Abstract
Premenopausal women and intact female rodents sustain smaller cerebral infarctions than males. Several sex-dependent differences have been identified as potential contributors, but many questions remain unanswered. Mice exhibit wide variation in native collateral number and diameter (collateral extent) that is dependent on differences in genetic background, aging, and other comorbidities and that contributes to their also-wide differences in infarct volume. Likewise, variation in infarct volume correlates with differences in collateral-dependent blood flow in patients with acute ischemic stroke. We examined whether extent of pial collateral arterioles and posterior communicating collateral arteries (PComAs) differ depending on sex in young, aged, obese, hypertensive, and genetically different mice. We combined new data with meta-analysis of our previously published data. Females of C57BL/6J (B6) and BALB/cByJ (BC) strains sustained smaller infarctions than males after permanent MCA occlusion. This protection was unchanged in BC mice after introgression of the B6 allele of Dce1, the major genetic determinant of variation in pial collaterals among mouse strains. Consistent with this, collateral extent in these and other strains did not differ with sex. Extent of PComAs and primary cerebral arteries also did not vary with sex. No dimorphism was evident for loss of pial collateral number and/or diameter (collateral rarefaction) caused by aging, obesity, and hypertension, nor for collateral remodeling after pMCAO. However, rarefaction was greater in females with long-standing hypertension. We conclude that smaller infarct volume in female mice is not due to greater collateral extent, greater remodeling, or less rarefaction caused by aging, obesity, or hypertension.
Keywords: Leptomeningeal collaterals, Posterior communicating artery, Sex, Ischemic stroke, Risk factors
Introduction
Enigmas remain regarding sex. One in five women between age 55 and 75 will have a stroke, compared to one in six men [1]. While most studies report that women suffer more severe strokes, experience worse outcomes, and have a higher mortality than men, some studies find that when corrected for factors such as age, comorbidities, and treatment options due to time elapsed between stroke and treatment, women and men under 70-year age have similar outcomes [2–7]. However, in older individuals, female sex is independently associated with poor functional outcome [4, 5]. This is partially because women experience first stroke at a later age, have a longer lifespan, are overrepresented in the elderly population and have increased incidence of certain risk factors such as atrial fibrillation [2–7]. In contrast, women have less incidence and severity of stroke prior to menopause, an effect that is partly ascribed to the neuroprotective, anti-apoptotic, vasodilatory, and anti-inflammatory effects of estrogen [6, 8 and references therein]. However, differences in sex hormones are not the only factor. Boys of neonatal through adolescent age have a higher incidence of ischemic stroke than girls [1], though no gender differences in fatality or neurological deficits have been identified [9]. In a meta-analysis of outcome measures for infants at risk for hypoxia-ischemia events, Smith et al. [10] found that females had better performance IQ outcomes than males. These data are consistent with other studies showing that females have a more favorable outcome after traumatic brain injury [11, 12] and premature birth [13, 14]. The mechanisms underlying this premenopausal “female advantage/protection” in overcoming hypoxic insult remain unclear.
A number of preclinical studies have reported sexual dimorphism in outcomes following ischemic stroke. Female rodents sustain reduced infarct volume after transient (t) or permanent (p) middle cerebral artery occlusion (MCAO), a difference that is diminished by ovariectomy and restored with estrogen replacement [15]. Similar findings have been reported in mice using a genetic approach to manipulate gonadal hormones [16]. Smaller infarction in females has been linked to estrogen-associated differences in inflammatory and apoptotic mechanisms and immune cell activity [6, 8, 17, 18]. However, hormone-independent findings have also been reported. For example, 16-month-old female mice have larger infarctions compared to age-matched males despite equivalent circulating estrogen [19]. Also, deletion of macrophage migration inhibitory factor increases infarct volume in intact and ovariectomized females to that seen in males [20]. Female rat pups show better recovery of auditory and cognitive functions after hypoxic brain injury despite similar or greater anatomical damage than in males [10]. In addition, sex-based differences in hypoxia-driven inflammatory and apoptotic pathways have been described in cells and neonatal animals independent of exposure to gonadal hormones [21–23], suggesting that sexual dimorphism in intrinsic cellular responses to tissue injury may contribute to differences in outcome after ischemic events. Despite the above findings, many questions remain regarding the mechanisms responsible for reduced infarction in females.
The major determinants of infarct volume caused by large-vessel acute ischemic stroke (AIS) distal to the circle of Willis are size of the territory at risk, duration of obstruction relative to oxygen demand, and the extent (i.e., number and diameter) of native pial (leptomeningeal) collaterals present before obstruction occurs. Pial collaterals are arteriole anastomoses that cross-connect a small number of the distal branches of adjacent arterial trees. Their extent varies widely in mice due to differences in genetic background, in particular for variants of the recently identified gene within the Dce1 locus, Rabep2, resulting in large differences in collateral-dependent blood flow and thus infarct volume after pMCAO [24–26]. As well, “environmental” factors, e.g., aging, endothelial dysfunction, hypertension, metabolic syndrome, diabetes, and obesity, cause loss of collateral number and/or a decrease in diameter of those that remain (collateral rarefaction), in association with an increase in infarct volume [27–30]. Indirect evidence based on imaging of collateral-dependent retrograde perfusion suggests extent of pial collaterals in AIS patients also varies widely [31] and negatively associates with aging, metabolic syndrome, and other comorbidities [32].
