Abstract
Blockers of the renin-angiotensin system are effective in the treatment of experimental and clinical diabetic nephropathy. An approach different from blocking the formation or action of angiotensin II(1-8) that could also be effective involves fostering its degradation. Angiotensin converting enzyme 2 (ACE2) is a monocarboxypeptidase than cleaves angiotensin II (1-8) to form angiotensin (1-7). Therefore, we examined the renal effects of murine recombinant ACE2 in mice with streptozotocin-induced diabetic nephropathy as well as that of amplification of circulating ACE2 using minicircle DNA delivery prior to induction of experimental diabetes. This delivery resulted in a long-term sustained and profound increase in serum ACE2 activity and enhanced ability to metabolize an acute angiotensin II (1-8) load. In mice with streptozotocin-induced diabetes pretreated with minicircle ACE2, ACE2 protein in plasma increased markedly and this was associated with a more than 100-fold increase in serum ACE2 activity. However, minicircle ACE2 did not result in changes in urinary ACE2 activity as compared to untreated diabetic mice. In both diabetic groups, glomerular filtration rate increased significantly and to the same extent as compared to non-diabetic controls. Albuminuria, glomerular mesangial expansion, glomerular cellularity and glomerular size, were all increased to a similar extent in minicircle ACE2-treated and untreated diabetic mice, as compared to non-diabetic controls. Recombinant mouse ACE2 given for 4 weeks by intraperitoneal daily injections in mice with streptozotocin-induced diabetic nephropathy also failed to improve albuminuria or kidney pathology. Thus, a profound augmentation of ACE2 confined to the circulation failed to ameliorate the glomerular lesions and hyperfiltration characteristic of early diabetic nephropathy. These findings emphasize the importance of targeting the kidney rather than the circulatory renin angiotensin system to combat diabetic nephropathy.
Keywords: Soluble ACE2, Minicircle delivery, Diabetic nephropathy, angiotensin II, angiotensin 1-7
INTRODUCTION
Clinical interventions targeting the renin-angiotensin system (RAS) in diabetic kidney disease center on the use of renin-angiotensin system (RAS) blockers1-6. While the therapeutic effect of RAS blockers is well established there is only incomplete response in terms of reducing proteinuria, kidney histology and preventing disease progression. Alternative and/or complementary approaches aimed at degrading Ang II (1-8) efficiently may be considered as newer therapeutic approaches aimed at RAS downregulation7, 8. ACE2 is a monocarboxypeptidase that enhances Ang II (1-8) degradation, and forms Ang (1-7), a peptide with beneficial anti-inflammatory and anti-proliferative actions that may confer renoprotection9-17. Genetic deletion of the Ang (1-7) receptor, the Mas receptor, has been reported to cause hyperfiltration and worsening of kidney disease13 whereas Ang (1-7) administration can improve experimental diabetic nephropathy (DN)17, 18. Amplification of ACE2 activity is therapeutically attractive in that it not only prevents accumulation of Ang II (1-8), the most active RAS peptide, but also fosters Ang (1-7) formation. Circulating ACE2 activity has been shown to be moderately augmented in rodent models of diabetes19-21, in humans with chronic kidney disease22, and diabetes accompanied by vascular complications23. This increase in ACE2 could serve as a compensatory mechanism to attenuate Ang II accumulation. Since the levels of ACE2 in plasma are low, however, the reported moderate increases in plasma ACE2 are likely not sufficient to exert a significant protective effect. We reasoned that achieving a large increase in ACE2 would ensure constant hydrolysis of Ang II (1-8) and formation of Ang (1-7) which, if sustained over time, could protect against the development of DN.
In its full-length form, ACE2 is a type 1 integral membrane glycoprotein that consists of three structural entities: the cytosolic, transmembrane and extracellular domains which together amount to a molecular weight of 120-130 kD24-27. The extracellular domain of ACE2, which confers its enzymatic activity, contains a single catalytic metallopeptidase unit28. ACE2 is mainly a tissue enzyme and its levels in the circulation, unlike the levels of ACE, are relatively low20, 29, 30. A form of ACE2 that is not tissue-bond, referred to as a soluble ACE2, is enzymatically active and has been found in the circulation, urine and cerebrospinal fluid21, 29, 31-33.
In mice with STZ model of diabetes19, 34 and in db/db mice35 administration of an ACE2 inhibitor caused worsening of albuminuria. In agreement with the studies where pharmacological ACE2 inhibitor was given to diabetic mice, the deletion of the Ace2 gene was reported to accentuate19 and the transgenic glomerular ACE2 over-expression ameliorated diabetes-related kidney lesions26. Moreover, a beneficial effect of human recombinant (r)ACE2 given by i.p. injections was reported to ameliorate albuminuria and diabetic kidney lesions in the Akita model of diabetic kidney disease36. This finding was surprising because human rACE2, when given to mice for more than 2 weeks, results in formation of neutralizing antibodies and the attendant loss of ACE2 activity8, 37. We therefore developed murine recombinant ACE237 and reasoned that ACE2 amplification using this rodent form of rACE2 would circumvent the problem of immunogenicity arising from chronic administration of xenogeneic human rACE2 to mice. Accordingly, in the present study we administered soluble mouse rACE2 protein by daily i.p. injections in mice that had been given STZ four weeks earlier to produce early DN.
To increase and sustain high levels of ACE2 activity for a much longer period, murine rACE2 was administered by mini-circle (Mc) ace2 DNA delivery. The Mc system utilizes a phiC31 integrase recombination event to remove the bacterial backbone elements of the plasmid that are required for plasmid amplification and replication in bacteria but contribute to gene silencing subsequently38, 39. By removing these sequences and isolating the circular expression cassette long term gene expression can be achieved from the Mc that is maintained as an extrachromosomal episome39. This approach also permitted to examine a potential preventative effect of preexisting high levels of circulating ACE2 on STZ-induced DN. Finally, the impact of markedly increasing ACE2 in the circulation on urinary ACE2 was examined not only in STZ treated mice with mild albuminuria but also in a model of CKD due to a Col4A3 gene deficiency that results in advanced alterations in the glomerular basement membrane and robust proteinuria40.
RESULTS
General and Kidney Parameters in Diabetic Mice treated with mrACE2 intraperitoneally
We have previously shown that when human rACE2 is administered to normal mice there is a loss of ACE2 activity by two weeks which is attributable to formation of neutralizing antibodies8. Therefore in this study we used mouse rACE2 which results in a sustainable increase in serum ACE2 activity for at least 4 weeks37. Studies using mrACE2 administration for four weeks by i.p. injections to STZ mice showed that mrACE2 does not have a protective effect on kidney pathology or urinary albumin excretion as compared to STZ-treated mice receiving PBS (Supplemental Results: Table S1 and Figure S1).
Characterization of ACE2 activity augmentation after minicircles ace2 DNA delivery
ACE2 mini-circle (Mc) was administered as a single injection38, 39 to mice in the FVB/N background. Two doses (10 ug and 30 ug) were used to determine whether Mc injection results in dose-dependent increases in serum ACE2 activity. At 3-9 days after Mc administration, serum ACE2 activity in female FVB/N mice that received 10 ug McACE2 (n=9) increased markedly as compared to controls (n=14) (138±48 vs. 0.5±0.1 RFU/uL/hr, p<0.01). In FVB/N mice that received 30 ug McACE2 (n=8), serum ACE2 activity increased even further (480±153 RFU/uL/hr, p<0.001).
