 ml/min/year, the median a slope of −9.1 ml/min/year, and the third quartile a slope of −5.8 ml/min/year. Thus, 80% of this cohort meets KDIGO guidelines’ definition of rapid progression, a rate of eGFR decline exceeding 5 ml/min/year.30
Figure 3.
Distribution of eGFR slopes in 364 patients in the Joslin ESRD cohort
Panel A: Histogram of the distribution of slopes of eGFR; insert – a trajectory of renal decline in a patient with the steepest slope(indicated with the arrow).
Panel B: Proposed categories for the slopes in Panel A
Re-analyzed data from Skupien et al. Diabete Care 2016.6
The dramatic difference between the steepness of eGFR slopes in this large cohort of T1D patients with ESRD, reported for the first time, and the slopes reported in the literature cannot have escaped the reader’s notice. Published findings were derived from follow-up studies of non-decliners mixed with decliners with quite variable rates of decline, including many with little chance of progressing to ESRD during life time. As a result, the median eGFR slope in the published studies was very shallow and varied from −4.0 to −1.5 ml/min/year30 Joslin data in Table 2.
Table 2.
Distribution of categories of progressive renal decline during 6–10 years of follow-up in patients with T1D (Panel A) or T2D (Panel B) in the Joslin Kidney Studies according to category of albuminuria at entry into follow-up. At entry, eGFR was normal in all patients.
| eGFR Loss per mL/yeara | Normo-Albuminuria | Micro-Albuminuria | Proteinuria | Total |
|---|---|---|---|---|
| % (N) | % (N) | % (N) | % (N) | |
| A. In Type 1 Diabetes | ||||
| <2.9 | 91% | 78% | 49% | 81% |
| 3–4.9 | 6% | 11% | 16% | 8% |
| 5–9.9 | 2% | 7% | 19% | 7% |
| > 10 | 1% | 4% | 16% | 4% |
| Total | 100% (932)b | 100% (525)b | 100% (275)c | 100% (1732) |
| B. In Type 2 Diabetes | ||||
| <2.9 | 80% | 67% | 32% | 72% |
| 3–4.9 | 13% | 18% | 17% | 15% |
| 5–9.9 | 6% | 12% | 30% | 10% |
| > 10 | 1% | 3% | 21% | 3% |
| Total | 100% (681) | 100% (418) | 100% (82) | 100% (1181)d |
Methods of serum creatinine determination and calibration for all patients involved in the Joslin Kidney Studies are described in Skupien et al. Diabetes Care 2016.4 eGFR slopes were estimated using linear regression and multiple serum creatinine measurments performed during 6–12 years follow-up observations.
Patients from the 1st and 2nd Joslin Kidney study in T1D were included. More information about these studies are provided in publications by Perkins et al10 and Krolewski et al.17
Patients from the Joslin Proteinuria Cohort, Skupien et al. Kidney Int. 2012.5
Patients from the 2nd Joslin Kidney study in T2D were included, (Krolewski et al. personal communication).
Not only did 4 out of 5 slopes in the Joslin ESRD cohort qualify as rapid progression according to the KIDGO guidelines, but they also spanned an extraordinarily wide range from, −72 to −5.0 ml/min/year. Such heterogeneity carries implications of an equally heterogeneous etiology and potentially diverse strategies for clinical management. So, we subdivided the whole range of rapid progression (in ml/min/year) into three partitions/categories: (−72 to −15), (−15 to −10) and (−10 to −5). The non-rapid progression (≥ −5 (ml/min/year) constitutes a fourth group. These divisions between categories are close to the exact quartile values −14.9, −9.1, −5.8 (ml/min/year) so the four groups are roughly equal. An alternative way of representing slopes is to express them as the estimated time needed to reach ESRD from a starting point such as an eGFR of 100 ml/min (Figure 3, Panel B). The ranges in the four categories of slopes, when translated into ranges of time to ESRD yielded: 2–6 years, 6–10 years, 10–20 years and 20–45 years. These values suggested names for the categories: Very Fast, Fast, Moderate, and Slow, respectively.
At present, we have no etiologic or pathophysiologic basis for categorizing the diversity of slopes. Transforming the ranges of slopes to ranges of time gives a helpful guide to thinking about the window of opportunity for interventions. For example, very fast and fast decliners have less than a decade from onset of decline to ESRD. This short window adds urgency to early diagnosis while renal function is normal. To be adequate, an intervention must be prompt and very effective. If efficient means can be developed to diagnose fast decliners early, aggressive “last resort” interventions will be well targeted.
Patient characteristics according category of renal decline
Clinical data were retrieved for almost all of Joslin T1D ESRD Cohort patients back to a time when their renal function was closest to normal. These are summarized in Table 1 according to the four categories of renal decline. It appears that neither sex, age at diabetes onset nor duration of T1D at entry into follow-up impacted the rate of renal decline. In contrast, higher HbA1c and ACR in fast and very fast decliners are both consistent with known associations of these covariates with the rate of decline.5, 31 Interestingly, the first quartile of the ACR (0.5g albumin/1g creatinine) was identical across the four categories, indicating that very fast and fast renal decline developed in a significant proportion of patients with only mild to moderate albuminuria. Paradoxically, median eGFR at entry was highest in very fast decliners and lowest in slow decliners. Most likely, the slow decliners’ low eGFR at entry reflects that the onset of their slow decline began many years before entry into the study and was not captured in our data. By design, the median eGFR slope was progressively less steep from the very fast to slow categories. As a reflection of that, time from entry to the onset of ESRD was progressively longer, and age at onset of ESRD was progressively older. This was in striking contrast to very similar age and duration of T1D at the entry/onset of renal decline in all categories of progressive renal decline.