Despite the wide variability in collateral extent arising from differences in genetic and environmental factors, no studies have examined whether extent differs with biological sex. We therefore combined newly obtained data with a “meta-like” analysis of our previously published studies [25, 26, 33] wherein data for female and male mice had been obtained but with n-sizes too small to permit statistical comparison. Our aim was to determine if the extent of pial collateral arterioles differ depending on sex in young adult, aged, obese, hypertensive, and genetically different mice. We also examined whether extent of the posterior communicating collateral arteries (PComAs), which are a major determinant of stroke severity when obstruction occurs within or proximal to the circle of Willis, differ with sex. This effort is in keeping with recent emphasis on including both sexes in animal experiments (NIH NOT-OD-15-102).
Materials and Methods
Mice
C57BL/6J (B6) mice were purchased from Jackson Laboratories and bred in-house. Eighteen- to 26-month-old B6 mice were from the National Institute of Aging. Congenic (CNG) B6 mice (CNGB6/B6) have varying amounts of B6 genotype spanning the Dce1 locus on chromosome 7 and are BALB/cByJ (BC) (Jackson, #001026) elsewhere [25, 26]. PCR markers were used to identify blocks of B6 genotype. Heterozygotes were mated to produce homozygous CNGB6/B6, control CNGBC/BC (essentially wild-type BC due to extensive backcrossing), and heterozygous CNGB6/BC lines [25, 26]. Previously published data from CNG lines 3, 4, 5, 6, and 7 were pooled and segregated by sex. Obese mice on a B6 background (Lepob/ob, Jackson, #006906) were fed a high-fat diet (D12079B, Research Diets Inc., New Brunswick, NJ) starting at 1.5-month age [30]. Hypertensive RTGMK renin transgenic mice (Rntg/+ and Rntg/tg) on a mixed 129SvEv;B6 background were generously provided by Dr. Kathleen Caron [30]. Controls were produced by intercrossing B6129S-F1/J mice (Jackson, #101043) to produce F2 hybrids. Procedures were approved by the University of North Carolina’s IACUC and were in accordance with NIH guidelines.
Angiography and Morphometry
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). The thoracic aorta was cannulated retrograde with stretched PE50 tubing, and the dorsal neocortex was exposed by craniotomy and imaged under a stereomicroscope. The vasculature was perfused with heparinized saline containing papaverine (40 μg/ml) and sodium nitroprusside (30 μg/ml), followed by a small bolus of Evans Blue (50 mg/ml). Yellow Microfil™ (FlowTech Inc., Carver, MA) was infused at a pressure sufficient to fill the precapillary vasculature. The dorsal cortex was treated topically with 4% paraformaldehyde and fixed overnight at 4 °C. Lumen diameter was measured for all MCA-to-ACA pial collaterals at their midpoints and averaged for each animal (ImageJ, NIH). Lumen diameters of the PComAs and primary cerebral arteries were averaged when bilaterally present.
Middle Cerebral Artery Occlusion
A ~4-mm incision was made caudal to the right eye and the temporalis was retracted. A ~2-mm2 craniotomy was made (18000-17, FST, Foster City, CA) over the MCA which was then cauterized (18010-00, FST, modified). The temporalis was reseated, and the incision was closed. Either 24 h or 6 days after MCA occlusion (see figures and/or legends for specific time) and euthanasia, the brain was removed and sliced into 1-mm coronal sections that were incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) in PBS at 37 °C and imaged. Total forebrain area and TTC-stained infarct area were measured for each section (ImageJ), summed, and percent infarct volume was calculated.
Statistics
Statistical analysis was performed with PASW Statistics 18 or Microsoft Excel. Values are expressed as mean ± SE. When bilaterally present, PComA and primary cerebral artery diameter was determined as the mean of both sides for each animal. t Tests for sex were one-tailed for infarct volume since in a smaller cohort we previously reported smaller infarctions in females [26] in agreement with other rodent studies; two-tailed for number and diameter of pial collaterals, PComAs, and primary cerebral arteries; and one-tailed for experimental versus control for the effect of comorbidities [30]. ANOVA was used to examine the interaction of sex and genotype for collateral number and diameter in hypertensive animals. PComA number was tested by χ2 test. p < 0.05 was considered significant. The following suggested STAIR criteria [34] were followed: investigators were blinded to genotype and sex; gene dose-response was evaluated by studying wild-type, heterozygous, and homozygous mutants; pMCAO was used to permanently recruit pial collaterals; and all negative results were reported.
Results
Female Mice Sustain Smaller Infarct Volumes
Twenty-four hours after pMCAO, infarct volume was 34% smaller in female B6, a strain with abundant large-diameter pial collaterals, and 8% smaller in female CNG BC/BC, a strain that is essentially wild-type BALB/cByJ and has a small number of small-diameter collaterals, thus larger infarctions (Fig. 1). The smaller infarct volume in females confirms previous reports in B6 and BC mice after tMCAO [e.g., 18, 35, 36] and rats and mice after pMCAO [37, 38]. Infarct volume trended (i.e., p ≥ 0.05) 10% smaller in female CNGB6/B6, a mouse line with the B6 allele of the Dce1 locus (chromosome 7:126,152,048–126,822,113) introgressed into the BC background. This genetic change causes them to form more pial collaterals of larger diameter during development (see below), resulting in infarct volumes similar to the B6 strain [25, 26]. The fact that infarct volume in females is similarly smaller in CNGB6/B6 (10%) and CNGBC/BC (8%) mice indicates that the effect of variants of the causal gene within the Dce1 locus, Rabep2, that is responsible for most of the difference in native collateral extent in inbred mouse strains [26], does not display sexual dimorphism.
Fig. 1.
