Abstract
Objective
We previously found in our embryonic studies that proper regulation of the chemokine CCL12 through its sole receptor CCR2, is critical for joint and growth plate development. In the present study, we examined the role of CCR2 in injury-induced-osteoarthritis (OA).
Method
We used a murine model of injury-induced-OA (destabilization of medial meniscus, DMM), and systemically blocked CCR2 using a specific antagonist (RS504393) at different times during disease progression. We examined joint degeneration by assessing cartilage (cartilage loss, chondrocyte hypertrophy, MMP-13 expression) and bone lesions (bone sclerosis, osteophytes formation) with or without the CCR2 antagonist. We also performed pain behavioral studies by assessing the weight distribution between the normal and arthritic hind paws using the IITS incapacitance meter.
Results
Testing early vs. delayed administration of the CCR2 antagonist demonstrated differential effects on joint damage. We found that OA changes in articular cartilage and bone were ameliorated by pharmacological CCR2 blockade, if given early in OA development: specifically, pharmacological targeting of CCR2 during the first 4 weeks following injury, reduced OA cartilage and bone damage, with less effectiveness with later treatments. Importantly, our pain-related behavioral studies showed that blockade of CCR2 signaling during early, 1–4wks post-surgery or moderate, 4–8wks post-surgery, OA was sufficient to decrease pain measures, with sustained improvement at later stages, after treatment was stopped.
Conclusions
Our data highlight the potential efficacy of antagonizing CCR2 at early stages to slow the progression of post-injury OA and, in addition, improve pain symptoms.
Keywords: Chemokines, Osteoarthritis, Joint disease, Animal Model, Cartilage, Bone
Introduction
Nearly half of subjects sustaining significant damage to ligaments, menisci, or articular surfaces will develop osteoarthritis (OA)(1, 2). After injury, clinically measurable OA can require decades to become symptomatic, therefore early diagnosis and intervention is thought to be crucial to slow or prevent subsequent OA (3).
Mutations in genes that are critical during development are thought to be associated with adult joint pathologies, including OA(4, 5). We previously reported that the chemokine CCL12 (a.k.a. monocyte chemoattractant protein-5, MCP-5) through its C-C Chemokine Receptor-2 (CCR2), regulates joint formation and limb ossification during development (6). Therefore, the rationale of the present study was to test whether the CCR2 signaling is dysregulated as consequence of joint injury and contributes to the cartilage and bone damage seen in post-injury OA.
In addition to CCL12, the C-C chemokine family includes several protein members such as CCL2, CCL8, CCL7, CCL13 (a.k.a. MCP-1, MCP2, MCP-3, MCP-4), that bind the same CCR2 receptor(7). CCR2 is expressed in haematopoietic and non-haematopoietic cells, including chondrocytes and osteoblasts(8, 9). Studies conducted in humans and rodents have shown that the CCR2 pathway is functionally implicated in different arthritis models(10–13) and may mediate OA pain-related behaviors(14–16).
Although features of cartilage destruction can be remarkable, subchondral bone changes, with increased remodeling and sclerosis, might be the first detectable sign of the OA process. Articular cartilage and subchondral bone are mechanically and biologically interdependent, but how these interdependent modifications contribute to the progression of OA is unknown. Recent studies have suggested an active role for CCR2 in bone remodeling and homeostasis(17).
These findings strongly suggest a key role for CCR2 signaling in OA, although it is still unclear whether there is any direct correlation with deterioration of the cartilage and bone structures. Therefore, we decided to investigate the role of CCR2 signaling in cartilage and bone degeneration after joint injury by systemically blocking CCR2 at different times during disease progression in a murine model of injury-induced OA (destabilization of medial meniscus, DMM), that allows a slow OA progression and is therefore more consistent with the human clinical disorder. We also established the correlation between OA progression with longitudinal analyses of pain behaviors.
Method
Materials
Rabbit polyclonal anti-CCL2 (orb13563), CCL8 (orb13565), CCL7 (orb13567), CCL13 (orb13285) and CCL12 (orb13284) antibodies were from Biorbyt; rabbit polyclonal anti-CCR2 was from Novusbio (NB100-701); anti-CollagenX (ab58632), anti-Osteocalcin (ab10911) and mouse monoclonal anti-MMP-13 (ab3208) antibodies were from Abcam; The CCR-2 antagonist RS-504393 was from Tocris Bioscience.
Induction of Experimental OA
Methods and results description follows Animal Research Reporting in Vivo Experiments guidelines. Experiments were approved by the UNC Animal Care and Use Committee. DMM was induced in twelve-week-old male C57BL/6 mice (Jackson) by transecting the menisco-tibial ligament, as originally described by Dr. Glasson(18–20). In the sham control, the ligament is visualized and left untouched. More details are described in the Supplementary Methods. Mice subjected to DMM/Sham were assessed for pain behaviors and euthanized 2, 4, 8 and 12 weeks (wks) post-surgery. Dissected knees were fixed, μCT scanned, then prepared for histology.
RS-504393 treatment
To block the CCR2 signaling we used a potent specific small antagonist molecule, RS-504393, following doses and route of administration that have been previously reported by us and previous investigators(6, 21, 22). More details on the experimental conditions are described in Supplementary Methods. RS-504393 does not display any affinity for CXCR1 or CCR3 receptors and a very low CCR1 binding (700-fold less than CCR2)(23). Therefore it has been widely used to specific antagonize CCR2-mediated signaling(6, 22, 24–26). Mice received RS-504393 (4mg/Kg/day, orally) or control vehicle (0.6% DMSO in water [vol./vol.]), at different time-points post-surgery, and for different periods, depending on the experimental setting, as shown in Fig. 2B, 5A and 6A (N=10 males treated with RS-504393 or control vehicle for each DMM/Sham surgery and each treatment). To determine whether RS-504393 treatment affected CCR2 activation in joint tissues, we followed CCR2 downstream signaling, such as Nuclear Factor ƙB p-65 (NF-ƙB-p65). We detected high levels of phosphorylated NF-ƙB-p65 in articular and calcified cartilage of DMM knees 12wks post-surgery (Supplementary Fig. S1); such levels were reduced in DMM/Sham knees treated with, after only 4 weeks of RS-504393 treatment.. Protein levels of CCLs did not seem to change with RS-504393 treatment (data not shown).
Figure 2. Blockade of CCR2 on cartilage DMM-induced damage measured at the early, moderate and severe stage.
(A) Scheme of RS-504393 treatment at different DMM-induced OA stages. (B) Protein levels of Collagen 10 (Col10), Osteocalcin (OCN) and MMP-13 were measured by IHC in medial femurs and tibiae obtained from mice 4 weeks after DMM/sham, with or without treatment with the CCR2 antagonist, RS-504393, from 1-to-4wks post-surgery. Images are representative of 6 different mice for each of the experimental points described (C) mRNA expression of Col10 and OCN was evaluated by in-situ hybridization (ISH) on DMM/sham knees, with or without RS-504393 treatment from 1-to-4wks post-surgery. Images are representative of 6 different mice for each of the experimental points described. (D) OARSI score was obtained from sections (ranging between 8 and 12 per mouse) from DMM-operated knees at 4, 8 and 12 weeks after surgery with or without RS-504393 treatment at the time indicated (N=6 for each of the experimental points described). The graph represents the mean ± standard deviation. Significance was set at P < 0.05. (E) Representative sections of OARSI score are shown at the indicated time points (N=6 for each of the experimental points described). Scale bars are 100 µm.