Does acute kidney injury (AKI) contribute to renal decline
AKI has been defined as a rapid decrease in kidney function as measured by increases in serum creatinine over a period ranging from hours to weeks and less than 3 months.32 The risk of AKI increases in the general population and affects older individuals disproportionally due to coexisting co-morbidities, including diabetes33. Patients who survived AKI have an increased risk of development of ESRD. Although little research has been done regarding this issue in diabetes, it is important to consider whether AKI could account for any of the findings presented in Figures 1 & 2 and in Table 1. The high density of measurements of serum creatinine in the Joslin T1D ESRD cohort allowed us to evaluate the occurrence of episodes of AKI in the trajectories of eGFR that ended up with ESRD. Two scenarios were possible.
First, a single severe AKI episode could have led to ESRD rapidly – within 3 months. However, none of the cases with ESRD in our cohort had such rapidly declining renal function. The fastest decliner, shown in Figure 2, needed 18 months to progress from eGFR 120 to 10 ml/min, and the trajectory of renal decline was linear based on multiple measurements of serum creatinine. Second, less severe AKI episode(s) could have resulted in rapid decreases in renal function with subsequent plateaus or even recoveries. The occurrence of such episodes would result in non-linear eGFR trajectories. Contrary to this pattern, most trajectories in our ESRD Cohort were linear. Representation of renal decline as a single slope was appropriate for the vast majority (i.e. 87%) of patients. Similarly, we found comparable results when we evaluated smoothed eGFR trajectories by a Bayesian approach.6
In summary, the empirical data obtained in the Joslin T1D ESRD Cohort do not support the hypothesis that AKI significantly contributes to renal decline leading to ESRD in T1D. This does not exclude the possibility that AKI is an important factor in the development of ESRD in T2D as some authors have postulated.27
4. When does fast decline begin and how frequent is it in patients with diabetes?
To determine how frequently fast decline occurs in patients with T1D and T2D in the clinic population, we recall our findings from the prospective 1st and 2nd Joslin Kidney Studies. A particular focus of these studies is to find means to identify such patients early, while renal function is normal, so interventions can be undertaken to stop or slow the decline. With that goal, we concentrated first on learning when fast decline begins. For this purpose, we studied decliners in normoalbuminuric patients with T1D.17 For decliners we plotted eGFR trajectories during the 6–12 years of follow-up. They were divided arbitrarily at the median entry eGFR (105 mL/min): above in panel A and below in panel B. The difference between panels is striking. Whereas almost all those above median had several years of stable eGFR (horizontal trajectories) before progressive decline developed, all of those below median were progressively declining from entry. This finding is novel and suggests that in patients with T1D, the onset of progressive renal decline (blue circle) happens suddenly when patients have normal renal function and for the majority normal urinary albumin excretion. Once decline begins, it continues at an almost constant rate so the majority of trajectories of eGFR decline can be represented as simple regression slopes. The slopes, however, varied tremendously among patients, ranging from a loss of 50 to a loss of only 3 ml/min per year. Fastest decliners would reach ESRD within 2–10 years, while the slowest might need 20–40 years. Note the red eGFR trajectories in two cases with very fast renal decline. While normoalbuminuria was present in all of these decliners at enrollment, microalbuminuria developed in half of them during follow-up and a few progressed to proteinuria.17
Frequencies of renal decline in T1D and T2D patients who were followed for 6–12 years in the 1st and 2nd Joslin Kidney Study are shown in Table 2 according to category of renal decline and category of albuminuria at enrollment. All patients had normal renal function (eGFR >60 ml/min) at enrollment. Among patients with normoalbuminuria, significant (at least 3 ml/min/year) progressive renal decline developed in 9% of T1D and 20% of T2D patients. Most declines were slow or moderate, and fast decline (eGFR slopes <−10.0 ml/min/year) was rare, about 1.0%. Among those with microalbuminuria or proteinuria at entry, significant decline developed more frequently, and fast decline developed in 3–5% of microlbuminurics and 17–21% in proteinurics. Interestingly among the fast decliners, ESRD developed in many within 10 years. Note that in the Joslin population, the proportion of fast decliners in T1D and T2D are very similar in each category of albuminuria. It has not been possible to evaluate how well these findings agree with other populations due to a lack of published data.
5. Mechanisms underlying renal decline in diabetes
Disease processes underlying the onset and the rate of progressive renal decline are unknown. Similarly unknown is which kidney compartment (tubules, interstitium, vasculature or glomerula) is initially involved and whether the processes change between early and late decline (Figure 2). In the absence of an animal model (34), answers to these unknowns have to be derived from studies in humans.
