Abstract
The Drosophila nephrocyte is a critical component of the fly renal system, and bears structural and functional homology to podocytes and proximal tubule cells of the mammalian kidney. Investigations of nephrocyte cell biological processes are fundamental to understanding the insect renal system. Nephrocytes are highly active in endocytosis and vesicle trafficking. Rab GTPases regulate endocytosis and trafficking, but specific functions of nephrocyte Rabs remain undefined. We analyzed Rab GTPase expression and function in Drosophila nephrocytes and found that 11 out of 27 Drosophila Rabs were required for normal activity. Rabs 1, 5, 7, 11, and 35 were most important. Nephrocyte specific Rab5 gene silencing eliminated all intracellular vesicles and the specialized plasma membrane structures essential for nephrocyte function; Rab7 silencing dramatically increased clear vacuoles and reduced lysosomes; Rab11 silencing increased lysosomes and reduced clear vacuoles. Our results suggest that Rab5 mediates endocytosis that is essential for maintenance of functionally critical nephrocyte plasma membrane structures, and that Rabs 7 and 11 mediate alternative downstream vesicle trafficking pathways leading to protein degradation and membrane recycling, respectively. Elucidating molecular pathways underlying nephrocyte function has the potential to yield important insights into human kidney cell physiology and mechanisms of cell injury that lead to disease. Limited treatment options and high incidence mean that discovering promising therapeutic targets in abnormal podocytes and proximal tubule cells is a priority. The Drosophila nephrocyte is emerging as a useful in vivo model system for molecular target identification and initial testing of therapeutic approaches.
Keywords: Drosophila, nephrocyte, Rab, vesicle trafficking, endosome
Introduction
The pericardial nephrocytes of Drosophila (hereafter, nephrocytes) are the components of the fly renal system that perform the analogous filtration and protein reabsorption functions of the mammalian glomerular podocytes and proximal tubule (PT) cells, respectively. Nephrocytes in fact share strikingly homologous molecular and structural attributes with both podocytes and PT cells (Cagan, 2011, Na and Cagan, 2013, Weavers, et al., 2009, Zhang, et al., 2013a, Zhang, et al., 2013b, Zhuang, et al., 2009). The principal function of the fly nephrocyte is to maintain the composition of the hemolymph through filtration and subsequent endocytosis of the filtrate. Nephrocytes form two rows of cells on either side of the heart tube, a contractile organ composed of cardiomyocytes. The proximity of the nephrocytes to the ostia, or heart inflow tracts, insures that filtered hemolymph enters the heart, which is then pumped for dispersal throughout the fly’s open circulatory system.
The plasma membrane (PM) surface area of the nephrocyte is expanded by extensive infoldings to form lacunar channels that are separated at the channel “mouth” by foot processes, across which the extracellular domains of membrane proteins form a filter structure termed the nephrocyte slit diaphragm (NSD). The NSD is molecularly, structurally, and functionally analogous to the slit diaphragm (SD) of the mammalian kidney and excludes larger hemolymph components from the lacunar channels (Weavers, Prieto-Sanchez, Grawe, Garcia-Lopez, Artero, Wilsch-Brauninger, Ruiz-Gomez, Skaer and Denholm, 2009). Reabsorption of filtered hemolymph proteins is associated with highly active endocytosis, reflected in the structure of the nephrocyte (Fig. 1A). Previously, we showed that the Drosophila homologs of Cubilin (Cubn) and Amnionless (AMN), major receptors for protein reabsorption in the PT, are specifically expressed in nephrocytes and function in protein uptake (Zhang, Zhao, Chao, Muir and Han, 2013a). Receptor-ligand complexes undergo clathrin-mediated endocytosis from the lacunar channel plasma membrane, and are then sorted to lysosomes and to recycling endosomes. Examination of dCubn- and dAMN-deficient nephrocytes demonstrated that endocytic activity was essential for maintaining the ultrastructural integrity of the cell and protecting the fly from ingested toxins.
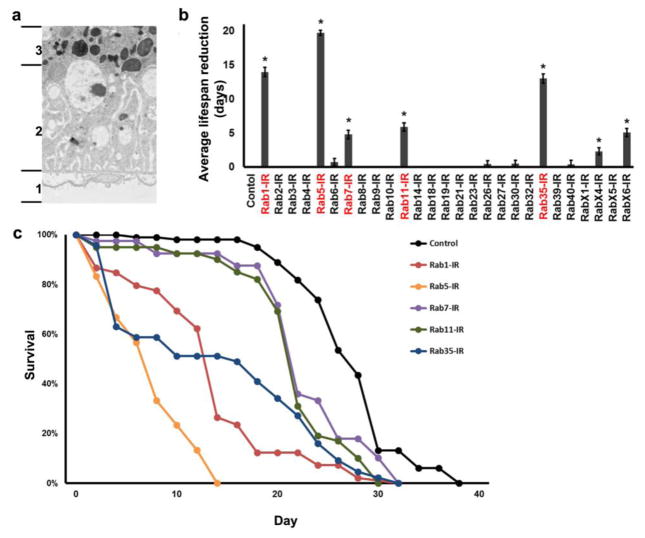
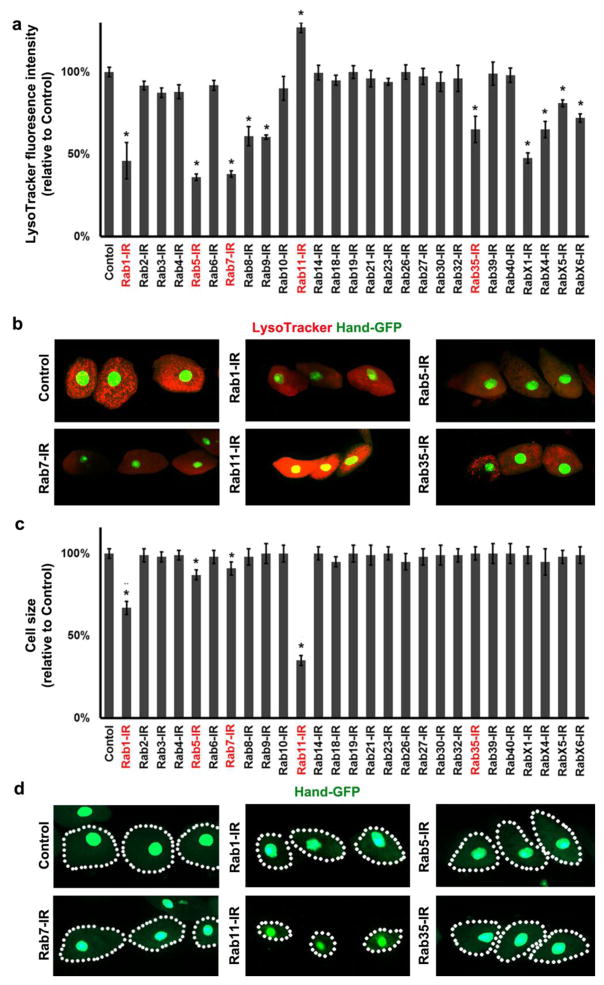
Fig. 1. Functional and cytoarchitectural domains of the Drosophila pericardial nephrocyte, adult fly mortality associated with Rab gene silencing, adult fly survival curves resulting from nephrocyte-specific silencing of genes encoding Rabs 1, 5, 7, 11, and 35.
a TEM of pericardial nephrocyte showing the three functional and cytoarchitectural domains: domain 1 composed of basement membrane, and “foot processes” spanned by nephrocyte diaphragms; domain 2 containing lacunar channels formed by invaginations of the plasma membrane, and clear vesicles; domain 3 composed largely of lysosomes. b Effect of nephrocyte-specific Rab gene silencing on average lifespan reduction (in days, relative to wild type control) of adult flies maintained under standard conditions. Three replicates were analyzed per genotype. Values are mean ± s.d. of 3 separate samples. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05. c Adult fly survival curves associated with nephrocyte-specific silencing of Rabs 1, 5, 7, 11, and 35.
