Abstract
To maintain potassium homeostasis, kidneys exert flow-dependent potassium secretion to facilitate kaliuresis in response to elevated dietary potassium intake. This process involves stimulation of calcium-activated large conductance maxi-K (BK) channels in the distal nephron, namely the connecting tubule and the collecting duct. Recent evidence suggests that the TRPV4 channel is a critical determinant of flow-dependent intracellular calcium elevations in these segments of the renal tubule. Here, we demonstrate that elevated dietary potassium intake (five percent potassium) increases renal TRPV4 mRNA and protein levels in an aldosterone-dependent manner and causes redistribution of the channel to the apical plasma membrane in native collecting duct cells. This, in turn, leads to augmented TRPV4-mediated flow-dependent calcium ion responses in freshly isolated split-opened collecting ducts from mice fed the high potassium diet. Genetic TRPV4 ablation greatly diminished BK channel activity in collecting duct cells pointing to a reduced capacity to excrete potassium. Consistently, elevated potassium intake induced hyperkalemia in TRPV4 knockout mice due to deficient renal potassium excretion. Thus, regulation of TRPV4 activity in the distal nephron by dietary potassium is an indispensable component of whole body potassium balance.
Keywords: distal nephron, flow-induced potassium secretion, BK channels, [Ca2+]i signaling, aldosterone
INTRODUCTION
Maintenance of whole body potassium homeostasis is of vital importance as extracellular [K+] is the major determinant of cell membrane potential. Since up to 98% of whole body potassium is stored in the intracellular compartments, even a one-time meal can double extracellular K+ content 1. An integrated whole body response to dietary potassium load includes rapid redistribution of K+ from the extracellular to intracellular compartments and elevated potassium excretion primarily in urine 2. Inability to properly control potassium balance during states of potassium loading can result in life-threating hyperkalemia leading to severe electrophysiological disturbances, such as neuronal and muscular hyperexcitability and cardiac arrhythmias 3.
Kidneys play a central role in maintaining potassium homeostasis by excreting approximately 90% of the dietary K+ load 1. Distal segments of renal nephron, including the connecting tubule (CNT) and the collecting duct (CD), are the primary sites of controlled potassium secretion into the lumen and, ultimately, K+ excretion in urine 4. Activity of the renal outer medullary K+ (ROMK) channels underlies potassium secretion at baseline conditions 5. Increases in dietary potassium intake promote expression of the large conductance maxi-K (also known as BK) channels in the distal tubular segments to accelerate urinary excretion of K+ 6, 7. Both aldosterone-dependent and -independent mechanisms account for stimulation of distal nephron potassium secretion 2. BK is a Ca2+ activated potassium channel comprised of a pore forming α subunit and regulatory β subunits (β1-β4), both of which bestow Ca2+ dependent properties to the functional channel 8, 9. Mice with deleted α or β BK subunits demonstrate impaired adaptation to elevated potassium intake exhibiting reduced urinary K+ excretion and hyperaldosteronism 7, 10.
Transient receptor potential vanilloid type 4 (TRPV4) channel is a Ca2+-permeable channel that can be activated in response to mechanical stress, such as elevated flow over the plasma membrane, in numerous cell types 11. While TRPV4 can be detected in several epithelial tissues, including lung, spleen, skin, sweat glands and some sensory neurons 12–16, expression of the channel is the most abundant in the kidney 15. Here, TRPV4 is predominantly localized to distal segments of the renal tubule, including the CD and CNT 17, 18. Recent experimental evidence demonstrate that genetic TRPV4 ablation disrupts [Ca2+]i responses to elevated tubular flow suggesting a central role of the channel as a flow sensor/transducer in the distal renal tubule 17. It is well documented that high dietary potassium intake increases fluid delivery to the distal tubular segments 9, and that renal K+ excretion exhibits a flow-dependent pattern 19, 20. This process is independent of ROMK and is largely mediated by BK channels, activated by a mechanosensitive flow-induced [Ca2+]i mobilization 19, 21, 22. Thus, TRPV4 is appropriately positioned to control flow-induced K+ secretion via regulation of [Ca2+]i levels. Consistent with this view, flow-dependent net K+ transport in the perfused cortical CD was shown to be abolished in TRPV4 deficient mice. Similarly, urinary potassium excretion in response to loop diuretics was found to be markedly impaired in TRPV4 knockouts 23. However, the physiological role of TRPV4 in the kidney for regulation of whole body potassium homeostasis remains poorly understood.
In this study, we demonstrate that renal TRPV4 function and expression pattern are controlled by variations in dietary potassium intake. This regulation is dependent on mineralocorticoid receptor (MR) activation suggesting a major role of the aldosterone axis. TRPV4 deletion markedly diminishes BK channel activity in freshly isolated split-opened CDs. Furthermore, TRPV4 −/− mice are able to maintain physiological levels of plasma potassium in unstressed conditions but become prominently hyperkalemic when dietary potassium intake is high. Overall, we demonstrate an essential role of renal TRPV4 in adaptation to elevated potassium load.
RESULTS
Renal TRPV4 expression is controlled by dietary potassium intake
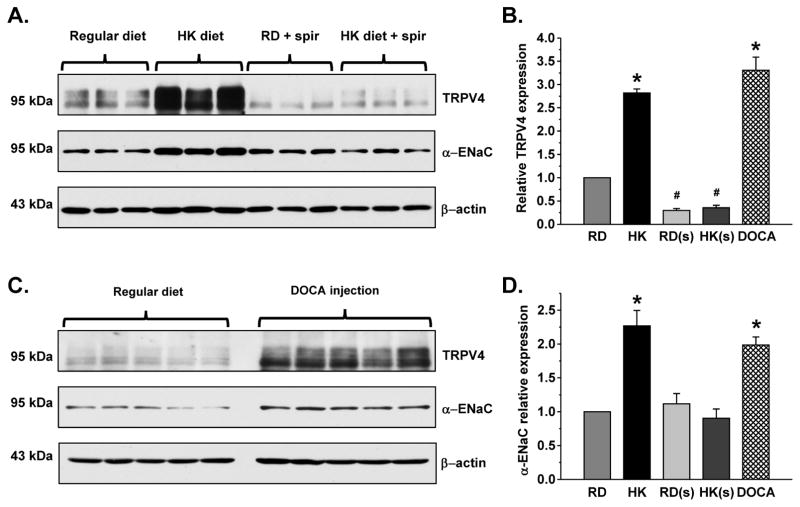
Ca2+-permeable TRPV4 channel is predominantly expressed in the distal tubular segments, namely CNT and CD, serving as a sensor/transducer of luminal flow at this site 17, 24. Elevated dietary potassium intake enhances tubular flow in the distal nephron to promote flow-induced potassium secretion, a process that is highly dependent on [Ca2+]i 9. Since TRPV4 can be involved in flow-dependent K+ secretion by controlling Ca2+-dependent BK channel activity, we hypothesized that TRPV4 expression and function in the kidney depend on systemic K+ status. Indeed, we found that renal TRPV4 protein (Figures 1A, B) and mRNA (Figure 1C) levels are upregulated in mice maintained on a high potassium (5% K+) diet for one week compared to those on a regular diet (0.9% K+). Specificity of the employed antibodies was verified in kidney homogenates from TRPV4 −/− mice (Supplementary Figure S1). This regulation is likely to be specific for TRPV4, since we did not detect any significant changes in abundance of the Ca2+-permeable TRPC3 channel, which is also expressed in the distal renal tubule 25 (Supplementary Figure S2).