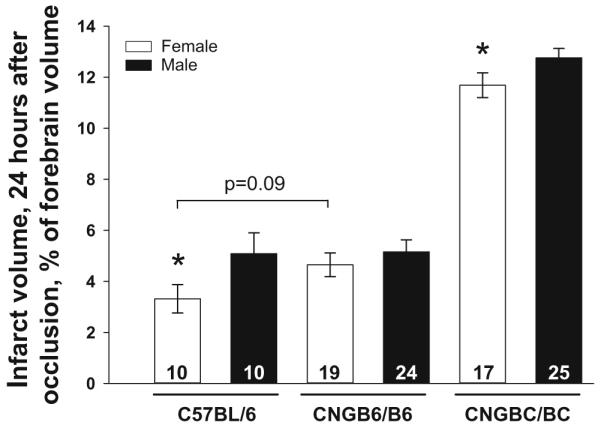
Infarct volume is reduced in females after permanent middle cerebral artery occlusion. Infarct volume in female mice is 34% smaller in high-collateral mice (C57BL/6J, B6), 8% smaller in low-collateral mice (CNGBC/BC; essentially wild-type BALB/cByJ, BC), and trends 10% smaller when the homozygous B6 allele of Determinant of collateral extent (Dce1) [25] is introgressed into BC mice (CNGB6/B6); see “Results” for details. Mice are 3–4 months old. For this and subsequent figures, values are mean ± SEM; number of animals is given in columns; *p < 0.05, **p < 0.01, ***p < 0.001 for male versus female; #p < 0.05, ##p < 0.01, ###p < 0.001 for same-sex comparison; p values are given if p = 0.05–0.10
Collateral Number and Diameter Do Not Vary with Sex
Variation in infarct volume after pMCAO among different strains of mice, including B6 and BC, are strongly dependent on differences in native pial collateral extent [24–26]. We therefore hypothesized that the smaller infarct volumes in females (Fig. 1) reflect a greater number and/or diameter of pial collaterals. However, number and diameter did not differ with sex (Fig. 2). We confirmed this for a large F2 hybrid population of B6 and BC mice that span the range of collateral extent present in B6 and BC mice (n = 232–240, ~half male and female, each genetically distinct) [33] (Supplemental Fig. I). This population also showed no difference between sexes in pial collateral remodeling (anatomic lumen enlargement) measured 3 days after pMCAO. Consistent with the above absence of difference in collateral extent, brain weight does not differ with sex in BC, B6, and six other strains of 4-month-old mice (http://phenome.jax.org/db/qp?rtn=views/measplot&brieflook=22702&projhint=Jaxpheno2). These data indicate that the smaller infarct volumes in female B6 and BC mice depend on factors other than collateral number and diameter.
Fig. 2.
Native pial collateral number and diameter, which vary with mouse strain, do not differ with sex. Number (for both hemispheres) and average diameter of MCA-to-ACA collaterals. Collaterals are denoted by asterisks in representative image. Mice are 3–4 months old
Aging-Induced Collateral Rarefaction Is Not Sex-Dependent
Male, 24- and 31-month-old B6 mice (~72 and 88 equivalent human years) have 11 and 22% fewer pial collaterals that are 24 and 30% smaller in diameter, respectively, than 3-month-old mice, in association with progressively larger infarct volumes at each age [27]. To determine if this age-induced collateral rarefaction differs with sex, we examined collateral extent in aged B6 and B6;129 F2 hybrid mice [30]. Rarefaction did not differ with sex (Fig. 3).
Fig. 3.
Aging-induced collateral rarefaction, i.e., decline in number and/or diameter, is independent of sex. Number and average diameter of MCA-to-ACA collaterals in 8- and 18–26-month-old B6, and 8- and 18-month-old F2 hybrid of B6 and Sv129 strains
Obesity-Induced Collateral Rarefaction Is Not Sex-Dependent
We previously reported that 8-month-old obese mice (Lepob/ob) had fewer collaterals of smaller diameter than age-matched wild-type mice [30]. No sexual dimorphism for obesity-induced collateral rarefaction was observed after dichotomizing these data for sex (Supplemental Fig. II). Female obese mice had smaller diameters than their female controls; however, the small n-sizes per group makes this finding uncertain.
Hypertension-Induced Collateral Rarefaction Is Not Sex-Dependent
Chronic renin-dependent hypertension causes rarefaction of pial collaterals, in association with increased infarct volume after pMCAO [30]. Mean arterial pressure in heterozygous Rntg/+ mice was 136, 139, and 140 mmHg at 4-, 8-, and 12-month age, compared to age-matched wild type (average of all ages = 110 mmHg), and was 153 mmHg in 4-month-old homozygous Rntg/tg mice [30]. After dichotomizing for sex, similar rarefaction was evident in 4-month-old mice of both sexes (Fig. 4). ANOVA revealed that loss of collateral number and diameter at 4-month age was dependent on level of hypertension (p = 0.09 and p < 0.001, respectively) but not sex (p = 0.74). However, rarefaction was greater in females with long-standing hypertension (8–12-month age). This cannot be attributed to higher arterial pressure, which instead was lower in females (135 versus 149 mmHg, p < 0.01) than males of this age (Supplemental Fig. III). This finding may have relevance to a previous report that 16-month-old female mice have larger infarctions compared to age-matched males [19].
Fig. 4.