Because Rab GTPases are master regulators, coordinators, and organizers of vesicle trafficking in virtually every eukaryotic cell (Schwartz, et al., 2007, Stenmark, 2009, Zerial and McBride, 2001), Rab proteins should be essential for nephrocyte function. A detailed understanding of fly renal physiology therefore requires a comprehensive examination of specific Rab activities in nephrocytes, a study that has not previously been described. The high degree of nephrocyte structural and functional homology to both podocytes and PT cells of the human kidney suggests that fundamental studies in the highly accessible fly system can also yield valuable insights into important cell biological processes underlying kidney function, with the potential to identify novel mechanisms of renal disease associated with abnormal filtration, receptor-mediated endocytosis, vesicle trafficking, and protein sorting. Blood filtration is performed by podocytes, highly specialized cells that form interdigitated processes spanned by slit diaphragm (SD) filtration structures (Grahammer, et al., 2013, Inoue and Ishibe, 2015, Pavenstadt, et al., 2003, Scott and Quaggin, 2015, Swiatecka-Urban, 2013). Intracellular membrane trafficking is critical for podocyte cell differentiation and development, maturation, and SD function (Steed, et al., 2010, Swiatecka-Urban, 2013, Tang and Brieher, 2012). In addition, podocytes actively take up and transcytose albumin and IgG, a process that prevents blockage of the SD (Akilesh, et al., 2008). It has also been shown that podocytes endocytose serum proteins quite actively, and that this is important for podocyte cell homeostasis (Bechtel, et al., 2013, Carson, et al., 2014, Dobrinskikh, et al., 2014). Proteins and other serum components that make it past the filtration barrier undergo reabsorption by PT cell receptor-mediated endocytosis (Christensen, et al., 2009, Nielsen and Christensen, 2010). Intracellular vesicle trafficking delivers endocytosed proteins to lysosomes, while the receptors are recycled (Gorvin, et al., 2013). The extensive and elaborate PT cell endocytic machinery indicates a high capacity for reabsorption (Christensen and Birn, 2002, Christensen, Verroust and Nielsen, 2009, Gorvin, Wilmer, Piret, Harding, van den Heuvel, Wrong, Jat, Lippiat, Levtchenko and Thakker, 2013, Nielsen, et al., 2007).
We performed a comprehensive in vivo analysis of Rab expression and function in Drosophila nephrocytes. We systematically assessed the effects of nephrocyte specific Rab gene silencing on adult fly longevity, developmental viability, hemolymph protein uptake, sequestration of ingested silver nitrate, and susceptibility to silver nitrate toxicity. We found that 11 of the 27 Drosophila Rabs were required for nephrocyte function, and that Rabs 1, 5, 7, 11, and 35 were particularly important. We further examined the effects of nephrocyte-specific silencing of these genes on lysosomes, cell size, and cell ultrastructure. We found that Rabs 5, 7, and 11 were essential for the regulation of endocytosis (Rab5), and key vesicle trafficking pathways involving protein degradation (Rab7) and membrane recycling (Rab11). Silencing of these genes profoundly disrupted vesicle organization and the integrity of cellular structures required for maintenance of filtration and subcellular structures involved in protein reabsorption.
Materials and Methods
Fly Strains
Flies were reared on standard food at 25°C. All UAS-Gal4 crosses were performed at 29°C to enhance Gal4 activity. The Dot-Gal4 strain was obtained from the Bloomington Drosophila Stock Center (BDSC). MHC-ANF-RFP was produced as described (Zhang et al., 2013a). Hand-GFP (Han, et al., 2006, Yi, et al., 2006, Zhang, Zhao, Chao, Muir and Han, 2013a) was used to label nephrocytes at all developmental stages. Transgenic RNAi lines were obtained from the BDSC or the Vienna Drosophila RNAi Center (VDRC). We tested two or more RNAi lines for each Rab gene (excepting Rabs 4, 14, 19, 26, and 30; only one line was available for each of these genes), and consistent phenotypes were observed for each line. YFP-Rab tagged fly lines (Dunst, et al., 2015) were obtained from the BDSC.