Renal impairment has been perceived as a consequence of progressive albuminuria. Recently, however, progressive renal decline is being recognized as the primary clinical manifestation of diabetic nephropathy that begins while patients have normal renal function and frequently in the absence of albuminuria.7, 17, 35 This together with wide individual variation in rate of decline prompted studies on genetics, metabolomics and proteomics profiles associated/predictive of progressive renal decline. Positive findings of such studies may inform us about mechanisms which underlie this decline. In our studies in the Joslin population we used various types of follow-up study designs in which biologic specimens were obtained at various points during follow-up and stored in biobanks. The specimens were analyzed with recently developed high through-put technologies that assay levels of large numbers of proteins, metabolites, mRNAs and miRNAs. The following are results of our studies regarding the role of TNF related proteins.
Circulating TNF related proteins and risk of fast decline to ESRD in diabetes
Almost 20 years ago Hasegawa et al. implicated TNFα in the pathogenesis of diabetic nephropathy.36 While subsequent studies in animal models have supported a causal role of TNFα in the development of diabetic nephropathy, 37, 38 animal models do not develop progressive renal decline.34 Clinical studies conducted in the early 2000s attempted to correlate serum and urinary levels of TNF related proteins with various manifestation of diabetic nephropathy and other diseases. The results were difficult to interpret. In some studies, serum TNFα was measured, while in others serum levels of TNF receptors (TNFR1 or TNFR2) were used as proxies for serum of TNFα.39–42 None were a comprehensive assessment of the independent effects of TNFα (free and bound) and TNFR1 and TNFR2 on risk of diabetic nephropathy in human studies.
To investigate these issues, we conducted a series of follow-up studies to examine the role of circulating TNF-related proteins (TNFα free, TNFα bound, TNFR1 and TNFR2) specifically in the development of progressive renal decline. In the first study Niewczas et al. showed that high levels of circulating TNFR1 or TNFR2 (levels of both highly correlated) predicted ESRD in T2D during 8–12 years of follow-up.19 The renal decline was fast in the majority of these cases of ESRD. While elevated circulating levels of free and total TNFα were also associated with the risk of ESRD in univariate analysis, those associations disappeared when examined in multivariate analysis with TNFR1 or TNFR2 included.19 The strong association of TNFRs with the risk of ESRD was replicated in our subsequent studies of T1D43 and T2D.44 Similar results were found in Pima Indian T2D, including a lack of association between serum levels of TNFα and risk of ESRD.45 Our findings were confirmed recently in other populations.46, 47 Elevated levels of circulating TNFR1 and TNFR2 are also predictors of early progressive renal decline in diabetes, but that association was not examined for its specificity for fast decliners.16, 17, 48
In summary, elevated levels of circulating TNFR1 and TNFR2 are strong predictors of fast renal decline to ESRD. Whether high levels of circulating TNFR1 and TNFR2 are causal factors or only markers of progressive renal decline in human diabetes is unknown. An important consideration is that the effects of high circulating levels of TNFR1 and TNFR2 on the risk of ESRD do not need to be mediated through TNFα, although that conclusion was drawn from animal studies. In all of our studies, the associations of circulating TNFα (free or bound) and renal decline disappeared when circulating levels of TNFR1 or TNFR2 were considered. The recent CRIC study may be a case of misinterpretation of the role of circulating TNFα. During 6 years of follow-up of patients with impaired renal function, the risk of significant renal decline was associated with serum levels of TNFα at baseline. Although many covariates were considered, the study did not measure and adjust the results for circulating levels of either TNFR1 or TNFR2.49
6. How to diagnose fast decliners?
Currently, two legacy tests are used to diagnose diabetic nephropathy: ACR and creatinine-based eGFR. However, they are of little value for distinguishing non-decliners from decliners, let alone which declines will be fast, moderate, or slow. Serial measurements of serum creatinine can distinguish decliners from non-decliners but only if the measurements are frequent (at least once a year) and extend over period of time (i.e. 3–5 years).5,30 These requirements limit the utility of this approach as a prognostic tool for a clinical setting, although it can be of value as an outcome measure in longitudinal studies and clinical trials.
Intensive research is underway to find an effective prognostic marker that identifies decliners at a single clinical encounter. Three goals motivate this effort. The first goal is to discover a marker that reveals a mechanism underlying progressive renal decline so appropriate therapy, existing or newly developed, can be applied (see discussion in section 5). The second goal is to identify a marker that distinguishes fast decliners from moderate and slow decliners. These may be used to develop prognostic tests that enable physicians to accurately predict whether the patient has a short or long interval of time to ESRD so adequate treatment can be selected (See Figure 6). The third goal is to develop from these markers enrollment criteria for selecting study groups for clinical trials evaluating new interventions to reduce the risk of ESRD. The effectiveness of prognostic markers for these goals needs to be considered separately for patients with normal (eGFR >60 ml/min) and patients with impaired renal function (eGFR <60 ml/min).