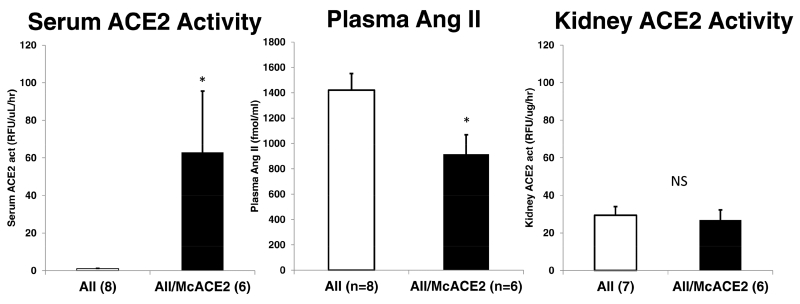
The marked increase in serum ACE2 activity achieved initially (3-9 days) was sustained over 10 weeks of observation in McACE2-treated (n=7) as compared to untreated mice (n=9) (108±46 vs.1.0±0.2 RFU/uL/hr, p<0.05, respectively). In urines from McACE2-treated mice, however, ACE2 activity was not significantly different than in urines from mice that did not receive McACE2 (3.5±0.9 vs. 4.4±0.6 RFU/ug creatinine/hr, respectively). At the end of this experiment (10-12 weeks after sham or McACE2 injection), mice were given an i.p. bolus of Ang II (0.2 ug/BW) or vehicle (PBS) to examine the ability to handle an acute Ang II load. Five minutes after the Ang II bolus ACE2 activity in serum and Ang II levels in plasma were evaluated from cardiac blood obtained at the sacrifice Plasma Ang II in samples obtained 5 minutes after Ang II bolus were significantly lower in McACE2 mice as compared to sham mice also infused with Ang II (915±154 vs. 1420±131 fmol/mL, p<0.05, respectively) (Figure 1, middle panel). Serum ACE2 activity was markedly higher in McACE2 mice (n=6) as compared to sham mice (n=8) (63.0±32.6 vs. 0.96±0.18 RFU/ul/hr, respectively) (Figure 1, left panel). Kidneys harvested after Ang II infusion, however, did not show any detectable increase in kidney ACE2 activity as compared to kidneys from vehicle treated mice (26.9±5.3 vs. 29.4±4.6 RFU/ug/hr) (Figure 1, right panel).
Figure 1.
Serum angiotensin-converting enzyme 2 (ACE2) activity (left), plasma angiotensin (Ang) II levels (middle), and kidney ACE2 activity (right) 5 minutes after a bolus of Ang II (AII) in mice pretreated with ace2 DNA minicircles (McACE2, black bars) and untreated (white bars). *P < 0.05 versus Ang II only; NS, not significant.
In additional experiments urinary Ang II(1-8) was evaluated after 1 week of Ang II administration. McACE2-pretreated (n=8) and sham-pretreated control mice (n=7) were administered Ang II (40 pmol/min) for seven days using subcutaneous osmotic minipumps. No significant differences in urinary Ang II levels were found between mice pretreated with McACE2 or not (1781±248 vs. 1794±166 fmol Ang II/mg creatinine, p=0.967, respectively). The levels of urinary Ang II in animals infused with this peptide were markedly higher than those in mice that did not receive Ang II infusion (n=9) (217±61 fmol Ang II/mg creatinine, p<0.001).
Altogether, these findings in wild type mice showed that McACE2 delivery results in a marked increase in serum ACE2 activity which facilitates lowering of plasma Ang II levels when this peptide is acutely infused. By contrast, urinary Ang II levels after 1 week of Ang II infusion are not significantly affected by prior McACE2 administration despite a large and sustained increase in serum ACE2 levels.
General and kidney parameters in mice with diabetes induced by STZ after minicircles DNA delivery of ACE2
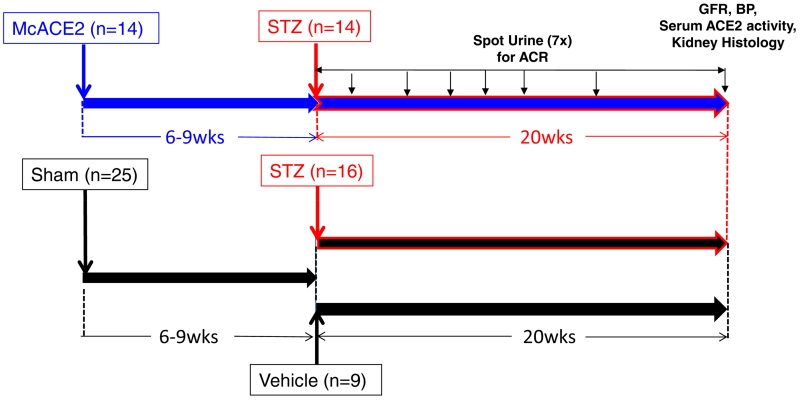
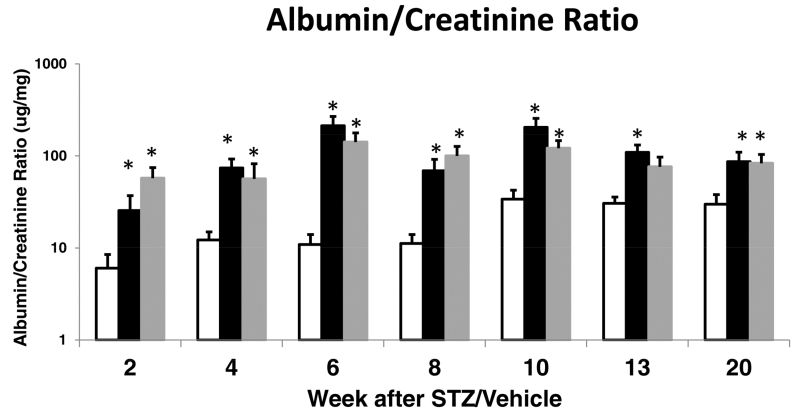
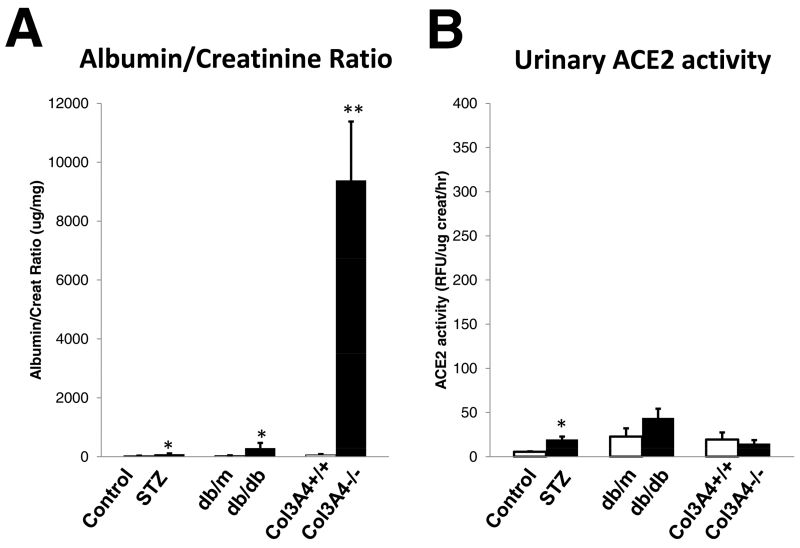
The scheme for ACE2 amplification via minicircle DNA delivery and later induction of diabetes by STZ is shown in Figure 2. Six to nine weeks after minicircle ace2 DNA injection or sham injection, STZ or vehicle was given. At 40 weeks of age, 20 weeks after diabetes induction and 26-29 weeks following ace2 DNA mini-circle or sham injection, the levels of blood glucose were similarly increased in both groups with STZ-induced diabetes (Table 1). The levels of serum ACE2 activity were profoundly increased in the diabetic group treated with ACE2 minicircles (n=14) as compared to untreated diabetic (n=15) and non-diabetic controls (n=9). Systolic blood pressure was not significantly different between STZ-McACE2 mice and STZ mice not treated with McACE2, (109±5 and 106±6 mm Hg, respectively). Body weight was similar in the three groups. Kidneys to body weight ratios were significantly higher in sham STZ and STZ/McACE2 as compared to a non-diabetic control group but not different from each other. The heart to body weights ratio was not significantly different between the three groups. No significant difference in the albumin/creatinine ratio (ACR) between the two diabetic groups was observed over the entire period of 20 weeks of observation (Figure 3).
