Abstract
Renal allografts from deceased African Americans with two apolipoprotein L1 gene (APOL1) renal-risk variants fail sooner than kidneys from donors with fewer variants. Kidney Donor Risk Index (KDRI) was developed to evaluate organ offers by predicting allograft longevity and includes African American race as a risk factor. Substituting APOL1 genotype for race may refine the KDRI. For 622 deceased African American kidney donors, we applied 10-fold cross-validation approach to estimate contribution of APOL1 variants to a revised KDRI. Cross-validation was repeated 10,000 times to generate distribution of effect size associated with APOL1 genotype. Average effect size was used to derive the revised KDRI weighting. Mean current-KDRI score for all donors was 1.4930 versus mean revised-KDRI score 1.2518 for 529 donors with 0/1 variant and 1.8527 for 93 donors with 2 variants. Original and revised KDRIs had comparable survival prediction errors after transplantation, but the spread in Kidney Donor Profile Index based on presence/absence of 2 APOL1 variants was 37 percentage points. Replacing donor race with APOL1 genotype in KDRI better defines risk associated with kidneys transplanted from deceased African American donors, substantially improves KDRI score for 85-90% of kidneys offered, and enhances the link between donor quality and recipient need.
Introduction
With the ongoing epidemic of chronic kidney disease, the need for transplantable kidneys continues to far exceed availability. In response, as part of the new kidney allocation system (KAS), the Kidney Donor Risk Index (KDRI) was developed as a tool to evaluate organ offers by predicting allograft longevity and potentially reduce discard rates and variability in access of hard-to-match transplant candidates, while also minimizing unrealized life-years and need for re-transplantation. This system, implemented in the United States in December 2014, is based on application of objective metrics to define individual donor quality that can be linked to recipient need and projected outcomes (1-5). In 2009, Rao and colleagues published an equation that provides the underpinnings for the KDRI (5). The equation incorporated 10 donor characteristics and 4 recipient/transplantation-procedure factors that are combined to estimate of the relative risk of post-transplant failure for a kidney from a given donor (5). Because recipient factors are not readily available at the time of an organ offer, a modified KDRI using only the donor characteristics was adopted for the KAS (6). KDRIs of all deceased kidney donors of a calendar year are ranked numerically, then divided into percentiles ranging from 0 to 100% (best to worse) to generate a table to map a KDRI to its Kidney Donor Profile Index (KDPI). The calculated KDRI for an individual donor is divided by the median KDRI in the prior available calendar year; that value is located on the mapping table to find its KDPI.
Among donor characteristics in the KDRI equation, African American (AA) race has a hazard ratio of 1.196, indicating an approximately 20% increased risk of allograft loss. Reasons for poorer outcomes with transplantation of kidneys from AA donors, relative to non-AA donors, remain unclear (7). Recently, our group reported that renal-risk variants (RRVs) in the donor apolipoprotein L1 gene (APOL1) are associated with outcomes of renal allografts from AA deceased donors (8-10). Kidneys from deceased AA donors with 2 APOL1 RRVs were at higher risk for early allograft failure than were kidneys from AA donors with fewer than 2 RRVs, recipients of which had allograft survival comparable to that for kidneys from European American (EA) donors (8-10). KDRI emerged from data incorporating race as a self-reported construct, and predates description of the APOL1 effect on renal-transplant outcomes. In the current study, we estimate the size of the effect of APOL1 genotype in the KDRI equation and its potential impact on KDPI-based allocation in the new KAS in the United States.
Materials and Methods
Study cohort
We retrieved all available archived DNA samples of AA donors whose kidneys were recovered and/or transplanted at 3 busy kidney-transplant programs with sizable populations of AA donors (Wake Forest, Emory University, and University of Alabama at Birmingham) and the Genomics of Deterioration of Kidney Allograft Function Genomics study that received samples from 9 Organ Procurement Organizations (10). A total of 1153 deceased-donor kidney transplantations were performed at 113 centers. The cohort includes 1,149 recipients of kidneys from 622 deceased AA donors (529 with 0/1 APOL1 RRV [979 transplants]; 93 with 2 RRVs [170 transplants]). Four recipients were excluded due to missing data for serum creatinine.
APOL1 genotyping
Genotyping was done with a custom array designed at Wake Forest School of Medicine to detect 2 single-nucleotide polymorphisms for APOL1 G1 RRV (rs73885319; rs60910145) and an insertion/deletion for G2 RRV (rs143830837) (10). Genotyping of 15 blind duplicates had a concordance rate of 100% and genotyping efficiency for the 3 variants comprising APOL1 G1 and G2 variants exceeded 99% for the 1,149 transplantations.
Clinical outcomes
Outcomes of transplantation were determined by using United Network of Organ Sharing identification numbers to link to data in the Scientific Registry of Transplant Recipients (SRTR) that include data on all donors, wait-listed candidates, and transplant recipients in the United States, as submitted by members of Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration in the United States Department of Health and Human Services oversees the activities of OPTN and SRTR contractors. The clinical and research activities reported are consistent with the Principles of the Declaration of Istanbul, outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Effects of APOL1 after adjusting for effects of donor race/ethnicity
Four thousand EA donors were randomly selected from the SRTR database and assigned 0 RRVs because APOL1 risk variants are virtually absent in this population (11,12). A model was fitted that included APOL1 and the 10 covariates in the current KDRI equation (including race/ethnicity). The likelihood ratio test was computed to compare the full model that included APOL1 as a covariate to the reduced model that did not include it. For comparison, a similar test was performed to evaluate the contribution of race/ethnicity in models that already contained the APOL1 genotypes as a covariate. Random selection of EA donors was repeated 10,000 times to generate the distribution of these tests, each time taking a different random sample of 4,000 EAs.
