Abstract
The multifunctional neuropeptide calcitonin gene-related peptide (CGRP) and its receptor are expressed throughout the gastrointestinal tract. Previous studies have shown that CGRP has roles in intestinal motility, water secretion, and inflammation. Furthermore, animal studies have demonstrated CGRP involvement in diarrhea secondary to C. difficile and food allergies. Diarrhea thus provides a convenient bioassay of CGRP activity in the GI system. In this proof of principle study, we report that prophylactic administration of an anti-CGRP antibody is able to block CGRP-induced diarrhea in mice. As a control, the CGRP-receptor antagonist olcegepant also attenuated the diarrhea response to CGRP. This preclinical study indicates that anti-CGRP antibodies may provide a new preventative therapy for gastrointestinal disorders involving CGRP.
Keywords: CGRP, Diarrhea, Colitis, Anti-CGRP antibody, Olcegepant, Mouse model
1. Introduction
CGRP is a multifunctional neuropeptide involved in a number of physiological and pathological processes in the body. CGRP is perhaps best known for its roles in vasodilation, nociception, and neurogenic inflammation (Brain and Grant, 2004; Russo, 2015; van Rossum et al., 1997; Wimalawansa, 1996). Less appreciated is the fact that CGRP is also a potent peptide in the gastrointestinal (GI) system. There are two (Hansson and Ronnback, 2003) isoforms of CGRP, α and β, which differ by only 3 amino acids in the human (Steenbergh et al., 1985), and have essentially identical activity (van Rossum et al., 1997). In the GI system, β-CGRP is the predominant isoform, but both are present (Mulderry et al., 1988; Schutz et al., 2004). The CGRP receptor is located throughout the GI system, including small and large intestines (Cottrell et al., 2012; Ozdemir et al., 1999). Within the GI system, CGRP has known roles in visceral nociception, blood flow, inflammation, motility, and secretion into the colon (Fargeas et al., 1985; Holzer, 2007; Holzer et al., 1989; Plourde et al., 1997; Rasmussen and Olesen, 1992; Rolston et al., 1989). Previous studies have demonstrated that CGRP can induce diarrhea in rodents (Bhargava et al., 2013; Keates et al., 1998; Yoshikawa et al., 2011).
In this study, we assessed the ability of anti-CGRP antibodies to block CGRP-induced diarrhea in both wild-type and CGRP-sensitized transgenic (nestin/hRAMP1) mice that overexpress CGRP receptors in the nervous system (Zhang et al., 2007). We reasoned that prophylactic pre-treatment with anti-CGRP antibodies could bind exogenous and/or endogenous-released CGRP preventing the onset of diarrhea. For comparison, we used acute co-administration of olcegepant, a potent antagonist of the CGRP receptor (Doods et al., 2000), that proved effective as an antimigraine drug in clinical trials (Olesen et al., 2004). This proof of principle study demonstrates that blocking CGRP actions is an effective pharmaceutical strategy for preventing or reducing diarrhea.
2. Materials and methods
2.1. Animals
C57BL/6J mice (Jackson Labs, Bar Harbor, ME) were either bred in our animal facilities or shipped at 9 weeks of age and acclimated for a minimum of 7 days prior to testing. The nestin/hRAMP1 mice have been described (Zhang et al., 2007). Approximately equal numbers of male and female mice were tested between 10 and 25 weeks of age. All animals were housed in groups of 2–5 per cage in standard conditions with access to water and food ad libitum. Animal care procedures were approved by the University of Iowa Animal Care and Use Committee and performed in accordance with NIH standards.