Figure 2.
Study design and time of injection of angiotensin-converting enzyme 2 (ACE2) minicircles (McACE2) or sham injection followed by streptozotocin (STZ) to induce diabetic nephropathy. Upper level: 6 to 9 weeks after ace2 DNA minicircles injection at a dose of 10 to 30 ug/mouse (n=14), mice were rendered diabetic by 2 STZ injections (5 days apart from each other). Lower level: 6 to 9 weeks after sham injection, 16 mice were rendered diabetic by 2 STZ injections while the remaining mice (n=9) received 2 injections of vehicle (Na-citrate; pH 4.5) and served as nondiabetic controls. ACR, albumin-to-creatinine ratio; BP, blood pressure; GFR, glomerular filtration rate.
Table 1.
General parameters in mice 20 weeks after diabetes induction with STZ and in vehicle-treated mice that served as a non-diabetic control group. Six to nine weeks prior to induction of diabetes with STZ, animals were either injected with ace2 DNA minicircle (McACE2) or given a sham injection.
| Parameter | Controls | STZ | STZ/McACE2 |
|---|---|---|---|
| Blood Glucose (mg/dL) | 177±16* | 457±36 | 449±32 |
| Body weight (g) | 24.3±0.4 | 25.9±0.6 | 26.1±0.6 |
| Serum ACE2 act (RFU/ul/hr) | 1.4±0.3* | 2.4±0.3 | 497±135** |
| L+R Kidney Weight (g) | 0.291±0.0087* | 0.437±0.0138 | 0.415±0.0204 |
|
Kidney/Body Weight Ratio
(mg/g) |
12.2±0.4* | 17.2±0.5 | 16.3±1.0 |
| Heart Weight (g) | 0.093±0.004 | 0.097±0.004 | 0.094±0.003 |
| Heart/Body Weight (mg/g) | 3.89±0.18 | 3.79±0.11 | 3.65±0.11 |
| SBP (mmHg) | 125±8 | 106±6 | 109±5 |
denotes significant differences between controls and both STZ and STZ/McACE2 (at least at p<0.05);
denotes a significant difference versus controls and STZ mice not pre-treated with McACE2 (p<0.05). Otherwise there were no significant differences between untreated and McACE2-treated STZ mice
Figure 3.
Urinary albumin-to-creatinine ratio (ACR) in streptozotocin (STZ) mice that were pretreated with angiotensin converting enzyme 2 (ace2) DNA minicircle (grey bars, n=14) or sham injected (black bars, n = 15–16). Vehicle-injected mice (white bars, that served as nondiabetic control group; n = 9) had ACR values significantly lower than both STZ-treated groups. There were no significant differences in ACR between sham STZ mice and minicircle angiotensin-converting enzyme (McACE2)-pretreated STZ mice at any of the time points measured. *Significantly different at least at a P < .05 level versus vehicle-injected nondiabetic controls.
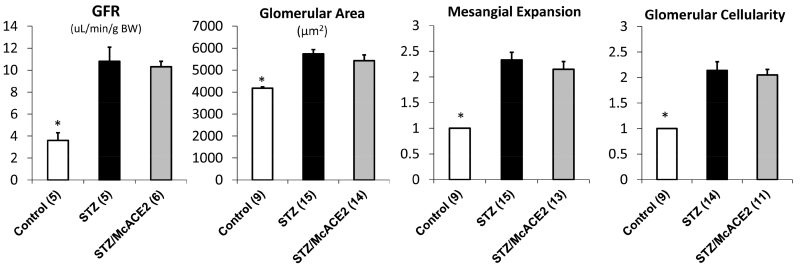
The GFR was markedly increased in both diabetic groups as compared to controls but not significantly different from each other (Figure 4). Consistent with hyperfiltration, glomerular surface area was increased in both diabetic groups but not significantly from each other. The remaining findings on histologic kidney evaluation are also summarized in Figure 4. Glomerular mesangial expansion was increased significantly in both diabetic groups as compared to controls but it was not significantly different from each other. No significant difference was found in glomerular cellularity between McACE2-treated and sham-treated diabetic mice.
Figure 4.
Kidney parameters in mice 20 weeks after diabetes induction with streptozotocin (STZ) and in vehicle-treated mice that served as a nondiabetic control group. Six to 9 weeks before induction of diabetes with STZ, animals were either injected with angiotensin converting enzyme 2 (ace2) DNA minicircle (McACE2) or given a sham injection (STZ). No significant differences were found in any of these parameters between STZ and STZ/McACE2. *Significant differences compared with both STZ and STZ/McACE2 (at least at a P < 0.05 level vs. vehicle control). BW, body weight.
Effect of plasma ACE2 overexpression on plasma, tissue and urinary ACE2 activity in mice with STZ-induced diabetes
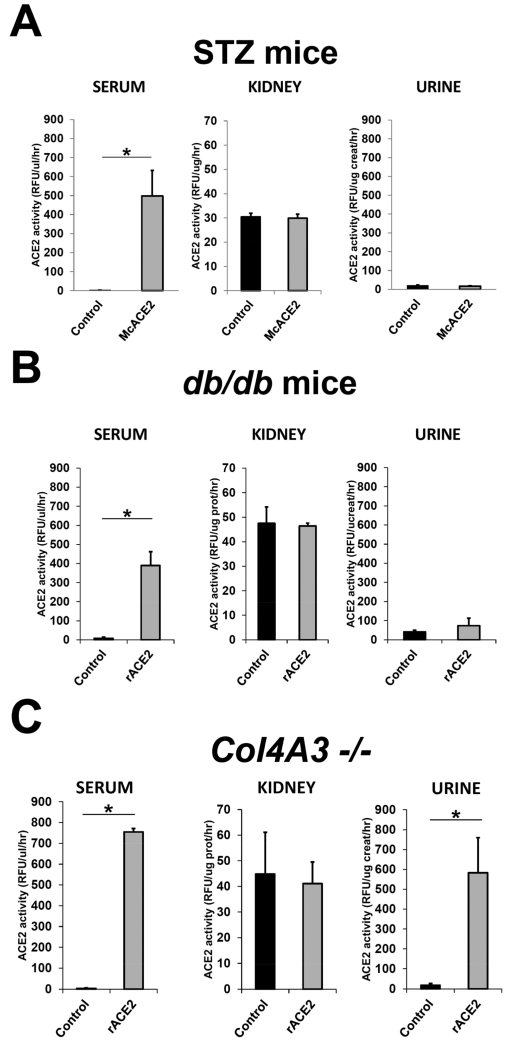
In the group with STZ-induced diabetes described above, a marked increase in serum ACE2 activity after a single ACE2 mini-circle injection was sustained for 26-29 weeks at which point animals were sacrificed (Table 1). The higher serum ACE2 activity could be attributed to the appearance of a large ACE2-immunoreactive band in the plasma of McACE2-treated diabetic mice (Figure 5). In lysates from thoracic aorta of McACE2-pretreated diabetic mice ACE2 enzyme activity was increased significantly (0.84±0.24 vs. 0.03±0.09 RFU/ug prot./hr, p<0.01). ACE2 activity was also significantly increased as compared to untreated diabetic mice in the heart (1.89±0.32 vs. 0.94±0.12 RFU/ug prot./hr, p<0.01) and in the liver (0.44±0.08 vs. 0.25±0.03 RFU/ug prot./hr, p<0.05, respectively). However, kidney ACE2 activity was not significantly different between the two groups (29.9±1.6 vs. 30.5±1.5 RFU/ug prot/hr respectively). McACE2 administration also did not produce any significant increase in urinary ACE2 activity (17±2 vs. 19.6±3.1 RFU/ug creat/hr) (Figure 6A).
