Abstract
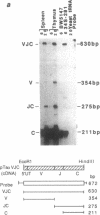
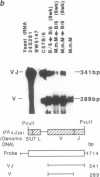
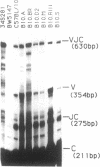
Nucleotide sequences of VJ (variable-joining) junctional regions of V14+ alpha-chain T-cell receptor genes show that most V alpha 14+ T cells use one alpha chain (V alpha 14J alpha 281 with a one-nucleotide N region, which is frequently used in keyhole limpet hemocyanin-specific suppressor T-cell hybridomas) in unprimed mice. Moreover, the frequency of this alpha-chain expression was greater than 1.5% of the total alpha chains found in laboratory strains, including B10 congenic mice. This is about 10(4) times higher than was expected. The V14J281 alpha-chain expression was relatively low but was significant in CD4+/CD8+ immature thymocytes and became quite high in mature single-positive T cells, implying that this alpha chain is selected during T-cell maturation. V14J281 expression increased with time after birth and reached a maximum at around 5 weeks of age. The ligand seems to be a self molecule and to be present in laboratory strains but to be absent in a wild mouse, Mus musculus molossinus, because bone marrow chimeras clearly showed that bone marrow cells derived from Mus musculus molossinus negative for this alpha chain raised V14J281-positive T cells in a C57BL/6 environment. The above results suggest that there are some selection mechanisms for this cell type other than those for conventional alpha beta T cells and also that the homogenous VJ junction of the V14J281 alpha chain plays a pivotal role in the selection of the T cell and its ligand reactivity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden B., Klotz J. L., Siu G., Hood L. E. Diversity and structure of genes of the alpha family of mouse T-cell antigen receptor. 1985 Aug 29-Sep 4Nature. 316(6031):783–787. doi: 10.1038/316783a0. [DOI] [PubMed] [Google Scholar]
- Asarnow D. M., Goodman T., LeFrancois L., Allison J. P. Distinct antigen receptor repertoires of two classes of murine epithelium-associated T cells. Nature. 1989 Sep 7;341(6237):60–62. doi: 10.1038/341060a0. [DOI] [PubMed] [Google Scholar]
- Asarnow D. M., Kuziel W. A., Bonyhadi M., Tigelaar R. E., Tucker P. W., Allison J. P. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988 Dec 2;55(5):837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Bandeira A., Larsson E. L., Forni L., Pereira P., Coutinho A. "In vivo" activated splenic T cells are refractory to interleukin 2 growth "in vitro". Eur J Immunol. 1987 Jul;17(7):901–908. doi: 10.1002/eji.1830170702. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Iwashima M., Kaplan K. B., Elliott J. F., Davis M. M. A new T-cell receptor gene located within the alpha locus and expressed early in T-cell differentiation. 1987 Jun 25-Jul 1Nature. 327(6124):677–682. doi: 10.1038/327677a0. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Rock E. P., Patten P. A., Davis M. M., Chien Y. H. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988 Feb 18;331(6157):627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- Garman R. D., Ko J. L., Vulpe C. D., Raulet D. H. T-cell receptor variable region gene usage in T-cell populations. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3987–3991. doi: 10.1073/pnas.83.11.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Engel I., McElligott D. L., Fink P. J., Hsu M. L., Hansburg D., Matis L. A. Selection of amino acid sequences in the beta chain of the T cell antigen receptor. Science. 1988 Mar 25;239(4847):1541–1544. doi: 10.1126/science.2832942. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Imai K., Kanno M., Kimoto H., Shigemoto K., Yamamoto S., Taniguchi M. Sequence and expression of transcripts of the T-cell antigen receptor alpha-chain gene in a functional, antigen-specific suppressor-T-cell hybridoma. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8708–8712. doi: 10.1073/pnas.83.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Wade T., White J., Kushnir E., Blackman M., Bill J., Roehm N., Marrack P. A T cell receptor V beta segment that imparts reactivity to a class II major histocompatibility complex product. Cell. 1987 Apr 24;49(2):263–271. doi: 10.1016/0092-8674(87)90567-8. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Koseki H., Imai K., Ichikawa T., Hayata I., Taniguchi M. Predominant use of a particular alpha-chain in suppressor T cell hybridomas specific for keyhole limpet hemocyanin. Int Immunol. 1989;1(6):557–564. doi: 10.1093/intimm/1.6.557. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F., Hausmann B., Chapman V. M. A new H-2-linked class I gene whose expression depends on a maternally inherited factor. Nature. 1983 Nov 24;306(5941):383–385. doi: 10.1038/306383a0. [DOI] [PubMed] [Google Scholar]
- Richards S., Bucan M., Brorson K., Kiefer M. C., Hunt S. W., 3rd, Lehrach H., Lindahl K. F. Genetic and molecular mapping of the Hmt region of mouse. EMBO J. 1989 Dec 1;8(12):3749–3757. doi: 10.1002/j.1460-2075.1989.tb08551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. E., Lacy M. J., McNeil L. K., Kranz D. M. Selection of variable-joining region combinations in the alpha chain of the T cell receptor. Science. 1988 Sep 9;241(4871):1354–1358. doi: 10.1126/science.2970673. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Sumida T., Sado T., Kojima M., Ono K., Kamisaku H., Taniguchi M. I-J as an idiotype of the recognition component of antigen-specific suppressor T-cell factor. Nature. 1985 Aug 22;316(6030):738–741. doi: 10.1038/316738a0. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Sumida T. "I-J" as an idiotypic marker on the antigen-specific suppressor T cell factor. Immunol Rev. 1985 Apr;83:125–150. doi: 10.1111/j.1600-065x.1985.tb00473.x. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]