Figure 1. TRPV4 expression in the kidney depends on dietary potassium intake.
(A) Representative Western blots from whole kidney lysates of C57BL/6 mice kept on regular (0.9% K+) and high (5% K+, HK) potassium diet for one week. The lysates were probed with anti-TRPV4 and anti-β-actin antibodies, respectively. The channel appears as a duplet of upper glycosylated and lower non-glycosylated forms. (B) Summary graph comparing TRPV4 expression from Western blots similar to that shown in (A). Intensities of the TRPV4-reporting reporting bands were normalized to the intensities of the respective actin bands. (C) Summary graph of relative TRPV4 mRNA levels in the kidney as detected by RT q-PCR in mice treated with regular (0.9% K+) and high (5% K+) potassium diet for one week. Mean TRPV4 cycle threshold values were normalized to the respective HPRT cycle threshold values. * - significant increase versus regular diet.
Regulation of renal TRPV4 by dietary potassium is aldosterone-dependent
Elevated potassium intake is known to augment mineralocorticoid aldosterone resulting in enhanced K+ secretion in the distal parts of renal tubule. Thus, we next asked whether stimulation of renal TRPV4 expression occurs in an aldosterone-dependent manner. As shown in the representative Western blot (Figure 2A) and the summary graph (Figure 2B), a high K+ diet fails to increase TRPV4 protein levels in mice treated with the mineralocorticoid receptor (MR) antagonist, spironolactone. MR inhibition also significantly reduces TRPV4 expression pointing to a constitutive nature of this regulation. Furthermore, stimulation of MR by administration of deoxycorticosterone acetate (DOCA) for 3 days increases renal TRPV4 levels (Figure 2C) to a similar extent as a high K+ diet (Figure 2B). The expression of full length α-ENaC (Figures 2A, C, and D), the most recognized aldosterone-responsive protein in the kidney, was used as a positive control for the treatments.
Figure 2. Aldosterone-MR signaling is essential for regulation of TRPV4 in the kidney by dietary K+ intake.
(A) Representative Western blots of whole kidney lysates from C57BL/6 mice kept on regular (0.9% K+) and high (5% K+) potassium (HK) diet for one week in the absence and presence of spironolactone (spir, 30 30 mg/kgBW) probed with anti-TRPV4 (upper), α-ENaC (middle), and β-actin (lower). (B) Summary graph comparing renal TRPV4 expression normalized to the respective intensity of β-actin in mice on regular diet (RD), high K+ diet (HK), regular diet + spironolactone (RD(s)), high K+ diet + spironolactone (HK(s)), and regular diet injected with Deoxycorticosterone acetate (DOCA). (C) Representative Western blots of whole kidney lysates from C57BL/6 mice kept on regular diet and injected with DOCA for 3 consecutive days (2.4 mg/injection/animal) probed with anti-TRPV4 (upper), α-ENaC (middle), and β-actin (lower). (D) Summary graph comparing full length α-ENaC expression normalized to the respective intensity of β-actin in mice on the same conditions as described in (B). * - significant increase versus RD; # - significant decrease versus RD.
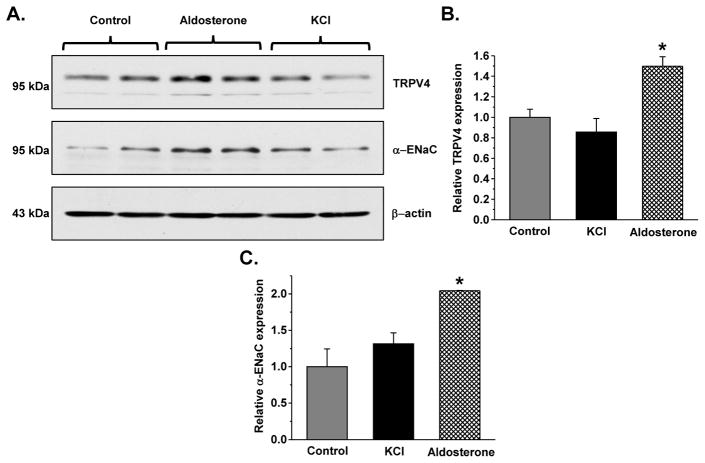
Since aldosterone-independent actions of extracellular K+ on electrolyte transport in the distal tubule have been recently documented 26, we next assessed the direct effects of aldosterone and increased extracellular potassium on TRPV4 expression in polarized mpkCCDc14 cells (mouse collecting duct cell line). Application of 1 μM aldosterone, but not elevation of extracellular KCl to 10 mM, from the basolateral side for 24 hours increases TRPV4 expression (Figures 3A, B) and [Ca2+]i responses to TRPV4 agonist, GSK1016790A (Supplementary Figure S3A). As expected, aldosterone also augments short-circuit current (Supplementary Figure S3B) and increases full length α-ENaC expression (Figure 3A, C). Overall, our results (Figures 2, 3) argue for the central role of aldosterone-MR axis in stimulation of renal TRPV4 expression during high K+ intake.
Figure 3. Aldosterone but not extracellular K+ increases TRPV4 abundance in mpkCCDc14 cells.
(A) Representative Western blots from mpkCCDc14 lysates in the control, after treatment with 1 μM aldosterone or addition of 5 mM KCl from the basolateral side for 24 hours probed with anti-TRPV4 (upper), α-ENaC (middle), and β-actin (lower). Summary graphs comparing TRPV4 (B) and full length α-ENaC (C) expression normalized to the respective intensity of β-actin in mice on the same conditions as described in (A). * - significant increase versus control.