2.2. Drug administration
For intracerebroventricular (icv) injections, 0.5 nmol (either human or rat) α-CGRP (Sigma) was administered in 2.0 μL Dulbecco phosphate-buffered saline (PBS) as the vehicle and olcegepant (BIBN-4096BS) was diluted in PBS and 2.5% DMSO (0.5 nmol). The rationale for using α-CGRP was that our initial observations of diarrhea were made during light aversion experiments with α-CGRP. While β-CGRP is more predominant than α-CGRP in the GI system (Mulderry et al., 1988; Schutz et al., 2004), we elected to continue with α-CGRP since both peptides act on the same receptors with essentially identical activity (van Rossum et al., 1997). The icv injections were done as previously described (Recober et al., 2009, 2010). For intraperitoneal (ip) injections, human α-CGRP was administered at 0.05 mg/kg. Two humanized anti-CGRP antibodies (Ab3 and Ab6), vehicle, and control antibody (anti-digoxin, isotype human IgG1 lacking N-glycosylation) were provided by Alder Biopharmaceuticals Inc. (Bothell, WA). Antibodies Ab3 and Ab6 have been described (US Patent Application No. 13476104). For experiments with Ab3, antibodies were administered via ip injection at 30 mg/kg. For experiments with Ab6, antibodies were administered via ip injection at 50 mg/kg. The two different antibodies were for different experiments based on limited antibody availability at the time of experiments; however, they have been shown to have same ability to bind CGRP and are effectively equivalent (see US Patent Application No. 13476104). Antibody injections were done 24 h prior to CGRP administration.
2.3. CGRP-induced diarrhea assessment
Mice were acclimated to the testing room (~22 °C) for at least 1 h with standard overhead fluorescent lighting (~200 lx inside the housing cage). Testing was performed between 0800 CST and 1430 CST. To assess the percentage of mice with diarrhea following CGRP administration, mice were placed on a white paper towel covering the floor of a clean cage and observed for 30 min. Their stool was either assessed as normal, formed pellets or as non-formed loose stool, which will be referred to as diarrhea, that stuck to the paper.
To quantify diarrhea, Whatman 3MM filter paper was weighed prior to placement in the cage. After 30 min, the paper was removed from the cage and dry, formed stools were removed by vertical displacement of the paper. The paper with any remaining stool and urine was then reweighed, and the initial weight of the paper was subtracted.
2.4. Statistical analysis
A trial refers to an independent experiment with the same experimental parameters, but separated by time with a unique cohort mice used for each trial. For calculation of differences between treatments, two different analyses were utilized. For analyses of the mean percent of mice with diarrhea per trial, a one-way repeated measures ANOVA (factor: treatment) was used. Where significant effects were observed, Bonferroni's multiple comparison test was used for post-hoc analysis comparing treatment groups. For analysis based on total number of mice with diarrhea per treatment regardless of trial, Fisher's exact test was used to compare two treatment groups. For mass of urine and stool, a two-way ANOVA (factors: icv treatment and ip Ab pre-treatment) was used. Where significant effects were observed, a Student's t-test for post-hoc analysis was used to compare between treatment groups. Data are reported as either percentage of animals or mean ± range or standard error of the mean (SEM). Data were analyzed using Prism software (GraphPad Software, San Diego, CA).
3. Results
3.1. CGRP-induced diarrhea in mice
Following icv CGRP administration, wild-type (C57BL/6J), transgenic (nestin/hRAMP1), and control littermate mice demonstrated a similar rate of CGRP-induced diarrhea (Table 1). Consequently, genotypes were combined. Administration of icv CGRP elicited diarrhea in 73% of the mice (n = 75) (Table 1). The effect was very consistent when measured between multiple trials (n = 7 trials), with 76% of mice per trial having diarrhea (Fig. 1). In contrast, no diarrhea was observed with vehicle administration. Consequently, the difference between icv CGRP and vehicle administration was significantly different both in terms of total number of mice (p < 0.001) and between trials (p < 0.001). However, while we observed diarrhea following icv injection of CGRP, this was likely due to peripheral leakage of CGRP during the injection, since diarrhea was not observed when CGRP was slowly injected icv via a cannula (not shown). The ability of peripherally administered CGRP to induce diarrhea was confirmed by ip CGRP injections, as described below. Thus, we surmise that icv CGRP induces diarrhea as the result of leakage of CGRP outside the brain and likely into the systemic circulation.