Hypertension-induced collateral rarefaction in young mice (ANOVA) does not differ with sex but is greater in 8–12-month-old female mice with chronic hypertension. Number and average diameter of MCA-to-ACA collaterals for 4- and 8–12-month-old wild-type (WT) and transgenic renin-overexpressing mice. Transgenic mice are mildly (Rntg/+, mean arterial pressure (MAP) = 135 mmHg) or moderately (Rntg/tg; MAP = 155 mmHg) hypertensive and are on a mixed B6;Sv129 background [30]
Extent of PCom Collateral Arteries and Diameter of the Primary Cerebral Arteries Do Not Vary with Sex
Variation in infarct volume after obstruction of a primary cerebral artery(s) within or proximal to the circle of Willis varies in association with differences in diameter of the PComAs and whether they are present unilaterally, bilaterally, or absent. These differences depend on genetic, environmental, and stochastic factors in mice [39]. To determine if sex also contributes to this variation and if the extent of PComAs in young females is greater and thus could contribute to their smaller infarct volumes, we measured the number and lumen diameter of PComAs in a new cohort of ~3-month-old B6 mice, and also obtained lumen diameter for their primary cerebral arteries. Number and diameter of PComAs and diameter of the primary cerebral arteries did not differ with sex, although both trended greater in females (Fig. 5). Female brains were larger (0.606 ± 11 vs 0.550 ± 4 g, p < 0.0001) despite smaller body weight (24 ± 0.7 vs 28 ± 0.8 g, p < 0.001). It is unlikely that the trend toward greater bilateral presence in females is due to their slightly greater age thus brain size, since variation in PCom presence is established at birth [39].
Fig. 5.
Diameter (b) and number (c) of posterior communicating collateral arteries (PComAs) and primary cerebral arteries do not vary with sex. a Representative image showing measurements were made 250 μm from bifurcations for ACA, MCA, 750 μm from bifurcation for ICA, and at midpoint of PCA and PComA. d Two-tailed χ2 test shows females trend toward a higher incidence of both PComAs being present. Female and male B6 mice, respectively, 3.7 and 3.3 months old (not significantly different), 0.606 ± 11 and 0.550 ± 4 g brain weight (p < 0.0001), and 24 ± 0.7 and 28 ± 0.8 g body weight (p < 0.001)
Discussion
We confirmed in two inbred strains of mice with large differences in pial collateral extent (B6, BC) that infarct volume after pMCAO is smaller in females than males. While this difference is well described using rodent models of tMCAO [16, 17, 35, 36, 40], it is less clear for pMCAO. A previous study reported that infarct volume in female B6 mice was smaller 24 h after tMCAO but similar after pMCAO [17]. In contrast, restoration of 17β-estradiol to physiological levels in ovariectomized rats and B6 mice reduced infarct volume after pMCAO, in association with delayed neuronal cell death [41]. In addition, initial and final lesions were smaller in female rats after pMCAO, while penumbral volume was not different (the latter supports our finding of no sex difference in pial collateral extent) [41]. The reasons for the discrepancies in mice are not apparent.
We found no evidence for greater pial collateral extent in female mice with different genetic backgrounds (B6, BC, F2 hybrids and congenics of these strains, and mixed B6;Sv129 mice) and young (3–4 months) and middle-aged mice (8–12-month age). Moreover, no sex-dependent differences were seen in rarefaction of pial collaterals caused by advanced aging (≥18-month age), obesity, or hypertension. In addition, extent of PComAs and lumen diameter of primary cerebral arteries in B6 mice did not vary with sex. However, females had slightly larger-diameter PComAs that were more frequently present bilaterally (58 vs 25 %), although neither difference reached significance. Since pMCAO increases flow across the PComAs, especially on the ipsilateral side [39], such an anatomic difference could contribute to smaller infarctions in females.
Our observation that native pial collateral extent and collateral remodeling after pMCAO were not greater in female mice focuses attention on other factors that impact collateral flow and differ with sex. Arterial pressure and the pressure gradient across collaterals are important determinants. For example, transient partial intra-aortic balloon occlusion increases cerebral blood flow by 35–52% in a non-ischemic porcine model [42], restores blood flow to baseline in the distal MCA tree in rodent models of MCAO [43], and is efficacious in patients with AIS [44]. Female B6 mice have as much as 10 mmHg higher blood pressure than males, which could contribute to their smaller infarctions (Fig. 1). However arterial pressure does not differ with sex in BC mice [45, 46].
After sudden occlusion of the trunk of an arterial tree like the MCA that possesses collaterals to the ACA and PCA trees, local myogenic and metabolic vasodilator mechanisms reduce resistance in the arterial tree downstream of the collaterals. These mechanisms, together with the occlusion-induced pressure gradient and flow across the collateral network, induce flow-mediated dilation (FMD) of arterioles/arteries upstream of the network, e.g., in the ACA and PCA trees. Few studies have examined whether sex differences in these intrinsic regulatory mechanisms exist in the cerebral vasculature. Females exhibit better myogenic reactivity of MCAs isolated from both normotensive and hypertensive rats [47]. More information is available for non-cerebral tissues [reviewed in 48]. For example, modulation of myogenic tone by endothelial-dependent factors differed with sex in murine mesenteric arteries [49] and FMD in skeletal muscle arterioles was greater in females [50]. No sex differences were observed for FMD in a large population-based study [51], nor for FMD or reactive hyperemia in patients with heart failure [52]. Besides the above local regulatory mechanisms, circulating estrogen has cerebral vasodilatory activity, including promotion of eNOS/NO activity and dilatory prostanoids [41, 53] that affect these mechanisms. Thus, females may be predisposed during acute cerebral ischemia to enhanced vasodilation and lower resistance above and below the collateral network, thus favoring increased collateral flow and rescue of penumbral tissue. Whether such sex-dependent differences in local hemodynamic regulation contribute to “female protection” in stroke is not known.