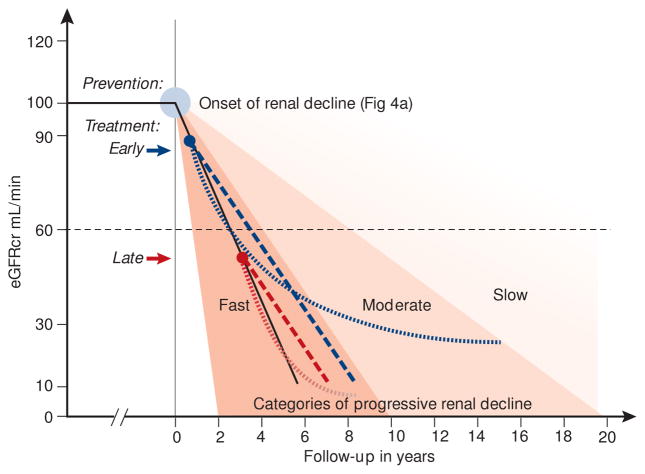
Figure 6.
Schematic representation of eGFR trajectories in fast decliners and their modifications in response to different interventions to reduce the rate of decline and postpone the risk of ESRD.
Intervention with a treatment that effectively reduces the rate of decline will postpone the onset of ESRD by a longer interval depending on how soon after the onset of decline the intervention occurs (dashed red and blue lines).
Evidence of an effective treatment may not be visible until after a lag interval (as described by Skupien et al. JASN 201431). That effect will not be realized, however, if the treatment is initiated too late (dotted red and blue lines).
In moderate and slow decliners, the effects of the treatments illustrated above, will be more pronounced. An immediate treatment effect (straight lines) would result in a greater delay in ESRD, and a lagged treatment effect (curves) would result in a higher residual eGFR. However, demonstrating these effects would require clinical trials longer than 3 years and different definition of end-point measures, e.g. deceleration of eGFR slopes or other more sensitive surrogates.
Consider the latter group of patients first. In our recent study, Yamanouchi et al. sought to develop criteria to identify fast decliners among diabetic patients with chronic kidney disease (CKD stages 3 or 4), so they could be enrolled into clinical trials.44 Toward this end, we examined baseline and follow-up data from patients enrolled into the T1D Joslin Proteinuria Cohort. A similar cohort of patients with T2D was used as a validation cohort. Fast decliners were patients who developed ESRD or lost ≥ 40% of baseline eGFR during 3 years of observation (typical duration of clinical trial).
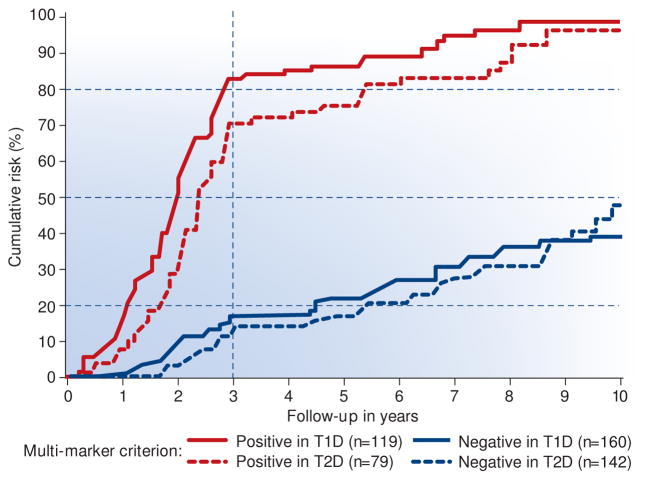
To search for optimal criteria we considered four markers measured at baseline: the two legacy markers, ACR and eGFR, plus two novel nephropathy markers, elevated serum TNFR1 or TNFR2. We used an example of machine learning methods, Classification and Regression Trees (CART), to identify markers and the cut-points in each of them that maximized separation of fast decliners from moderate, slow and non-decliners. Only two markers were important. The highest cumulative risk of ESRD within the 3-year follow-up was for patients with baseline serum TNFR1 >4.3 ng/ml (cut-point selected by CART) regardless of the values of other markers. Similarly, the cumulative risk of ESRD was high for patients with baseline serum TNFR1 between 2.9 and 4.3 ng/ml if baseline ACR was >1.9 g/g. These two sub-groups were combined into one high-risk group, and all other patients comprised the low risk group (Figure 6). The multi-marker (composite) criterion had a sensitivity of 72% and prognostic value of 81%, a very efficient prognostic test to identify fast decliners. Its application would increase the efficiency of clinical trials of new interventions for preventing or postponing onset of ESRD.
The cumulative risk of ESRD is shown in Figure 5 according to duration of follow-up and whether the multi-marker criterion was positive and negative. The cumulative risk of ESRD after the 3rd year reached 70–80% and 15%, respectively. Within 10 years, ESRD developed in 100% of the criterion-positive group, while it remained in the 35–45% range in the criterion-negative group. Note that risk of ESRD was almost identical for T1D and T2D in the criterion-positive and -negative groups. As of now, for moderate decliners who developed ESRD during the 4th to 10th years of follow-up, we do not have an efficient prognostic criterion to distinguish them from slow or non-decliners who remained without ESRD beyond the 10th year. Undoubtedly, such prognostic test would help physician to stratify patients according to risk of ESRD and would foster clinical trials that would focus on development of interventions in moderate decliners.
Figure 5.
Cumulative risk of ESRD during 10 year follow-up in the two Joslin cohorts with chronic kidney disease according to value of multi-marker criterion at entry into the follow-up.