Estimation of KDRI coefficient beta for APOL1 genotype
We adopted a 10-fold cross-validation approach: nine tenths of available observations were used to estimate a KDRI coefficient beta for APOL1 2-RRV genotype in a Cox proportional hazard regression. This estimated parameter was combined with published KDRI beta components for the remaining 9 donor characteristics to compute the modified KDRI. This result was used to test for association with survival outcome in the remaining one tenth of the sample. The split-sample approach for estimating and validating the revised KDRI was repeated 10,000 times to generate the sampling distributions of the estimated parameter associated with APOL1 genotype and the estimated c-index. The mean of the coefficients associated with APOL1 was used for all comparisons with the original KDRI.
Calculation of KDRI
Parameter estimates associated with the 10 donor characteristics have been determined in 69,440 adult, first-time recipients of deceased-donor kidneys in the United States from 1995-2005 (5). KDRI parameter estimates for age and serum creatinine are provided at specific knots (18 and 50 years for age, 1.5 mg/dL for serum creatinine); age, height and weight are centered at 40 years, 170 cm and 80 kg, respectively, to improve the fit of the model (13). KDRI is calculated as the exponential of the fitted (predicted) score. KDRI = exp(Xβ), in which X represents the vector of observations for the 10 variables and β is the vector of parameter estimates. We compared the current KDRI equation with our proposed revision: in the latter, APOL1 is coded as a binary variable under a recessive mode of inheritance; AAs with 2 APOL1 RRVs are coded as 1 and donors with fewer RRVs are coded as 0. This coding implies that the parameter estimate for donors with 0/1 RRV is 0, leading to renal-allograft survival rates equivalent to those for kidneys from EA donors (10).
Assessment of KDRI to predict clinical outcomes
Efforts to evaluate KDRI's capacity to associate donor characteristics with kidney-allograft outcomes can be seen as independent efforts to validate the index. However, the number of kidney transplants from AA deceased donors with APOL1 genotype data is limited, with virtually no independent validation dataset. Thus, we opted for a split-sample approach; a fraction of the sample was used to determine overall contribution of APOL1 (i.e., xbeta component) in a model that included the other donor characteristics as covariates.
Discrimination and calibration measures, including overall and time-dependent estimates of c-index, specificity and sensitivity, and confidence intervals, were obtained for the original and revised KDRIs under the Cox proportional hazard model for time-to-allograft failure and competing-risk model that treated death with functional allograft as a competing risk. Overall c-index and predictive ability of both KDRIs were evaluated using bootstrap-based internal calibration and validation approaches (14). These approaches were implemented in the R-package regression modeling strategies (“rms”) (15). Time-dependent measures, including area under the receiver operating characteristic (ROC) curve or c-index, true-positive rate (TPR), false-positive rate (FPR), positive-predictive value (PPV), and negative-predictive value (NPV), were obtained for 1, 3, and 5 years after transplantation using a cutoff value of 1.2218 that corresponded to the median KDRI in 2014. There are several statistical approaches for estimating these parameters. Key differences among them include the manner in which censored observations are treated, ability to derive a closed-form equation for estimating standard error associated for the estimates, and range of models that could be considered. For example, few of these approaches accommodate competing-risk and correlated-survival outcomes, as when 2 recipients receive a kidney from 1 donor. Estimates for prognostic accuracy of the current and revised KDRIs were calculated under the inverse probability weighting approach and under the Cox proportional odds model (16). We derived predictive accuracy estimates under a competing-risk model (17). This approach was implemented in R: http://CRAN.R-project.org/package=timeROC. Estimates were generated under 2 alternative definitions of event of interest. Area under the curve (AUC) 1 refers to area under the ROC curve relative to a ‘main event’. Subjects who have experienced this event are compared to those who are event-free, i.e., subjects who are alive with functional allograft before or at the time point of interest. AUC 2 focuses on each specific type of event; subjects with event of interest are contrasted with those who did not have the event and were at risk before or at the time-point of interest.
Determination of KDPI
We divided KDRIs calculated using the current KDRI equation and the equation with APOL1 genotype instead of race by 1.2218 (median KDRI [scaling factor] of 2014), to generate KDRIs_Median. KDPI was determined by finding the KDPI assigned to the KDRI_Median in the 2014 KDRI-to-KDPI mapping table.
Results
Relative impacts of APOL1 and race on KDRI
To determine relative effects of donor APOL1 genotype versus race/ethnicity on allograft survival, a model containing APOL1 genotype (2 versus 0/1 RRVs) and ethnicity was fitted. This model contained all other covariates and was compared to a similar model that was restricted to only ethnicity. Over the 10,000 iterations, the mean chi-squared statistic for this test was 4.88 for models that tested for the significance of APOL1 when it was added last, compared to 2.87 when race/ethnicity was added last, p-values of 0.03 and 0.09, respectively. Thus, effect of race was no longer significant once APOL1 genotype was included in the model. In this repeated sampling experiment, effect of APOL1 was statistically significant in 99.9% of the random samples, in contrast to only 24.8% for race/ethnicity in models that also contained APOL1 genotype.