Resistance downstream of an occlusion can also increase from thrombotic and hemostatic changes and thereby reduce collateral blood flow. Marked worsening in neurological outcome after the initial ischemic event occurs in 8–38% of stroke patients [54, 55]. This has been associated with insufficient collateral-dependent flow, early reocclusion, reduced body temperature, clot progression, and other metabolic factors and comorbidities. Many of these factors affect the clotting cascade. In rodents, estrogen-related sex differences in thrombosis and hemostasis have been reported, with studies finding, although not without some controversy, that females clot more slowly than males [56, 57]. In B6 mice, this is associated with differences in hepatic growth hormone secretion and clotting factor production [58] and lower concentrations of fibrogen (BC) and fibrinogen (B6) [59, 60].
Sex-dependent cellular responses to oxygen and nutrient deprivation may also influence infarct volume [reviewed in 61]. Cells within penumbral tissue evidence delayed apoptosis that varies with sex [61–63]. Apoptosis occurs through different interdependent pathways involving overproduction of neuronal nitric oxide, peroxynitrite formation, DNA damage, and overactivation of the DNA repair enzyme, poly-ADP ribose polymerase-1 (PARP-1), which triggers translocation of apoptosis-inducing factor (AIF) from mitochondria to the nucleus, leading to caspase-independent apoptosis [63 and references therein]. Female mice deficient in neuronal nitric oxide synthase sustained larger infarction after pMCAO, whereas males experienced neuroprotection. This result was independent of blood flow in the ischemic territory [63]. Furthermore, loss of PARP-1 did not affect males but caused increased infarction in females. Genetically, female (XX) neurons displayed lower in vitro sensitivity to ectoposide- and staurine-induced apoptosis than male (XY) neurons, and XX neurons activated a cytochrome c-dependent apoptotic pathway while XY neurons initiated an AIF-dependent apoptotic pathway [64]. Caspase inhibition after tMCAO reduced infarct volume and improved neurological outcome in female B6 mice but had no effect in males [36].
Inflammatory responses, which can be both beneficial and harmful to cells and impact stoke outcome, display sex differences [65–67]. Ischemia-induced endothelial adhesion and leukocyte accumulation in the dependent vascular tree can lead to increased resistance and “sludging,” followed by release of inflammatory cytokines and chemokines by leukocytes that disrupt the blood-brain barrier and promote neuronal death [65, 66]. Ovariectomized rats displayed increased adhesion at baseline and after tMCAO compared to intact controls [67], suggesting that estrogen-mediated attenuation of leukocyte adhesion may contribute to better stroke outcome. Microglia activated by ischemia produce M1 (pro-inflammatory) or M2 (anti-inflammatory) responses [see 66, 68], the former involving release of factors including reactive oxygen species and matrix metalloproteinases that recruit other immune cell types. While directing removal of cell debris and release of trophic factors to counter cell damage, the M1 response also expands local tissue damage. In contrast, the M2 response involves release of IL-4 and IL-10 and other factors that favor tissue repair. Estrogen inhibits microglial proliferation and activation and favors M2 macrophage infiltration [8, 69, 70]. Genetic deletion of IL-4 in mice is accompanied by worse outcomes in both sexes and abolishes female neuroprotection [70]. Females with deletion of macrophage migration inhibitory factor have more vigorous macrophage activation and larger infarct volumes than intact controls [20]. T-lymphocytes from male mice isolated 24 h after tMCAO and stimulated with a Nox2 activator produce sevenfold greater superoxide compared to females [17].
This study has several limitations. Several data groups that we reported previously, but without dichotomization for sex [30], resulted in n-sizes of four after dichotomization. Unfortunately, we cannot examine additional animals because we extinguished these lines. We used pMCAO to induce sustained flow across the pial collateral network, while evidence suggests that sex differences in infarct volume are enhanced in the tMCAO model [e.g., 17]. However, after first confirming that females have smaller infarctions, our objectives were to examine whether a sex difference exists: for extent of native pial collaterals, PComAs, and the primary cerebral arteries; for rarefaction of pial collaterals caused by aging, obesity, and hypertension; and for pial collateral remodeling after occlusion. Remodeling requires sustained flow across the collateral network for many hours-to-days, thus necessitating the use of pMCAO.
As yet, there is no single strain or group of mouse strains that best models the variation of anatomic collaterals that may exist in humans. In Fig. 2, we quantified, according to sex, the number and lumen diameter (i.e., “extent”) of pial collaterals that connect the MCA and ACA trees, per hemisphere, in B6 and BC inbred mice. These two strains approach the extremes in differences in pial collateral extent among 25 strains that we have studied in the past [25]. We also did the same for two congenic strains that we constructed with collateral extents that are intermediate to the B6 and BC strains (Fig. 2). We further did this for a B6-BC/F2 population of 242 mice that we constructed that span the range of collateral number (0 to 15) and lumen diameter (8 to 28 μm) that we have found exists among inbred strains (Supplemental Fig. I). There were no differences in collateral extent, per sex, in any of these mice. Owing to technical limitations, no studies have quantified extent of pial collaterals to test whether such variation also exists in humans. However, collateral score/status measured at baseline in patients with acute M1/M2-MCA stroke, which correlates with relative pial collateral-dependent blood flow, is well-known to exhibit a wide range—from at or near-zero to the highest score quantified (a value of 3 or 22, depending on the scoring system) [71]. Yet, many factors besides anatomic collaterals can contribute to differences in collateral blood flow, although the former has the greatest potential impact. We are unaware of any studies that have determined whether collateral score exhibits sexual dimorphism in humans.