Table 1.
CGRP induces diarrhea in mice.
| Treatmenta | Genotype | Diarrhea (n) |
Total (n) |
% | p b |
|---|---|---|---|---|---|
| Veh | C57BL/6J | 0 | 21 | 0 | |
| nestin/hRAMP1 | 0 | 10 | 0 | ||
| Control littermates |
0 | 15 | 0 | ||
| Total | 0 | 46 | 0 | – | |
| CGRP | C57BL/6J | 18 | 23 | 78.3 | |
| nestin/hRAMP1 | 19 | 26 | 73.1 | ||
| Control littermates |
18 | 26 | 69.2 | ||
| Total | 55 | 75 | 73.3 | Veh: p < 0.001 | |
| olceg | nestin/hRAMP1 | 0 | 6 | 0 | |
| Control littermates |
0 | 6 | 0 | ||
| Total | 0 | 12 | 0 | n.s. | |
| olceg + CGRP |
nestin/hRAMP1 | 8 | 19 | 42.1 | |
| Control littermates |
6 | 17 | 35.3 | ||
| Total | 14 | 36 | 38.9 | Veh, CGRP: p < 0.001; olceg: p < 0.05 |
Treatment with icv injection of vehicle (veh), CGRP, olcegepant (olceg), or co-administration of olcegepant and CGRP (olceg + CGRP).
p values calculated from Fisher's exact test compared to vehicle treated mice, CGRP-treated mice, or olcegepant-treated mice, as indicated (n.s. = not significant).
Fig. 1.
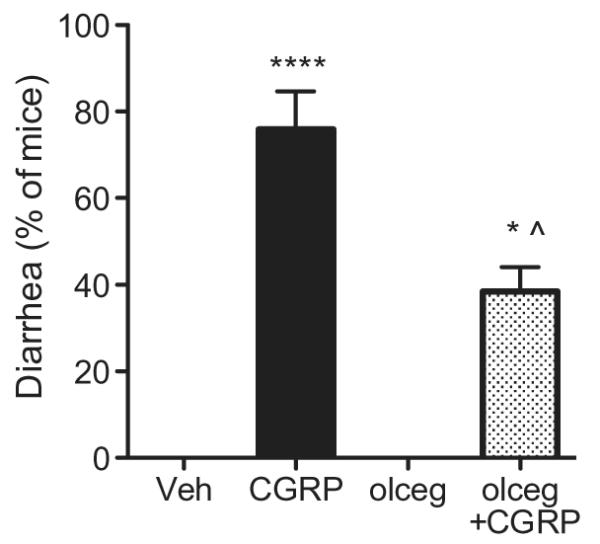
Olcegepant attenuates CGRP-induced diarrhea in mice. Mice administered icv vehicle (Veh) did not demonstrate diarrhea (n = 5 trials), whereas a high percentage of mice administered icv CGRP had diarrhea (****p < 0.0001; n = 7 trials). Co-administration of olcegepant (olceg) (n = 4 trials) reduced the percentage of animals with CGRP-induced diarrhea compared to CGRP-treated mice (*p < 0.05; n = 4 trials), and was significantly more (^p < 0.05) than olcegepant alone (n = 2 ). Means ± range are given. Each trial included 4–18 mice.
3.2. Olcegepant attenuates CGRP-induced diarrhea
To test the effect of acute treatment with a CGRP receptor antagonist, we used a potent small molecule antagonist, olcegepant (Doods et al., 2000; Olesen et al., 2004). Co-administration of icv olcegepant with CGRP appeared to reduce the percentage of mice demonstrating CGRP-induced diarrhea to 39% of mice per trial (n = 4) (Fig. 1), which is again very comparable to the percent of total mice when trials are combined (Table 1). This reduction was significant based on number of mice (p < 0.01) and per trial (p < 0.05). No diarrhea was observed following icv olcegepant alone. Thus, icv CGRP can induce diarrhea in mice, and olcegepant can significantly decrease the percentage of mice with CGRP-induced diarrhea. The observation that olcegepant yielded only partial blockage likely reflects the relatively low affinity of this antagonist for rodent compared to human CGRP receptors (Doods et al., 2000). Of note, we suspect that olcegepant efficacy stems from leakage into the peripheral circulation and blocks endogenous rodent CGRP-receptors within the GI tract.