Figure 5.
Blood plasma (0.25 uL/well) from 6 untreated streptozotocin mice (STZ) and 6 STZ mice that were pretreated with ace2 minicircle DNA (McACE2 STZ). The samples were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and probed in Western blot using a specific anti–angiotensin-converting enzyme 2 (ACE2) antibody. The Western blot shows an ACE2 immunoreactive band at around 100 kDa in plasma samples from each of the McACE2 STZ-pretreated mice, which was not observable in the plasma from untreated STZ mice.
Figure 6.
Serum, kidney, and urine angiotensin-converting enzyme 2 (ACE2) activity in streptozotocin (STZ) mice overexpressing ACE2 after ace2 minicircle DNA injection and in acutely soluble mouse recombinant ACE2 (rACE2)-treated db/db mice or Col4A3−/− mice (grey bars) compared with the respective untreated control mice (black bars). (a) In McACE2-treated STZ mice (n = 14), serum ACE2 activity was augmented markedly (P < 0.01), whereas kidney and urine ACE2 activity were similar to that of untreated STZ mice (n = 15). (b) In acutely rACE2-treated db/db mice (n = 3), a robust increase in serum ACE2 activity (P < 0.01) was associated with unchanged kidney and urinary ACE2 activity compared with the noninjected db/db mice (n = 3). (c) Same parameters for Col4A3−/− mice treated acutely with murine rACE2 or controls. In acutely rACE2–treated Col4A3−/− mice (n = 3), the increase in serum ACE2 activity compared with noninjected mice (n=3) was associated with a marked increase in urinary ACE2 activity. *Significant differences (at least at P < 0.05 level).
Effect of rACE2 administration to db/db mice and to proteinuric Col4A3−/− mice
The db/db model of type 2 diabetes was also used to examine whether or not urinary ACE2 can increase after rACE2 infusion in another diabetic model that exhibits a ‘microalbuminuric’ range of albumin creatinine ratio (Figure 7A). For comparison purposes Col4A3−/− mice, a model of CKD with marked proteinuria40-42, was also studied. Unlike in STZ and db/db mice, with only a modest increase in ACR, in Col4A3−/− mice (n=11) ACR was markedly elevated (9387±1993 ug/mg) as compared to the corresponding WT controls of the same age and sex (n=5, 63±23 ug/mg) (p<0.001) (Figure 7A). Urinary ACE2 activity was not significantly different between the two groups (14.9±3.8 vs. 19.4±8.0 RFU/ug creat/hr, respectively) (Figure 7B). Consistent with previous reports, in urines from STZ and db/db diabetic mice, ACE2 activity at baseline were higher or tended to be higher than in their respective non-diabetic controls (Figure 7B).
Figure 7.
Albumin creatinine ratio (ACR) and urinary angiotensin-converting enzyme 2 (ACE2) activity in streptozotocin (STZ)-treated mice, db/db mice, and in Col4A3−/− mice (filled bars) compared with their respective controls (empty bars) under baseline conditions. (a) In Col4A3−/− mice (n = 11), ACR was increased markedly compared with Col4A3+/+ (n = 5), STZ (n = 15), and db/db mice (n = 3) (**P < 0.001). ACR was increased significantly in STZ and db/db mice compared with their respective nondiabetic controls (*P < 0.05). (b) Urine ACE2 was increased in STZ (*P < 0.05), but not significantly in db/db mice, compared with their respective nondiabetic controls. Urine ACE2 was decreased, but not significantly in Col4A3−/− mice, compared with Col4A3+/+ mice of the same age (8–10 wk).
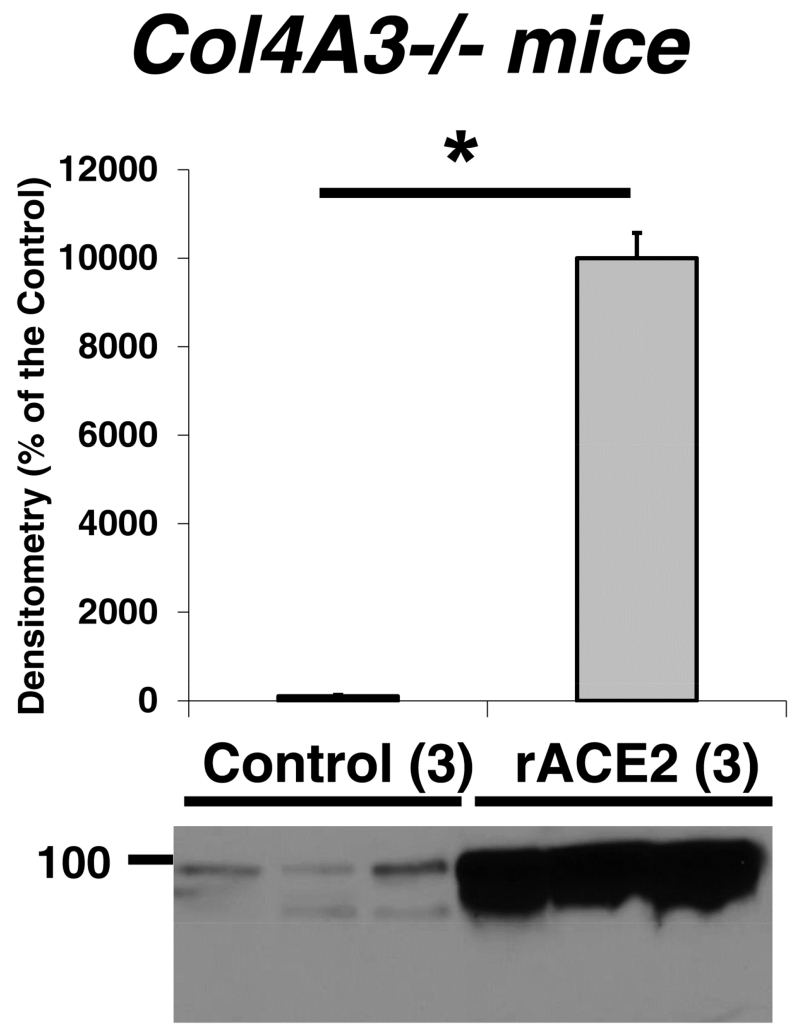
Similar to the STZ model, in db/db mice the acute infusion of rACE2 increased serum ACE2 activity markedly without any increase in urine ACE2 activity (Figure 6B). A single bolus injection of soluble rACE2 to the Col4A3−/− mice caused a marked elevation in serum ACE2 activity similar to that seen in STZ-treated mice pretreated with ACE2 via minicircle DNA delivery or db/db mice that received rACE2 acutely (compare Figure 6AB with 6C, left panels). After infusion of mrACE2 in Col4A3−/− mice, urinary ACE2 activity increased markedly (Figure 6C, right panel). This is in sharp contrast with the lack of increase in urine ACE2 activity in STZ-treated mice (Figure 6A, right panel) and db/db mice (Figure 6B, right panel). ACE2 protein by Western blot also increased in urines from Col4A3−/− mice (Figure 8). Kidney ACE2 activity (Figure 6C, middle panel) and ACE2 protein abundance by Western blot (not shown) were not significantly affected by ACE2 infusion.