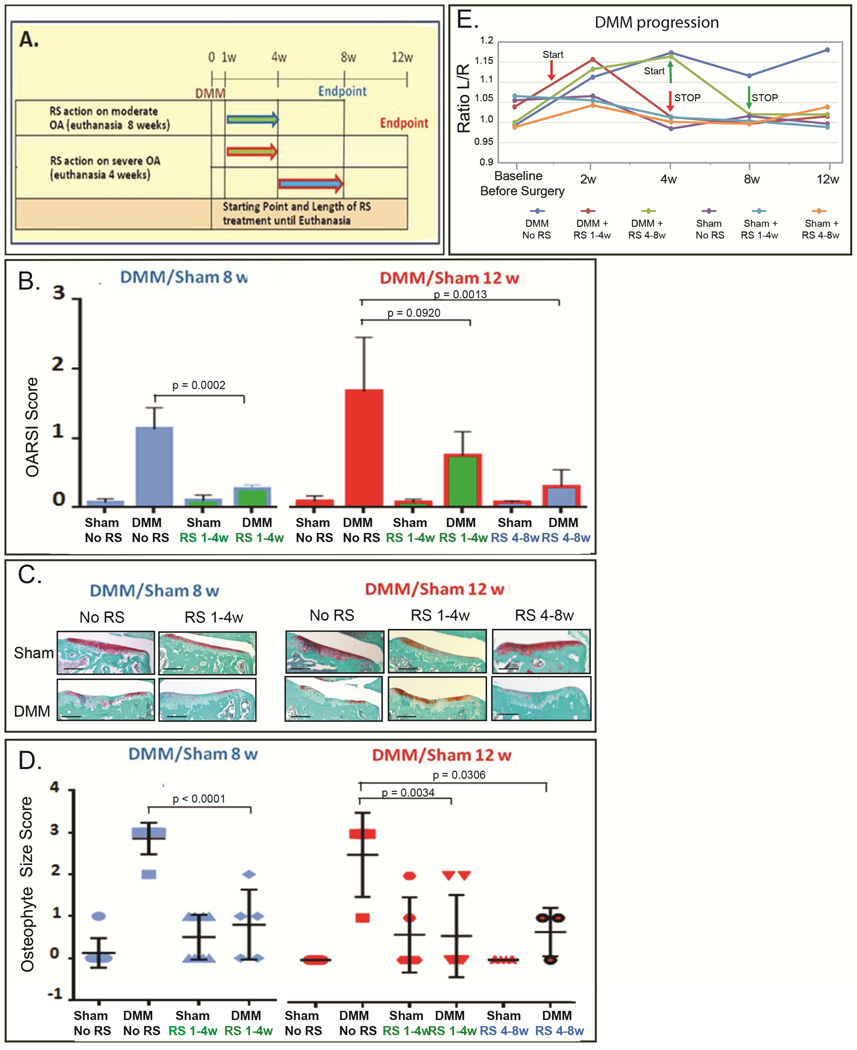
Figure 5. Effect of longer and sustained CCR2 blockade on cartilage and bone damage.
(A) Scheme of RS-504393 treatment at different DMM-induced OA stages. (B) OARSI scoring (N=6 for each of the experimental points described) with (C) representative sections at each time point (scale bars 100 µm), (D) bone sclerosis by μ-CT (for untreated samples, N=5 for both Sham and DMM at each of the time points analyzed; for RS-504393 treated samples, N= 5 for both Sham and DMM at each of the time points analyzed) and (E) osteophyte size scoring (N=6 for each of the experimental points described) were obtained from DMM/Sham-operated knees at 8 and 12 weeks after surgery, treated with or without RS-504393 at the time indicated in scheme A. All graphs represent the mean ± standard deviation. Significance was set at P < 0.05.
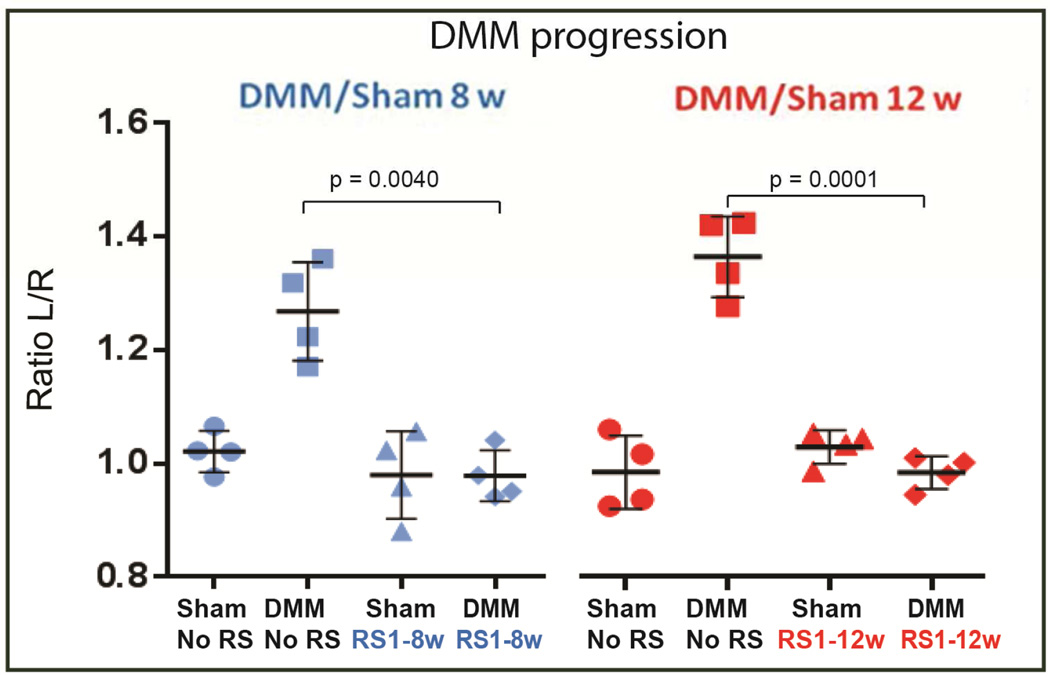
Figure 6. Effect of longer and sustained CCR2 blockade on pain response.
Pain measures (N=4 for each of the experimental points described) were obtained by assessing the weight shift from the operated (right) limb to the un-operated (left) limb, in mice 8 and 12 wks after DMM/sham surgery, with or without RS-504393 treatment at the time indicated in Fig. 5A (1-to-8wks or 1-to-12wks) (Incapacitance meter). The graph represents the mean ± standard deviation. Significance was set at P < 0.05.
Pain behavioral assessment
Weight distribution between the un-operated and operated paws was measured with the IITS incapacitance meter(27) (Life Science). In this system, the weight distribution on the paws was measured as the force exerted by each limb on a transducer plate on the floor over a given time. The measurement of weight bearing is static, as involved restraining the animal. Each test was timed over 10-seconds and repeated for a series of 12 tests. Results for each time point were expressed as the ratio of weight distribution between each limb (Left/Normal vs Right/Arthritic). As a result of OA-induced pain, the weight bearing on the operated knee is decreased compared to the un-operated, resulting in a different ratio between the two paws.