3.3. Anti-CGRP antibodies block CGRP-induced diarrhea in mice
To determine if CGRP-binding antibodies could inhibit CGRP-induced diarrhea, animals received ip injection of vehicle, control antibody, or anti-CGRP antibody 24 h prior to administration of icv CGRP. Both anti-CGRP antibodies used (Ab3 and Ab6) have equivalent cross-reaction with rat, mouse, and human CGRP (not shown). One trial was performed with wild-type mice and another with nestin/hRAMP1 mice. Since, as in the previous study, both genotypes responded to CGRP in the same manner, data have been combined. CGRP induced diarrhea in animals pre-treated with vehicle (87%, n = 15 mice, 2 trials) (Table 2), at comparable levels in both trials (85%) (Fig. 2). Pretreatment with control antibody did not significantly affect CGRP activity (Table 2 and Fig. 2). However, anti-CGRP antibody (Ab3) completely inhibited CGRP-induced diarrhea (0%; n = 22 mice, 2 trials), which was significantly different from vehicle or control antibody treated animals based on analysis by trial and total number of mice (p < 0.001) (Fig. 2, Table 2).
Table 2.
Prevention of CGRP-induced diarrhea by anti-CGRP antibodies.
| Pre-treatmenta | Treatmentb | Diarrhea (n) |
Total (n) |
% | p c |
|---|---|---|---|---|---|
| Veh | icv CGRP | 13 | 15 | 86.7 | – |
| Con Ab | 15 | 21 | 71.4 | n.s. | |
| Anti-C Ab | 0 | 22 | 0 | Veh, Con Ab: p < 0.001 |
|
| Con Ab | ip CGRP (0.05 mg/kg) |
7 | 9 | 77.8 | – |
| Anti-C Ab | 0 | 8 | 0 | Con Ab: p < 0.005 |
Pre-treatment with ip injection of vehicle (Veh), control antibody (Con Ab), or anti-CGRP antibody (anti-C Ab).
Treatment with CGRP by icv or ip injections. Note that ip injected animals had been injected with icv CGRP 3 days prior.
p values calculated from Fisher's exact test compared to vehicle-treated mice, or control antibody-treated mice, as indicated (n.s. = not significant).
Fig. 2.
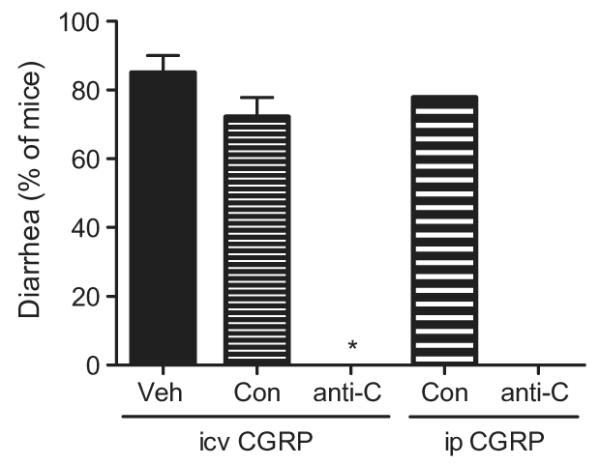
Anti-CGRP antibody blocks CGRP-induced diarrhea in mice. CGRP-induced diarrhea was completely blocked by ip anti-CGRP (anti-C) (30 mg/kg, Ab3) antibody in both wildtype mice given human CGRP and nestin/hRAMP1 mice given rat CGRP, which was significantly less (*p < 0.001) than mice pre-treated by ip antibody vehicle (Veh) or ip control (Con) antibody (n = 2 trials). Anti-CGRP antibody also blocked subsequent CGRP-induced diarrhea at the 0.05 mg/kg ip human CGRP dose (n = 1 trial). All trials included 9–12 mice per trial except ip Veh + icv CGRP trials, which included 5–10 mice per trial. Means ± range are given.