Estimation and significance of KDRI coefficient beta for APOL1 2-renal-risk-variant genotype
Table 1 shows distributions of the 9 characteristics used as donor factors in calculating KDRI between the 2 groups of donors, based on APOL1 genotype. The groups differed only in frequency of history of diabetes (less common in donors with 2 APOL1 RRVs).
Table 1.
Distribution of donor characteristics used in the KDRI equations, grouped by number of APOL1 renal-risk variants
| 0 or 1 APOL1 variant | 2 APOL1 variants | ||
|---|---|---|---|
| Donors, n | 529 | 93 | |
| Kidney transplants, n | 979 | 170 | |
| Variable | P value | ||
| Age, yr | 35.7 (17.3) 37.0 | 35.9 (15.5) 40.0 | 0.9 |
| <18 yr, n (%) | 83 (15.7%) | 10 (10.8%) | 0.2 |
| >50 yr, n (%) | 125 (23.6%) | 19 (20.4%) | 0.5 |
| Height, cm | 167.5 (21.0) 172.7 | 169.2 (19.6) 172.7 | 0.4 |
| Weight, kg | 77.9 (27.1) 77.2 | 79.5 (23.9) 79.4 | 0.3 |
| Weight <80 kg, n (%) | 290 (54.8%) | 47 (50.5%) | 0.4 |
| History of hypertension, n (%) | 184 (34.8%) | 39 (41.9%) | 0.2 |
| History of diabetes, n (%) | 44 (8.3%) | 2 (2.2%) | 0.04 |
| Cause of death=stroke, n (%) | 222 (42.0%) | 45 (48.4%) | 0.2 |
| Serum creatinine, mg/dL | 1.3 (1.1) 1.1 | 1.3 (0.8) 1.1 | 0.9 |
| Serum creatinine >1.5 mg/dL | 125 (23.6%) | 17 (18.3%) | 0.3 |
| HCV-positive, n (%) | 15 (2.8%) | 4 (4.3%) | 0.4 |
| Donation after circulatory death, n (%) | 21 (4.0%) | 3 (3.2%) | 0.7 |
Data for age, height, weight and serum creatinine shown as mean (SD) median
APOL1, apolipoprotein L1 gene; HCV, hepatitis C virus; KDRI, kidney donor risk index
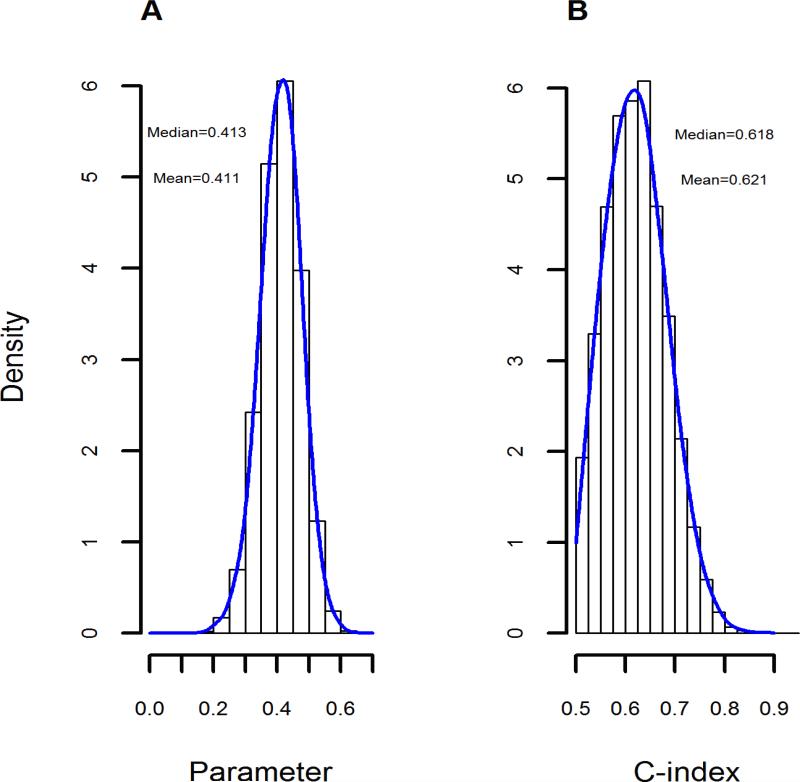
The observed c-index with the currently formulated KDRI tested with full data was 0.608, matching a previously published estimate (13). Distribution of observed APOL1 genotype-revised KDRI c-indices ranged from 0.50 to 0.90. Observed mean was 0.621 and median was 0.618 (Figure 1). APOL1-revised KDRI c-index was higher than observed c-index for the currently formulated KDRI in 57% of 10,000 replications, indicating the revised KDRI has at least equivalent predictive ability for transplant outcomes. Distribution of the KDRI coefficient betas for the APOL1 2-RRV genotype had a similar shape, with mean 0.411 (standard deviation (SD) 0.18) and median 0.413 (Figure 1). We used the mean value to calculate revised KDRI scores.
Figure 1. Parameter estimates and c-indices associated with the revised KDRI equation.
(A) Observed distribution of the KDRI coefficient betas for APOL1 2-RRV genotype in the 10,000 replications of the cross-validation effort. The mean value of 0.411 as the antilog function (base e) denotes a hazard ratio for allograft failure of 1.508 for kidneys from AA donors with 2 APOL1 RRVs compared to kidneys from AA donors with no or 1 APOL1 RRV. (B) Corresponding c-index estimated from the model that tested for an association between the revised KDRI and the observed renal-allograft survival outcome.