ANF-RFP Uptake Assay
The assay was performed as previously described (Zhang et al., 2013b). Briefly, 10 female flies carrying MHC-ANF-RFP, Hand-GFP, Dot-Gal4 transgenes were crossed to 5 male flies carrying a UAS-RabX-RNAi targeting transgene at 25°C. Two days after crossing, flies were transferred to small egg collection cages with grape juice agar plates for 24 hr at 25°C. The collected embryos were maintained at 29°C, and RFP fluorescence in pericardial nephrocytes was recorded for second instar larvae and newly emerged adults (within 24 hr of eclosion). For quantification, 20 nephrocytes were analyzed from each of 3 larvae or flies per genotype. The results are presented as mean±s.d. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
AgNO3 Uptake Assay
Flies of the appropriate genotype were allowed to lay eggs on standard apple juice agar plates for 24 hr. Freshly emerged first instar larvae were transferred to agar-only plates supplemented with regular yeast paste containing AgNO3 (2.0 g yeast in 3.5 ml 0.0005% AgNO3 solution) and allowed to develop at 29°C until adulthood. AgNO3 uptake by pericardial nephrocytes was assessed in adult flies one day post-emergence by dissecting heart tissues into Drosophila Schneider’s Medium (ThermoFisher) and examining cells by phase contrast microscopy. For quantification, 20 nephrocytes were analyzed from each of 3 female flies per genotype. The results are presented as mean±s.d. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
Survival assay
Within 24 hr of egg laying, Drosophila larvae were transferred from 25°C to 29°C to enhance Gal4 driven UAS-transgene expression. Adult male flies were maintained in vials at 29°C in groups of 15 or fewer. 50 flies were assayed per genotype. Three replicates were analyzed per genotype. Values are mean ± s.d. of 3 separate samples. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
Toxin Stress Assay
AgNO3 toxicity assay was performed essentially as described (Weavers, Prieto-Sanchez, Grawe, Garcia-Lopez, Artero, Wilsch-Brauninger, Ruiz-Gomez, Skaer and Denholm, 2009). Flies with the appropriate genotype were allowed to lay eggs on standard apple juice agar plates for 24 hrs. After aging for 24 hr, 20 newly emerging first instar larvae from this plate were transferred to agar-only plates supplemented with regular yeast paste or with yeast paste containing AgNO3 (2.0 g yeast in 3.5 ml of 0.005% AgNO3 solution), and allowed to develop at 29°C until adult stage. The percentage of emerging adults was recorded. Three replicates of 20 flies each were analyzed per genotype. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
qRT-PCR Analysis
RNA was isolated using Trizol Reagent (Invitrogen, Carlsbad, CA) from 100 dissected (pooled) adult hearts and 20 intact adult flies carrying the indicated Rab-RNAi silencing transgene. RNA purity and concentration were determined using a Nanodrop-1000 (Thermo Scientific, Wilmington, DE). Total RNA (1 μg) was reverse transcribed using Superscript IV (Invitrogen). SYBR Green based real-time qPCR (Power Cyber Mastermix; Applied Biosystems, Carlsbad, CA) was performed using a StepOne Plus (Applied Biosystems). Table S1 lists primer pairs and expected product sizes. Quantitative values were determined using the 2−ΔΔCT method (Livak and Schmittgen, 2001), normalizing to GAPDH. Values are mean ± s.d. of 3 separate samples. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
LysoTracker Dye Fluorescence
Larvae and adult flies were dissected and heart tissue was fixed for 10 minutes in 4% paraformaldehyde in phosphate-buffered saline (PBS). LysoTracker (Red DND-99, ThermoFisher Scientific) was used according to manufacturer’s instructions. For quantification, 20 nephrocytes were analyzed from each of 3 larvae per genotype. The results are presented as mean±s.d. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
Confocal Imaging, Image Quantification, and Transmission Electron Microscopy
Confocal imaging was performed with a Zeiss ApoTome.2 microscope using a 20× Plan-Apochromat 0.8 N.A. air objective. For quantitative comparisons of fluorescence intensity, common settings were chosen to avoid oversaturation. ImageJ Software Version 1.49 was used for image processing. Transmission electron microscopy was carried out using standard procedures. Briefly, third instar wandering larvae of the indicated genotypes were fixed using Sorensen phosphate buffer containing 4% paraformaldehyde and 2.5% glutaraldehyde. The processed samples were analyzed using a Philips CM100 TEM.
Results
Nephrocyte structural and functional domains
The Drosophila nephrocyte is a relatively large cell that is structurally and functionally ideally suited, by virtue of its high endocytic activity, to study essential roles of Rab GTPases in orchestrating the vesicle trafficking required for endocytosis, targeting of endocytosed materials to degradation and recycling pathways, and maintenance of the filtration apparatus and plasma membrane. Figure 1a shows the three functional and cytoarchitectural domains of the pericardial nephrocyte: an apical domain 1 consisting of basement membrane, and “foot processes” spanned by nephrocyte slit diaphragms (NSD); domain 2 consisting of plasma membrane (PM) invaginations forming lacunar channels, and clear vesicles; and domain 3 composed largely of lysosomes.
Rab gene silencing effects on fly lifespan
Because nephrocytes remove metabolic waste products and toxins from the hemolymph (Cagan, 2011) we hypothesized that nephrocyte-specific silencing of essential Rab genes would attenuate the lifespan of the fly. We produced strains in which UAS-Rab-RNAi gene silencing transgenes targeting each of the 27 Drosophila Rab genes (Dunst, Kazimiers, von Zadow, Jambor, Sagner, Brankatschk, Mahmoud, Spannl, Tomancak, Eaton and Brankatschk, 2015) were expressed in nephrocytes using a Dot-Gal4 driver construct (Zhang, Zhao, Chao, Muir and Han, 2013a, Zhang, Zhao and Han, 2013b). We found that in addition to pericardial nephrocytes, our Dot-Gal4 enhancer-driver was active only in a subset of ventral ganglion neurons (data not shown). Figure 1b shows the average number of adult life-days lost (relative to wild type control) as a result of silencing of the indicated Rab gene expression in nephrocytes. Detrimental effects were observed for flies in which the Rab genes 1, 5, 7, 11, 35, X4, and X6 were silenced. Although essential for nephrocyte-influenced adult viability, RabX4 and RabX6 show very low or no homology, respectively, with any human Rab and thus were not subjected to detailed analysis beyond initial comprehensive screening. Rab1, Rab5, Rab7, Rab11, and Rab35 are all highly conserved from flies to humans (FlyBase). Indeed, Rabs 1, 5, 7, and 11 are included in a set of core Rabs that have been maintained in virtually all eukaryotes (Dunst, Kazimiers, von Zadow, Jambor, Sagner, Brankatschk, Mahmoud, Spannl, Tomancak, Eaton and Brankatschk, 2015, Pereira-Leal and Seabra, 2001). Figure 1c shows the adult survival curves for fly lines in which Rab genes 1, 5, 7, 11, 35 were silenced in nephrocytes. Rab5 silencing produced the most severe effect on viability, with all flies dead before day 15. Because Rab5 is associated with early endocytic processes, this result strongly suggested that endocytosis is an essential nephrocyte function.
Rab gene expression in nephrocytes
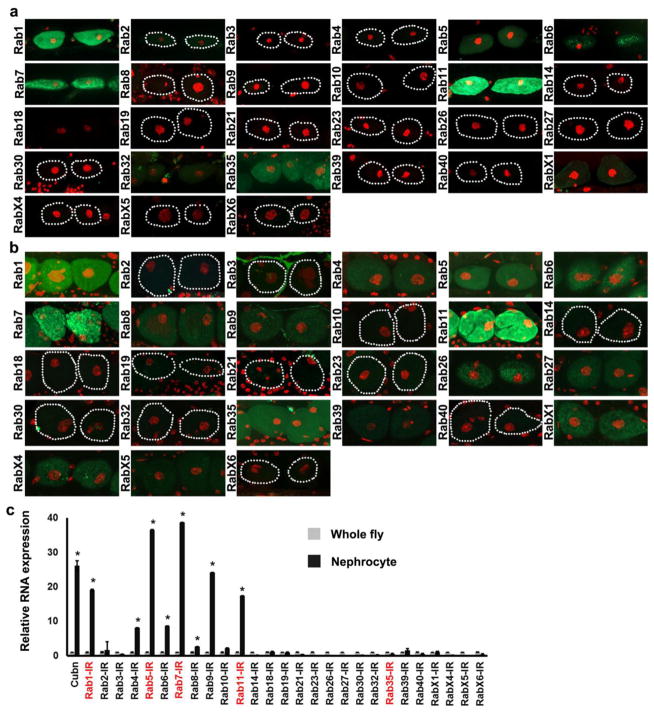
We comprehensively assessed nephrocyte expression of endogenously tagged Drosophila YFP-Rab proteins (Dunst, Kazimiers, von Zadow, Jambor, Sagner, Brankatschk, Mahmoud, Spannl, Tomancak, Eaton and Brankatschk, 2015). Figure 2a,b show larval and adult nephrocyte Rab expression, respectively. Consistent with gene silencing effects on adult viability, tagged Rabs 1, 5, 7, 11, and 35 were detectable by fluorescence microscopy in nephrocytes of third instar larvae (Fig. 2a) and adult flies (Fig. 2b). We also used qRT-PCR to measure heart-specific Rab RNA abundance relative to intact, whole flies. Dissected heart tissue consisted primarily of nephrocytes and cardiomyocytes, in which Rab 1, 5, 7, 9, and 11 RNA levels were substantially elevated compared to whole flies (Fig. 2c), approaching or exceeding levels of the nephrocyte-specific Cubilin (Cubn) RNA that served as a positive control in this analysis (Zhang, Zhao, Chao, Muir and Han, 2013a).