Micro-computed tomography (μ-CT) analysis
Dissected knees were fixed (4% paraformaldehyde) overnight at 4°C. Following a rinse in PBS, specimens were scanned using μCT (Scanco Medical μCT 40) at UNC Biomedical Research Imaging Center Small Animal Imaging Facility. Scans were performed in 70% ethanol at 55kVp, 145 µA, 300 ms integration time, and a 6-µm isotropic voxeledge. The medial tibial plateau was defined as the region of the tibia superior to the growth plate, posterior to the insertion of the anterior cruciate ligament, and medial to the midline of the intercondylar notch. Peripheral osteophytes were excluded. We evaluated subchondral bone sclerosis and remodeling in this region with a direct-model morphometric measure of bone volume (BV) over Total Volume (TV). BV segmentation was performed based on a calibration curve derived from manufacturer-supplied phantoms containing known hydroxyapatite (HA) composition: voxels with a linear attenuation coefficient ≥ 1.76 cm−1 (corresponding to 330 mg HA/cm3) were considered mineralized tissue. A Gaussian segmentation filter with kernel of 2 and standard deviation of 1.2 voxels was uniformly applied to the volume of interest.
Histologic assessment of arthritis and osteophyte scoring
Fixed knees were decalcified in 10% (weight/volume) EDTA/PBS for 14 days, embedded in paraffin, and frontal sections (6µm) through the entire joint were cut. Sections at 70µm intervals were subjected to Safranin-O/Fast Green staining and images were taken with an Olympus BX51 microscope and a DP71 camera. For OA grading, we used the OARSI histopathology scoring system for OA in mouse(28). Multiple sections (ranging between 8 and 12) from each joint were graded from 0 (normal) to 6 (≥ 75% cartilage loss) in a blinded manner, assessing all quadrants of the joint separately. Results were expressed as the average of scores in all quadrants in all sections. For osteophyte grading, we used the histological scoring system described by Little et al, developed to score both osteophyte size (from 1 to 3) and osteophyte maturity (from 1 to 3), with the latter reflecting the osteophyte tissue composition(29). Details are described in the Supplementary Methods. Because osteophytes in the DMM are predominately localized on the medial-tibial plateau, only sections from this region were used for osteophyte grading.
Immunohistochemistry (IHC) and in-situ hybridization (ISH) studies
Histological sections adjacent to the ones used for OARSI Scoring, were used for IHC and ISH studies. For IHC, Vectastain ABC kit (Vector Laboratories) was used according to the manufacturer’s instructions. ISH was performed as previously described (30). The plasmid carrying the cDNA insertion for mouse Osteocalcin (Ocn) was provided by G. Karsenty (Columbia University); the probe for Collagen-X-α-1 was provided by D.G. Mortlock (Vanderbilt University). Images were taken with an Olympus BX51 microscope and a DP71 camera. Detailed experimental conditions for each antibody/probe are described in Supplementary Methods.
Statistics
Data are expressed as mean ± SD. Statistical analyses were performed using a two-way ANOVA followed by Tukey’s post hoc test for multiple comparisons by Prism 6 (Graphpad Software). Statistical significance was set at p ≤ 0.05.
Results
CCL12 is up-regulated in the articular cartilage of mouse osteoarthritic knees in a murine model of OA
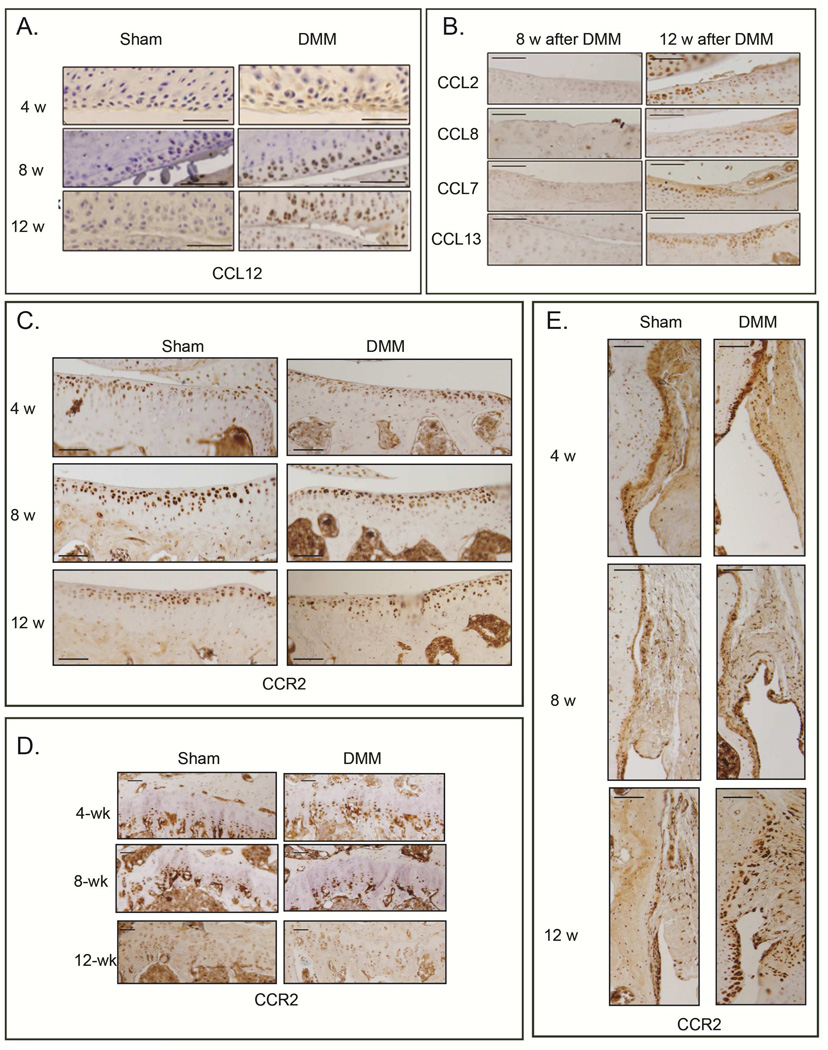
In light of our previous finding supporting a role for CCR2 signaling in joint development, we investigated a potential involvement of CCR2 signaling in injury-induced OA, using DMM as a model. We assessed the protein level of CCLs chemokines in the articular cartilage of knees following DMM/Sham surgery (Fig. 1A). CCL12 was detectable in cartilage from DMM knees but not sham controls, and levels increased with OA severity, from early (4wks post-surgery) to more severe stages (8wks and 12wks). Notably, we found that mouse CCL2, CCL8, CCL7 and CCL13 were not detected in the articular cartilage up to 8wks post-surgery (Fig. 1B). Interestingly, immunostaining for the CCR2 receptor was evident in the articular cartilage and calcified cartilage, as well as in hypertrophic chondrocytes of the growth plate and in the synovium: however, following surgery, CCR2 protein level did not seem to significantly change in such compartments at any OA stage (Fig 1C, 1D and 1E).