AA, African American; APOL1, apolipoprotein L1 gene; KDRI, kidney donor risk index; RRV, renal-risk variant
APOL1-based revision of KDRI improved the score for the 85% of AA donors who possessed 0/1 RRV: median 1.1633 versus 1.3913 with the current equation. For two AA donors with identical characteristics except for APOL1 genotype, 2 APOL1 RRVs conferred a hazard ratio that averaged 1.51 times that associated with 0/1 RRV (KDRI coefficient beta 0.411 versus 0.000; Table 2). Among donors with 0/1 APOL1 RRV, distribution of revised KDRIs had mean of 1.2518 (SD 0.4237) and median of 1.1633 versus 1.8527 (SD 0.5609) and 1.8154, respectively, among donors with 2 APOL1 RRVs. The difference between revised KDRIs was significant (P<0.001).
Table 2.
Comparison of KDRIs and KDPIs, grouped by number of APOL1 renal-risk variants
| 0 or 1 APOL1 variant | 2 APOL1 variants | |||
|---|---|---|---|---|
| KDRI equation | Current | Revised | Current | Revised |
| Race | AA | AA | AA | AA |
| Coefficient beta | ||||
| Race | 0.179 | NA | 0.179 | NA |
| APOL1 genotype | NA | 0.000 | NA | 0.411 |
| Hazard ratio | ||||
| Race | 1.196 | NA | 1.196 | NA |
| APOL1 genotype | NA | 1.000 | NA | 1.508* |
| KDRI | 1.4972 | 1.2518 | 1.4689 | 1.8527** |
| Scaling factor (for 2014) | 1.2218 | 1.2218 | 1.2218 | 1.2218 |
| KDRI scaled | 1.2254 | 1.0246 | 1.2023 | 1.5164 |
| KDPI | 71% | 53% | 69% | 88% |
KDRIs were calculated for subgroups of AA deceased donors, based on number of APOL1 RRVs. The calculations used the weightings (coefficient beta) for the 9 non-race/ethnicity characteristics, with coefficient beta for race/ethnicity of 0.179 in the current KDRI equation and coefficient beta of 0.411 for donors with 2 APOL1 RRVs in the revised KDRI equation. The resultant KDRI was divided by the scaling factor for 2014 (1.2218) to calculate KDRI scaled; that number converted to KDPI by locating it on the KDRI-to-KDPI mapping table for 2014.
KDRIs and KDRIs scaled shown as means
P = 0.01 for comparison of hazard ratios for African Americans based on genotype;
P <0.001 for comparison of KDRIs for African Americans based on genotype
AA, African American; APOL1, apolipoprotein L1 gene; KDRI, kidney donor risk index; KDPI, kidney donor profile index; NA, not applicable
Prognostic capacity of KDRI
Table 3 compares prognostic ability of KDRIs at 1, 3, and 5 years after transplantation using a cutoff value of 1.2218 corresponding to the median KDRI in 2014. Overall, both KDRI equations had similar performances for c-indices, and positive and negative predicted values. Estimated true-positive rates (TPR) did not significantly differ. The revised KDRI had a significantly lower false-positive rate at all post-engraftment time-points. Overall, the current and revised KDRI equations had similar prediction errors (Nagelkerke r-squares, mean prediction error, and integrated Brier scores (IBS), Table 4). r-squares and prediction errors were estimated using the “rms” package. IBS was estimated using a prediction-error curve (“pec”) (18). These estimates were generated with a bootstrapping approach. Predictive power of the KDRI equations did not differ.
Table 3.
Prognostic accuracy measures and 95% confidence intervals estimated at 1, 3, and 5 years after transplantation
| Measure | One year after transplantation | Three years after transplantation | Five years after transplantation | |||
|---|---|---|---|---|---|---|
| Current KDRI | Revised KDRI | Current KDRI | Revised KDRI | Current KDRI | Revised KDRI | |
| AUC | 0.589(0.553,0.625) | 0.600(0.561,0.638) | 0.592(0.551,0.633) | 0.602(0.565,0.639) | 0.594(0.554,0.634) | 0.604(0.556,0.653) |
| TPR | 0.700(0.657,0.743) | 0.626(0.574,0.677) | 0.694(0.642,0.746) | 0.618(0.569,0.666) | 0.689(0.643,0.736) | 0.611(0.547,0.676) |
| FPR | 0.582(0.553,0.611) | 0.481(0.458,0.503)* | 0.571(0.541,0.600) | 0.467(0.436,0.499)* | 0.562(0.527,0.596) | 0.457(0.423,0.491)* |
| PPV | 0.074(0.059,0.090) | 0.080(0.062,0.097) | 0.178(0.153,0.203) | 0.190(0.157,0.223) | 0.252(0.218,0.285) | 0.267(0.225,0.309) |
| NPV | 0.954(0.944,0.965) | 0.954(0.944,0.964) | 0.887(0.864,0.910) | 0.887(0.870,0.904) | 0.837(0.809,0.865) | 0.837(0.811,0.863) |
AUC: Area under the curve
TPR: True positive rate
FPR: False positive rate
PPV: Positive predictive value
NPV: Negative predictive value
Denotes that the 95% confidence intervals for the results for the current KDRI and the revised KDRI at that interval after transplantation did not overlap.