Increased dietary potassium load promotes TRPV4 translocation to the apical plasma membrane to augment flow- mediated [Ca2+]i responses in native CD cells
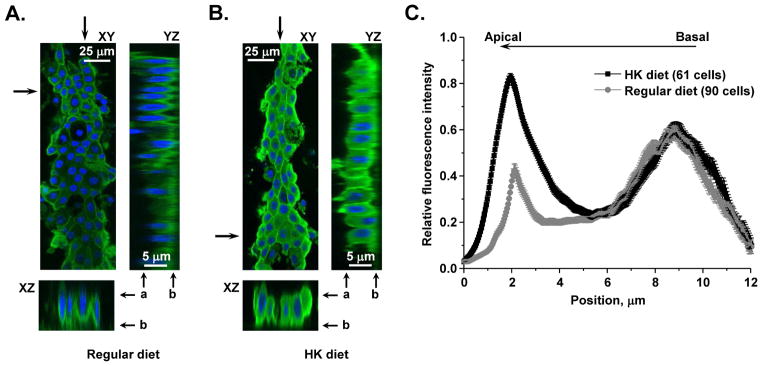
We further examined whether elevated potassium intake affects subcellular TRPV4 distribution using immunofluorescent confocal microscopy in split-opened CDs. Consistently, we observed a significantly stronger TRPV4-reporting signal in the CDs isolated from mice maintained on a high K+ diet as compared to the controls on a regular potassium regimen (Figure 4A, B). High K+ intake also induces a prominent accumulation of TRPV4 at the apical region of CD cells, as indicated by the relative fluorescent signals along the z-axis (Figure 4C).
Figure 4. High K+ dietary intake induces apical TRPV4 accumulation in native CD cells.
(A) A representative confocal micrograph demonstrating TRPV4 abundance and distribution (pseudocolor green) in the CD from C57BL/6 mouse kept on regular (0.9 % K+) intake. Here and below, representative XZ and YZ plane projections (location of the cross-section is marked by arrows), showing TRPV4 distribution along the basal-apical axis, were reconstructed from Z-stacks of confocal images. Nuclear DAPI staining is shown in pseudocolor blue. Position of the apical and basal sides is shown with “a” and “b”, respectively. (B) A representative confocal micrograph visualizing TRPV4 abundance and distribution (pseudocolor green) in the CD from C57BL/6 mouse kept on a high potassium (5% K+) diet. (C) Distribution of averaged relative fluorescent signals representing TRPV4 localization along Z-axis in CD cells from C57BL/6 mice kept on regular and high potassium intake. For each individual cell, the fluorescent signal was normalized to its corresponding maximal value. At least 6 CDs from 3 different mice were used to obtain statistics for any given treatment.
We next used ratiometric Fura 2-based [Ca2+]i imaging in freshly isolated split-opened CDs (Figure 5 inset) to test whether high K+-induced TRPV4 translocation leads to augmented channel activity. Compelling experimental evidence suggest a pivotal role of TRPV4 in flow-mediated [Ca2+]i elevations in distal nephron cells 17, 24, 27. Consistently, the magnitude of [Ca2+]i mobilization elicited by high luminal flow is significantly larger in the CD cells from animals maintained on elevated potassium intake (Figures 5A, B). Importantly, increased flow has no measurable effect on [Ca2+]i in CD cells from TRPV4 −/− mice on both regular and high K+ intake verifying the TRPV4-dependent nature of these responses (Figure 5A, B). Overall, our results demonstrate that variations in dietary potassium intake promote robust changes in renal TRPV4 expression, subcellular localization and activity.
Figure 5. High dietary potassium intake augments TRPV4-dependent [Ca2+]i elevations in the CD cells.
(A) The average time course of relative changes in Fura 2 F340/F380 ratio in split-opened CDs in response to 10x elevation in flow (shown with a bar on top) from apical side. CDs were isolated from WT mice kept on control (squares) and high potassium (circles) diet, as well as TRPV4 −/− mice kept on control (triangles) and high potassium (inverted triangles) diet. The insert contains representative micrographs of a typical split-opened CD after loading with Fura-2 taken with bright-field illumination (left) and 380 nm excitation (right). (B) The summary graph of the magnitudes of flow-induced Ca2+ responses in CD cells for the groups in (A). * - significant increase versus control.
BK channel activity in the CD is drastically diminished upon TRPV4 ablation
BK-dependent flow-induced K+ secretion in the CD is a critical part of augmented kaliuresis in response to elevated dietary K+ intake 19, 21, 22. The BK channels rely on [Ca2+]i mobilization, arising from TRPV4 activation. Thus, we next hypothesized that TRPV4 deficiency leads to diminished BK activity. To probe this concept, we employed patch clamp electrophysiology to monitor BK activity at the single channel level in split-opened CDs from wild type (WT) and TRPV4 −/− mice. We did not detect BK activity when both groups of mice were kept on a regular K+ diet (data not shown). In contrast, we reliably monitored single channel BK activity in CD cells from mice maintained on a high K+ diet. We observed active BK channels in 31 out of 145 patches in WT mice, but only in 2 out of 36 patches in TRPV4 knockouts (Figure 6A, B). BK channel activity was similar in principal (polygonal) and intercalated (round) cells (Supplementary Figure S4). Furthermore, the number of functional BK channels is greatly decreased upon TRPV4 ablation (Figure 6C). Importantly, this was not associated with reduced BK channel expression levels in TRPV4 −/− compared to WT mice (Supplementary Figure S5). Altogether, these results provide direct support to the idea that TRPV4 serves as a source of elevated [Ca2+]i for BK activation in the CD and TRPV4 dysfunction diminishes BK-dependent K+ excretion by the kidney.
Figure 6. TRPV4 −/− mice have impaired BK channel activity in the CD.
(A) Representative current traces of single channel BK activity in CD cells from WT (top) and TRPV4−/− mice (bottom) kept on high (5% K+) potassium diet for 1 week. The patches were held at a test potential of Vh=−Vp=+100 mV. Outward K+ currents are upward. Dashed lines indicate the respective current state with c denoting the closed state. (B) Pie charts representing the frequency of observing patches with active channels (f) for the conditions described in (A). (C) Summary graph of functional BK levels (fN) for WT and TRPV4 −/− mice kept on high potassium diet. * – significant decrease vs WT.