Figure 8.
Western blot densitometry results of angiotensin converting enzyme 2 (ACE2) protein detected in urine from Col4A3−/− mice that received recombinant ACE2 (rACE2) (n = 3, grey) or did not receive it (n = 3, black). The corresponding Western blot image is shown at the bottom. *P < 0.001.
The above findings show that in a model of Col4A3 gene deficiency associated with profound proteinuria due to increased permeability of the glomerular basement membrane40, 41, infused ACE2 can be filtered and thus recovered in the urine. This is in sharp contrast to mice with STZ induced diabetes and db/db mice with modest albuminuria where infused rACE2 cannot be filtered and therefore cannot be recovered in the urine.
Effect of chronic ACE2 overexpression by minicircle delivery on plasma RAS peptides in mice with STZ-induced diabetes
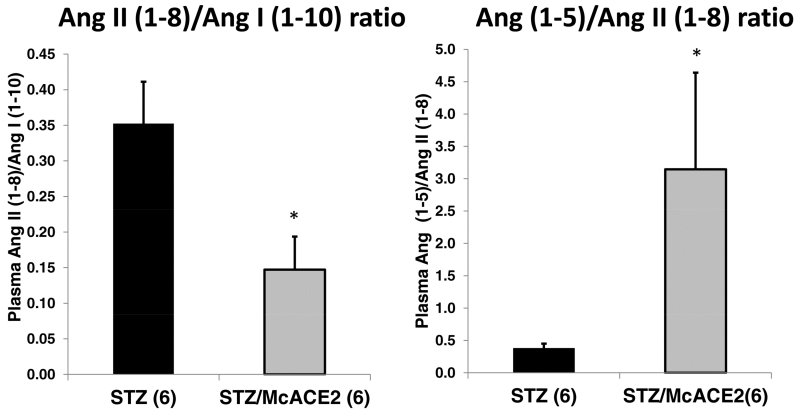
The effect of serum ACE2 overexpression by minicircle delivery on plasma RAS peptides was examined using LC/MS-MS (see Supplemental Results). In plasma from McACE2-treated STZ mice, the levels of Ang II (1-8) were lower than those observed in the plasma of the control animals injected with STZ but the difference did not achieve statistical significance (Table S2). Ang II (1-8) levels are influenced by the precursor peptide, Ang I (1-10), and its degradation. Therefore, the ratio between Ang II (1-8) and Ang I (1-10) was used to assess the relation between these peptides43-45. Angiotensin II (1-8)/Ang I (1-10) ratio was significantly lower in diabetic mice pretreated with ACE2 minicircles as compared to sham-treated diabetic mice (0.147±0.047 vs. 0.352±0.059, p<0.05) (Figure 9). This shows that for a given amount of the precursor peptide Ang I (1-10), there is a lower level of Ang II (1-8) in McACE2 treated than in untreated mice which is consistent with increased degradation of Ang II (1-8) by excess of circulating ACE2.
Figure 9.
Plasma Ang II (1-8)/Ang I (1-10) and Ang (1-5)/Ang II (1-8) ratio in untreated STZ mice (black, n = 6) and in STZ mice pretreated with ace2 DNA minicircles (grey, n = 6). The bars represent means ± SE. Plasma Ang peptides were measured by LC-MS/MS as described in the Materials and Methods section. *P <0.05 vs. untreated STZ mice.
In the McACE2 group, plasma Ang (1-5) levels were higher than in sham diabetic mice but the difference did not reach statistical significance (6.2±2.4 vs. 3.5±1.5, respectively.) As an index of Ang II (1-8) conversion to Ang (1-7), a ratio of Ang (1-5)/Ang II (1-8) was used. The Ang (1-5)/Ang II (1-8) ratio was markedly and significantly increased in McACE2-treated mice as compared to untreated mice (3.145±1.494 vs. 0.376±0.075, p<0.05, respectively) (Figure 9). Overall these findings are consistent with increased conversion of Ang II (1-8) to Ang (1-7), and then conversion of Ang(1-7) to Ang (1-5) in the McACE2-treated group.
No differences between the two groups were found in regards to any of the other six angiotensin peptides measured as well as plasma renin or plasma angiotensinogen (see Supplementary material for detailed information).
DISCUSSION
This study examined the question as to whether a sustained increase in circulating ACE2 activity prevents or attenuates kidney damage in mice with STZ induced diabetic nephropathy (DN). For this, two approaches to deliver ACE2 were used: a) i.p. administration daily for 4 weeks to male mice with STZ-induced early DN which resulted in a moderate increase in “trough” serum ACE2 levels and b) an ace2 minicircle DNA single injection approach to female mice to increase circulating ACE2 prior to induction of DN by STZ. This approach resulted in a very large and sustained increase in levels of serum ACE2 and allowed for a long-term evaluation of STZ-induced diabetes (20 weeks) on kidney parameters.
The hypothesis that a sustained chronic increase in serum ACE2 can afford protection against DN was not supported by our findings in mice made diabetic by STZ. Neither the administration of ACE2 by DNA minicircles, nor by daily i.p injections of murine rACE2 conferred any significant renoprotective effect in terms of either sustained reduction in albuminuria (Figure 3) or improvement in the glomerular lesions typically seen in mice with early DN such as an increase glomerular size, matrix deposition and cellularity (Figure 4). The elevated GFR of the STZ-treated mice was not reduced by increasing the levels of circulating ACE2 (Figure 4). Thus, the characteristic hyperfiltration of early DN was also not affected by augmentation of serum ACE2. Suppression of ACE2 activity in mice with a selective inhibitor of this enzyme has been shown to prevent diabetes-associated hyperfiltration19, 46. A similar hemodynamic pattern to that caused by pharmacologic ACE2 inhibition was reported in diabetic ACE2KO mice suggesting that ACE2 may be involved in the induction and/or maintenance of diabetic hyperperfusion19, 46. In our studies, we attribute the lack of an effect of rACE2 on hyperfiltration or glomerular lesions of diabetes, despite the marked and sustained increase in systemic ACE2, to the failure to deliver the large ACE2 protein to the kidney (see below). The lack of effect of ACE2 amplification on blood pressure is not surprising since hypertension is not a feature of STZ-induced DN47, 48.