In summary, we find that smaller infarct volume in female mice after pMCAO is not due to a greater extent of pial collaterals. Collateral number and diameter showed no sexual dimorphism in young-adult, middle-, and old-aged mice, or in strains of mice with genetic-determined differences in native collaterals. The amount of collateral rarefaction caused by aging, obesity, or hypertension also did not differ with sex. However, females exhibited greater rarefaction with long-standing hypertension, a finding which could explain their larger infarctions when examined at 16-month age, compared to age-matched males [19]. Extent of the PComAs also showed no sexual dimorphism, nor were there sex differences in diameter of the primary cerebral arteries. In addition, collateral remodeling after pMCAO did not differ with sex. These findings indicate that factors other than anatomic differences in the pial and Willisian collateral circulations underlie the reduced severity of stroke in female mice.
Supplementary Material
Acknowledgements
The authors wish to thank Dr. Steven Warach, Seton/UT Southwestern Clinical Research Institute, for discussions that encouraged this study.
Compliance with Ethical Standards This study was funded by NIH-NHLBI HL111070 and NIH-NINDS NS083633 (JEF).
Abbreviations
- ACA
Anterior cerebral artery
- AIS
Acute ischemic stroke
- BA
Basilar artery
- BC
BALB/cByJ inbred mouse strain
- B6
C57BL/6J inbred mouse strain
- Dce1
Congenetic locus on chromosome 7, denoted Determinant of collateral extent-1
- ICA
Internal carotid artery
- Lepob/ob
Leptin-deficient mouse model of obesity
- MCA
Middle cerebral artery
- pMCAO
Permanent MCA occlusion
- PCA
Posterior cerebral artery
- PComA
Posterior communicating collateral artery
- Rntg
Renin-overexpressing transgenic mouse model of hypertension
- tMCAO
Transient MCA occlusion
- WT
Wild-type strain
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12975-016-0508-0) contains supplementary material, which is available to authorized users.
Author Contributions JEF designed the study, analysis, and figures; JEF wrote the manuscript, JLL assisted; HZ, SMM, AA, and JLL performed the experiments and statistics.
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Barrett KM, Brott TG, Brown RD, Jr, Frankel MR, Worrall BB, Silliman SL, Case LD, Rich SS, Meschia JF. Sex differences in stroke severity, symptoms, and deficits after first-ever ischemic stroke. J Stroke Cerebrovasc Dis. 2007;16(1):34–9. doi: 10.1016/j.jstrokecerebrovasdis.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehme AK, Siegler JE, Mullen MT, Albright KC, Lyerly MJ, Monlezun DJ, Jones EM, Tanner R, Gonzales NR, Beasley TM, Grotta JC, Savitz SI, Martin-Schild S. Racial and gender differences in stroke severity, outcomes, and treatment in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23(4):e255–61. doi: 10.1016/j.jstrokecerebrovasdis.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irie F, Kamouchi M, Hata J, Matsuo R, Wakisaka Y, Kuroda J, Ago T, Kitazono T. Sex differences in short-term outcomes after acute ischemic stroke: the Fukuoka stroke registry. Stroke. 2014;46(2):471–6. doi: 10.1161/STROKEAHA.114.006739. [DOI] [PubMed] [Google Scholar]
- 5.Lewsey JD, Gillies M, Jhund PS, Chalmers JW, Redpath A, Briggs A, Walters M, Langhorne P, Capewell S, McMurray JJ, Macintyre K. Sex differences in incidence, mortality, and survival in individuals with stroke in Scotland, 1986 to 2005. Stroke. 2009;40(4):1038–43. doi: 10.1161/STROKEAHA.108.542787. [DOI] [PubMed] [Google Scholar]
- 6.Turtzo LC, McCullough LD. Sex differences in stroke. Cerebrovasc Dis. 2008;26(5):462–74. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, McCullough LD. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochem Int. 2012;61(8):1255–65. doi: 10.1016/j.neuint.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golomb MR, Fullerton HJ, Nowak-Gottl U, Deveber G. Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke. 2009;40(1):52–7. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- 10.Smith AL, Alexander M, Rosenkrantz TS, Sadek ML, Fitch RH. Sex differences in behavioral outcome following neonatal hypoxia ischemia: insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp Neurol. 2014;254:54–67. doi: 10.1016/j.expneurol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Donders J, Hoffman NM. Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology. 2002;16(4):491–9. doi: 10.1037//0894-4105.16.4.491. [DOI] [PubMed] [Google Scholar]
- 12.Morrison WE, Arbelaez JJ, Fackler JC, De Maio A, Paidas CN. Gender and age effects on outcome after pediatric traumatic brain injury. Pediatr Crit Care Med. 2004;5(2):145–51. doi: 10.1097/01.pcc.0000112373.71645.2a. [DOI] [PubMed] [Google Scholar]
- 13.Kent AL, Wright IM, Abdel-Latif ME. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics. 2012;129(1):124–31. doi: 10.1542/peds.2011-1578. [DOI] [PubMed] [Google Scholar]