TRPV4 −/− mice develop hyperkalemia due to renal K+ retention in response to elevated potassium intake
We next assessed whether diminished BK activity in TRPV4 deficient mice perturbs systemic K+ balance during high potassium intake. WT and TRPV4 −/− mice have virtually identical plasma [K+] levels (4.07±0.19 mM and 4.19±0.22 mM, respectively) under basal conditions (Figure 7A). One week of a high potassium diet has no appreciable effect on plasma [K+] levels in WT animals (4.15±0.10 mM), but elicits apparent hyperkalemia (5.61±0.37 mM) in mice lacking the TRPV4 channel (Figure 7A). Fecal K+ excretion is not significantly different in WT and TRPV4 −/− (Figure 7B) pointing to renal K+ retention as the underlying cause for the elevated plasma K+ levels in mutant mice. After one week on a high K+ diet, urinary excretion of K+ and Na+ is comparable in WT and TRPV4−/− (Supplementary Figure S6) suggesting that a new steady state has been reached at the expense of elevated plasma [K+]. Serum aldosterone elevation induced by augmented potassium intake is significantly higher in TRPV4 knockouts when compared to WT controls (Figure 7C). Thereby, mutant mice fail to eliminate the excess of potassium despite elevated circulating aldosterone levels. The capacity to excrete K+ with urine is practically identical in WT and TRPV4 −/− mice under basal conditions. As expected, high K+ diet induced significant increase in urinary flow rate in both mouse strains (Supplementary Figure S7). However, TRPV4 mutants lag behind WT controls with respect to kaliuretic response during the first 2 days of a high potassium load (Figure 8), indicating that TRPV4 ablation delays arrival at the new steady state, where potassium intake equals its output. The assessed parameters from metabolic cage studies are summarized in the Supplementary Table 1.
Figure 7. TRPV4−/− mice exhibit impaired adaptation to elevated potassium intake.
(A) Summary graph of plasma K+ levels in WT (light grey) and TRPV4 −/− (dark grey) mice kept on control and elevated K+ (5%) diet for 7 days. (B) Summary graph of K+ excretion with feces in WT (light grey) and TRPV4 −/− (dark grey) mice kept on control and elevated K+ (5%) diet for 7 days. (C) Summary graph of plasma aldosterone concentrations in WT (light grey) and TRPV4 −/− (dark grey) on low (0.01%) and high (5%) K+ diet for 7 days. * - significant increase vs WT HK diet.
Figure 8. Renal potassium excretion is delayed in TRPV4−/− mice fed a high K+ diet.
Summary graph visualizing K+ excretion with urine in WT (light grey) and TRPV4 −/− (dark grey) mice maintained on a regular K+ diet and in response to elevated potassium load (5% K+) for 1 or 2 days. * – significant decrease vs a respective value in WT mice.
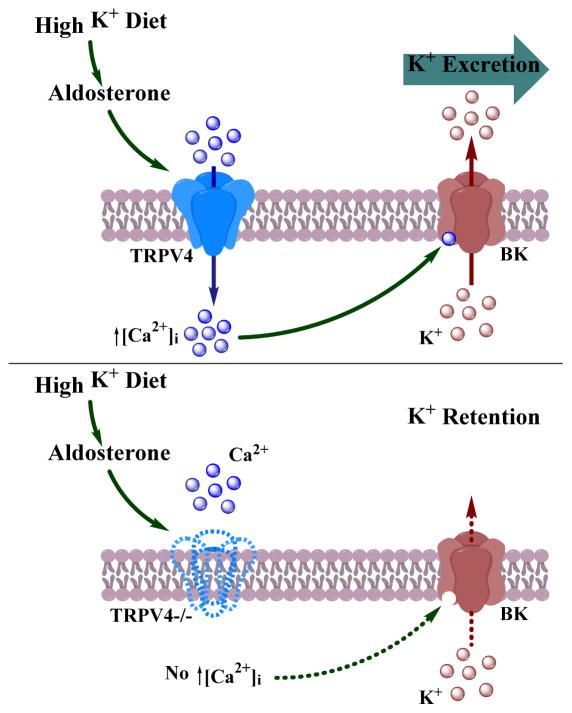
Overall, our results demonstrate that intact TRPV4 function is essential for the maintenance of whole body potassium homeostasis in adaptation to elevated potassium intake. Elevated dietary K+ increases TRPV4 expression in the distal tubule in an aldosterone-dependent manner and augmented TRPV4-dependent [Ca2+]i influx is required to stimulate BK-dependent K+ secretion (Figure 9). TRPV4 ablation reduces BK activity leading subsequently to renal K+ retention and hyperkalemia in response to dietary potassium load.
Figure 9.
The proposed role of TRPV4 in regulation of BK-mediated K+ secretion during high potassium intake.
DISCUSSION
In this study, we identified a previously unrecognized regulation of TRPV4 in the distal renal tubule by dietary potassium intake. We demonstrated that elevated dietary K+ causes multi-component upregulation of renal TRPV4 function including increased mRNA and protein levels, translocation to the apical plasma membrane and enhanced TRPV4-mediated [Ca2+]i responses to flow (Figures 1–5). We further put forward the evidence that this is a critical mechanism for stimulation of flow-induced K+ secretion via BK channels (Figure 6) serving as a component of the integrative response to dietary potassium load. Disruption of this regulation in TRPV4 −/− mice leads to compromised systemic K+ balance with hyperkalemia and impaired renal K+ excretion in the presence of high K+ diet (Figure 7).
Our results support a dominant role of aldosterone-MR signaling in controlling TRPV4 activity in the kidney by dietary K+ intake. TRPV4 expression positively correlated with the functional status of MR in intact kidneys as well as in cultured mpkCCDc14 cells (Figures 2, 3). Steroid hormones, namely dexamethasone, were reported to upregulate TRPV4 mRNA and channel activity in extrarenal tissues by targeting glucocorticoid receptors (GR) 28, 29. In contrast, we found that inhibition of MR virtually precludes TRPV4 regulation in the kidney by dietary potassium which is consistent with the distal tubule, a site with most prominent TRPV4 expression, being a MR, but not a GR responsive tissue. Aldosterone exerts its well-recognized stimulatory effects on distal nephron K+ secretion via activation of serum glucocorticoid kinase 1 (SGK1) to increase activity of ROMK1 30, 31. Indeed, SGK1 deficient animals have impaired renal K+ excretion in response to dietary K+ load 30. It was recently proposed that SGK1 is able to also directly phosphorylate TRPV4 at Ser824 residue and this interaction seems to increase channel activity and Ca2+ influx in over-expression systems 32. It is quite possible that aldosterone increases TRPV4 abundance in the distal tubule in an SGK1-dependent manner. On the other side, several aldosterone-independent mechanisms have been proposed as contributing factors for adaptation to the dietary K+ load. The existing experimental evidence suggests that the amount of dietary K+ is already sensed in the gut and/or the portal vein even prior to increased plasma K+ 33. The exact mechanism of how this stimulates kaliuresis is yet to be elucidated. Furthermore, changes in extracellular K+ levels per se can also directly regulate ion transport in the distal nephron. K+ gavage induces rapid de-phosphorylation of the sodium chloride cotransporter (NCC) before plasma aldosterone levels rise to promote potassium secretion in CNT and CCD 34. Furthermore, adrenalectomy, while blunting, does not disrupt stimulation of distal nephron K+ secretion by dietary potassium intake 35. While our results in cultured mpkCCDc14 cells failed to demonstrate the direct effect of increased extracellular K+ on TRPV4 expression (Figure 3), this does not exclude contribution of aldosterone-independent mechanisms in regulation of renal TRPV4 by dietary potassium. Thus, both aldosterone-dependent 36 and -independent 21 regulation of BK-mediated K+ secretion in CDs were reported. The observations that mice with impaired BK activity have compromised renal K+ excretion 7, 10 emphasize the physiological relevance of this mechanism for whole body K+ homeostasis.