Minicircle ace2 DNA delivery was remarkably successful in achieving a consistent and profound increase in serum ACE2 activity that lasted for several months. The minicircle approach of minicircle DNA delivery of ACE2 provides therefore a model of gene delivery that is resistant to gene silencing and consequently allows for long-term overexpression of proteins of interest. As such it is not labor intensive and it is faster than having to create a transgenic rodent model to test similar questions related to overexpression of protein of interest. This model of gene delivery has been applied so far to a few proteins38, 39, 49 enabling a sustained transgene expression for up to 1 year without apparent adverse effects50. The profound increase in serum ACE2 activity achieved by the minicircle delivery resulted in enhanced ability to dispose of an infused Ang II load as compared to non-treated animals (Figure 1B). This shows that an excess of plasma Ang II can be metabolized more rapidly when the levels of serum ACE2 are markedly increased chronically and is consistent with previous studies by us infusing ACE2 acutely8, 37. The single injection of minicircle containing coding sequence for soluble mouse ACE2 (amino acids 1-740) also resulted in an increased ACE2 expression in the aorta and liver as demonstrated by an increase in enzymatic activity in these tissues. Despite the large increase in serum ACE2 activity, however, neither kidney ACE2 activity nor protein increased above baseline values.
ACE2 is a large 100-110 kD protein that is not filterable by normal glomeruli. In STZ-induced diabetes and in db/db mice, glomerular permeability is only modestly altered, as judged by the modest degree of albuminuria51. In these two models, urinary ACE2 did not increase despite large increases in serum ACE2 activity (Figure 6). By contrast, in a mouse model of profound proteinuria, the Col4A3−/− mice40 an acute infusion of murine rACE2 resulted in a marked recovery of ACE2 protein and ACE2 activity in the urine. Our findings show that soluble rACE2 is not likely to be effective to treat early kidney disease with mild albuminuria, such that seen in STZ, db/db, Akita and most other rodent models of diabetic nephropathy52-55. Soluble rACE2 administration, however, could be effective in models with overt proteinuria where marked alterations in glomerular permeability should allow the kidney delivery of a large molecule such as ACE2. Future studies should be undertaken in models that have higher albumin excretion rates, such as OVE-2656 or BTBRob/ob mice57. From the above-mentioned it can also be extrapolated that if a therapeutic effect of administered ACE2 on DN had been observed it would have been primarily related to increased levels of ACE2 activity in the circulation where large increases in serum ACE2 activity were achieved. In this respect, the potential therapeutic benefit should depend, at least in part, on the status of the circulating RAS. In diabetic kidney disease, plasma renin activity is usually reduced l58, 59. Rather the activation of the RAS in diabetic kidney disease is local at the kidney level59-62. By extrapolation, our data suggest that the efficacy of widely used RAS blockers in the treatment of DN must be the result of an inhibitory effect exerted directly at the kidney level, ACE inhibitors and AT1 receptor blockers are small compounds that are freely filtered. ACE inhibitors are well known to decrease kidney ACE activity very effectively63. In a model of a podocyte-specific ACE2-transgenic, partial protection against STZ-induced glomerular damage has been reported26. This further illustrates the importance of targeting ACE2 directly to the kidney to achieve a therapeutic benefit.
The effect of a large increase in circulating ACE2 activity achieved by McACE2 gene delivery on plasma levels of Ang II and other Ang peptides was examined by LC/MS-MS analysis (see Supplement Table S2 and Figure 9). In the plasma from McACE2-treated STZ mice, the levels of Ang II (1-8) were lower than those observed in the plasma of the control animals injected with STZ but the difference did not achieve statistical significance. Ang II/Ang I ratio, however, was significantly lower in STZ-McACE2 as compared to STZ sham mice which is consistent with enhanced Ang II degradation. In plasma, Ang (1-7) has a very short half-life64, 65 and is quickly converted to Ang (1-5) by ACE65. This explains, in part, that plasma levels of Ang (1-7), the direct product of ACE2 cleavage of Ang II, were very low and below the level of detection in some cases. Therefore, we used the Ang 1-5/Ang II (1-8) ratio as an index of Ang II (1-8) degradation. This ratio was markedly higher in McACE2-treated diabetic mice than in sham-treated diabetic mice suggesting a degradation of Ang II(1-8) as a result of plasma ACE2 overexpression with formation of Ang (1-7), and later Ang (1-5) from the rapid degradation of Ang(1-7). This analysis suggests that the large increase in circulating ACE2 in McACE2-treated mice is associated with enhanced metabolism of Ang II(1-8) as reflected by an increase in the Ang (1-5)/Ang II(1-8) ratio. Of note, the activities of other enzymes that contribute to Ang II formation from Ang I (ACE) or its degradation (APA) were not significantly affected by the overexpression of ACE2 in plasma achieved by McACE2 (Supplemental Results).
A possibility that needs to be considered is that long-term and profound augmentation of circulating ACE2 in McACE2-treated mice with the resulting enhanced Ang II(1-8) metabolism could have resulted in up-regulation of plasma renin activity and depletion of AOG leading to a reduced formation of RAS peptides. Plasma total renin protein levels tended to be higher in McACE2 mice but not significantly as compared to sham STZ-treated mice. Neither total nor active serum AOG levels were different in McACE2 diabetic mice as compared to untreated diabetic mice (Figure S2). These findings therefore show that profound amplification of circulating ACE2 activity for long periods of time (26-29 weeks) does not result in exhaustion of circulating AOG levels possibly due to enhanced synthesis of this protein in the liver.
In summary, minicircle ace2 DNA delivery induced a profound and sustained increase in plasma ACE2 activity but did not affect urinary ACE2 activity in normal mice or mice with STZ induced mild DN. Urinary albumin excretion, glomerular mesangial expansion, glomerular cellularity and glomerular size, were all increased to a similar extent in McACE2-treated and untreated diabetic mice, as compared to non-diabetic controls. In addition, GFR was increased markedly as compared to non-diabetic controls but unaffected by minicircle ace2 DNA delivery. We conclude that a profound augmentation of ACE2 activity confined to the circulation is not sufficient to attenuate albuminuria, glomerular hyperfiltration and the glomerular pathology characteristic of mice with STZ-induced early DN. A large enzyme such as ACE2 cannot be filtered under normal conditions or after STZ-induced moderate glomerular injury. By contrast, when glomerular permeability is sufficiently altered as is the case in the Col4A3−/− model of CKD with robust proteinuria, a rapid increase in serum ACE2 results in a marked increase in urinary ACE2 protein and activity. Recombinant ACE2 based therapies may find a niche for the therapy of advanced proteinuric CKD when glomerular filtration barrier is sufficiently disrupted allowing the filtration of this large enzyme or by developing smaller compounds that are ACE2 activators that can be easily filtered by the kidney.
METHODS
Intraperitoneal injections of mrACE2 to diabetic mice
Description included in Supplemental Methods.
ACE2 minicircle preparation and injection
Description included in Supplemental Methods.
Induction of diabetes using STZ and measurements performed
Description included in Supplemental Methods.
Acute administration of soluble recombinant ACE2 to Col4A3−/− and db/db mice
Female Col4A3−/− at 6-10 weeks of age were used as a proteinuric model of kidney disease to study the effect of acute rACE2 administration on serum, urinary and kidney ACE2 activity. As a model of type 2 diabetes, db/db mice (C57BLKS/JLepr) were used. Immediately after voiding urine, mice were administered an intraperitoneal bolus of recombinant ACE2 (4 mg/kg). Urine was then collected in metabolic cages over 3 h after rACE2 intraperitoneal bolus. Mice were then given an overdose of Euthasol and blood was rapidly drawn by cardiac puncture. Mice were perfused with PBS (25ml/mouse) to flush out the remaining blood from organs, and kidneys were collected.
Kidney histology
Description included in Supplemental Methods.