Figure 1. Protein levels of CCL12, CCR2 and other CCR2 ligands during DMM-induced OA.
Levels of (A) CCL12 and (B) other’s CCR2 ligands (CCL2, CCL8, CCL7, CCL13) were measured by immunohistochemistry (IHC) in articular cartilage sections obtained from medial knee joints of mice at the indicated time points after DMM or sham control surgery. Brown is immunopositive staining. DMM-operated knees at 4, 8 and 12 weeks post-surgery were immunostained with antibodies to CCR2 and protein levels where visualized in (C) medial tibial cartilage, (D) chondrocytes of the growth plate and (E) synovium. Images are representative of 6 different mice for each of the experimental points described. Scale bars are 100 µm.
Early systemic blockade of CCR2 during OA development decreases articular cartilage damage
In order to determine whether CCR2 plays a role in injury-induced OA development, we used a small CCR2 antagonist, RS-504393, that specifically blocks its binding site to CCLs ligands (but not the CCR1, CCR3 or any of CXCRs)(23). We treated mice at different time-points after DMM surgery (Fig. 2A) and analyzed the effect on early (4wks), moderate (8wks) and severe (12wks) OA. Blockade of CCR2 during early stages (from 1-to-4wks) led to decreased protein and mRNA levels of Collagen10 (Fig. 2B and 2C), and decreased MMP-13 protein levels (Fig. 2B); in addition, we found decreased OA-associated pathological changes in DMM articular cartilage measured at week-4 by OARSI score as well as by histomorphometric quantification of articular cartilage (Fig. 2D, 2E and Supplementary Fig. S2). Dosing the CCR2 antagonist from 4-to-8wks also reduced articular cartilage loss measured at week-8 (Fig. 2D, 2E and S2). However, when CCR2 antagonist was delayed until 8wks post-surgery, cartilage damage measured at week-12 was reduced but was not statistically significant from the untreated DMM joints (Fig. 2D–E and S2). These data suggest that CCR2 signaling enhances early progression of cartilage damage during injury-induced OA, with less effect at later time points.
Early systemic blockade of CCR2 prevents bone sclerosis and osteophyte formation in injury-induced OA
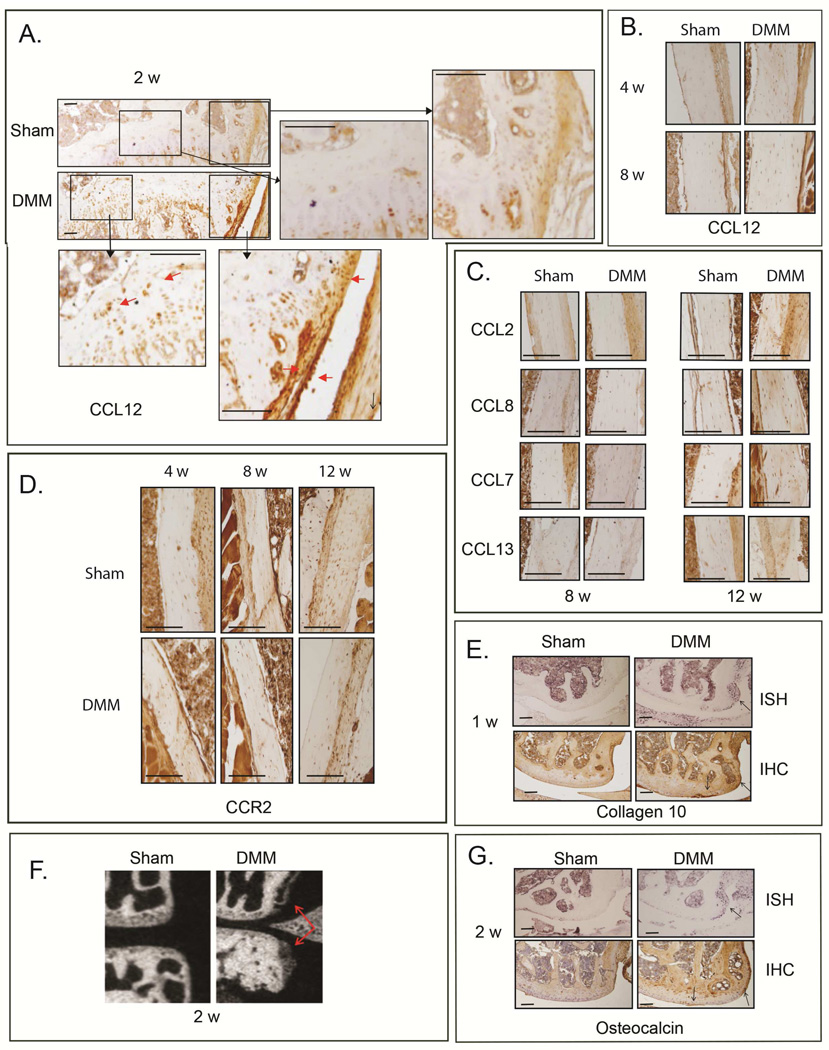
Bone damage may precede cartilage degeneration during injury-induced OA(31–33). We found that CCL12 is normally present in osteoblasts, periosteum and hypertrophic chondrocytes; however, as early as two weeks after DMM, higher levels of CCL12 protein were detected in these tissues, compared to sham (Fig. 3A); such increase in bone tissues was still present at 4 and 8wks post-surgery, compared to sham controls (Fig. 3B). In contrast, other’s MCPs ligands showed no significant changes in osteoblasts up to 8wks, but their protein levels seemed to increase only at the more severe OA stage (12wks post-surgery, Fig. 3C). Similarly to the articular cartilage, CCR2 protein levels in osteoblasts did not seem to change with OA progression (Fig 3D) One week post-surgery, hypertrophic chondrocytes can be detected in the area where an osteophyte is forming based on CollagenX protein and mRNA expressions, (Fig. 3E). By two weeks, the osteophytes have begun to mature, as revealed by μCT and osteocalcin expression (Fig. 3F and 3G). We also noticed increased subchondral bone sclerosis in the central portion of the medial tibia plateau of DMM knees (μCT, Fig. 3F) compared to sham controls by 2wks post-surgery, which becomes significant by 4wks (Fig. 4A).
Figure 3. Protein levels of CCL12, CCR2 and other CCR2 ligands in bone cell compartments during DMM-induced OA.
Protein levels of CCL12 were visualized by IHC in the bone tissues (A) 2 wks after DMM/sham, as well as (B) 4 and 8 wks post-surgery. Protein levels of (C) CCL2, CCL8, CCL7, CCL13, as well as (D) CCR2 were visualized in bone tissue by IHC at the indicated time points. (E) Protein levels and mRNA expression of Col10 were visualized by IHC and ISH, respectively, in medial femurs obtained from mice 1wk after DMM/sham surgery. Osteophytes were visualized by (F) μCT analyses and (G) OCN protein/mRNA levels (IHC and ISH) in medial femurs and tibiae obtained from mice 2wks after DMM/sham surgery. Images are representative of 6 different mice for each of the experimental points described. Scale bars are 100 µm.