The pattern of regulation of TRPV4 in the distal nephron by dietary K+ intake in the current study virtually mimics the effects observed for BK channel: increased mRNA abundance, translocation of the channel to the apical plasma membrane and increased activity 37. This implicates that both channels could be up-regulated by the same mechanism to facilitate excretion of potassium in the presence of high dietary K+. Recent cumulative evidence documented a pivotal role of With-No-Lysine (K) kinase (WNK) signaling network in the distal tubule in regulation of multiple transporting systems (NCC, ROMK, ENaC, BK) during variations in dietary K+ intake 26, 38. WNK4 was shown to inhibit macroscopic K+ currents in BKα transfected HEK293T cells and this was reversed by SGK1 co-expression 39, 40. Interestingly, WNK4 similarly downregulates TRPV4 function by decreasing channel plasma membrane levels 41. Further studies are required to test whether WNK signaling cascade coordinates expression and function of both BK and TRPV4 in the distal tubule depending on dietary K+ intake.
The current study provides direct evidence of functional coupling between TRPV4 and BK channels in the CD. The BK channel was shown to be expressed at the apical border of both principal and intercalated cells of the CNT/CD 42, 43. We did not detect major differences in BK function in these cells types in WT mice fed with high potassium diet. Consistently, TRPV4 expression and TRPV4-mediated [Ca2+]i responses to elevated flow were also comparable in these cell types, as we documented previously 17. Therefore, the mechanism of flow-induced K+ secretion via BK channels is most likely similar in principal and intercalated cells. Importantly, we report here that genetic TRPV4 ablation greatly diminishes BK single channel activity (Figure 6). This strongly supports the view that TRPV4 serves as a route to increase [Ca2+]i to promote BK activation. Our results are coherent with the observation that TRPV4−/− mice have reduced renal K+ excretion in response to stimulation of urinary production with furosemide 23. Importantly, TRPV4 deletion also leads to hyperkalemia with renal K+ retention in response to high K+ diet (Figure 7) as was described for BK deficient models 7, 10. A similar functional interplay between TRPV4 and Ca2+-dependent K+ channels was also revealed in vascular resistive vessels. Here, TRPV4 is expressed in endothelial cells and contributes to control of vascular tone by promoting vasodilation 44–46. Activation of TRPV4 by flow-induced shear stress leads to activation of Ca2+-dependent small conductance SK3 (KCa2.3) channel and hyperpolarization 44, 47, 48. This is transmitted to the smooth muscle cell to activate voltage-stimulated Ca2+ channels and, in turn, activation of BK in the smooth muscle cell. The BK-induced hyperpolarization subsequently inhibits the voltage-activated Ca2+ channel causing smooth muscle relaxation and vessel dilation. Consistently, TRPV4 was recently proposed to be involved in Angiotensin II-dependent endothelial dysfunction 49.
In summary, this study unravels a physiologically relevant role of TRPV4 in the kidney in maintaining K+ homeostasis of the whole organism. We demonstrate that TRPV4 ablation impairs adaptation to changes in dietary potassium intake that cannot be compensated by other mechanisms. From a clinical perspective, genetic polymorphisms in TRPV4 in humans associated with partial loss-of-function may indicate an increased susceptibility to develop hyperkalemia. On the other side, the results of our study suggest that stimulation of TRPV4 activity can be potentially beneficial in treatment of various pathological states associated with increased plasma K+ levels.
MATERIALS AND METHODS
Reagents
All chemicals and materials were from Sigma (St. Louis, MO), VWR (Radnor, PA), and Tocris (Ellisville, MO) unless noted otherwise and were of reagent grade.
Animal studies
Animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of the University of Texas Health Science Center at Houston. For experiments, 6–8 weeks old C57BL/6 (Charles Rivers Laboratories, Wilmington, MA) and TRPV4 −/− (having C57BL/6 background and described previously 16, 17) mice were used. To examine effects of dietary potassium intake, animals were provided chow containing regular (0.9% K+, TD.7912), and high potassium (5% K+, TD 150699) for 7 days. All diets were purchased from Envigo (former Harlan Teklad; Madison, WI, USA). Spironolactone USP (30 mg/kgBW; Amneal Pharmaceutical) was added to drinking water for 7 days. Upon necessity, mice were injected with Deoxycorticosterone acetate (DOCA) for 3 consecutive days (2.4 mg/injection/animal) prior to the experimentation similarly to what we have done previously50.
RT q-PCR
Total RNA was harvested from freshly isolated kidney. Samples were homogenized with TRIzol Reagent (Ambion, Life technologies). Chloroform was added to the homogenates, and RNA precipitated with isopropanol. cDNA was prepared from 40 ng of total RNA using AffinityScript reverse transcriptase (Agilent Technologies) and oligo-dt primers. PCR master mix containing either TRPV4 or HPRT primers and probe (TRPV4 primer 1: 5’-TCGTCACAGACCTTCATGTTG-3’, primer 2: 5’-CCTCTTCAAAGACCTCTTCCG-3’ and probe: 5’-/56- FAM/CCTGCTTGT/ZEN/GTACCTGCTCTTCATGA/31ABkFQ/-3’. HPRT primer 1: 5’-AACAAAGTCTGGCCTGTATTC-3’, primer 2: 5’-CCCCAAAATGGTTAAGGTTGC-3’ and probe: 5’-/56-FAM/CTTGCTGGT/ZEN/GAAAAGGACCTCTCGAA/31ABkFQ/-3’) were subsequently added. TRPV4 and HPRT expression was determined in triplicates by RT q-PCR analysis using a Roche Lightcycler 480 instrument II following cycling conditions for a total of 40 cycles. Mean TRPV4 cycle threshold values were normalized to the respective HPRT cycle threshold values.