Fig. 2. Rab protein expression in nephrocytes, and relative RNA abundance in heart tissue.
Fluorescence micrographs showing a third instar larvae and b adult nephrocyte expression of endogenously tagged YFP-Rab proteins (green). Nephrocyte nuclei labeled with DAPI (red). Dotted lines indicate outlines of nephrocytes. c qRT-PCR analysis of Rab RNA enrichment in dissected adult fly heart tissue (nephrocytes and cardiomyocytes) relative to whole fly. Cubilin (Cubn) was previously shown to be expressed predominantly in nephrocytes (Zhang, Zhao, Chao, Muir and Han, 2013a) and was thus used as a positive control. Values are mean ± s.d. of 3 separate samples. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
Rab gene silencing effects on nephrocyte function
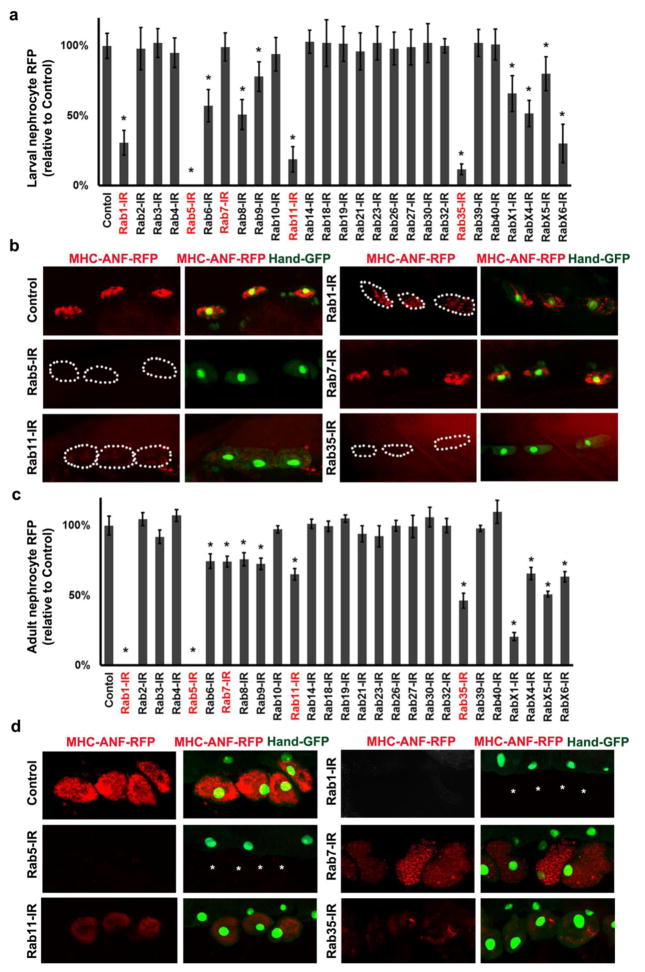
In order to directly measure functional effects of specific Rab gene silencing we assayed nephrocyte uptake of hemolymph protein (Zhang, Zhao and Han, 2013b). In this assay, an MHC-ANF-RFP transgene is introduced into Dot-Gal4; UAS-Rab-RNAi fly lines. In these flies the myosin heavy chain (MHC) promoter directs muscle cell expression of a rat atrium natriuretic factor (ANF) - red fluorescent protein (RFP) fusion protein (ANF-RFP) that is secreted into the hemolymph. The ANF-RFP is taken up and easily visualized in pericardial nephrocytes. Figure 3a,b and 3c,d show ANF-RFP uptake in nephrocytes of second instar larvae and recently emerged adult flies, respectively, expressing RNAi transgenes silencing Drosophila Rabs. Silencing Rab5 completely abolished ANF-RFP uptake by larval nephrocytes (Fig. 3a,b) and led to absence of the pericardial nephrocytes in adult flies (Fig. 3d). Similarly, Rab1 silencing led to reduced ANF-RFP uptake in larvae (Fig. 3a,b) and absence of adult nephrocytes (Fig. 3d). Rab11 and Rab35 silencing dramatically reduced ANF-RFP uptake in larvae (Fig. 3a,b), but the effect was significantly less severe in adult nephrocytes (Fig. 3c,d). Interestingly, silencing Rab7 had no effect or relatively minor effects on ANF-RFP uptake in larvae and adults, respectively. The most significant effect on adult nephrocyte uptake of ANF-RFP resulted from silencing of RabX1 (Fig. 3c), although compromised nephrocyte protein uptake was not associated in this case with decreased adult survival (Fig. 1b).
Fig. 3. Nephrocyte uptake of hemolymph protein.