Table 4.
Prediction errors associated with currently formulated KDRI and revised KDRI equation
| Measure | Current KDRI | Revised KDRI |
|---|---|---|
| R2 | 0.018 | 0.024 |
| Mean PE at 1 year after transplantation | 0.003 | 0.001 |
| Mean PE at 3 years after transplantation | 0.009 | 0.009 |
| Mean PE at 5 years after transplantation | 0.009 | 0.005 |
| Overall IBS | 0.154 | 0.155 |
| IBS at 1 year after transplantation | 0.039 | 0.039 |
| IBS at 3 years after transplantation | 0.073 | 0.073 |
| IBS at 5 years after transplantation | 0.101 | 0.100 |
IBS, integrated Brier scores; KDRI, kidney donor risk index; PE, prediction error
Table 5 presents the estimated discriminatory measures for a competing-risk model that treats death with functional allograft as competing with allograft failure. The pattern is similar to results with the model that treats death with functional allograft as a censored observation. The KDRIs had comparable performances with overlapping confidence intervals, with the revised equation providing slightly higher (better) point estimates in most cases. Point estimates of the c-indices for death were all lower than observed c-indices for allograft failure, independent of time-point considered and version of KDRI equation, consistent with our previous work indicating APOL1 genotype as a better predictor of allograft failure than recipient death (9,10).
Table 5.
C-indices and 95% confidence intervals estimated at 1, 3, and 5 years after transplantation under a competing-risk model, treating death with allograft function as a competing outcome
| Measure | One year after transplantation | Three years after transplantation | Five years after transplantation | |||
|---|---|---|---|---|---|---|
| Current KDRI | Revised KDRI | Current KDRI | Revised KDRI | Current KDRI | Revised KDRI | |
| AUC 1 for allograft failure | 0.674(0.591,0.757) | 0.674(0.593,0.755) | 0.629(0.567,0.692) | 0.639(0.578,0.700) | 0.646(0.583,0.710) | 0.657(0.593,0.721) |
| AUC 2 for allograft failure | 0.671(0.588,0.755) | 0.672(0.591,0.753) | 0.618(0.556,0.68) | 0.629(0.568,0.69) | 0.626(0.564,0.688) | 0.633(0.57,0.696) |
| AUC 1 for death with a functioning graft | 0.579(0.47,0.688) | 0.567(0.462,0.673) | 0.627(0.546,0.708) | 0.62(0.541,0.699) | 0.642(0.566,0.719) | 0.670(0.591,0.749) |
| AUC 2 for death with a functioning allograft | 0.568(0.464,0.672) | 0.556(0.447,0.664) | 0.608(0.53,0.685) | 0.598(0.518,0.678) | 0.611(0.534,0.687) | 0.636(0.562,0.710) |
AUC 1 and AUC 2 are defined following Blanche and colleagues (17). AUC 1 refers to the estimated c-index, assuming controls are free of either event (subjects who are alive with a functioning allograft) before the time point of interest. AUC 2 focuses on each specific type of failure; therefore, an AUC 2 estimate for allograft failure (e.g., at 3 years) assumes that controls are alive at 3 years after transplantation.
AUC, area under the curve
Effect of APOL1 genotype on KDPI
Table 2 shows KDPIs corresponding to mean values of the currently formulated KDRIs for the donors, grouped by number of APOL1 RRVs. Substituting APOL1 genotype for race in the equation showed a profound effect. The KDPI for an AA deceased kidney donor with 0/1 APOL1 renal-risk variants would decrease by 18 percentage points, whereas the KDPI for AA donors with 2 renal-risk variants would increase by 19 points, a net impact of 37 percentage points. For the former, the KDPI would approximate the median for all deceased kidney donors. In contrast, the KDPI for the latter group reaches the range for donors whose kidneys are likely to fare poorly, and substantially impact recipient choice.
Table 6 illustrates effects of substituting APOL1 genotype for race in the KDRI equation on KDRI scores and KDPIs in four clinical situations. Four deceased donors are compared: an EA, an AA with 0 APOL1 RRV, an AA with 1 APOL1 RRV, and an AA with 2 APOL1 RRVs. In the current KDRI, the 3 AAs are assigned identical race risk, a coefficient beta of 0.179. In the revised KDRI equation using APOL1 genotype, AAs with 0/1 APOL1 RRV receive no adverse weighting for the characteristic because survival of such kidneys was equivalent to that for kidneys from EAs who do not carry an APOL1 RRV (10). Example #1: replacement of race by APOL1 genotype for AAs with 0/1 RRV reduces a KDPI of 86% to 71%, kidneys now subject to standard allocation. Example #2: the revised KDRI equation for an AA donor with 2 RRVs increases KDPI to the 85% threshold for consideration for expanded-criteria-donor allocation. However, for AAs with 0/1 RRV, KDPI deceases to 46%. Example #3: use of APOL1 genotype for an AA donor with 0/1 RRV and a current KDPI of 30% reduced KDPI to 13%, within a range suitable for the best candidates. Example #4: using the revised KDRI formula widely separates AA donors with KDPI 18% with the current KDRI equation to those with an outstanding KDPI of 4% (0/1 RRV) or a mid-range KDPI of 42% (2 RRVs). In each of these 4 scenarios, use of APOL1 genotype dramatically impacts recipient selection.
Table 6.