The following markers at baseline were considered: ACR, eGFR, TNFR1 and TNFR2 to develop the multi-marker criterion to identify patients (fast decliners) at risk of ESRD during the first 3 year of follow-up using data from T1D cohort. The performance of the criterion was replicated in the data from T2D cohort.
Positive criterion: at baseline serum TNFR1 >4.3 ng/ml disregarding the other markers, or serum TNFR1 between 2.9 and 4.3 ng/ml and ACR >1.9 g albumin/g creatinine in urine.
Negative criterion: at baseline serum TNFR1 <4.3 ng/ml and ACR <1.9 g/albumin/1 g creatinine in urine, or serum TNFR1 <2.9 ng/ml disregarding values of other markers.
It was extraordinary that the multi-marker criterion that was developed in the T1D cohort produced almost identical stratification according to ESRD risk in T2D cohort. The T2D cohort had very different clinical characteristics than the T1D cohort.
Re-analyzed data from Yamanouchi M et al. Kidney Int 2016 (under review).44
Regarding diabetic patients with normal renal function, we do not have a good prognostic test to identify fast and moderate decliners. Several new markers that are predictors of progressive renal decline have been identified in these patients 15, 16–18. However, none of the markers have good prognostic performance, singly or together. With these markers we were able to design a prognostic test to identify patients with fast and moderate renal decline that has 50% sensitive and 58% positive predictive value (our studies in cohort described in Table 2B, Krolewski et al. personal communication). More efficient prognostic markers are needed to identify patients with fast and moderate progressive renal decline when they have still normal renal function. Such a tool is critical to the development of early interventions against ESRD in diabetes (see Figure 6).
7. Decliners and interventions
Intervention strategies for modifying the natural history of progressive renal decline are outlined in Figure 6.
Prevention of onset of early renal decline
At present interventions to prevent onset at this stage are few. In the DCCT/EDIC study of healthy T1D patients, CKD stage ≥3 developed in 5% of the standard treatment group between the 10th and 20th years of follow-up. In the experimental group with intensified glycemic control, the onset of CKD stage ≥3 was delayed by 5 years. It developed in the first case after 15 years, in 2.5% after 20 years, and in 5% after 25 years of follow-up.50 Since the cumulative risk curves were paralleled but shifted by 5 years, one may postulate the intensive glycemic control delayed the onset of early renal decline (see Figure 4A) but did not change the distribution of rates of renal decline. Overall this intervention, although successful, was very expensive when applied to everybody before their risk status was known. If a patient’s risk status could be determined beforehand, the effort and expense could be allocated more efficiency.
Figure 4.
eGFR trajectories in T1D patients with normoalbuminuria who developed progressive renal decline during 6–10 years of follow-up, plotted according to whether baseline eGFR was above (Panel A) or below (Panel B) the median (105 mL/min). Onset of early progressive renal decline was observed only in patients with eGFR above 105 mL/min.
Lines in red indicate patients with very fast renal decline. Proteinuria developed in them after the onset of early renal decline. Microalbuminuria developed in half of the decliners in both panels by the end of follow-up.
Adapted from Krolewski et al. Diabetes Care 2014.17
The other global strategy postulated in the 90s was to treat all diabetic patients with RAS inhibition as a preventive measure against nephropathy. Despite high enthusiasm, however, this strategy was shown to be unsuccessful in T1D.51, 52 In T2D, a meta-analysis of trials was able to detect a reduced risk for microalbuminuria, but it is unknown whether this intervention postponed the onset of early renal decline or the rate of decline.53 Recently, treatment of T2D patients with Empagliflozin (SGLT2 inhibitor) has been shown to stabilize renal function.54 Remarkably, the eGFR tracing became a horizontal line over the 4-year trial duration, while it declined progressively in the placebo arm. It is unclear whether this intervention delayed the onset of early renal decline as well as reducing the rate of decline. It has not been tested in T1D.
Early vs. late treatment of patients with renal decline
Another effect of intervention is a slowing of the rate of eGFR loss in patients who already have progressive renal decline. Since renal function decline seems to be linear, early implementation of an effective treatment, i.e. in patients with normal renal function, would result in greater postponement of the onset of ESRD in comparison with implementation of the same treatment in patients with CKD stage ≥3 (see Figure 6). An illustrative example was recently reported. The combined data from several studies in T2D revealed that early vs late RAS intervention delayed ESRD by 4.2 years as compared to 1.4 years, respectively.55 Since average values were reported it is unknown whether this intervention’s effect was equal across the spectrum of rates of decline or differed in very fast, fast, moderate and slow decliners.
Evaluation of effectiveness of therapies according to early and late treatment is hampered by the FDA regulations regarding the reliable end-points for trials in diabetic kidney diseases. The approved outcome measures (doubling of serum creatinine or significant postponement of the onset of ESRD) for use in clinical trials may not be sensitive enough to detect effects that are less than dramatic, particularly in the most relevant patients with early renal function decline and well preserved GFR. The proper outcome measure is a change in the rate of renal decline, as in the ongoing study Preventing Early Renal function loss in Diabetes (PERL) clinical trial which investigates the effectiveness of allopurinol treatment in reducing the rate of GFR loss in T1D.56 The challenge being the need for 3 years of follow-up with GFR determinations with precise GFR measurements (iohexol plasma clearance).