a Second instar larvae nephrocytes in which the indicated Rab gene was silenced were quantitatively assessed for uptake of ANF-RFP fusion protein from the hemolymph. Red fluorescent protein (RFP) fused to rat atrium natriuretic factor (ANF) is expressed in and secreted by muscle cells from a transgene driven by the MHC enhancer. ANF-RFP fluorescence in nephrocytes expressed as percent of WT control. b Fluorescence microscopy showing ANF-RFP uptake (red) by nephrocytes (GFP nuclear expression) in which the indicated Rab gene expression was silenced. Dotted lines indicate outlines of nephrocytes. c Adult flies in which the indicated Rab gene was silenced were quantitatively assessed for uptake of ANF-RFP fusion protein from the hemolymph. ANF-RFP in nephrocytes expressed as percent of WT control. d Fluorescence microscopy showing ANF-RFP (red) uptake by nephrocytes (GFP nuclear expression) in which the indicated Rab gene expression was silenced. Asterisks indicate missing nephrocytes as a result of Rab1 and Rab5 silencing. For quantification, 20 nephrocytes were analyzed from each of 3 larvae or flies per genotype. The results are presented as mean±s.d. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
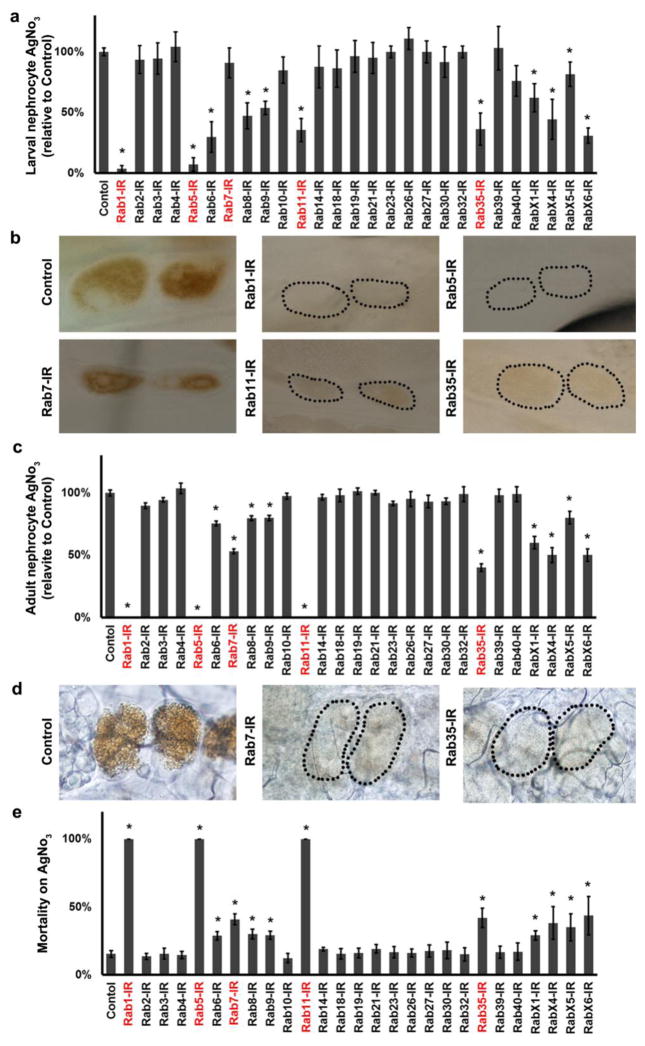
To further directly assess the importance of individual Rab proteins for nephrocyte function, we exploited the role of the pericardial nephrocytes in uptake and sequestration of toxins (Cagan, 2011). Ingested silver nitrate (AgNO3) is normally sequestered in pericardial nephrocytes (Figures 4a–d). We silenced the 27 Drosophila Rab proteins in nephrocytes of larvae (Fig. 4a,b) and adult flies (Fig. 4c,d) and compared AgNO3 levels to wild type controls. Rab 1 and Rab 5 gene silencing virtually abolished nephrocyte AgNO3 sequestration in larvae (Fig. 4a,b). In adults silencing of Rab1 and Rab5 results in absence of nephrocytes. Very dramatic reductions in AgNO3 levels were observed, in larval and adult nephrocytes, from silencing of Rab11 and Rab35. Larval nephrocytes in which Rab7 was knocked down were able to sequester AgNO3 normally, but in adults this function was reduced by approximately 40% (Fig. 4a–d). In adults, silencing of either RabX4 or RabX6 reduced nephrocyte AgNO3 sequestration to 50% of wild type control levels (Fig. 4c), and this functional deficit correlated with significant reductions in average lifespan (Fig. 1b).
Fig. 4. AgNO3 Uptake and Developmental Toxicity Assays.
a Third instar larvae nephrocytes in which the indicated Rab gene was silenced were quantitatively assessed for sequestration of ingested AgNO3. AgNO3 in nephrocytes expressed as percent of WT control. b Micrographs showing sequestration of ingested AgNO3 in larval pericardial nephrocytes in which the indicated Rab gene expression was silenced. Dotted lines indicate outlines of nephrocytes. c Adult nephrocytes in which the indicated Rab gene was silenced were quantitatively assessed for sequestration of ingested AgNO3. AgNO3 in nephrocytes expressed as percent of WT control (note that Rab1 and Rab5 silencing result in absence of adult nephrocytes). d Micrographs showing sequestration of ingested AgNO3 in adult pericardial nephrocytes in which the indicated Rab gene expression was silenced. Dotted lines indicate outlines of nephrocytes. e Developmental toxicity from ingestion of dietary AgNO3. Mortality index is expressed as the % of second instar larvae of the indicated Rab silencing genotype that fail to develop into adult flies. For quantification, 20 nephrocytes were analyzed from each of 3 larvae or flies per genotype. The results are presented as mean±s.d. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
We further comprehensively analyzed the effect of Rab gene silencing on AgNO3 exposure-induced developmental toxicity (developmental mortality) from second instar larvae to the emergence of adult flies from pupae. As shown in Figure 4e, while 15–20% of wild type controls fail to produce adults in the presence of AgNO3, silencing of Rab1, Rab5, and Rab11 led to complete (100%) developmental lethality. Rab7 and Rab35 silencing also significantly increased developmental lethality (Figure 4e).
Rab silencing effects on lysosomes and cell size
Endocytosed hemolymph proteins are normally trafficked to lysosomes for degradation, and we reasoned that the integrity of this subcellular compartment should be essential for normal nephrocyte function and influenced significantly by Rab protein expression and activity. To examine the effects of specific Rab gene silencing on nephrocyte lysosomes, we quantitatively analyzed LysoTracker dye fluorescence intensity in nephrocytes in which Rab genes were silenced. As shown in Figure 5a,b Rab5 and Rab7 knockdown led to the most significant reductions in LysoTracker dye fluorescence intensity, indicating reduced lysosome function. Substantial reductions were also observed as a consequence of Rab1 and Rab35 silencing. Uniquely, silencing of Rab11 led to a significant increase in LysoTracker fluorescence intensity. In the course of these studies we noted that silencing of Rab11 was associated with dramatic reductions in nephrocyte cell size (Fig. 5c,d). Systematic analysis revealed that knockdown of Rab1, Rab5, and Rab7 also led to smaller nephrocytes.
Fig. 5. Nephrocyte-specific gene silencing effects on lysosomes and nephrocyte cell size.
a Quantitative analysis of Rab gene silencing effects on LysoTracker (lysosome marker) fluorescence intensity in nephrocytes of third instar larvae. Fluorescence intensity expressed as percent of WT control. b Micrographs showing LysoTracker dye fluorescence (red) in pericardial nephrocytes (green, labeled with nuclear GFP fluorescence from expression of Hand-GFP transgene) of larvae in which the indicated Rab gene was silenced. c Quantitation of larval nephrocyte cell size as a function of Rab gene silencing. Bar graphs shows cell area as percent of WT control nephrocytes. d Micrographs showing cell size of Rab-silenced larval nephrocytes (labeled by nuclear GFP fluorescence from expression of Hand-GFP transgene). Dotted lines delineate cell boundaries. For quantification, 20 nephrocytes were analyzed from each of 3 larvae per genotype. The results are presented as mean±s.d. Results were analyzed by Student’s t-test. Statistical significance was defined as P<0.05.