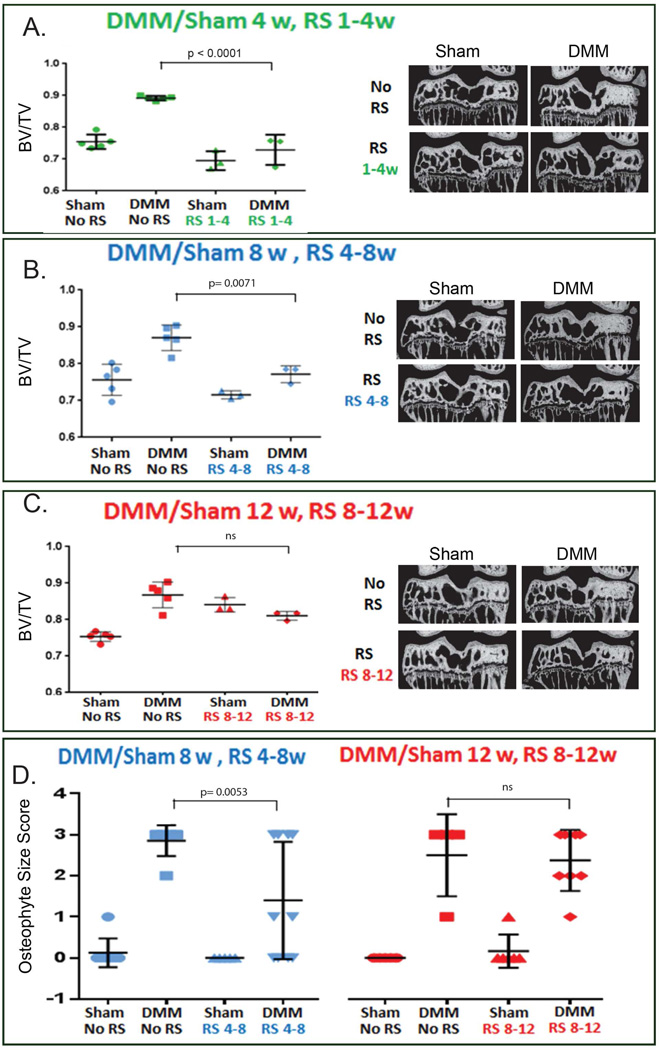
Figure 4. Effect of blockade of CCR2 on bone after DMM-induced OA measured at the early, moderate and severe stage.
Bone sclerosis induced by DMM (BV/TV) was measured by μ-CT at the medial tibia plateau (A) 4wks, (B) 8wks and (C) 12wks after DMM/sham, treated with (N=3, for both Sham and DMM at each of the time points analyzed) and without RS-504393 (N=5, for both Sham and DMM at each of the time points analyzed) at the times indicated in Fig. 2A (A, 1-to-4wks; B, 4-to-8wks; C, 8-to-12wks). Representative mid-coronal sections are presented with a segmentation mask equivalent to that applied during quantitative bone morphometry analysis. (D) Osteophyte size scoring was measured from sections (4/mouse) obtained from the anterior /medial margin of the tibia from DMM-operated knees at 8 weeks (N= 6 for both untreated Sham and DMM; for RS-504393 treated samples, N= 5 for Sham and N=6 for DMM) and 12 weeks (for untreated samples, N=5 for Sham and N=4 for DMM; for RS-504393 treated samples, N= 6 for both Sham and DMM) after surgery, with or without RS-504393 treatment at the time indicated in Fig. 2A (4-to-8wks or 8-to-12wks). All graphs represent the mean ± standard deviation. Significance was set at P < 0.05.
To analyze the role of the CCL12/CCR2 system in bone during OA, we analyzed the DMM-induced bone-related changes in early (4wks), moderate (8wks) and severe (12wks) OA following CCR2 blockade by RS-504393 antagonist. Blockade of CCR2 showed a protective effect on bone-related OA changes when treatment was delivered during early OA stages. Specifically, CCR2 blockade from 1-to-4wks led to a significant decrease in osteocalcin protein levels (Fig. 2B) and bone sclerosis (μCT, Fig. 4A). Although osteophytes can be visible at 2wks (Fig. 3F) and 4wks, differences between DMM and shams at this early stage did not reach statistical significance to perform osteophyte grading of RS-504393 treated samples. When evaluated in the setting of moderate OA (8wks), CCR2 antagonist treatment (4-to-8wks) in DMM knees was able to reduce bone sclerosis (Fig. 4B, μ-CT) and osteophyte size, although some large osteophytes were still detected in a few samples (osteophyte size score, Fig. 4D). In contrast, CCR2 blockade at the late time point, with CCR2 antagonist begun 8wks post-surgery, was ineffective in preventing OA bone sclerosis (Fig. 4C) or reducing osteophyte size (Fig. 4D) measured at the severe stage (12wks). No significant changes in osteophyte tissue composition (maturity score) were detected at either the moderate (8wks) or severe (12wks) time points, although a trend in reduction of the maturity score was noticed in RS-treated DMM knees (1-to-4wks and 4-to-8wks post-surgery, data not shown).
Longer and continuous CCR2 blockade does not prevent cartilage and bone damage in moderate and severe OA, but decreases pain responses
In order to determine whether an earlier and longer CCR2 inhibition would better protect joints from cartilage and bone degeneration measured at the moderate and severe stages, we administered RS-504393 starting one-week post-surgery and continued the treatment until euthanasia, at 8 or 12wks post-surgery (Fig. 5A). Surprisingly, such prolonged treatment led to different outcomes compared to short-term treatment, as neither cartilage damage (Fig 5B–5C and Supplementary Fig. S3) nor DMM-induced bone changes measured as bone sclerosis (Fig. 5D) and osteophyte size (Fig. 5E) were reduced. Similar effects were noticed when scoring for osteophyte maturity, with no significant differences between treated and untreated groups (data not shown). These results, obtained at both the moderate (8wks) and severe (12wks) stages, suggest that longer CCR2 blockade may be ineffective in protecting from joint degeneration.
Measurements of weight bearing have been used to grade OA pain in arthritis models(27, 34). We measured the shift of weight from the operated knee (right) to the contralateral (left). Although joint damage was not reduced, prolonged and continuous treatment with the CCR2 antagonist (Fig. 5A), starting 1wk post-surgery and lasting until moderate (8wks) or severe (12wks) stages, significantly reduced pain measures (Fig. 6).
Early and transient targeting of CCR2 is sufficient to prevent long term joint damage and reduce pain responses
The results obtained above suggested that timing of CCR2 inhibition is crucial to prevent, or slow, the progression of cartilage and bone responses leading to injury-induced OA. Therefore, we next investigated the potential efficacy of antagonizing CCR2 at early time points but for short and transient time-frames, and assessed OA at more advanced stages, after treatment had been stopped (Fig. 7A). Dosing the CCR2 antagonist from 1-to-4wks led to reduction of injury-induced cartilage damage (Fig. 7B, 7C and Supplementary Fig. S4) and osteophyte formation (Fig. 7D) measured at the moderate stage (8wks). Similarly, antagonizing CCR2 either from 1-to-4wks or 4-to-8wks post-surgery and examining the joint at 12wks, resulted in a reduced cartilage degeneration and osteophyte size score (Fig. 7B, 7C, S4 and 7D). A slight but not statistical significant reduction in osteophyte maturity was also noticed in both treatments (data not shown). These results suggest that CCR2 signaling enhances early progression of joint damage during OA and transient inhibition of CCR2 for 3–4 weeks is sufficient to reduce the severity of OA at later time points.