Current standards of care in diabetic nephropathy emphasize good control of glycaemia, blood pressure (including RAS inhibition) and lipids and cessation of smoking.57 Multifactorial intervention in T2D delayed ESRD and reduced mortality 58 and clinical data showed similar effects in T1D59, but these findings need to be replicated in different settings.
Delayed effect of therapies to treat renal decline
The disease process that underlies progressive renal decline to ESRD is not only complex but also long lasting. Effective interventions in this process may be followed by a lag period of several years before the therapeutic effect can be demonstrated (see Figure 6 dotted lines). For example, implementation of an effective treatment that has a lag period in patients with CKD stage 3 will not be demonstrable in fast decliners for the disease will outrun the response. On the other hand, the effect of the same treatment initiated in patients with normal renal function would be detectable in a clinical trial that was carried out long enough. Furthermore, the effects will be more pronounced in moderate and slow decliners, as we recently demonstrated for the effect of improved glycemic control on postponing onset of ESRD in T1D patients with proteinuria 31.
New therapies to treat renal decline
Application of markers to enrich the study population for fast decliners and/or responders to intervention would increase efficiency. In an ongoing study in albuminuric T2D patients of the effect of treatment with a selective endothelin A receptor antagonist on hard renal endpoints, all patients are initially receiving an active study drug before randomization. So the study is enriched for patients responding with a decline in albuminuria (Clinical Trial identifier: NCT01858532).
If the underlying pathophysiology of kidney disease in diabetes varies among patients, treatment may need to be more personalized in the future. The lack of targeting interventions to appropriately enriched study groups may have contribute to the failure of several recent trails in diabetic kidney disease. A number of new treatment strategies affecting different pathways, such as inflammation, fibrosis, and oxidative stress, are being tested for effectiveness in diabetic kidney disease.60 In order to address fast renal decline we may have to learn from the approaches being developed in other areas such as oncology. For the most aggressive cancer cases where a treatment is not known, which could be compared to the patients with very fast progressive renal decline, phase 1 trial units have been established where patients are profiled with tumor and germline genomics, tumor transcriptomics, and potentially other “omics” in an attempt to characterize the pathophysiology of the disease and subsequently identify a potential (new) drug fitting to the patient’s individual profile, thereby maximizing the likelihood of success.61
Transcriptomic profiles in kidney biopsies from patients with diabetic kidney disease and animal models of diabetic kidney disease pointed to the JAK/STAT pathway as a key pathway in diabetic kidney disease. A subsequent clinical study of intervention with the JAK/STAT inhibitor baricitinip reduced albuminuria.62 If this approach to identifying new pathways for intervention is taken to the individual level, it may be an option for aggressive precision medicine in patients with fast renal function decline.63 We are therefore aiming at identifying diagnostic markers and putative targets that can be the basis for personalized treatment of diabetic nephropathy.
Acknowledgments
We are grateful for the support from: the Novo Nordisk Foundation grant NNF14OC0013659 (PROTON: PeRsOnalising Treatment Of diabetic Nephropathy) to PR and ASK: the JDRF grant “Biomarkers of Diabetic Nephropathy Collaborative Research Initiative (DN-BIO)” No.3-SRA-2015-106-Q-R for sub-project “Predictors of progressive renal decline in Type 1 diabetes” to ASK and the NIH grant DK-041526 to ASK.
Footnotes
Disclosures:
ASK is a co-inventor of the TNFR1 and TNFR2 patent for predicting risk of ESRD. This patent was licensed by the Joslin Diabetes Center to EKF Diagnostics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krolewski M, Eggers PW, Warram JH. Magnitude of end-stage renal disease in IDDM: a 35 year follow-up study. Kidney Int. 1996;50:2041–2046. doi: 10.1038/ki.1996.527. [DOI] [PubMed] [Google Scholar]
- 2.Finne P, Reunanen A, Stenman S, et al. Incidence of end-stage renal disease in patients with Type 1 diabetes. JAMA. 2005;294:1782–1787. doi: 10.1001/jama.294.14.1782. [DOI] [PubMed] [Google Scholar]
- 3.LeCaire TJ, Klein BEK, Howard KP, et al. Risk for end-stage renal disease over 25 years in the population-based WESDR cohort. Diabetes Care. 2014;37:381–388. doi: 10.2337/dc13-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosolowsky ET, Skupien J, Smiles AM, et al. Risk of ESRD in Type 1 Diabetes Remains High in Spite of Renoprotection. J Am Soc Nephrol. 2011;22(3):545–553. doi: 10.1681/ASN.2010040354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skupien J, Warram JH, Smiles AM, et al. Early Renal Function Decline Predicts Risk of ESRD: 5–18 year Follow-up of Patients with Type 1 Diabetes and Proteinuria. Kidney Int. 2012;82(5):589–97. doi: 10.1038/ki.