Abnormal ultrastructure and vesicle composition associated with Rab gene silencing
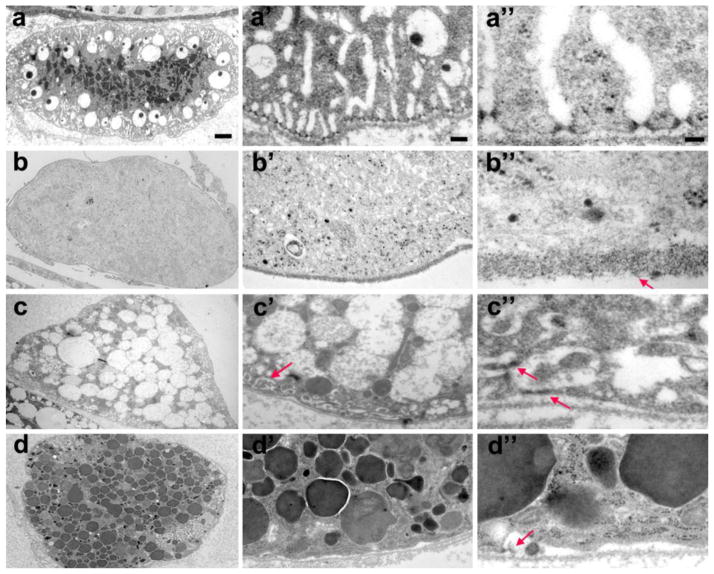
To further examine Rab effects on nephrocyte function and cell structure we carried out a TEM analysis on nephrocytes of third instar larvae in which Rab5, Rab7 and Rab11 were silenced (Fig. 6a–d). Rab 5 knockdown resulted in virtually complete elimination of intracellular structures necessary for nephrocyte filtration and reabsorption functions (Fig. 6a,b). Absent were the NSDs, lacunar channels, clear vesicles, and lysosomes characteristic of normal nephrocytes (Fig. 1a, 6a). Rab5 silencing resulted in a relatively homogeneous and opaque cytoplasm lacking recognizable intracellular structures (Figure 6b,b’), and a significantly thickened basement membrane with deposits of electron-dense material (Figure 6b”, arrow). In Rab7 RNAi knockdown nephrocytes, by contrast, we observed a dramatic expansion of large clear endocytic vesicles, and an almost complete absence of lysosomes (Fig. 6c, compare to 6a). Interestingly, Rab11 RNAi knockdown produced the opposite phenotype: dramatically increased numbers of lysosomes and virtual absence of clear vesicles (Fig. 6d, compare to 6a,c). Rab5 is associated with clathrin-coated pits (McLauchlan, et al., 1998) and endosome fusion (Gorvel, et al., 1991). Our observations indicated that in pericardial nephrocytes disruption of these processes blocked the major endocytic pathways through which hemolymph components are directed, via late endosomes, to lysosomes for degradation and receptor and membrane components are recycled back to the lacunar plasma membrane. Rab5 silencing also resulted in the complete breakdown of filtration and reabsorption structural features comprising NSDs and lacunar channels (Fig. 6b’,b”). Rab7 is associated with late endosome formation and lysosome fusion (Wang, et al., 2011), whereas Rab11 is required for formation of recycling endosomes (Nebenfuhr, 2002, Pfeffer, 2001). Our observations suggest that the Rab11-dependent clear vesicles and the Rab7-dependent dark lysosomes represent the two major endocytic pathways corresponding to the key nephrocyte functional activities analogous to vertebrate PT cells of storage/sequestration and reabsorption/degradation, respectively. Both pathways are initiated through Rab5-dependent clathrin-coated vesicles and early endosome fusion. As observed for Rab5, knock down of Rab7 and Rab11 was also associated with loss or severe disruption of NSDs and lacunar channel structures (Fig. 6c’,c”,d’,d”).
Fig. 6. Rab5, Rab7 and Rab11 gene expression is required for normal third instar larvae nephrocyte ultrastructure and vesicle composition.
a-a” In wild type control nephrocytes the lysosomes are concentrated in the interior of the cell, surrounded by clear vacuoles. NSDs and lacunar channels are precisely arrayed around the cell periphery. b-b” Rab5 gene silencing eliminated NSDs, lacunar channels, vacuoles, and lysosomes. The basement membrane was substantially thickened (arrow, b”). c-c” Rab7 gene silencing led to the accumulation of large clear vacuoles, and absence of lysosomes. Lacunar channels were greatly reduced in size and morphologically abnormal (arrow, c’), while NSDs were mislocalized and structurally deformed (arrows, c”). d-d” Rab11 gene silencing dramatically increased the number of lysosomes, at the expense of large clear vacuoles. Lacunar channels and NSDs were virtually absent, except for occasional remnants (arrow, d”). Scale bars: 2 microns (a–d), 0.2 micron (a’–d’), and 0.05 micron (a”–d”).
Discussion
We found that 11 out of the 27 Drosophila Rabs were required for nephrocyte function, with the most significant effects on adult longevity from knock down of Rabs 1, 5, 7, 11, and 35 (Fig. 1). An essential role for nephrocytes in fly survival, however, is controversial as flies lacking nephrocytes due to mutation of Klf15 exhibit a normal lifespan (Ivy, et al., 2015). We note, however, that our longevity studies were conducted under different conditions. Also, it is not unreasonable to propose that fly survival may be more severely compromised by the abnormal physiology of dysfunctional nephrocytes as seen in our study, compared to the absence of nephrocytes in the Klf15 mutants. Rabs 1, 5, 7, 11, and 35 are highly conserved from flies to humans, and with the exception of Rab35 are highly expressed in the heart (Fig. 2, and FlyBase). Rab40 silencing also significantly increased adult fly mortality (Fig. 1) and this Rab is also highly conserved, though expressed only at low levels in the heart (Fig. 2, and FlyBase). Rab40 silencing was not, however, associated with significant effects in subsequent functional assays (and see below). These included quantitative assessments of hemolymph protein uptake, and uptake and sequestration of toxic AgNO3 from the diet. We also measured the effect of Rab gene silencing on developmental mortality in the presence of dietary AgNO3. Rab1, Rab 5, Rab11, and Rab35 silencing was associated with significant mortality prior to emergence of adult flies. In the absence of AgNO3 only Rab 5, 7, 11, and 35 silencing induced developmental mortality, at lower levels of phenotypic severity than observed in the presence of the toxin. We did not observe effects of Rab7 silencing in comprehensive functional screening assays for protein and toxin uptake, though Rab7 knock down was associated with reduced adult fly longevity and some degree of abnormal developmental mortality. Conversely, we observed that compromised protein or toxin uptake was not invariably correlated with reduced adult fly longevity or developmental lethality (e.g. Rab27).