In a follow-up experiment to directly test peripheral administration of CGRP, the wild-type mice from one of the trials were re-tested using an ip injection of CGRP. The injection was done three days after the prior icv injections at which point any preexisting CGRP-induced diarrhea had resolved. There was no additional injection of antibodies. Following 0.05 mg/kg ip CGRP administration, the mice that had received anti-CGRP antibody 3 days earlier did not exhibit any diarrhea (Table 2 and Fig. 2). In contrast, 77.8% of control antibody-treated mice had diarrhea, which was significantly more than anti-CGRP antibody-treated mice (p < 0.005) (Table 2).
To further quantify CGRP-induced diarrhea, we weighed excretions from nestin/hRAMP1 mice injected with CGRP 24 h after being given either control or anti-CGRP antibodies. After icv CGRP administration, animals were allowed to recover in a clean cage with filter paper. After 30 min, dry stools were removed from the paper by vertical suspension of the paper, leaving wet and non-formed stools as well as any urine absorbed by the paper. Anti-CGRP antibody (Ab6) significantly blocked the CGRP-induced increase in excretion mass by 76.6% compared to control antibody treated-mice (p < 0.0001) (Fig. 3). The amount of excrement in CGRP-treated mice that received anti-CGRP antibody prior was comparable to vehicle-treated mice (p = 0.30) (Fig. 3). Based on visual observation and the wet excrement weights, nearly all (10 out of 12) mice given CGRP with control antibody had diarrhea, which was not observed for any of the mice given CGRP with anti-CGRP antibody, nor for any mice given vehicle.
Fig. 3.
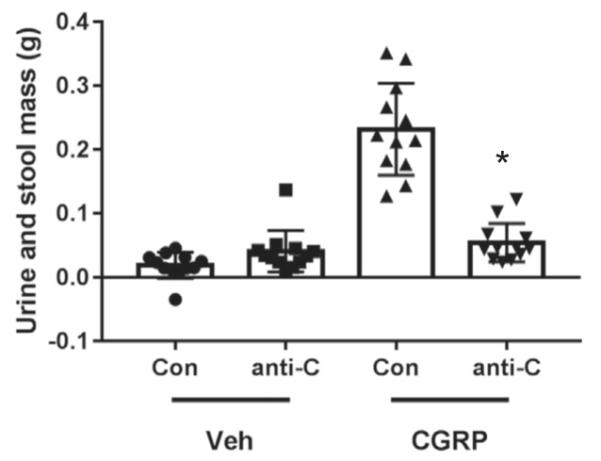
Anti-CGRP antibody reduced CGRP-induced excrement. Mass of urine and non-formed stool excreted by individual nestin/hRAMP1 mice following icv CGRP or vehicle. Prophylactic ip administration of anti-CGRP antibody (50 mg/kg, Ab6) 24 h prior prevented rat CGRP-induced diarrhea as compared to control antibody (*p < 0.0001). Both antibody and CGRP injection had significant effects (F1,44 = 38.44, p < 0.0001; F1,44 = 80.88, p < 0.0001, respectively). For all groups, n = 12. Data for individual mice with means ± SEM are given.
4. Discussion
This proof-of-principle study demonstrates that systemic administration of anti-CGRP antibodies prevents the development of CGRP-induced diarrhea. Diarrhea was completely blocked by prophylactic administration of anti-CGRP antibodies 24 h prior to CGRP administration. As a control, CGRP-induced diarrhea could be attenuated by co-administration of olcegepant, a CGRP receptor antagonist. Thus, the easily observed diarrhea provides a convenient bioassay of CGRP activity in the GI system, which can be completely blocked by prophylactic administration of anti-CGRP antibodies.