Western blotting
Immediately after dissection kidneys were placed on ice, decapsulated and homogenized in 3 volumes of ice-cold hypotonic lysis buffer containing 50mM Tris, 1% Triton X-100, 5mM EDTA (pH=7.4) supplemented with Complete Mini protease and PhosSTOP phosphatase inhibitor cocktails (Roche Diagnostics, Indianapolis, IN, USA). Protein concentration was determined with a Bradford assay using bovine serum albumin as a standard. The samples were diluted with hypotonic lysis buffer, denatured and reduced in Laemmli buffer supplemented with 5% μ-ME at +75°C for 10 min to obtain the final protein concen tration of 4 mg/ml. The samples (10 μg/lane) were separated on 12% polyacrylamide gels at 150 V for 105 min and transferred to nitrocellulose membrane for 135 min at 100 V. Subsequently the nitrocellulose membrane was incubated with either anti-TRPV4 (1:1000, Alomone labs, Jerusalem, Israel), anti-αENaC (1:1000, Stress Marq, Victoria, Canada), anti-TRPC3 (1:500, Alomone Labs, Jerusalem, Israel) or anti-BK β4 (1:200, Alomone Labs, Jerusalem, Israel) antibodies for 1.5 hours followed by incubation with secondary anti-actin (1:5000, Abcam, Cambridge, UK) antibodies for 1.5 hours at room temperature. Blots were quantified using ImageJ 1.49s software (NIH, USA). The intensities for TRPV4, TRPC3, BK β4 and α-ENaC protein bands were normalized to the intensities of the corresponding actin bands, used as a loading control.
Isolation of individual collecting ducts
The procedure for isolation of the CDs from C57BL/6 (WT) and TRPV4 −/− mice suitable for Ca2+-imaging, patch clamp electrophysiology and immunofluorescent microscopy closely follows the protocols previously published by our laboratory 17, 27, 51. Kidneys were cut into thin slices (< 1 mm) with slices placed into ice-cold physiologic saline solution (PSS: 150 NaCl, 5 mM KCl, 1 CaCl2, 2 MgCl2, 5 glucose and 10 HEPES (pH 7.35)). CDs were visually identified by their morphological features (pale color; coarse surface and, in some cases, bifurcations) and were mechanically isolated from kidney slices by micro-dissection using watchmaker forceps under a stereomicroscope. Isolated CDs were attached to 5 x 5 mm cover glasses coated with poly-L-lysine. A cover-glass containing a CD was placed in a perfusion chamber mounted on an inverted Nikon Eclipse Ti microscope and perfused with a bath solution at room temperature. CDs were split-opened with two sharpened micropipettes, controlled with different micromanipulators, to gain access to the apical membrane. The tubules were used within 2 hours of isolation.
[Ca2+]i imaging
Intracellular calcium levels were measured in individual cells within split-opened area of freshly isolated CDs using Fura 2 fluorescence ratiometric imaging as described previously 24, 27, 51. Briefly, split-opened CDs were loaded with Fura-2 by incubation with 2 μM Fura-2/AM in a bath solution for 40 min at room temperature. Subsequently, tissue samples were washed and incubated for additional 10–15 minutes prior to experimentation. CDs were placed in an open-top imaging study chamber (RC-26GLP; Warner Instruments, Hamden, CT, USA) with a bottom coverslip viewing window and the chamber attached to the microscope stage of a Nikon Ti-S Wide-Field Fluorescence Imaging System (Nikon Instruments, Melville, NY, USA) integrated with Lambda XL light source (Sutter Instrument, Novato, CA, USA) and QIClick 1.4 megapixel monochrome CCD camera (QImaging, Surrey, BC, Canada) via NIS Elements 4.3 Imaging Software (Nikon Instruments, Melville, NY, USA). Cells were imaged with a 40X Nikon Super Fluor objective and regions of interest (ROIs) were drawn for individual cells. The Fura 2 fluorescence intensity ratio was determined by excitation at 340 nm and 380 nm and calculating the ratio of the emission intensities at 511 nm in the usual manner every 5 seconds. The changes in the ratio are reported as an index of changes in intracellular calcium, as well documented previously 52. No significant Fura 2 bleaching and leakage were detected during the timeline of experiments. At least 3 individual CDs (~10 cells in each) from at least 3 mice were used for each experimental set. To test the effect of elevated flow on [Ca2+]i, the rate of perfusion was instantly increased from 1.5 ml/min (~15 mm H2O) to 15 ml/min (~80 mmH2O) producing shear stress of approximately 3 dyn/cm2 17. This value fits well within the physiological range of shear stress present in the rat and mouse CD, as was assessed previously 53.
Single channel recordings
Single channel activity of maxi-K (BK) channels on the apical membrane of CD cells was determined in a cell-attached configuration under voltage-clamp conditions (−Vp = +100 mV). Events were inspected visually prior to acceptance. BK activity was analyzed over a span of 180 sec for each experimental condition. Recording pipettes had resistances of 8–10 megaOhms. Gap-free single channel current data from gigaohm seals were acquired (and subsequently analyzed) with an Axopatch 200B (Molecular Devices, Sunnyvale, CA, USA) patch clamp amplifier interfaced via a Digidata 1440 (Molecular Devices, Sunnyvale, CA, USA) to a PC running the pClamp 10.4 suite of software (Molecular Devices, Sunnyvale, CA, USA). The frequency of observing patches with active channels (f) was calculated as a ratio of the number of patches with at least one active channel to the total number of patches. To assess functional BK levels (fN), the frequency of observing patches with active channels (f) was multiplied by the average number of active channels in a patch (N) from a given strain/experimental condition. BK was identified by its large conductance (>150 pS), selectivity to K+, and activation upon excision of a cell-attached patch (i.e. increased Ca2+ concentration from the inner side). Bath and pipette solutions were (in mM): 150 NaCl, 5 mM KCl, 1 CaCl2, 2 MgCl2, 5 glucose and 10 HEPES (pH 7.35); and 150 mM KCl, 2 mM MgCl2, 10 mM HEPES (pH 7.35).
Immunofluorescent microscopy
Freshly-isolated split-open CD were fixed with 4% paraformaldehyde in PBS (pH = 7.4) for 15 min at RT. After fixation the samples were permeabilized by addition of 0.1% Triton X-100 in PBS for 10 min and washed in PBS 3 times for 5 min. Nonspecific staining was blocked with 10% normal goat serum (NGS, Jackson Immunoresearch, USA) in PBS for 30 min at RT. After washing with PBS (3 times for 5 min) the samples were incubated for 3 hr at room temperature in dark with anti-TRPV4 tagged with ATTO 550 (1:50, Alomone Labs, Israel; Cat. # ACC-034-AO) in 2% serum in PBS. After washing with PBS (3 times for 5 min) the samples were stained with DAPI (1.5 μM concentration, Calbiochem, San Diego, CA, USA) to visualize nuclei. Subsequently the samples were dehydrated, and mounted with permanent mounting medium (SouthernBiotech, Birmingham, AL, USA). Labeled tissue samples were examined with an inverted Nikon A1R confocal laser microscope using a 60X Plan-Fluor oil-immersion (1.3 NA) objective. Samples were excited with 405 and 561.7 nm laser diodes and emission captured with a 16-bit Cool SNAP HQ2 camera (Photometrics, Tucson, AZ, USA) interfaced to a computer running Nikon NIS Elements 4.3 elements software. 3-D stacks of split-open CD were generated from series of confocal plane images with 0.25 μm step.