Vesicle trafficking activity disrupted by Rab knock down should be reflected in altered vesicle constituents. We demonstrated using LysoTracker that silencing of Rabs 5, 7, and 11 had the most significant effects on lysosome abundance. Rabs 5 and 7 appeared to promote lysosome formation or stability. Rab11 could be interpreted to function either by inhibiting lysosome formation, promoting lysosome turnover, or promoting an alternative vesicle trafficking pathway. Some endocytosed proteins are directed from sorting endosomes to lysosomes for degradation. The resulting amino acids can be utilized by the nephrocytes or secreted into the hemolymph for use by different tissues. Other absorbed proteins (including toxin-binding proteins), are trafficked to storage vacuoles where toxins like AgNO3 and deleterious metabolic byproducts are neutralized by sequestration. The major nephrocyte functions are carried out, then, through two alternative vesicle trafficking pathways. Our TEM analysis showed that these pathways diverge downstream of Rab5-dependent endocytosis and early endosome fusion. Subsequently, Rab7 was critical for the lysosomal trafficking pathway: when Rab7 was knocked down lysosomes were eliminated because all vesicle traffic was abnormally directed to clear storage vacuoles, which became super abundant. Thus, silencing Rab7 in nephrocytes did not produce phenotypes in which RFP or AgNO3 levels were reduced (Figs. 2 and 3), because these markers of nephrocyte function were stored/sequestered in clear vacuoles. Abnormal vesicle trafficking in Rab7-silenced nephrocytes did reduce adult fly longevity, but with the lowest phenotypic severity (Fig. 1c). Rab11, by contrast, is required for the storage vacuole trafficking pathway. Rab11 knock down led to the virtual disappearance of clear endocytic vacuoles, and the hyper accumulation of lysosomes. Thus Rab11 silencing reduced AgNO3 sequestration (Fig. 3a) and enhanced degradation of RFP (Fig. 2a).
Silencing of Rab5 not only eliminated lysosomes and clear vesicles, but also the NSDs and lacunar channels, and led to abnormal thickening of the basement membrane. This result highlights the critical roles of endocytosis and vesicle trafficking in maintaining the integrity of nephrocyte features that are structurally and functionally analogous to those of vertebrate kidney podocytes (filtration) and PT cells (reabsorption). That these functions are critical for viability was dramatically apparent from our assays that showed high adult mortality and developmental lethality resulting from Rab5 knock down. We are also intrigued by the possibility that nephrocyte basement membrane thickening in the absence of Rab5 models the proposed requirement for endocytosis in the prevention of podocyte slit diaphragm/glomerular basement membrane blockage due to protein accumulation (Akilesh, Huber, Wu, Wang, Hartleben, Kopp, Miner, Roopenian, Unanue and Shaw, 2008). In Rab7 knockdown nephrocytes, although the degradation pathway was blocked, the recycling pathway (by which membrane components, including receptors, are directed back into the plasma membrane) was apparently still present, possibly even enhanced and thus leading to abnormal, highly compressed lacunar structures densely packed near the plasma membrane (Fig. 6c’, arrow). NSD structures were present, but highly disorganized (Fig. 6c”, arrows). In Rab11 knockdown nephrocytes, lacunar structures were almost completely absent from most plasma membrane regions (Fig. 6d’), while nephrocyte diaphragms disappeared or had only a few remnants remaining (Fig. 6d”, arrow), suggesting that Rab11 is required for recycling pathway mediated maintenance of NSDs and lacunar channels. By this reasoning, blocking the recycling pathway might also be expected to result in dramatically reduced cell size, as we observed for Rab11 silencing, through alteration of the normal relationship between membrane uptake via endocytosis and membrane recycling. Figure 7 presents a simplified model of the nephrocyte roles of Rabs 5, 7, and 11 in orchestrating key steps in endocytosis and vesicle trafficking to lysosomes, recycling endosomes, and return of membrane constituents to the PM. The specific roles of other Rabs identified in this study as important for nephrocyte function are currently being investigated.
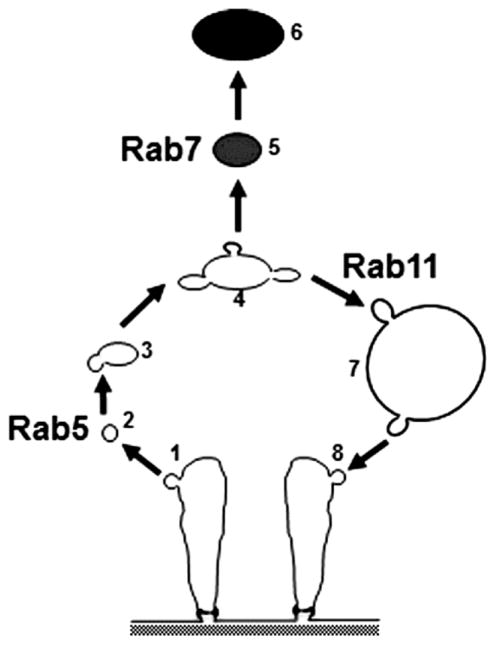
Fig. 7. A model highlighting the central roles of Rab5, Rab7, and Rab11 in endocytic membrane trafficking in pericardial nephrocytes.
Rab5 regulates the endocytosis of Clathrin-coated vesicles (1) from the plasma membrane of lacunar channels containing hemolymph components that have been filtered by the NSD. Vesicle uncoating (2) is followed by fusion of early endosomes (3) and formation of late and sorting endosomes (4). Rab7 directs vesicle cargo targeted for degradation (5) to the lysosomes (6). Rab11 regulates recycling of membrane lipids and membrane receptors via clear vacuoles (7) and recycling vesicles (8) back to the lacunar channel PM. In the absence of Rab5 the normal vesicle organization of the nephrocyte was abolished. In addition, the highly ordered cell surface structures featuring NSDs and lacunar channels were no longer present, indicating that endocytosis is essential for the formation and/or maintenance of the ultrastructural features required for nephrocyte hemolymph filtration, reabsorption of proteins, and sequestration of toxins. Silencing of Rab7 prevented the formation of lysosomes and promoted the proliferation and expansion of clear vacuoles. This was associated with disruption of the normal NSD and lacunar channel ultrastructure, though components of these structures were still visible in a disordered and compacted state at the cell periphery. Knockdown of Rab11, by contrast, led to superabundance of lysosomes and disappearance of clear vacuoles. This phenotype was associated with loss of NSDs and lacunar channels, presumably reflecting the requirement for Rab11 in recycling of materials to maintain these structures.
Earlier work indicated that the Drosophila nephrocyte shares remarkable structural and functional homology with both podocytes and PT cells (Simons and Huber, 2009, Weavers, Prieto-Sanchez, Grawe, Garcia-Lopez, Artero, Wilsch-Brauninger, Ruiz-Gomez, Skaer and Denholm, 2009, Zhang, Zhao, Chao, Muir and Han, 2013a, Zhang, Zhao and Han, 2013b, Zhuang, Shao, Guo, Trimble, Pearce and Abmayr, 2009). Nephrocytes, by combining the features of these two renal cell types, present a highly useful system for in vivo studies of fundamental kidney processes, studies that cannot readily be performed in a mammalian model. The critical role of endocytosis in serum protein reabsorption by PT cells is well documented, and more recent discoveries have shown the importance of endocytosis in podocyte biology (Akilesh, Huber, Wu, Wang, Hartleben, Kopp, Miner, Roopenian, Unanue and Shaw, 2008, Bechtel, Helmstadter, Balica, Hartleben, Kiefer, Hrnjic, Schell, Kretz, Liu, Geist, Kerjaschki, Walz and Huber, 2013, Carson, Okamura, Wakashin, McFann, Dobrinskikh, Kopp and Blaine, 2014, Dobrinskikh, Okamura, Kopp, Doctor and Blaine, 2014). Thus the study of nephrocyte Rab GTPases, as key regulators of vesicle trafficking, can shed light on physiological processes occurring in human kidney cells, with the potential to identify novel molecular targets for therapeutic approaches addressing important kidney diseases.