Examples of effect APOL1 genotype on KDRIs and KDPIs
| Examples | ||||
|---|---|---|---|---|
| #1 | #2 | #3 | #4 | |
| Age, yr | 53 | 49 | 30 | 20 |
| Height, cm | 163 | 173 | 188 | 178 |
| Weight, kg | 70.5 | 77.3 | 93.2 | 81.8 |
| History of hypertension | No | No | No | No |
| History of diabetes | No | No | No | No |
| Death, cause | CNS tumor | Head trauma | Anoxia | Other |
| SCr, mg/dL | 1.0 | 1.2 | 1.2 | 1.0 |
| HCV status | Negative | Negative | Negative | Negative |
| DCD status | Yes | No | No | No |
| Current KDRI | Revised KDRI | |||
|---|---|---|---|---|
| Race or Genotype: | European American | African American | APOL1 renal-risk variants | |
| 0 or 1 | 2 | |||
| Example #1 | ||||
| KDRI | 1.4987 | 1.7925 | 1.4987 | 2.2605 |
| KDRI scaled | 1.2266 | 1.4671 | 1.2266 | 1.8502 |
| KDPI | 71% | 86% | 71% | 97% |
| Example #2 | ||||
| KDRI | 1.1713 | 1.4008 | 1.1713 | 1.8181 |
| KDRI scaled | 0.9586 | 1.1465 | 0.9586 | 1.4459 |
| KDPI | 46% | 64% | 46% | 85% |
| Example #3 | ||||
| KDRI | 0.8459 | 1.0117 | 0.8459 | 1.2635 |
| KDRI scaled | 0.6924 | 0.8281 | 0.6924 | 1.0443 |
| KDPI | 13% | 30% | 13% | 55% |
| Example #4 | ||||
| KDRI | 0.7466 | 0.8930 | 0.7466 | 1.1262 |
| KDRI scaled | 0.6111 | 0.7309 | 0.6111 | 0.9217 |
| KDPI | 4% | 18% | 4% | 42% |
APOL1, apolipoprotein L1 gene; CNS, central nervous system; DCD, donation after circulatory death; HCV, hepatitis C virus; KDRI, kidney donor risk index; KDPI, kidney donor profile index; SCr, serum creatinine
Discussion
It has long been known that kidneys donated by AAs fare worse after transplantation than kidneys from non-African Americans, regardless of race of the recipient (19-22). Epidemiologic data are consistent enough that donor race, despite its weakness as a biologic construct, was incorporated into the KDRI equation (5,6). An important and growing body of evidence now indicates that poor outcomes attributable to race may better associate with donor APOL1 genotype. Five years after engraftment, kidneys from AA deceased donors with 2 APOL1 RRVs were more than twice as likely to have failed than those from donors with fewer RRVs (9,10). Renal histology in most failed allografts from donors with 2 APOL1 RRVs displayed APOL1-associated lesions (8). In contrast, for kidneys from AA deceased donors with 0/1 APOL1 RRV, long-term outcomes appeared to be equivalent to those for kidneys from EA deceased donors (8-10). The KDRI equation was developed before the APOL1 effect in deceased-donor kidney transplantation was identified; in the face of emerging evidence in this regard, and the vagaries associated with inclusion of a variable termed “race,” reassessment of current equations is indicated (5,6). We propose inclusion of APOL1 genotyping in KDPI-based allocation as a significant advance in both objectivity and predictive utility.
In the current analysis, the effect of 2 APOL1 RRVs on allograft survival was substantial. The calculated KDRI coefficient beta 0.411 and corresponding hazard ratio 1.508 contrast with values for AA race in the current KDRI equation of 0.1790 and 1.196, respectively, and the weighting for 2 APOL1 RRVs exceeds that of the other 9 donor characteristics (5,6). The c-statistic in our models ranged from 0.58 to 0.65, numbers that coincide with the predictive value of the current KDRI equation. While the comparative performance of the revised KDRI is modest, more kidneys from AA donors would likely be available for transplantation. In the interval 2011-2015, kidneys for transplantation have been recovered from 5,752 AAs (23). A KDRI calculated with the substitution of race by APOL1 places a higher penalty on renal allografts from the 13% of AA deceased donors with 2 APOL1 RRVs, while removing the unnecessary penalty applied to the AA deceased donors who carry fewer RRVs. This change means that as many as 10,000 kidneys from about 5004 donors over the past 5 years would have been likely offered to patients with significantly more favorable prognoses and thus better utilized. Notably, this change in the formula did not reduce the predictive and discriminatory powers of the KDRI. Likelihood ratio tests comparing models that included APOL1 genotype and race to models that had only race/ethnicity as a covariate revealed that APOL1 genotypes consistently and significantly impacted allograft survival after deceased-donor kidney transplantation. In this dataset, the effect of race in conjunction with APOL1 was less clear with only 1 of 4 models showing a significant effect, suggesting that APOL1 may account for the preponderance of what has been termed the “race effect” associated with AA donors. Certainly, more data are needed to fully estimate the residual contribution of race beyond APOL1.