Systemic measurements
Blood samples (approximately 500 μl) were taken by terminal cardiac puncture in anesthetized animals. Serum was separated by centrifugation at 1300g in Vacutainer Plus SST plastic tubes with clot activator and gel for serum separation (Prod. No. 367988; BD, Franklin Lakes, NJ, USA). Urinary pH was measured in fresh spot urine samples using MI-410 pH microelectrode (Microelectrodes Inc., New Hampshire, NH, USA). To assess urinary potassium excretion mice were acclimated for 3 days in metabolic cages (3600M021; Techniplast, West Chester, PA, USA) with free access to water and regular diet. Following acclimation, 24-hour urine samples were collected in mice maintained on the same regular potassium diet. After that, the mice were challenged with a high potassium diet (5% K+) for 2 consecutive days. To estimate the amount of potassium excreted with urine on a daily basis, urinary potassium concentration was normalized on the 24-hour urine volume. To minimize circadian effects, urine collections were conducted around 10–11 a.m. Serum and urinary electrolyte concentrations were measured using Jenway PFP7 Flame photometer (Bibby Scientific, Burlington, NJ, USA). Urinary creatinine concentration was assessed with QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA, USA) utilizing improved Jaffe method as we used previously 54. Aldosterone was purified from serum samples with chloroform extraction and measured using an enzymatic immunoassay kit (Cat. No. 501090; Cayman Chemical, Ann Arbor, MI, USA), in accordance with the vendor’s protocol.
Cell culture
Immortalized mouse cortical collecting duct (mpkCCDc14) principal cells were grown on permeable supports (Costar Transwells, 0.4-μm pore, 24-mm diameter) until polarization and development of avid Na+ transport, as described previously 55, 56. Growth medium for mpkCCDc14 principal cells was composed of equal volumes of DMEM and Ham's F-12, 50 nM dexamethasone, 2% FBS, and 1% Pen/Strep. Equivalent short circuit current (Isc) across the mpkCCDc14 cell monolayer was calculated using Ohm's law as the quotient of trans-epithelial voltage (VT) to trans-epithelial electrical resistance (RT) under open circuit conditions using a EVOM2 voltmeter with dual Ag/AgCl pellet electrodes (World Precision Instruments, Sarasota, FL, USA). Twenty four hours prior to experimentation, the medium was replaced with minimal medium that contained only DMEM, Ham's F-12 and antibiotics. Vehicle, Aldosterone (1 μM) and KCl (5 mM) were added from the basolateral side for 24 hours.
Statistics
All summarized data are reported as mean ± SEM. All statistical comparisons were made using one-way ANOVA. A P value of less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
This research was supported by NIH-NIDDK DK095029 (to O. Pochynyuk), AHA-15SDG25550150 (to M. Mamenko), and NIH-DIDDK DK098401 (to R. G. O’Neil). Carol Stavinoha (UTHSC at Houston) is recognized for the technical assistance with experimental animals.
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381–401. doi: 10.1146/annurev.physiol.010908.163241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer BF. Regulation of Potassium Homeostasis. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:1050–1060. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nature reviews Nephrology. 2014;10:653–662. doi: 10.1038/nrneph.2014.168. [DOI] [PubMed] [Google Scholar]
- 4.Wang WH, Giebisch G. Regulation of potassium (K) handling in the renal collecting duct. Pflugers Archiv : European journal of physiology. 2009;458:157–168. doi: 10.1007/s00424-008-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert SC, Desir G, Giebisch G, et al. Molecular diversity and regulation of renal potassium channels. Physiological reviews. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holtzclaw JD, Grimm PR, Sansom SC. Role of BK channels in hypertension and potassium secretion. Current opinion in nephrology and hypertension. 2011;20:512–517. doi: 10.1097/MNH.0b013e3283488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieg T, Vallon V, Sausbier M, et al. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney international. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- 8.Rothberg BS. The BK channel: a vital link between cellular calcium and electrical signaling. Protein Cell. 2012;3:883–892. doi: 10.1007/s13238-012-2076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen D, Cornelius RJ, Sansom SC. Interacting influence of diuretics and diet on BK channel-regulated K homeostasis. Curr Opin Pharmacol. 2014;15:28–32. doi: 10.1016/j.coph.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm PR, Irsik DL, Settles DC, et al. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11800–11805. doi: 10.1073/pnas.0904635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol. 2010;103:2–17. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuyama R, Vriens J, Voets T, et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell metabolism. 2008;8:257–265. doi: 10.1016/j.cmet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Muramatsu S, Wakabayashi M, Ohno T, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. The Journal of biological chemistry. 2007;282:32158–32167. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- 15.Liedtke W, Choe Y, Marti-Renom MA, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. ProcNatlAcadSciUSA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berrout J, Jin M, Mamenko M, et al. Function of TRPV4 as a mechanical transducer in flow-sensitive segments of the renal collecting duct system. JBiolChem. 2012;287:8782–8791. doi: 10.1074/jbc.M111.308411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamenko M, Zaika O, Boukelmoune N, et al. Deciphering physiological role of the mechanosensitive TRPV4 channel in the distal nephron. American journal of physiology Renal physiology. 2015;308:F275–286. doi: 10.1152/ajprenal.00485.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woda CB, Bragin A, Kleyman TR, et al. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. AmJPhysiol Renal Physiol. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman JS, Hamburger RJ. Potassium transport in the connecting tubule. Miner Electrolyte Metab. 1996;22:242–247. [PubMed] [Google Scholar]
- 21.Liu W, Morimoto T, Woda C, et al. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. American journal of physiology Renal physiology. 2007;293:F227–235. doi: 10.1152/ajprenal.00057.2007. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol. 1998;164:35–45. doi: 10.1007/s002329900391. [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi J, Tsuruoka S, Mizuno A, et al. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. AmJPhysiol Renal Physiol. 2007;292:F667–F673. doi: 10.1152/ajprenal.00458.2005. [DOI] [PubMed] [Google Scholar]
- 24.Zaika O, Mamenko M, Berrout J, et al. TRPV4 dysfunction promotes renal cystogenesis in autosomal recessive polycystic kidney disease. Journal of the American Society of Nephrology : JASN. 2013;24:604–616. doi: 10.1681/ASN.2012050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goel M, Sinkins WG, Zuo CD, et al. Identification and localization of TRPC channels in the rat kidney. American journal of physiology Renal physiology. 2006;290:F1241–1252. doi: 10.1152/ajprenal.00376.2005. [DOI] [PubMed] [Google Scholar]