Figure 7. Effect of transient and short CCR2 blockade on cartilage and bone damage, as well as on pain response.
(A) Scheme of RS-504393 treatment at different DMM-induced OA stages. (B) OARSI scoring (N=6 for each of the experimental points described) was measured from sections from DMM/sham knees at 8 and 12 weeks after surgery with or without RS-504393 treatment at the time indicated and (C) representative sections are shown at each time point (scale bars 100 µm). (D) Osteophyte size scoring was measured from sections (4/mouse) obtained from the anterior/medial margin of the tibia from DMM/sham knees at 8 weeks (N=6 for both untreated and RS-504393 treated samples) and 12 weeks after surgery (for untreated samples, N=5 for Sham and N=4 for DMM; for samples RS-504393 treated from 1–4wks, N=5 for Sham and N=6 for DMM; for samples RS-504393 treated from 4–8wks, N=6 for Sham and N=3 for DMM), with or without RS-504393 treatment at the time indicated. (E) Longitudinal pain measures were obtained by assessing the weight shift from the operated (right) limb to the un-operated (left) limb, in mice at 2, 4, 8 and 12wks after DMM/sham surgery, with or without RS-504393 treatment at the time indicated using the Incapacitance meter (N=6 for each of the experimental points described). All graphs represent the mean ± standard deviation. Significance was set at P < 0.05.
To determine whether pain responses with such transient treatment were improved, we assessed differences in weight bearing during DMM-induced OA, as described above(27). As shown in Figure 7E, longitudinal evaluation of pain measures over 12wks post-surgery, showed that transient CCR2 blockade from 1-to-4wks or 4-to-8wks, significantly decreased pain responses, even after treatment was stopped. These results highlight the potential efficacy of antagonizing CCR2 at early time points to decrease the progression of cartilage and bone responses that lead to OA and, in addition, improve pain symptoms. We also tested other behavioral parameters following DMM, such as motor coordination (by accelerated RotaRod) and measures of locomotor activity (by photobeam breaks in Open Field locomotor chamber). No differences in motor coordination (RotaRod Performance, Supplementary Fig. S5) or locomotor activity (Open Field, data not shown) were found in DMM and sham controls at the time-points evaluated in this study. Therefore these latter methods may not be adequate to assess the overall mobility of the mouse during DMM-induced OA, at least until the time points addressed in this study, perhaps due to a compensation with the forelimbs.
Discussion
After joint injury, clinically measurable OA can require decades to become symptomatic, therefore early diagnosis and intervention is crucial to slow OA damage before advanced symptomatic disease occurs(3). Here we show, using a model of injury-induced OA, that early CCR2 inhibition can significantly reduce OA damage in a time-dependent manner.
We found that the CCR2 ligand CCL12, increases in articular cartilage and bone tissues during the early phases of OA development, between 2 and 4wks post-surgery. At later stages, also other CCR2 ligands (CCL2, CCL8, CCL7 and CCL13) are detectable in articular cartilage. These findings are consistent with human studies that noted increased human CCL2 and CCR2 in patients with OA(35, 36).
Consistent with studies suggesting a role as a mediator of OA pain (14,15, 36), we were also able to demonstrate that CCR2 signaling inhibition reduced pain by examining joint loading as a measure of pain response.
Our data show that early inactivation of CCR2 (from 1-to-4wks) decreased articular cartilage degradation that was associated with reduced Collagen × and MMP-13 protein levels, markers of chondrocyte hypertrophy and cartilage degradation, respectively. In addition, the bone changes associated with OA progression were reduced, including a reduction of OCN expression, bone sclerosis and osteophyte size. Similar results were obtained when analyzed in the setting of moderate OA (8wks): blocking CCR2 from 4-to- 8wks post-surgery reduced cartilage damage; bone sclerosis and osteophyte size were also significantly reduced, although to a lesser extent, and some large osteophytes were still evident in a few samples, suggesting a possible temporal-dependency of CCR2 action on bone damage.
In a recent study, using the DMM model, Miller et al. demonstrated up-regulation of CCR2 in the dorsal root ganglia with consequent reduction of pain response in Ccr2-null mice(15). They also measured OA changes at 8wks post-surgery in the Ccr2 null mice and found OA reduced scores, although not statistically significant from wild-type controls. While at a first sight this finding may seem to contradict our results, in fact it mirrors our subsequent data obtained with sustained CCR2 blockade. Unlike results with transient blockade given for 4 weeks, we did not see a significant reduction in cartilage damage, bone sclerosis or osteophytes when mice were treated from 1–8wks or 1–12wks post-surgery. Like Miller et al (16), we did see a reduction in pain response.
One explanation for such a different outcome for short vs long-term inhibition may be found in the known chemokine cooperativity, which is the ability of distantly related chemokines to enhance each other’s function(37–41). Recently, Verkar et al. demonstrated that chemokine cooperativity is not strictly dependent on the receptor of the cooperative chemokine, but can originate from chemokine heterodimerization or from their binding to membrane-tethered glycosaminoglycans (GAGs), which establishes a chemokine concentration gradient(42). In this respect, the CCR2 ligands CCL2, binds to several GAGs with relatively high affinity(37–41). Chemokine cooperativity would allow other chemokines to activate their cognate receptors at lower chemokine concentrations; therefore, longer CCR2 blockage may result in an increased local concentration of CCR2-ligands in multiple cell compartments that over time may significantly extend the range for leukocyte recruitment by chemokine cooperativity. Interestingly, studies conducted in models of inflammatory arthritis using Ccr2-null mice showed that CCR2 may instead have a protective role in the pathogenesis of autoimmune arthritis, where CCR2 absence was associated with altered bone remodeling by osteoclasts(43, 44). Therefore, an early increased concentration of inflammatory cells and altered bone remodeling in the global Ccr2-null mouse, may have masked the protective action on OA.
In contrast with other more severe post-traumatic models, the slow OA cartilage and bone degeneration induced by DMM can proceed with little to no inflammatory cell infiltration into the synovium (20, 45). A carefully done time-course study of the DMM model reported mild but significant synovial hyperplasia around 4wks post-surgery in DMM compared to sham control knees, although synovial scores were much lower than other more inflammatory models(46). We did not detect any significant synovial changes or increases in TRAP positive cells in the joint tissue sections up to 12wks post-surgery (data not shown).Therefore, the CCR2 contribution to cartilage and bone changes as OA severity increases might reflect a more complex process in those tissues that is independent of effects on infiltration of inflammatory cells. Notably, although CCR2 serves as a receptor for multiple CCLs(7, 47, 48), neither CCR2 receptor nor CCLs, other than CCL12, were significantly altered in cartilage and bone with DMM until later points (≥8wk). Therefore, we speculate that rising cartilage and bone levels of CCL12 may play a role in mediating the early DMM-induced OA damage, although we cannot completely exclude that circulating levels of other CCLs from other tissues (such as the synovium) may also be involved in early OA damage.