There are potential benefits of revising the current KDRI equation as suggested. As implied above, utilizing donor APOL1 genotype rather than race is likely to improve the allocation of better quality kidneys to patients projected to have longer post-transplant survival (estimated post-transplant survival, EPTS), enhancing a major stated goal of the KAS. For kidneys from AA deceased donors currently assessed as being of sufficiently poor quality (KDPI ≥ 85%) to merit allocation to patients with limited EPTS or consideration for discard, substitution of APOL1 genotype for race in the KDRI equation would substantially improve the KDRI for 85-90% these donors. Beyond improving accuracy of allocation, it would also likely reduce discard of such kidneys. Furthermore, our proposed revision may reduce racial disparities in renal-allograft survival. Kidneys recovered from AAs are more likely to be transplanted into AA than EA recipients because of relative differences in distribution of blood types and HLA specificities (24,25). If the KDRI equation were adjusted by replacing race with APOL1 genotype, young AAs awaiting kidney transplantation may be more likely to receive a kidney with a better predicted outcome because the number of such kidneys would increase.
Conversely, substitution of APOL1 genotype for race in the KDRI equation will increase the KDRI score and the resultant KDPI for about 13% of AA donors with currently estimated KDPIs as low as 15-19%. Use of a revised KDRI would change assessment of kidneys from donors with 2 RRVs to a category suitable for many recipients, but less desirable for those within the top 20% for EPTS. For kidneys from donors with 2 RRVs and KDPI bordering on the 85th percentile, a reformulated KDRI more accurately reflect prognosis, allowing them to be more confidently offered to recipients with less favorable EPTS. While very high KDPIs may prompt consideration for discard, it is important to take into account that most allografts from donors with 2 APOL1 RRVs did not quickly develop APOL1-associated damage (10).
There are several limitations implicit in our findings. Our new equation alters only the impact of race in the KDRI equation; the parameter estimates for the other 9 factors in the equation remain unchanged. The current KDRI was derived from data for 69,440 adult, single-kidney, first-time, deceased-donor kidney-transplant recipients in the United States from 1995-2005 (5). Substantially more donors with APOL1 genotyping and recipient follow-up data in a national prospective study would be needed to confirm that donors who differ by number of APOL1 RRVs are otherwise comparable regarding the other 9 characteristics, and to re-estimate the other parameters and validate the revised KDRI equation. Though our work indicates no adverse impact on allograft survival of kidneys with 0 or 1 APOL1 RRV, a larger experience might (or might not) identify an effect on outcome. Data we utilized to estimate the APOL1 effect lacked prospective follow-up and some potentially important clinical data, including histology of failed allografts, dosing and exposure to immunosuppressive medications, frequency and type of viral infections, presence of donor-specific anti-HLA antibodies, and occurrence of acute rejection or delayed allograft function (10). However, data utilized to derive KDRI also lack this granularity. Furthermore, prior studies have not obtained genotype data for APOL1 for recipients or other loci for donors; these data are needed to robustly test whether renal allograft survival is the consequence of donor APOL1 genotype or mere association. Again, however, two parameters already incorporated in KDRI (race and age) also do not link cause and effect.
There may be potential practical drawbacks to replacing race in KDRI with APOL1 genotype, though their merit may be diminishing. As noted earlier, evidence supporting the impact of 2 APOL1 RRVs on kidney disease and allograft survival is expanding rapidly. The statistical effect documented in our work is strong, and defines only potential benefit on allocation and allograft survival without indication of adverse impact on either endpoint. Clinical availability of APOL1 genotyping is also growing, and is now a standard offering from many commercial laboratories. The costs of APOL1 genotyping are declining and relatively modest, especially when considering the likelihood of an increased pool of donor kidneys and potential for improved allograft survival by better matching donors and recipients. Finally, even our own concern about tight turn-around restraints associated with organ recovery from deceased donors is increasingly alleviated with broad incorporation of polymerase chain reaction testing for viral infections now standard as part of the United States Public Health Service guidelines across the country. Adding APOL1 genotyping would seem a minor hurdle. Despite these trends, we recognize the limitations of our efforts, and the need for additional work and modeling prior to incorporation of APOL1 status into the organ allocation process (26,27). By pointing out both the scope and potential benefit of such a change, perhaps these other efforts can be hastened.
In conclusion, the effect of adjusting the KDRI equation by using APOL1 genotype instead of race is substantial and likely to be beneficial. Revision of the KDRI formula would favorably impact the stated goals of the new KAS and enhance the precision of organ allocation, with the potential to improve overall allograft survival. We believe confirmation of our findings and their subsequent incorporation into national policy to represent significant advances in this era of organ shortage, of benefit not only for minority candidates but all stakeholders.
Acknowledgments
We thank Dr. Vera Hauptfeld for processing of DNA samples from kidney donors in Alabama and Dr. Michael Gautreaux for processing DNA samples from kidney donors at Wake Forest.
Preliminary findings of this work were presented as an abstract at the Annual Meeting of the American Society of Nephrology on November 6, 2015 in San Diego, CA.
This work was supported by grants from the National Institutes of Health R01 DK070941 (B.I.F.), R01 DK084149 (B.I.F.), R01 MD009055 (J.D., B.I.F., and B.A.J) and 5U19-AI070119 (A.K.I. and B.A.J.). No person with the funding sources had any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. The data analyzed here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients.