Our finding that administration of CGRP leads to diarrhea in mice is fully consistent with prior reports (Bhargava et al., 2013; Gao and Drew, 2016; Keates et al., 1998; Yoshikawa et al., 2011), as well as the ability of CGRP to affect intestinal motility (Fargeas et al., 1985; Holzer et al., 1989; Rasmussen and Olesen, 1992) and water secretion (Rolston et al., 1989). In this regard, it is important to reiterate that diarrhea following icv CGRP was not due to central actions, but rather was a consequence of peripheral leakage during the injection procedure. Interestingly, the rate of CGRP-induced diarrhea was similar between wildtype mice and nestin/hRAMP mice, despite the latter being sensitized to CGRP. However, nestin/hRAMP mice have overexpression of human RAMP1, a subunit of the CGRP receptor, only within the nervous system, so these findings are consistent with CGRP actions at receptors on non-neural cells within the GI system.
Previously, we have used CGRP administration to induce light aversion and cutaneous allodynia in mice as a model of migraine (Kaiser et al., 2012; Marquez de Prado et al., 2009; Recober et al., 2009, 2010). Multiple Phase 2 clinical trials have recently demonstrated that anti-CGRP monoclonal antibodies are safe and efficacious as a preventative agent for reducing the headache burden in episodic and chronic migraine (Bigal et al., 2015a, 2015b; Dodick et al., 2014a, 2014b; Sun et al., 2016). Diarrhea and other digestive symptoms are commonly associated symptoms of migraine, with about 22% of migraineurs reporting gastrointestinal issues (Kelman, 2004). Furthermore, increasing headache frequency has been found to be associated with a higher prevalence of gastrointestinal symptoms including nausea and diarrhea (Aamodt et al., 2008). Our results predict that CGRP may directly play a role in migraine-related gastrointestinal symptoms.
Beyond migraine, the efficacy of anti-CGRP antibodies predicts that blocking CGRP may offer a novel means to clinically manage colitis and other inflammatory diseases involving diarrhea. Reducing CGRP activity either by knockdown of the CLR subunit of the CGRP receptor or pre-treatment with the antagonist CGRP (8–37) reduced intestinal secretion and enteritis induced by Clostridium difficile toxin (Bhargava et al., 2013; Keates et al., 1998). C. difficile is a frequent hospital-acquired infection secondary to antibiotic use and leads to severe diarrhea. Furthermore, mice lacking the RAMP1 subunit of the CGRP receptor had a lower incidence of diarrhea induced by ovalbumin in a food allergy model (Yoshikawa et al., 2011). Moreover, CGRP has been suggested to play a role in visceral nociception (Plourde et al., 1997), GI blood flow, inflammation, motility, and secretion into the colon (Holzer, 2007), which may contribute to chronic conditions including inflammatory bowel diseases (Holzer, 1998). In a mouse model of colitis using dextran sodium sulfate, a TRPM8 agonist was shown to block CGRP release (Ramachandran et al., 2013). Taken together, these data indicate that CGRP is a mediator of colitis and enteritis in inflammatory bowel diseases. In addition, a recent study reported that olcegepant prevented post-operative ileus, suggesting that blocking CGRP could be useful following GI surgery (Glowka et al., 2015) and that CGRP likely plays multiple roles in the GI system. Thus, we predict that attenuating CGRP activity in the GI system with CGRP-blocking antibodies could have multiple beneficial effects.
5. Conclusion
Overall, this study suggests that CGRP antibodies may provide a novel therapeutic tool in the management of diarrhea and other GI pathologies involving CGRP. In particular, the use of anti-CGRP antibodies or CGRP receptor antibodies would be ideal for long-term treatments, analogous to their role for prophylactic treatment of migraine (Bigal et al., 2015a, 2015b; Dodick et al., 2014a, 2014b; Sun et al., 2016). This preclinical proof of concept study raises the possibility that anti-CGRP antibodies may also diminish diarrhea secondary to various gastrointestinal disorders including infections such as C. difficile and inflammatory bowel disorders.