- 14.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71(3):305–10. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 15.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29(1):159–65. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 16.Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab. 2015;35(2):221–9. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, Guida E, Broughton BR, Drummond GR, Sobey CG. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab. 2010;30(7):1306–17. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong X, Xu L, Wei L, White RE, Ouyang YB, Giffard RG. IL-4 is required for sex differences in vulnerability to focal ischemia in mice. Stroke. 2015;46(8):2271–6. doi: 10.1161/STROKEAHA.115.008897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29(4):792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turtzo LC, Li J, Persky R, Benashski S, Weston G, Bucala R, Venna VR, McCullough LD. Deletion of macrophage migration inhibitory factor worsens stroke outcome in female mice. Neurobiol Dis. 2013;54:421–31. doi: 10.1016/j.nbd.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renolleau S, Fau S, Charriaut-Marlangue C. Gender-related differences in apoptotic pathways after neonatal cerebral ischemia. Neuroscientist. 2008;14(1):46–52. doi: 10.1177/1073858407308889. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Eghbali-Webb M. Gender-related differences in basal and hypoxia-induced activation of signal transduction pathways controlling cell cycle progression and apoptosis, in cardiac fibroblasts. Endocrine. 2002;18(2):137–45. doi: 10.1385/ENDO:18:2:137. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci U S A. 2009;106(39):16692–7. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30(5):923–34. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sealock R, Zhang H, Lucitti JL, Moore SM, Faber JE. Congenic fine-mapping identifies a major causal locus for variation in the native collateral circulation and ischemic injury in brain and lower extremity. Circ Res. 2014;114:660–71. doi: 10.1161/CIRCRESAHA.114.302931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucitti JL, Sealock R, Buckley BK, Zhang H, Xiao L, Dudley AC, Faber JE. Variants of Rab GTPase-effector binding protein-2 cause variation in the collateral circulation and severity of stroke. Stroke. 2016 doi: 10.1161/STROKEAHA.116.014160. doi: 10.1161/STROKEAHA.116.014160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. 2011;31(8):1748–56. doi: 10.1161/ATVBAHA.111.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecht N, He J, Kremenetskaia I, Nieminen M, Vajkoczy P, Woitzik J. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke. 2012;43(11):3052–62. doi: 10.1161/STROKEAHA.112.653204. [DOI] [PubMed] [Google Scholar]
- 29.Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res. 2010;106(12):1870–81. doi: 10.1161/CIRCRESAHA.109.212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore SM, Zhang H, Maeda N, Doerschuk CM, Faber JE. Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury. Angiogenesis. 2015;18(3):265–81. doi: 10.1007/s10456-015-9465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheth SA, Liebeskind DS. Collaterals in endovascular therapy for stroke. Curr Opin Neurol. 2015;28(1):10–5. doi: 10.1097/WCO.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 32.Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013;74:241–8. doi: 10.1002/ana.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Zhang H, Dai X, Sealock R, Faber JE. Genetic architecture underlying variation in extent and remodeling of the collateral circulation. Circ Res. 2010;107(4):558–68. doi: 10.1161/CIRCRESAHA.110.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RIGOR Improving the quality of NINDS-supported preclinical and clinical research through rigorous study design and transparent reporting. 2012 http://www.ninds.nih.gov/funding/transparency_in_reporting_guidance.pdf.
- 35.Broughton BR, Brait VH, Kim HA, Lee S, Chu HX, Gardiner-Mann CV, Guida E, Evans MA, Miller AA, Arumugam TV, Drummond GR, Sobey CG. Sex-dependent effects of G protein-coupled estrogen receptor activity on outcome after ischemic stroke. Stroke. 2014;45(3):835–41. doi: 10.1161/STROKEAHA.113.001499. [DOI] [PubMed] [Google Scholar]
- 36.Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40(5):1842–8. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baskerville TA, Macrae IM, Holmes WM, McCabe C. The influence of gender on ‘tissue at risk’ in acute stroke: a diffusion-weighted magnetic resonance imaging study in a rat model of focal cerebral ischaemia. J Cereb Blood Flow Metab. 2016;36(2):381–6. doi: 10.1177/0271678X15606137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elzer JG, Muhammad S, Wintermantel TM, Regnier-Vigouroux A, Ludwig J, Schutz G, Schwaninger M. Neuronal estrogen receptor-alpha mediates neuroprotection by 17beta-estradiol. J Cereb Blood Flow Metab. 2010;30(5):935–42. doi: 10.1038/jcbfm.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faber JE, Rzechorzek W, Dai KZ, Summers BT, Blazek CN, Hedges SJ, Zhang H. Genetic, environmental and stochastic factors contribute to variation in the posterior communicating collaterals of the circle of Willis. Stroke. 2017 doi: 10.1007/s12975-018-0626-y. (in press, International Stroke Conference 2017) [DOI] [PubMed] [Google Scholar]
- 40.Dotson AL, Wang J, Chen Y, Manning D, Nguyen H, Saugstad JA, Offner H. Sex differences and the role of PPAR alpha in experimental stroke. Metab Brain Dis. 2016;31(3):539–47. doi: 10.1007/s11011-015-9766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147(6):3076–84. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- 42.Hammer M, Jovin T, Wahr JA, Heiss WD. Partial occlusion of the descending aorta increases cerebral blood flow in a nonstroke porcine model. Cerebrovasc Dis. 2009;28(4):406–10. doi: 10.1159/000235628. [DOI] [PubMed] [Google Scholar]
- 43.Winship IR, Armitage GA, Ramakrishnan G, Dong B, Todd KG, Shuaib A. Augmenting collateral blood flow during ischemic stroke via transient aortic occlusion. J Cereb Blood Flow Metab. 2014;34(1):61–71. doi: 10.1038/jcbfm.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schellinger PD, Shuaib A, Kohrmann M, Liebeskind DS, Jovin T, Hammer MD, Sen S, Huang DY, Solander S, Gupta R, Leker RR, Saver JL. Reduced mortality and severe disability rates in the SENTIS trial. AJNR Am J Neuroradiol. 2013;34(12):2312–6. doi: 10.3174/ajnr.A3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deschepper CF, Olson JL, Otis M, Gallo-Payet N. Characterization of blood pressure and morphological traits in cardiovascular-related organs in 13 different inbred mouse strains. J Appl Physiol (1985) 2004;97(1):369–76. doi: 10.1152/japplphysiol.00073.2004. [DOI] [PubMed] [Google Scholar]