Abbreviations
- AA
African American
- APOL1
apolipoprotein L1 gene
- AUC
area under the curve
- EPTS
estimated post-transplant survival
- FPR
false-positive rate
- IBS
integrated Brier score
- KAS
kidney allocation system
- KDPI
kidney donor profile index
- KDRI
kidney donor risk index
- NPV
negative predictive value
- OPTN
Organ Procurement and Transplantation Network
- PPV
positive predictive value
- ROC
receiver operating characteristic
- RRV
renal-risk variant
- SRTR
Scientific Registry of Transplant Recipients
- TPR
true-positive rate
Footnotes
Disclaimer
The interpretation and reporting of these data are the responsibilities of the authors and in no way should be considered as an official policy of or interpretation by the Scientific Registry of Transplant Recipients or the United States Government.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described in the American Journal of Transplantation. Wake Forest University Health Sciences and B.I.F. have filed for a patent related to APOL1 genetic testing. B.I.F. receives research support from Novartis Pharmaceuticals and serves as a consultant for Ionis Pharmaceuticals. S.O.P. has served as a consultant for Dompe Pharmaceuticals. The other authors have no conflicts of interest to disclose.
References
- 1.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25:1842–1848. doi: 10.1681/ASN.2013070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyberg SL, Matas AJ, Rogers M, et al. Donor scoring system for cadaveric renal transplantation. Am J Transplant. 2001;1:162–170. [PubMed] [Google Scholar]
- 3.Nyberg SL, Matas AJ, Kremers WK, et al. Improved scoring system to assess adult donors for cadaver renal transplantation. Am J Transplant. 2003:3715–721. doi: 10.1034/j.1600-6143.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 4.Schold JD, Kaplan B, Baliga RS, Meier-Kriesche HU. The broad spectrum of quality in deceased donor kidneys. Am J Transplant. 2005;5(4 Pt 1):757–765. doi: 10.1111/j.1600-6143.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 5.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 6. https://optn.transplant.hrsa.gov/resources/allocation-calculators/kdpi-calculator/
- 7.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343:1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 8.Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11:1025–1030. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman BI, Julian BA, Pastan SO, et al. Apolipoprotein L1 gene variants in decreased organ donor are associated with renal allograft failure. Am J Transplant. 2015;15:1615–1622. doi: 10.1111/ajt.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman BI, Pastan SO, Israni AK, et al. APOL1 genotype and kidney transplantation outcomes from deceased African American donors. Transplantation. 2016;100:194–202. doi: 10.1097/TP.0000000000000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Seaghdha CM, Parekh RS, Hwang SJ, et al. The MYH9/APOL1 region and chronic kidney disease in European-Americans. Hum Mol Genet. 2011;20:2450–2456. doi: 10.1093/hmg/ddr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke JN, Bostrom MA, Hicks PH, et al. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol Dial Transplant. 2012;27:1505–1511. doi: 10.1093/ndt/gfr522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. https:optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf.
- 14.Harrell FE., Jr . Regression Modeling Strategies. Springer-Verlag New York, Inc.; Secaucus, NJ, USA: pp. 517–520. ©2006 ISBN:0387952322. [Google Scholar]
- 15.Harrell Frank E., Jr. rms: Regression Modeling Strategies. R package version 4.3-1. 2015 http://CRAN.R-project.org/package=rms.
- 16.Zheng Y, Cai T, Brown M. survAccuracyMeasures: Estimate accuracy measures for risk prediction markers from survival data. R package version 1.2. 2014 http://CRAN.R-project.org/package=survAccuracyMeasures.
- 17.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic ROC curves for censored event times with competing risks. Stat Med. 2013;32:5381–5397. doi: 10.1002/sim.5958. [DOI] [PubMed] [Google Scholar]
- 18.Mogensen UB, Ishwaran H, Gerds TA. Evaluating random forests for survival analysis using prediction error curves. J Stat Softw. 2012;50:1–23. doi: 10.18637/jss.v050.i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58:1311–1317. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 20.Swanson SJ, Hypolite IO, Agodoa LY, et al. Effect of donor factors on early graft survival in adult cadaveric renal transplantation. Am J Transplant. 2002;2:68–75. doi: 10.1034/j.1600-6143.2002.020112.x. [DOI] [PubMed] [Google Scholar]
- 21.Chakkera HA, O'Hare AM, Johansen KL, et al. Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol. 2005;16:269–277. doi: 10.1681/ASN.2004040333. [DOI] [PubMed] [Google Scholar]
- 22.Callender CO, Cherikh WS, Traverso P, Hernandez A, Oyetunji T, Chang D. Effect of donor ethnicity on kidney survival in different recipient pairs: an analysis of the OPTN/UNOS database. Transplant Proc. 2009;41:4125–4130. doi: 10.1016/j.transproceed.2009.06.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#.
- 24.Roberts JP, Wolfe RA, Bragg-Gresham JL, et al. Effect of changing the priority for HLA matching on the rates and outcomes of kidney transplantation in minority groups. N Engl J Med. 2004;350:545–551. doi: 10.1056/NEJMoa025056. [DOI] [PubMed] [Google Scholar]
- 25.Cannon RM, Brock GN, Marvin MR, Slakey DP, Buell JF. The contribution of donor quality to differential graft survival in African American and Caucasian renal transplant recipients. Am J Transplant. 2012;12:1776–1783. doi: 10.1111/j.1600-6143.2012.04091.x. [DOI] [PubMed] [Google Scholar]
- 26.Freedman BI, Julian BA. Should kidney donors be genotyped for APOL1 risk alleles? Kidney Int. 2015;87:671–673. doi: 10.1038/ki.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojo A, Knoll GA. APOL1 genotyping of African American deceased organ donors: not just yet. Am J Transplant. 2015;15:1457–1458. doi: 10.1111/ajt.13230. [DOI] [PubMed] [Google Scholar]