Acknowledgements
This work was supported by National Institutes of Health grants NS075599 and F30 NS067887 and a grant from Alder Biopharmaceuticals. We thank Annie Tye for help with experiments and members of the Russo lab for stimulating discussions. E.A.K. designed, performed, and analyzed the antibody experiments and wrote the manuscript. B.J.R. and A.K. assisted with all experiments. A.R. and A.K. performed the olcegepant studies. B.K. and L.G-M. provided the antibodies and advice on their use. A.F.R. helped design experiments and write the manuscript. B.K. was and L.G-M. is an employee and A.F.R is a consultant for Alder Biopharmaceuticals. E.A.K., A.K., A.R., and A.F.R. are on a patent application on therapeutic uses of CGRP antibodies.
Footnotes
The other authors declare no competing financial interests.
References
- Aamodt AH, Stovner LJ, Hagen K, Zwart JA. Comorbidity of headache and gastrointestinal complaints. The Head-HUNT Study. Cephalalgia. 2008;28:144–151. doi: 10.1111/j.1468-2982.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- Bhargava A, Clifton MS, Mhaske P, Liao M, Pothoulakis C, Leeman SE, Grady EF. Local injection of dsRNA targeting calcitonin receptor-like receptor (CLR) ameliorates Clostridium difficile toxin A-induced ileitis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:731–736. doi: 10.1073/pnas.1219733110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Dodick DW, Rapoport AM, Silberstein SD, Ma Y, Yang R, Loupe PS, Burstein R, Newman LC, Lipton RB. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015a;14:1081–1090. doi: 10.1016/S1474-4422(15)00249-5. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Edvinsson L, Rapoport AM, Lipton RB, Spierings EL, Diener HC, Burstein R, Loupe PS, Ma Y, Yang R, Silberstein SD. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015b;14:1091–1100. doi: 10.1016/S1474-4422(15)00245-8. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Alemi F, Kirkland JG, Grady EF, Corvera CU, Bhargava A. Localization of calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1) in human gastrointestinal tract. Peptides. 2012;35:202–211. doi: 10.1016/j.peptides.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J, investigators, A.L.D.s Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014a;13:1100–1107. doi: 10.1016/S1474-4422(14)70209-1. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014b;13:885–892. doi: 10.1016/S1474-4422(14)70128-0. [DOI] [PubMed] [Google Scholar]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargeas MJ, Fioramonti J, Bueno L. Calcitonin gene-related peptide: brain and spinal action on intestinal motility. Peptides. 1985;6:1167–1171. doi: 10.1016/0196-9781(85)90445-0. [DOI] [PubMed] [Google Scholar]
- Gao YR, Drew PJ. Effects of voluntary locomotion and calcitonin gene-related peptide on the dynamics of single dural vessels in awake mice. J. Neurosci. 2016;36:2503–2516. doi: 10.1523/JNEUROSCI.3665-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowka TR, Steinebach A, Stein K, Schwandt T, Lysson M, Holzmann B, Tsujikawa K, de Jonge WJ, Kalff JC, Wehner S. The novel CGRP receptor antagonist BIBN4096BS alleviates a postoperative intestinal inflammation and prevents postoperative ileus. Neurogastroenterol. Motil. 2015;27:1038–1049. doi: 10.1111/nmo.12584. [DOI] [PubMed] [Google Scholar]
- Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- Holzer P. Implications of tachykinins and calcitonin gene-related peptide in inflammatory bowel disease. Digestion. 1998;59:269–283. doi: 10.1159/000007504. [DOI] [PubMed] [Google Scholar]
- Holzer P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr. Opin. Pharmacol. 2007;7:563–569. doi: 10.1016/j.coph.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Bartho L, Matusak O, Bauer V. Calcitonin gene-related peptide action on intestinal circular muscle. Am. J. Phys. 1989;256:G546–G552. doi: 10.1152/ajpgi.1989.256.3.G546. [DOI] [PubMed] [Google Scholar]
- Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J. Neurosci. 2012;32:15439–15449. doi: 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keates AC, Castagliuolo I, Qiu B, Nikulasson S, Sengupta A, Pothoulakis C. CGRP upregulation in dorsal root ganglia and ileal mucosa during Clostridium difficile toxin A-induced enteritis. Am. J. Phys. 1998;274:G196–G202. doi: 10.1152/ajpgi.1998.274.1.G196. [DOI] [PubMed] [Google Scholar]
- Kelman L. The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache. 2004;44:865–872. doi: 10.1111/j.1526-4610.2004.04168.x. [DOI] [PubMed] [Google Scholar]
- Marquez de Prado B, Hammond DL, Russo AF. Genetic enhancement of calcitonin gene-related peptide-induced central sensitization to mechanical stimuli in mice. J. Pain. 2009;10:992–1000. doi: 10.1016/j.jpain.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulderry PK, Ghatei MA, Spokes RA, Jones PM, Pierson AM, Hamid QA, Kanse S, Amara SG, Burrin JM, Legon S, et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25:195–205. doi: 10.1016/0306-4522(88)90018-8. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Ozdemir FA, Berghofer P, Goke R, Goke B, McGregor GP. Specific calcitonin gene-related peptide binding sites present throughout rat intestine. Peptides. 1999;20:1361–1366. doi: 10.1016/s0196-9781(99)00142-4. [DOI] [PubMed] [Google Scholar]
- Plourde V, St-Pierre S, Quirion R. Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am. J. Phys. 1997;273:G191–G196. doi: 10.1152/ajpgi.1997.273.1.G191. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Hyun E, Zhao L, Lapointe TK, Chapman K, Hirota CL, Ghosh S, McKemy DD, Vergnolle N, Beck PL, Altier C, Hollenberg MD. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7476–7481. doi: 10.1073/pnas.1217431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BK, Olesen J. Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia. 1992;12:221–228. doi: 10.1046/j.1468-2982.1992.1204221.x. (discussion 186) [DOI] [PubMed] [Google Scholar]
- Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J. Neurosci. 2009;29:8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58:156–165. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston RK, Ghatei MA, Mulderry PK, Bloom SR. Intravenous calcitonin gene-related peptide stimulates net water secretion in rat colon in vivo. Dig. Dis. Sci. 1989;34:612–616. doi: 10.1007/BF01536340. [DOI] [PubMed] [Google Scholar]
- Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu. Rev. Pharmacol. Toxicol. 2015;55:533–552. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz B, Mauer D, Salmon AM, Changeux JP, Zimmer A. Analysis of the cellular expression pattern of beta-CGRP in alpha-CGRP-deficient mice. J. Comp. Neurol. 2004;476:32–43. doi: 10.1002/cne.20211. [DOI] [PubMed] [Google Scholar]
- Steenbergh PH, Hoppener JW, Zandberg J, Lips CJ, Jansz HS. A second human calcitonin/CGRP gene. FEBS Lett. 1985;183:403–407. doi: 10.1016/0014-5793(85)80820-6. [DOI] [PubMed] [Google Scholar]
- Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M, Saper J, Cady R, Chon Y, Dietrich J, Lenz R. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:382–390. doi: 10.1016/S1474-4422(16)00019-3. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci. Biobehav. Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr. Rev. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- Yoshikawa R, Mikami N, Otani I, Kishimoto T, Nishioka S, Hashimoto N, Miyagi Y, Takuma Y, Sueda K, Fukada S, Yamamoto H, Tsujikawa K. Suppression of ovalbumin-induced allergic diarrhea by diminished intestinal peristalsis in RAMP1-deficient mice. Biochem. Biophys. Res. Commun. 2011;410:389–393. doi: 10.1016/j.bbrc.2011.05.141. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J. Neurosci. 2007;27:2693–2703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