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skupien J, Warram J, Smiles A, et al. Patterns of Estimated Glomerular Filtration Rate Decline Leading to End-Stage Renal Disease in Type 1 Diabetes. Diabetes Care. 2016 doi: 10.2337/dc16-0950. Published online September, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krolewski AS. Progressive Renal Decline: The New Paradigm of Diabetic Nephropathy in Type 1 Diabetes. Diabetes Care. 2015;38(6):1–9. doi: 10.2337/dc15-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller PM, Rogus JJ, Cleary PA, et al. The Genetics of Kidneys in Diabetes (GoKinD) Study: A genetics collection available for identifying the genetic susceptibility factors for diabetic nephropathy in type 1 diabetes mellitus. J Am Soc Neph. 2006;17(7):1782–1790. doi: 10.1681/ASN.2005080822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wanic K, Placha G, Dunn J, et al. Exclusion of polymorphisms in carnosinase genes (CNDP1 & CNDP2) as cause of diabetic nephropathy in type 1 diabetes mellitus. Results of large case – control and follow – up studies. Diabetes. 2008;57(9):2547–2551. doi: 10.2337/db07-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pezzolesi MG, Poznik GD, Mychaleckyj JC, et al. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes. 2009;58(6):1403–1410. doi: 10.2337/db08-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krolewski AS, Laffel LM, Krolewski M, et al. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;332(19):1251–5. doi: 10.1056/NEJM199505113321902. [DOI] [PubMed] [Google Scholar]
- 12.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348(23):2285–93. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 13.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and risk of early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 14.Ficociello LH, Perkins BA, Roshan B, et al. Renal hyperfiltration and the development of microalbuminuria in type 1 diabetes. Diabetes Care. 2009;32(5):889–893. doi: 10.2337/dc08-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ficociello LH, Rosolowsky ET, Niewczas M, et al. High serum uric acid and early progressive renal function loss in patients with type 1 diabetes. Results of 6-year follow-up. Diabetes Care. 2010;33(6):1337–1343. doi: 10.2337/dc10-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF Receptors 1 and 2 predict stage 3 of CKD in Type 1 diabetes. J Am Soc Nephrol. 2012;23(3):507–515. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krolewski AS, Niewczas MA, Skupien J, et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care. 2014;37(1):226–234. doi: 10.2337/dc13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak N, Skupien J, Niewczas MA, et al. Increased plasma Kidney Injury Molecule-1 suggests early progressive renal decline in non-proteinuric patients with Type 1 diabetes. Kidney Int. 2015;89:459–467. doi: 10.1038/ki.2015.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF Receptors 1 and 2 Predict ESRD in Type 2 diabetes. J Am Soc Nephrol. 2012;23(3):507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niewczas MA, Sirich TL, Mathew AV, et al. Uremic solutes and risk of end-stage renal disease in type 2 diabetes. Kidney Int. 2014;85(5):1214–1224. doi: 10.1038/ki.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitch WE, Walser M, Buffington GA, et al. A simple method of estimating progression of chronic renal failure. Lancet. 1976;2: 1326–1328. doi: 10.1016/s0140-6736(76)91974-7. [DOI] [PubMed] [Google Scholar]
- 22.Jones RH, Hayakawa H, Mackay JD, et al. Progression of diabetic nephropathy. Lancet. 1979;1:1105–1106. doi: 10.1016/s0140-6736(79)91788-4. [DOI] [PubMed] [Google Scholar]
- 23.Bleyer AJ. A reciprocal graph to plot the reciprocal serum creatinine over time. Am J Kidney Dis. 1999;34: 576–578. doi: 10.1016/s0272-6386(99)70089-2. [DOI] [PubMed] [Google Scholar]
- 24.Mogensen CE. Progression of nephropathy in long-term diabetics with proteinuria and effect of initial anti-hypertensive treatment. Scand J Clin Lab Invest. 1976;36: 383–388. doi: 10.1080/00365517609055274. [DOI] [PubMed] [Google Scholar]
- 25.Parving HH, Smidt UM, Friisberg B, et al. A prospective study of glomerular filtration rate and arterial blood pressure in insulindependent diabetics with diabetic nephropathy. Diabetologia. 1981;20:457–461. doi: 10.1007/BF00253407. [DOI] [PubMed] [Google Scholar]
- 26.Viberti GC, Bilous RW, Mackintosh D, et al. Monitoring glomerular function in diabetic nephropathy. A prospective study. Am J Med. 1983;74:256–264. doi: 10.1016/0002-9343(83)90624-1. [DOI] [PubMed] [Google Scholar]
- 27.Kelly KJ, Dominguez JH. Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am J Nephrol. 2010;32:469–475. doi: 10.1159/000320749. [DOI] [PubMed] [Google Scholar]
- 28.Shah BV, Levey AS. Spontaneous changes in the rate of decline in reciprocal serum creatinine: errors in predicting the progression of renal disease from extrapolation of the slope. J Am Soc Nephrol. 1992;2:1186–1191. doi: 10.1681/ASN.V271186. [DOI] [PubMed] [Google Scholar]
- 29.Szeto CC, Leung CB, Wong TY, et al. Extrapolation of reciprocal creatinine plot is not reliable in predicting the onset of dialysis in patients with progressive renal insufficiency. J Intern Med. 2003;253:335–342. doi: 10.1046/j.1365-2796.2003.01121.x. [DOI] [PubMed] [Google Scholar]
- 30.KDIGO. Definition, identification, and prediction of CDK progression. Kidney Int. 2013;(suppl 3):63–72. doi: 10.1038/kisup.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skupien J, Warram JH, Smiles A, et al. Improvement of glycemic control reduces risk of ESRD in Type 1 diabetes with proteinuria. J Am Soc Nephrol. 2014;25(12):2916–2925. doi: 10.1681/ASN.2013091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–21. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–73. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breyer MD, Böttinger E, Brosius FC, 3rd, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16(1):27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 35.Perkins BA, Ficociello LH, Roshan B, et al. In patients with Type 1 diabetes and new onset micro-albuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010;77:57–64. doi: 10.1038/ki.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa G, Nakano K, Sawada M, et al. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991;40(6):1007–1012. doi: 10.1038/ki.1991.308. [DOI] [PubMed] [Google Scholar]