Western blot
Description included in Supplemental Methods.
Enzymatic activities of angiotensinases
The activities of the following angiotensinases were evaluated: ACE2, ACE, and aminopeptidase A, as previously described in detail21, 66-68.
Measurements of serum angiotensinogen and total renin protein levels
Active and total AOG was measured using ELISA kits (IBL)69. Mouse total renin protein was measured using ELISA kit from Ray Biotech.
Blood pressure measurements
(see Supplemental Methods).
Statistical analysis
The data are reported as the arithmetic mean ± standard error of the mean (SEM), unless stated otherwise. Differences between two groups were analyzed using a two-tailed Student’s t-test, and for non-normally distributed data (e.g. urinary ACR), log transformation was performed for the subsequent statistical analysis. For multiple comparisons ANOVA was used followed by LSD post hoc test. The IBM SPSS software (version 23) was used for the statistical analyses.
Supplementary Material
Acknowledgments
Sources of Support
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK080089 and R01DK104785), and a gift to Northwestern University by the Joseph and Bessie Feinberg Foundation. YK received a National Institute of Diabetes and Digestive and Kidney Diseases grant (R01DK060635).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Nothing to disclose
References
- 1.Raij L. The pathophysiologic basis for blocking the renin-angiotensin system in hypertensive patients with renal disease. Am J Hypertens. 2005;18:95S–99S. doi: 10.1016/j.amjhyper.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 2.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Seminars in nephrology. 2007;27:144–152. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 3.de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921–927. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 4.Bichu P, Nistala R, Khan A, et al. Angiotensin receptor blockers for the reduction of proteinuria in diabetic patients with overt nephropathy: results from the AMADEO study. Vascular health and risk management. 2009;5:129–140. [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. The New England journal of medicine. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 6.Remuzzi G, Ruggenenti P, Perna A, et al. Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: a post hoc analysis of the RENAAL trial results. J Am Soc Nephrol. 2004;15:3117–3125. doi: 10.1097/01.ASN.0000146423.71226.0C. [DOI] [PubMed] [Google Scholar]
- 7.Batlle D, Wysocki J, Soler MJ, et al. Angiotensin-converting enzyme 2: enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int. 2012;81:520–528. doi: 10.1038/ki.2011.381. [DOI] [PubMed] [Google Scholar]
- 8.Wysocki J, Ye M, Rodriguez E, et al. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55:90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dilauro M, Burns KD. Angiotensin-(1-7) and its effects in the kidney. The Scientific World Journal. 2009;9:522–535. doi: 10.1100/tsw.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmerman DL, Zimpelmann J, Xiao F, et al. The effect of angiotensin-(1-7) in mouse unilateral ureteral obstruction. The American journal of pathology. 2015;185:729–740. doi: 10.1016/j.ajpath.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Zimpelmann J, Cheng K, et al. The role of angiotensin converting enzyme 2 in the generation of angiotensin 1-7 by rat proximal tubules. Am J Physiol Renal Physiol. 2005;288:F353–362. doi: 10.1152/ajprenal.00144.2004. [DOI] [PubMed] [Google Scholar]
- 12.Ye M, Wysocki J, Naaz P, et al. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension. 2004;43:1120–1125. doi: 10.1161/01.HYP.0000126192.27644.76. [DOI] [PubMed] [Google Scholar]
- 13.Pinheiro SV, Ferreira AJ, Kitten GT, et al. Genetic deletion of the angiotensin-(1-7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009;75:1184–1193. doi: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- 14.Moon JY, Tanimoto M, Gohda T, et al. Attenuating effect of angiotensin-(1-7) on angiotensin II-mediated NAD(P)H oxidase activation in type 2 diabetic nephropathy of KK-A(y)/Ta mice. Am J Physiol Renal Physiol. 2011;300:F1271–1282. doi: 10.1152/ajprenal.00065.2010. [DOI] [PubMed] [Google Scholar]
- 15.Benter IF, Yousif MH, Dhaunsi GS, et al. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. American journal of nephrology. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 16.Varagic J, Ahmad S, Nagata S, et al. ACE2: angiotensin II/angiotensin-(1-7) balance in cardiac and renal injury. Current hypertension reports. 2014;16:420. doi: 10.1007/s11906-014-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giani JF, Burghi V, Veiras LC, et al. Angiotensin-(1-7) attenuates diabetic nephropathy in Zucker diabetic fatty rats. Am J Physiol Renal Physiol. 2012;302:F1606–1615. doi: 10.1152/ajprenal.00063.2012. [DOI] [PubMed] [Google Scholar]
- 18.Mori J, Patel VB, Ramprasath T, et al. Angiotensin 1-7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol. 2014;306:F812–821. doi: 10.1152/ajprenal.00655.2013. [DOI] [PubMed] [Google Scholar]
- 19.Tikellis C, Bialkowski K, Pete J, et al. ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes. 2008;57:1018–1025. doi: 10.2337/db07-1212. [DOI] [PubMed] [Google Scholar]
- 20.Yamaleyeva LM, Gilliam-Davis S, Almeida I, et al. Differential regulation of circulating and renal ACE2 and ACE in hypertensive mRen2.Lewis rats with early-onset diabetes. Am J Physiol Renal Physiol. 2012;302:F1374–1384. doi: 10.1152/ajprenal.00656.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wysocki J, Garcia-Halpin L, Ye M, et al. Regulation of urinary ACE2 in diabetic mice. Am J Physiol Renal Physiol. 2013;305:F600–611. doi: 10.1152/ajprenal.00600.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts MA, Velkoska E, Ierino FL, et al. Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28:2287–2294. doi: 10.1093/ndt/gft038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soro-Paavonen A, Gordin D, Forsblom C, et al. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. Journal of hypertension. 2012;30:375–383. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- 24.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 25.Lew RA, Warner FJ, Hanchapola I, et al. Characterization of angiotensin converting enzyme-2 (ACE2) in human urine. Int J Pept Res Ther. 2006;12:283–289. doi: 10.1007/s10989-006-9031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadarajah R, Milagres R, Dilauro M, et al. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int. 2012;82:292–303. doi: 10.1038/ki.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert DW, Yarski M, Warner FJ, et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 29.Rice GI, Jones AL, Grant PJ, et al. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48:914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- 30.Levy A, Yagil Y, Bursztyn M, et al. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1953–1961. doi: 10.1152/ajpregu.90592.2008. [DOI] [PubMed] [Google Scholar]
- 31.Kawajiri M, Mogi M, Higaki N, et al. Angiotensin-converting enzyme (ACE) and ACE2 levels in the cerebrospinal fluid of patients with multiple sclerosis. Mult Scler. 2009;15:262–265. doi: 10.1177/1352458508097923. [DOI] [PubMed] [Google Scholar]