- 26.Terker AS, Zhang C, McCormick JA, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell metabolism. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamenko M, Zaika OL, Boukelmoune N, et al. Discrete control of TRPV4 channel function in the distal nephron by protein kinases A and C. The Journal of biological chemistry. 2013;288:20306–20314. doi: 10.1074/jbc.M113.466797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JW, Ku SK, Han MH, et al. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. International journal of molecular medicine. 2015;36:29–42. doi: 10.3892/ijmm.2015.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boychuk CR, Zsombok A, Tasker JG, et al. Rapid Glucocorticoid-Induced Activation of TRP and CB1 Receptors Causes Biphasic Modulation of Glutamate Release in Gastric-Related Hypothalamic Preautonomic Neurons. Frontiers in neuroscience. 2013;7:3. doi: 10.3389/fnins.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang DY, Wulff P, Volkl H, et al. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. Journal of the American Society of Nephrology : JASN. 2004;15:885–891. doi: 10.1097/01.asn.0000120368.59693.a8. [DOI] [PubMed] [Google Scholar]
- 31.Ring AM, Leng Q, Rinehart J, et al. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin SH, Lee EJ, Hyun S, et al. Phosphorylation on the Ser 824 residue of TRPV4 prefers to bind with F-actin than with microtubules to expand the cell surface area. Cellular signalling. 2012;24:641–651. doi: 10.1016/j.cellsig.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Greenlee M, Wingo CS, McDonough AA, et al. Narrative review: evolving concepts in potassium homeostasis and hypokalemia. Ann Intern Med. 2009;150:619–625. doi: 10.7326/0003-4819-150-9-200905050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorensen MV, Grossmann S, Roesinger M, et al. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney international. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 35.Muto S, Sansom S, Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting ducts from adrenalectomized rabbits. The Journal of clinical investigation. 1988;81:376–380. doi: 10.1172/JCI113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen D, Cornelius RJ, Yuan Y, et al. Regulation of BK-alpha expression in the distal nephron by aldosterone and urine pH. American journal of physiology Renal physiology. 2013;305:F463–476. doi: 10.1152/ajprenal.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estilo G, Liu W, Pastor-Soler N, et al. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. American journal of physiology Renal physiology. 2008;295:F780–788. doi: 10.1152/ajprenal.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoorn EJ, Nelson JH, McCormick JA, et al. The WNK kinase network regulating sodium, potassium, and blood pressure. Journal of the American Society of Nephrology : JASN. 2011;22:605–614. doi: 10.1681/ASN.2010080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Subramanya AR, Satlin LM, et al. Regulation of large-conductance Ca2+-activated K+ channels by WNK4 kinase. American journal of physiology Cell physiology. 2013;305:C846–853. doi: 10.1152/ajpcell.00133.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue P, Zhang C, Lin DH, et al. WNK4 inhibits Ca(2+)-activated big-conductance potassium channels (BK) via mitogen-activated protein kinase-dependent pathway. Biochimica et biophysica acta. 2013;1833:2101–2110. doi: 10.1016/j.bbamcr.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Y, Subramanya A, Rozansky D, et al. WNK kinases influence TRPV4 channel function and localization. American journal of physiology Renal physiology. 2006;290:F1305–1314. doi: 10.1152/ajprenal.00391.2005. [DOI] [PubMed] [Google Scholar]
- 42.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. American journal of physiology Renal physiology. 2004;287:F1030–1037. doi: 10.1152/ajprenal.00169.2004. [DOI] [PubMed] [Google Scholar]
- 43.Pluznick JL, Wei P, Grimm PR, et al. BK-{beta}1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. American journal of physiology Renal physiology. 2005;288:F846–854. doi: 10.1152/ajprenal.00340.2004. [DOI] [PubMed] [Google Scholar]
- 44.Sonkusare SK, Bonev AD, Ledoux J, et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sukumaran SV, Singh TU, Parida S, et al. TRPV4 channel activation leads to endothelium-dependent relaxation mediated by nitric oxide and endothelium-derived hyperpolarizing factor in rat pulmonary artery. Pharmacological research : the official journal of the Italian Pharmacological Society. 2013;78:18–27. doi: 10.1016/j.phrs.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. Journal of cardiovascular pharmacology. 2013;61:113–119. doi: 10.1097/FJC.0b013e318279ba42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma X, Du J, Zhang P, et al. Functional role of TRPV4-KCa2. 3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertension. 2013;62:134–139. doi: 10.1161/HYPERTENSIONAHA.113.01500. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan MN, Earley S. TRP channel Ca(2+) sparklets: fundamental signals underlying endothelium-dependent hyperpolarization. American journal of physiology Cell physiology. 2013;305:C999–C1008. doi: 10.1152/ajpcell.00273.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishijima Y, Zheng X, Lund H, et al. Characterization of blood pressure and endothelial function in TRPV4-deficient mice with l-NAME- and angiotensin II-induced hypertension. Physiol Rep. 2014;2:e00199. doi: 10.1002/phy2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamenko M, Zaika O, Prieto MC, et al. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension. 2013;62:1111–1122. doi: 10.1161/HYPERTENSIONAHA.113.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mamenko M, Zaika O, O'Neil RG, et al. Ca2+ Imaging as a tool to assess TRP channel function in murine distal nephrons. Methods Mol Biol. 2013;998:371–384. doi: 10.1007/978-1-62703-351-0_29. [DOI] [PubMed] [Google Scholar]
- 52.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. The Journal of biological chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 53.Wu L, Gao X, Brown RC, et al. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. AmJPhysiol Renal Physiol. 2007;293:F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 54.Mamenko M, Zaika O, Doris PA, et al. Salt-dependent inhibition of epithelial Na+ channel-mediated sodium reabsorption in the aldosterone-sensitive distal nephron by bradykinin. Hypertension. 2012;60:1234–1241. doi: 10.1161/HYPERTENSIONAHA.112.200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pochynyuk O, Bugaj V, Vandewalle A, et al. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. American journal of physiology Renal physiology. 2008;294:F38–46. doi: 10.1152/ajprenal.00403.2007. [DOI] [PubMed] [Google Scholar]
- 56.Zaika O, Mamenko M, O'Neil RG, et al. Bradykinin acutely inhibits activity of the epithelial Na+ channel in mammalian aldosterone-sensitive distal nephron. American journal of physiology Renal physiology. 2011;300:F1105–1115. doi: 10.1152/ajprenal.00606.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.