- 37.Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 2003;64(4):1208–1213. doi: 10.1046/j.1523-1755.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 38.Awad AS, You H, Gao T, et al. Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury. Kidney Int. 2015;88(4):722–733. doi: 10.1038/ki.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridker PM, Rifai N, Pfeffer M, et al. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101(18): 2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 40.Tonelli M, Sacks F, Pfeffer M, et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68(1): 237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 41.Keller C, Katz R, Cushman M, et al. Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA) BMC Nephrol. 2008;9(1):9. doi: 10.1186/1471-2369-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niewczas MA, Ficociello LH, Johnson AC, et al. Serum concentrations of markers of TNFalpha and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2009;4(1): 62–70. doi: 10.2215/CJN.03010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skupien J, Warram JH, Niewczas MA, et al. Synergism between circulating tumor necrosis factor receptor 2 and HbA(1c) in determining renal decline during 5–18 years of follow-up in patients with type 1 diabetes and proteinuria. Diabetes Care. 2014;37(9):2601–2608. doi: 10.2337/dc13-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamanouchi M, Skupien J, Niewczas MA, et al. Improved clinical trial enrollment criteria to identify patients with diabetes at risk of ESRD. Kidney Int. 2016 doi: 10.1016/j.kint.2017.02.010. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavkov ME, Nelson RG, Knowler WC, et al. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015;87(4):812–819. doi: 10.1038/ki.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saulnier PJ, Gand E, Ragot S, et al. Association of serum concentration of TNFR1 with all-cause mortality in patients with type 2 diabetes and chronic kidney disease: follow-up of the SURDIAGENE Cohort. Diabetes Care. 2014;37(5):1425–1431. doi: 10.2337/dc13-2580. [DOI] [PubMed] [Google Scholar]
- 47.Forsblom C, Moran J, Harjutsalo V, et al. Added value of soluble tumor necrosis factor-alpha receptor 1 as a biomarker of ESRD risk in patients with type 1 diabetes. Diabetes Care. 2014;37(8):2334–2342. doi: 10.2337/dc14-0225. [DOI] [PubMed] [Google Scholar]
- 48.Miyazawa I, Araki S, Obata T, et al. Association between serum soluble TNF alpha receptors and renal dysfunction in Type 2 diabetic patients without proteinuria. Diabetes Res Clin Pract. 2011;92:174–180. doi: 10.1016/j.diabres.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Amdur RL, Feldman HI, Gupta J, et al. Inflammation and Progression of CKD: The CRIC Study. Clin J Am Soc Nephrol. 2016;11(9):1546–1556. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Boer IH, Sun W, Cleary PA, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bilous R, Chaturvedi N, Sjolie AK, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med. 2009;151(1):11–20. doi: 10.7326/0003-4819-151-1-200907070-00120. [DOI] [PubMed] [Google Scholar]
- 53.Persson F, Lindhardt M, Rossing P, et al. Prevention of microalbuminuria using early intervention with renin-angiotensin system inhibitors in patients with type 2 diabetes: A systematic review. J Renin Angiotensin Aldosterone Syst. 2016;17(3) doi: 10.1177/1470320316652047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wanner C, Inzucchi SE, Lchin JM, et al. Empagliflozin and progression of kidney disease in Type 2 diabetes. N Engl J Med. 2016;375:323–34. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 55.Schievink B, Kröpelin T, Mulder S, et al. Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2016;18:64–71. doi: 10.1111/dom.12583. [DOI] [PubMed] [Google Scholar]
- 56.Maahs DM, Caramori L, Cherney DZ, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13:550–559. doi: 10.1007/s11892-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Diabetes A. Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34:3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 59.Andresdottir G, Jensen ML, Carstensen B, et al. Improved prognosis of diabetic nephropathy in type 1 diabetes. Kidney Int. 2015;87:417–426. doi: 10.1038/ki.2014.206. [DOI] [PubMed] [Google Scholar]
- 60.Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nature Reviews Disease Primers. 2015;1:15018. doi: 10.1038/nrdp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tuxen IV, Jonson L, Santoni-Rugiu E, et al. Personalized oncology: genomic screening in phase 1. APMIS. 2014;122:723–733. doi: 10.1111/apm.12293. [DOI] [PubMed] [Google Scholar]
- 62.Brosius FC, Tuttle KR, Kretzler M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia. 2016;59:1624–1627. doi: 10.1007/s00125-016-4021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komorowsky CV, Brosius FC, III, Pennathur S, et al. Perspectives on systems biology applications in diabetic kidney disease. J Cardiovasc Transl Res. 2012;5:491–508. doi: 10.1007/s12265-012-9382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]