Supplementary Material
Table S1. Rab gene specific primers used for qRT-PCR analysis and anticipated amplification product sizes.
Acknowledgments
Funding
We thank the Bloomington stock center and the VDRC for Drosophila stocks. We thank the Microscopy and Image Analysis Laboratory at the University of Michigan for their technical support for TEM. We want to specially thank Dotty Sorenson and Sasha Meshinchi for their assistance in electron microscopy. Z. H. was supported by grant R01-DK098410 from the NIH.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:967–972. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel W, Helmstadter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, Schell C, Kretz O, Liu S, Geist F, Kerjaschki D, Walz G, Huber TB. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. Journal of the American Society of Nephrology : JASN. 2013;24:727–743. doi: 10.1681/ASN.2012070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagan RL. The Drosophila nephrocyte. Current Opinion in Nephrology and Hypertension. 2011;20:409–415. doi: 10.1097/MNH.0b013e328347ae02. [DOI] [PubMed] [Google Scholar]
- Carson JM, Okamura K, Wakashin H, McFann K, Dobrinskikh E, Kopp JB, Blaine J. Podocytes degrade endocytosed albumin primarily in lysosomes. PloS One. 2014;9:e99771. doi: 10.1371/journal.pone.0099771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nature Reviews Molecular Cell Biology. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Verroust PJ, Nielsen R. Receptor-mediated endocytosis in renal proximal tubule. Pflugers Archiv : European Journal of Physiology. 2009;458:1039–1048. doi: 10.1007/s00424-009-0685-8. [DOI] [PubMed] [Google Scholar]
- Dobrinskikh E, Okamura K, Kopp JB, Doctor RB, Blaine J. Human podocytes perform polarized, caveolae-dependent albumin endocytosis. American Journal of Physiology Renal Physiology. 2014;306:F941–951. doi: 10.1152/ajprenal.00532.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunst S, Kazimiers T, von Zadow F, Jambor H, Sagner A, Brankatschk B, Mahmoud A, Spannl S, Tomancak P, Eaton S, Brankatschk M. Endogenously tagged rab proteins: a resource to study membrane trafficking in Drosophila. Developmental Cell. 2015;33:351–365. doi: 10.1016/j.devcel.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gorvin CM, Wilmer MJ, Piret SE, Harding B, van den Heuvel LP, Wrong O, Jat PS, Lippiat JD, Levtchenko EN, Thakker RV. Receptor-mediated endocytosis and endosomal acidification is impaired in proximal tubule epithelial cells of Dent disease patients. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7014–7019. doi: 10.1073/pnas.1302063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahammer F, Schell C, Huber TB. The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nature Reviews Nephrology. 2013;9:587–598. doi: 10.1038/nrneph.2013.169. [DOI] [PubMed] [Google Scholar]
- Han Z, Yi P, Li X, Olson EN. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development. 2006;133:1175–1182. doi: 10.1242/dev.02285. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ishibe S. Podocyte endocytosis in the regulation of the glomerular filtration barrier. American Journal of Physiology Renal Physiology. 2015;309:F398–405. doi: 10.1152/ajprenal.00136.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy JR, Drechsler M, Catterson JH, Bodmer R, Ocorr K, Paululat A, Hartley PS. Klf15 is critical for the development and differentiation of Drosophila nephrocytes. PLoS One. 2015;10:e0134620. doi: 10.1371/journal.pone.0134620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Current Biology. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Na J, Cagan R. The Drosophila nephrocyte: back on stage. JASN. 2013;24:161–163. doi: 10.1681/ASN.2012121227. [DOI] [PubMed] [Google Scholar]
- Nebenfuhr A. Vesicle traffic in the endomembrane system: a tale of COPs, Rabs and SNAREs. Curr Opin Plant Biol. 2002;5:507–512. doi: 10.1016/s1369-5266(02)00303-5. [DOI] [PubMed] [Google Scholar]
- Nielsen R, Christensen EI. Proteinuria and events beyond the slit. Pediatric Nephrology. 2010;25:813–822. doi: 10.1007/s00467-009-1381-9. [DOI] [PubMed] [Google Scholar]
- Nielsen R, Courtoy PJ, Jacobsen C, Dom G, Lima WR, Jadot M, Willnow TE, Devuyst O, Christensen EI. Endocytosis provides a major alternative pathway for lysosomal biogenesis in kidney proximal tubular cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5407–5412. doi: 10.1073/pnas.0700330104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiological Reviews. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. Journal of Molecular Biology. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. Journal of Cell Science. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- Scott RP, Quaggin SE. Review series: The cell biology of renal filtration. The Journal of Cell Biology. 2015;209:199–210. doi: 10.1083/jcb.201410017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Huber TB. Flying podocytes. Kidney International. 2009;75:455–457. doi: 10.1038/ki.2008.653. [DOI] [PubMed] [Google Scholar]
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends in Cell Biology. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature Reviews Molecular Cell Biology. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Swiatecka-Urban A. Membrane trafficking in podocyte health and disease. Pediatric Nephrology. 2013;28:1723–1737. doi: 10.1007/s00467-012-2281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang VW, Brieher WM. alpha-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. The Journal of Cell Biology. 2012;196:115–130. doi: 10.1083/jcb.201103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Ming Z, Xiaochun W, Hong W. Rab7: role of its protein interaction cascades in endo-lysosomal traffic. Cell Signal. 2011;23:516–521. doi: 10.1016/j.cellsig.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Weavers H, Prieto-Sanchez S, Grawe F, Garcia-Lopez A, Artero R, Wilsch-Brauninger M, Ruiz-Gomez M, Skaer H, Denholm B. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature. 2009;457:322–326. doi: 10.1038/nature07526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi P, Han Z, Li X, Olson EN. The mevalonate pathway controls heart formation in Drosophila by isoprenylation of Ggamma1. Science. 2006;313:1301–1303. doi: 10.1126/science.1127704. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nature Reviews Molecular Cell Biology. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhao Y, Chao Y, Muir K, Han Z. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. JASN. 2013a;24:209–216. doi: 10.1681/ASN.2012080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhao Y, Han Z. An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. JASN. 2013b;24:191–197. doi: 10.1681/ASN.2012080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development. 2009;136:2335–2344. doi: 10.1242/dev.031609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Rab gene specific primers used for qRT-PCR analysis and anticipated amplification product sizes.