- 32.Marshall AC, Shaltout HA, Pirro NT, et al. Antenatal betamethasone exposure is associated with lower ANG-(1-7) and increased ACE in the CSF of adult sheep. Am J Physiol Regul Integr Comp Physiol. 2013;305:R679–688. doi: 10.1152/ajpregu.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chappell MC. Of diabetic mice and ACE2: a new biomarker of renal disease? Am J Physiol Renal Physiol. 2013;305:F970–972. doi: 10.1152/ajprenal.00403.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soler MJ, Wysocki J, Ye M, et al. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614–623. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- 35.Ye M, Wysocki J, William J, et al. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 36.Oudit GY, Liu GC, Zhong J, et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–538. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye M, Wysocki J, Gonzalez-Pacheco FR, et al. Murine Recombinant Angiotensin-Converting Enzyme 2: Effect on Angiotensin II-Dependent Hypertension and Distinctive Angiotensin-Converting Enzyme 2 Inhibitor Characteristics on Rodent and Human Angiotensin-Converting Enzyme 2. Hypertension. 2012;60:730–740. doi: 10.1161/HYPERTENSIONAHA.112.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen ZY, He CY, Ehrhardt A, et al. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Molecular therapy: the journal of the American Society of Gene Therapy. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 39.Osborn MJ, McElmurry RT, Lees CJ, et al. Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of alpha-L-iduronidase in mice with mucopolysaccharidosis type I. Molecular therapy: the journal of the American Society of Gene Therapy. 2011;19:450–460. doi: 10.1038/mt.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosgrove D, Meehan DT, Grunkemeyer JA, et al. Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes & development. 1996;10:2981–2992. doi: 10.1101/gad.10.23.2981. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Asada M, Higashi AY, et al. Loss of the BMP antagonist USAG-1 ameliorates disease in a mouse model of the progressive hereditary kidney disease Alport syndrome. The Journal of clinical investigation. 2010;120:768–777. doi: 10.1172/JCI39569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gross O, Beirowski B, Koepke ML, et al. Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int. 2003;63:438–446. doi: 10.1046/j.1523-1755.2003.00779.x. [DOI] [PubMed] [Google Scholar]
- 43.Juillerat L, Nussberger J, Menard J, et al. Determinants of angiotensin II generation during converting enzyme inhibition. Hypertension. 1990;16:564–572. doi: 10.1161/01.hyp.16.5.564. [DOI] [PubMed] [Google Scholar]
- 44.Campbell DJ, Alexiou T, Xiao HD, et al. Effect of reduced angiotensin-converting enzyme gene expression and angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptide levels in mice. Hypertension. 2004;43:854–859. doi: 10.1161/01.HYP.0000119190.06968.f1. [DOI] [PubMed] [Google Scholar]
- 45.van Kats JP, Duncker DJ, Haitsma DB, et al. Angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade prevent cardiac remodeling in pigs after myocardial infarction: role of tissue angiotensin II. Circulation. 2000;102:1556–1563. doi: 10.1161/01.cir.102.13.1556. [DOI] [PubMed] [Google Scholar]
- 46.Tikellis C, Brown R, Head GA, et al. Angiotensin-converting enzyme 2 mediates hyperfiltration associated with diabetes. Am J Physiol Renal Physiol. 2014;306:F773–780. doi: 10.1152/ajprenal.00264.2013. [DOI] [PubMed] [Google Scholar]
- 47.Katoh M, Ohmachi Y, Kurosawa Y, et al. Effects of imidapril and captopril on streptozotocin-induced diabetic nephropathy in mice. European journal of pharmacology. 2000;398:381–387. doi: 10.1016/s0014-2999(00)00320-4. [DOI] [PubMed] [Google Scholar]
- 48.Candido R, Allen TJ, Lassila M, et al. Irbesartan but not amlodipine suppresses diabetes-associated atherosclerosis. Circulation. 2004;109:1536–1542. doi: 10.1161/01.CIR.0000124061.78478.94. [DOI] [PubMed] [Google Scholar]
- 49.Dietz WM, Skinner NE, Hamilton SE, et al. Minicircle DNA is superior to plasmid DNA in eliciting antigen-specific CD8+ T-cell responses. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:1526–1535. doi: 10.1038/mt.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viecelli HM, Harbottle RP, Wong SP, et al. Treatment of phenylketonuria using minicircle-based naked-DNA gene transfer to murine liver. Hepatology. 2014;60:1035–1043. doi: 10.1002/hep.27104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurley SB, Clare SE, Snow KP, et al. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290:F214–222. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- 52.Brosius FC, 3rd, Alpers CE, Bottinger EP, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurley SB, Mach CL, Stegbauer J, et al. Influence of genetic background on albuminuria and kidney injury in Ins2(+/C96Y) (Akita) mice. Am J Physiol Renal Physiol. 2010;298:F788–795. doi: 10.1152/ajprenal.90515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang JH, Paik SY, Mao L, et al. Diabetic kidney disease in FVB/NJ Akita mice: temporal pattern of kidney injury and urinary nephrin excretion. PloS one. 2012;7:e33942. doi: 10.1371/journal.pone.0033942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang JH, Gurley SB. Assessment of diabetic nephropathy in the Akita mouse. Methods Mol Biol. 2012;933:17–29. doi: 10.1007/978-1-62703-068-7_2. [DOI] [PubMed] [Google Scholar]
- 56.Zheng S, Noonan WT, Metreveli NS, et al. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes. 2004;53:3248–3257. doi: 10.2337/diabetes.53.12.3248. [DOI] [PubMed] [Google Scholar]
- 57.Hudkins KL, Pichaiwong W, Wietecha T, et al. BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol. 2010;21:1533–1542. doi: 10.1681/ASN.2009121290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price DA, Porter LE, Gordon M, et al. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol. 1999;10:2382–2391. doi: 10.1681/ASN.V10112382. [DOI] [PubMed] [Google Scholar]
- 59.Hollenberg NK. Direct renin inhibition and the kidney. Nat Rev Nephrol. 2010;6:49–55. doi: 10.1038/nrneph.2009.201. [DOI] [PubMed] [Google Scholar]
- 60.Anderson S. Role of local and systemic angiotensin in diabetic renal disease. Kidney international Supplement. 1997;63:S107–110. [PubMed] [Google Scholar]
- 61.Fisher ND, Price DA, Litchfield WR, et al. Renal response to captopril reflects state of local renin system in healthy humans. Kidney Int. 1999;56:635–641. doi: 10.1046/j.1523-1755.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 62.Velez JC. The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol. 2009;5:89–100. doi: 10.1038/ncpneph1015. [DOI] [PubMed] [Google Scholar]
- 63.Schlueter W, Keilani T, Batlle DC. Tissue renin angiotensin systems: theoretical implications for the development of hyperkalemia using angiotensin-converting enzyme inhibitors. The American journal of the medical sciences. 1994;307(Suppl 1):S81–86. [PubMed] [Google Scholar]
- 64.Iyer SN, Chappell MC, Brosnihan KB, et al. Role of AT1 and AT2 receptors in the plasma clearance of angiotensin II. Journal of cardiovascular pharmacology. 1998;31:464–469. doi: 10.1097/00005344-199803000-00019. [DOI] [PubMed] [Google Scholar]
- 65.Yamada K, Iyer SN, Chappell MC, et al. Converting enzyme determines plasma clearance of angiotensin-(1-7) Hypertension. 1998;32:496–502. doi: 10.1161/01.hyp.32.3.496. [DOI] [PubMed] [Google Scholar]
- 66.Wysocki J, Ye M, Batlle D. Plasma and Kidney Angiotensin Peptides: Importance of the Aminopeptidase A/Angiotensin III Axis. Am J Hypertens. 2015;28:1418–1426. doi: 10.1093/ajh/hpv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wysocki J, Ye M, Soler MJ, et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 68.Haber PK, Ye M, Wysocki J, et al. Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: studies in vivo, ex vivo, and in vitro. Hypertension. 2014;63:774–782. doi: 10.1161/HYPERTENSIONAHA.113.02856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsusaka T, Niimura F, Pastan I, et al. Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int. 2014;85:1068–1077. doi: 10.1038/ki.2013.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.