Another explanation for the discrepancy between short-term CCR2 blockade vs global gene ablation, may be found in CCR2 involvement in growth plate development during embryogenesis. We previously reported that transient ex-vivo and in-vivo inactivation during embryonic stages, altered joint formation and ossification of the growth plate(6). Although pre-natal chemokine redundancy may have balanced the CCR2 deficiency leading to no apparent macroscopic changes in joint phenotype in the global Ccr2-null mouse, an alteration in chemokine distribution as well as a possible small change in bone turnover may render a global Ccr2-null mutant less able to be protected from OA damage in adult life. A protective role for CCR2 antagonism in OA by in-vivo pharmacologic inhibition, has been recently reported in a rat post-traumatic OA model, although the effect was more pronounced at later stages(24). Such discrepancy in the timing of CCR2 action, may be due to the more severe rat model, involving the knee anterior cruciate ligament (ACL) transection and partial meniscectomy(24). Differently from DMM, ACL transection may be associated with patellar maltracking or dislocation of the patella, with much faster progression of joint degeneration and additional intra-articular inflammation(49). Therefore, a sustained myeloid infiltration by macrophages and osteoclasts following severe injury may play a significant role on CCR-2-mediated bone changes and may reflect different temporal stages compared to DMM.
The efficacy of early and transient targeting of the CCR2 axis to delay OA progression is further suggested by our experiments where we windowed CCR2 blockage from 1-to-4wks or 4-to-8wks post-surgery, and measured joint damage at 12wks. Such selective timing was sufficient to halt cartilage and bone degeneration measured at the severe stage (12wks), even though treatment had been stopped. Our finding highlights the potential efficacy of antagonizing CCR2 soon after trauma and for a relatively short time to possibly prevent, or slow, OA cartilage and bone responses.
The efficacy of CCR2 inhibition is also supported by data correlating CCR2 targeting to decrease of pain. In accordance with results obtained in the Ccr2-null mouse(15), we found that CCR2 antagonism significantly decreased pain responses when the inhibitor was administered continuously from 1-to-8wks and 1-to-12wks post-trauma, although joint degeneration was not rescued. In their report, Miller et al.(16) correlated CCR2 inactivation in the sensory neurons of the dorsal root ganglia with decreased pain response in the DMM model. Therefore, the decreased pain due to CCR2 antagonism could reflect the systemic action of the CCR2 inhibitor on target cells that do not contribute to joint structure. However, we demonstrated that transient CCR2 targeting at both the early (1-to-4wks) and moderate (4-to-8wks) stages, was sufficient to drastically decrease pain perception measured at later stages (12wks), after treatment had been stopped for 4/8 weeks, suggesting amelioration of joint dysfunction.
By addressing the early molecular responses that lead to OA after joint injury, these results can help in leading to strategies for early treatments to prevent the symptoms and long-term sequelae of OA.
Supplementary Material
Acknowledgments
We thank the Histology Research Core Facility (Kirk McNaughton and J. Ashley Ezzell) and the Biomedical Research Imaging Center for their technical assistance.
Role of the funding source
This work has been sponsored by the National Institute of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (1R03AR063232 to Dr. Longobardi and 7R01AR057042-06 to Dr. Spagnoli).
This work was also sponsored by the Arthritis National Research Foundation (13-2389 to Dr. Longobardi).
Dr. D’Onofrio’s work was supported by the Second University of Naples.
Sponsors had no involvement in the study design, collection, analysis, interpretation of data or in the manuscript writing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Study conception and design: L.L., R.F.L., A.S. Acquisition of data: L.L., J.D.T., L.T., H.W., A.E., N.D., E.S., H.O., V.U. Data analysis and interpretation: L.L., J.D.T., L.T., T.L., T.J.M., M.L.B., V.U., R.F.L., A.S. Obtaining of funding: L.L. and A.S. have obtained funding to support the work presented in the manuscript.
- Drafting and revision of manuscript: all authors have contributed to the draft and revision of the manuscript and their comments have been added to the final version when appropriate.
- Final Approval of the Manuscript: all authors have reviewed the final version of the manuscript and approved the version to be published.
Lara Longobardi takes full responsibility for data integrity and analysis, from inception to finished article (lara_longobardi@med.unc.edu).
Competing interests
Authors do not have conflicts of interest to disclose
References
- 1.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 2.Dirschl DR, Marsh JL, Buckwalter JA, Gelberman R, Olson SA, Brown TD, et al. Articular fractures. J Am Acad Orthop Surg. 2004;12(6):416–423. doi: 10.5435/00124635-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(6):802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longobardi L, Li T, Tagliafierro L, Temple JD, Willcockson HH, Ye P, et al. Synovial joints: from development to homeostasis. Current osteoporosis reports. 2015;13(1):41–51. doi: 10.1007/s11914-014-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139(2):541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longobardi LLT, Myers TJ, O’Rear L, Ozkan H, Li Y, Contaldo C. And Spagnoli A.. TGF-β Type II Receptor/MCP-5 Axis: at the Crossroad between Joint and Growth Plate Development. Developmental Cell. 2012 doi: 10.1016/j.devcel.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med. 1997;185(1):99–109. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charo IF. CCR2: from cloning to the creation of knockout mice. Chem Immunol. 1999;72:30–41. doi: 10.1159/000058724. [DOI] [PubMed] [Google Scholar]
- 9.Muller K, Ehlers S, Solbach W, Laskay T. Novel multi-probe RNase protection assay (RPA) sets for the detection of murine chemokine gene expression. Journal of immunological methods. 2001;249(1–2):155–165. doi: 10.1016/s0022-1759(00)00354-9. [DOI] [PubMed] [Google Scholar]
- 10.De Benedetti F, Pignatti P, Bernasconi S, Gerloni V, Matsushima K, Caporali R, et al. Interleukin 8 and monocyte chemoattractant protein-1 in patients with juvenile rheumatoid arthritis. Relation to onset types, disease activity, and synovial fluid leukocytes. The Journal of rheumatology. 1999;26(2):425–431. [PubMed] [Google Scholar]
- 11.Hayashida K, Nanki T, Girschick H, Yavuz S, Ochi T, Lipsky PE. Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res. 2001;3(2):118–126. doi: 10.1186/ar149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruth JH, Rottman JB, Katschke KJ, Jr, Qin S, Wu L, LaRosa G, et al. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis and rheumatism. 2001;44(12):2750–2760. doi: 10.1002/1529-0131(200112)44:12<2750::aid-art462>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Szekanecz Z, Halloran MM, Volin MV, Woods JM, Strieter RM, Kenneth Haines G, 3rd, et al. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant-induced arthritis. Arthritis and rheumatism. 2000;43(6):1266–1277. doi: 10.1002/1529-0131(200006)43:6<1266::AID-ANR9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 14.Dawes JM, Kiesewetter H, Perkins JR, Bennett DL, McMahon SB. Chemokine expression in peripheral tissues from the monosodium iodoacetate model of chronic joint pain. Mol Pain. 2013;9:57. doi: 10.1186/1744-8069-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(50):20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82(1):51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- 17.Wu AC, Morrison NA, Kelly WL, Forwood MR. MCP-1 expression is specifically regulated during activation of skeletal repair and remodeling. Calcif Tissue Int. 2013;92(6):566–575. doi: 10.1007/s00223-013-9718-6. [DOI] [PubMed] [Google Scholar]