- 46.Svenson K, Smith R. Mouse Phenome Database web site. The Jackson Laboratory; Bar Harbor, Maine USA: 2016. Systolic blood pressure and pulse rate survey in 14 inbred strains of mice. http://phenome.jax.org. [Google Scholar]
- 47.Ibrahim J, McGee A, Graham D, McGrath JC, Dominiczak AF. Sex-specific differences in cerebral arterial myogenic tone in hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2006;290:H1081–9. doi: 10.1152/ajpheart.00752.2005. [DOI] [PubMed] [Google Scholar]
- 48.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation. 2004;11:9–38. doi: 10.1080/10739680490266162. [DOI] [PubMed] [Google Scholar]
- 49.Chan MV, Bubb KJ, Noyce A, Villar IC, Duchene J, Hobbs AJ, Scotland RS, Ahluwalia A. Distinct endothelial pathways underlie sexual dimorphism in vascular auto-regulation. Br J Pharmacol. 2012;167:805–17. doi: 10.1111/j.1476-5381.2012.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin J, Kandhi S, Froogh G, Jiang H, Luo M, Sun D, Huang A. Sexually dimorphic phenotype of arteriolar responsiveness to shear stress in soluble epoxide hydrolase-knockout mice. Am J Physiol Heart Circ Physiol. 2015;309:H1860–6. doi: 10.1152/ajpheart.00568.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnabel RB, Wild PS, Prochaska JH, Ojeda FM, Zeller T, Rzayeva N, Ebrahim A, Lackner J, Beutel ME, Pfeiffer N, Sinning CR, Oertelt-Prigione S, Regitz-Zagrosek V, Binder H, Münzel T, Blankenberg S, Gutenberg Health Study Investigators Sex differences in correlates of intermediate phenotypes and prevalent cardiovascular disease in the general population. Front Cardiovasc Med. 2015;2:15. doi: 10.3389/fcvm.2015.00015. doi: 10.3389/fcvm.2015.00015. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paine NJ, Hinderliter AL, Blumenthal JA, Adams KF, Jr, Sueta CA, Chang PP, O’Connor CM, Sherwood A. Reactive hyperemia is associated with adverse clinical outcomes in heart failure. Am Heart J. 2016;178:108–14. doi: 10.1016/j.ahj.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol (1985) 2006;101(4):1252–61. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 54.Nacu A, Bringeland GH, Khanevski A, Thomassen L, Waje-Andreassen U, Naess H. Early neurological worsening in acute ischaemic stroke patients. Acta Neurol Scand. 2016;133(1):25–9. doi: 10.1111/ane.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsivgoulis G, Apostolidou N, Giannopoulos S, Sharma VK. Hemodynamic causes of deterioration in acute ischemic stroke. Perspectives in Medicine. 2012;1:177–84. [Google Scholar]
- 56.Lemini C, Jaimez R, Franco Y. Gender and inter-species influence on coagulation tests of rats and mice. Thromb Res. 2007;120(3):415–9. doi: 10.1016/j.thromres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Roy-O’Reilly M, McCullough LD. Sex differences in stroke: the contribution of coagulation. Exp Neurol. 2014;259:16–27. doi: 10.1016/j.expneurol.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong JH, Dukes J, Levy RE, Sos B, Mason SE, Fong TS, Weiss EJ. Sex differences in thrombosis in mice are mediated by sex-specific growth hormone secretion patterns. J Clin Invest. 2008;118(8):2969–78. doi: 10.1172/JCI34957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gregorova S, Divina P, Storchova R, Trachtulec Z, Fotopulosova V, Svenson KL, Donahue LR, Paigen B, Forejt J. Mouse consomic strains: exploiting genetic divergence between Mus M. musculus and Mus M. Domesticus subspecies. Genome Res. 2008;18(3):509–15. doi: 10.1101/gr.7160508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters LL, Cheever EM, Ellis HR, Magnani PA, Svenson KL, Von Smith R, Bogue MA. Large-scale, high-throughput screening for coagulation and hematologic phenotypes in mice. Physiol Genomics. 2002;11(3):185–93. doi: 10.1152/physiolgenomics.00077.2002. [DOI] [PubMed] [Google Scholar]
- 61.Herson PS, Palmateer J, Hurn PD. Biological sex and mechanisms of ischemic brain injury. Transl Stroke Res. 2013;4(4):413–9. doi: 10.1007/s12975-012-0238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62(4):329–39. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- 63.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25(4):502–12. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 64.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279(37):38563–70. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 65.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–89. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory disequilibrium in stroke. Circ Res. 2016;119(1):142–58. doi: 10.1161/CIRCRESAHA.116.308022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santizo RA, Anderson S, Ye S, Koenig HM, Pelligrino DA. Effects of estrogen on leukocyte adhesion after transient forebrain ischemia. Stroke. 2000;31(9):2231–5. doi: 10.1161/01.str.31.9.2231. [DOI] [PubMed] [Google Scholar]
- 68.Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ganter S, Northoff H, Mannel D, Gebicke-Harter PJ. Growth control of cultured microglia. J Neurosci Res. 1992;33(2):218–30. doi: 10.1002/jnr.490330205. [DOI] [PubMed] [Google Scholar]
- 70.Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann Neurol. 2005;58(2):317–21. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- 71.Qazi E, Al-Ajlan FS, Najm M, Menon BK. The role of vascular imaging in the initial assessment of patients with acute ischemic stroke. Curr Neurol Neurosci Rep. 2016;16:32. doi: 10.1007/s11910-016-0632-y. doi: 10.1007/s11910-016-0632-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