- 18.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 19.Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis and rheumatism. 2004;50(8):2547–2558. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- 20.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15(9):1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, et al. Gene therapy expressing amino-terminal truncated monocyte chemoattractant protein-1 prevents renal ischemia-reperfusion injury. Journal of the American Society of Nephrology : JASN. 2003;14(4):1066–1071. [Google Scholar]
- 22.Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. The American journal of pathology. 2004;165(1):237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirzadegan T, Diehl F, Ebi B, Bhakta S, Polsky I, McCarley D, et al. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle. J Biol Chem. 2000;275(33):25562–25571. doi: 10.1074/jbc.M000692200. [DOI] [PubMed] [Google Scholar]
- 24.Appleton CT, Usmani SE, Pest MA, Pitelka V, Mort JS, Beier F. Reduction in disease progression by inhibition of transforming growth factor alpha-CCL2 signaling in experimental posttraumatic osteoarthritis. Arthritis & rheumatology. 2015;67(10):2691–2701. doi: 10.1002/art.39255. [DOI] [PubMed] [Google Scholar]
- 25.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. Journal of the American Society of Nephrology : JASN. 2003;14(10):2503–2515. doi: 10.1097/01.asn.0000089563.63641.a8. [DOI] [PubMed] [Google Scholar]
- 26.Yang SJ, IglayReger HB, Kadouh HC, Bodary PF. Inhibition of the chemokine (C-C motif) ligand 2/chemokine (C-C motif) receptor 2 pathway attenuates hyperglycaemia and inflammation in a mouse model of hepatic steatosis and lipoatrophy. Diabetologia. 2009;52(5):972–981. doi: 10.1007/s00125-009-1309-8. [DOI] [PubMed] [Google Scholar]
- 27.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2003;11(11):821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 28.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis and rheumatism. 2009;60(12):3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spagnoli A, O'Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, et al. TGF-beta signaling is essential for joint morphogenesis. J Cell Biol. 2007;177(6):1105–1117. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burr DB. Increased biological activity of subchondral mineralized tissues underlies the progressive deterioration of articular cartilage in osteoarthritis. The Journal of rheumatology. 2005;32(6):1156–1158. discussion 8–9. [PubMed] [Google Scholar]
- 32.Kouri JB, Aguilera JM, Reyes J, Lozoya KA, Gonzalez S. Apoptotic chondrocytes from osteoarthrotic human articular cartilage and abnormal calcification of subchondral bone. J Rheumatol. 2000;27(4):1005–1019. [PubMed] [Google Scholar]
- 33.Zamli Z, Robson Brown K, Tarlton JF, Adams MA, Torlot GE, Cartwright C, et al. Subchondral bone plate thickening precedes chondrocyte apoptosis and cartilage degradation in spontaneous animal models of osteoarthritis. Biomed Res Int. 2014:606870. doi: 10.1155/2014/606870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen KD, Mata BA, Gabr MA, Huebner JL, Adams SB, Jr, Kraus VB, et al. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res Ther. 14(2):R78. doi: 10.1186/ar3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levinger I, Levinger P, Trenerry MK, Feller JA, Bartlett JR, Bergman N, et al. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis and rheumatism. 2011;63(5):1343–1348. doi: 10.1002/art.30287. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Jiang BE. Serum and synovial fluid chemokine ligand 2/monocyte chemoattractant protein 1 concentrations correlates with symptomatic severity in patients with knee osteoarthritis. Ann Clin Biochem. 2014 doi: 10.1177/0004563214545117. [DOI] [PubMed] [Google Scholar]
- 37.Bai Z, Hayasaka H, Kobayashi M, Li W, Guo Z, Jang MH, et al. CXC chemokine ligand 12 promotes CCR7-dependent naive T cell trafficking to lymph nodes and Peyer's patches. Journal of immunology. 2009;182(3):1287–1295. doi: 10.4049/jimmunol.182.3.1287. [DOI] [PubMed] [Google Scholar]
- 38.Gouwy M, Struyf S, Catusse J, Proost P, Van Damme J. Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. Journal of leukocyte biology. 2004;76(1):185–194. doi: 10.1189/jlb.1003479. [DOI] [PubMed] [Google Scholar]
- 39.Gouwy M, Struyf S, Noppen S, Schutyser E, Springael JY, Parmentier M, et al. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Molecular pharmacology. 2008;74(2):485–495. doi: 10.1124/mol.108.045146. [DOI] [PubMed] [Google Scholar]
- 40.Kuscher K, Danelon G, Paoletti S, Stefano L, Schiraldi M, Petkovic V, et al. Synergy-inducing chemokines enhance CCR2 ligand activities on monocytes. European journal of immunology. 2009;39(4):1118–1128. doi: 10.1002/eji.200838906. [DOI] [PubMed] [Google Scholar]
- 41.Paoletti S, Petkovic V, Sebastiani S, Danelon MG, Uguccioni M, Gerber BO. A rich chemokine environment strongly enhances leukocyte migration and activities. Blood. 2005;105(9):3405–3412. doi: 10.1182/blood-2004-04-1648. [DOI] [PubMed] [Google Scholar]
- 42.Verkaar F, van Offenbeek J, van der Lee MM, van Lith LH, Watts AO, Rops AL, et al. Chemokine cooperativity is caused by competitive glycosaminoglycan binding. Journal of immunology. 2014;192(8):3908–3914. doi: 10.4049/jimmunol.1302159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poo YS, Nakaya H, Gardner J, Larcher T, Schroder WA, Le TT, et al. CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J Virol. 2014;88(12):6862–6872. doi: 10.1128/JVI.03364-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rampersad RR, Tarrant TK, Vallanat CT, Quintero-Matthews T, Weeks MF, Esserman DA, et al. Enhanced Th17-cell responses render CCR2-deficient mice more susceptible for autoimmune arthritis. PloS one. 2011;6(10):e25833. doi: 10.1371/journal.pone.0025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takebe K, Rai MF, Schmidt EJ, Sandell LJ. The chemokine receptor CCR5 plays a role in post-traumatic cartilage loss in mice, but does not affect synovium and bone. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(3):454–461. doi: 10.1016/j.joca.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson MT, Moradi B, Zaki S, Smith MM, McCracken S, Smith SM, et al. Depletion of protease-activated receptor 2 but not protease-activated receptor 1 may confer protection against osteoarthritis in mice through extracartilaginous mechanisms. Arthritis & rheumatology. 2014;66(12):3337–3348. doi: 10.1002/art.38876. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Zepeda EA, Combadiere C, Rothenberg ME, Sarafi MN, Lavigne F, Hamid Q, et al. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and-3. Journal of immunology. 1996;157(12):5613–5626. [PubMed] [Google Scholar]
- 48.Kurihara T, Bravo R. Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. J Biol Chem. 1996;271(20):11603–11607. doi: 10.1074/jbc.271.20.11603. [DOI] [PubMed] [Google Scholar]
- 49.Teeple E, Jay GD, Elsaid KA, Fleming BC. Animal models of osteoarthritis: challenges of model selection and analysis. The AAPS journal. 2013;15(2):438–446. doi: 10.1208/s12248-013-9454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.