Abstract
Background/Aims
The use of genetic probes for the diagnosis of pulmonary tuberculosis (TB) has been well described. However, the role of these assays in the diagnosis of intestinal tuberculosis is unclear. We therefore assessed the diagnostic utility of the Xpert Mycobacterium tuberculosis/rifampicin (MTB/RIF) assay, and estimated the prevalence of multidrug-resistant (MDR) TB in the Indian population.
Methods
Of 99 patients recruited, 37 had intestinal TB; two control groups comprised 43 with Crohn's disease (CD) and 19 with irritable bowel syndrome. Colonoscopy was performed before starting any therapy; mucosal biopsies were subjected to histopathology, acid-fast bacilli staining, Lowenstein-Jensen culture, and nucleic acid amplification testing using the Xpert MTB/RIF assay. Patients were followed up for 6 months to confirm the diagnosis and response to therapy. A composite reference standard was used for diagnosis of TB and assessment of the diagnostic utility of the Xpert MTB/RIF assay.
Results
Of 37 intestinal TB patients, the Xpert MTB/RIF assay was positive in three of 37 (8.1%), but none had MDR-TB. The sensitivity, specificity, positive predictive value, and negative predictive value of the Xpert MTB/RIF assay was 8.1%, 100%, 100%, and, 64.2%, respectively.
Conclusions
The Xpert MTB/RIF assay has low sensitivity but high specificity for intestinal TB, and may be helpful in endemic tuberculosis areas, when clinicians are faced with difficulty differentiating TB and CD. Based on the Xpert MTB/RIF assay, the prevalence of intestinal MDR-TB is low in the Indian population.
Keywords: Tuberculosis, multidrug-resistant; Crohn disease; Xpert MTB/RIF assay
INTRODUCTION
Extrapulmonary tuberculosis (EPTB) with abdominal involvement can occur in isolation or along with a pulmonary focus, similar to that observed in patients with disseminated tuberculosis (TB). The recent human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome pandemic has resulted in a changing epidemiology and has once again increased the magnitude of the burden of EPTB. EPTB constitutes approximately 15% to 20% of all cases of TB in immunocompetent patients and accounts for more than 50% of the cases in HIV-positive individuals.1 The lymph nodes are the most common sites of involvement, followed by the lungs (i.e., pleural effusion), and virtually any site of the body can be affected.2 The intestines and peritoneum are the sixth most frequent site of EPTB, and intestinal and peritoneal TB comprise up to 5% of all cases of TB.2
Within the gastrointestinal (GI) tract, the ileocecal area is the most common site of involvement and is affected in 75% of cases.3 Other locations in the GI tract in the order of decreasing frequency are the ascending colon, the jejunum, appendix, duodenum, stomach, esophagus, sigmoid colon, and rectum. Segmental ulceration with colitis is the most typical presentation.4 However, simultaneous involvement of multiple areas of the bowel can also occur. Despite a high index of suspicion, intestinal TB can be difficult to diagnose. Symptoms are vague, signs are non-specific, and the clinical features closely mimic many other diseases such as CD. Conventional diagnostic methods for abdominal TB have a poor yield, and diagnosis is often delayed.5
The major etiology of ulceroconstrictive diseases of the small and large intestines in India until now has been intestinal tuberculosis (ITB) caused by Mycobacterium tuberculosis (MTB). However, there has been a change in the disease pattern over the last two decades. There is an apparent increase in the incidence of CD in India, which was once considered to be an uncommon disease.6 Both CD and ITB are seen commonly in India.7,8,9 The phenotypic presentation of both diseases is quite similar, and there is no gold standard to distinguish between the two diseases.10,11,12,13 In such situations, physicians often resort to a trial of antitubercular therapy (ATT), and the response is used to differentiate between intestinal TB and CD.14 Diagnostic tests that can address this confusion are therefore needed. Xpert MTB/rifampicin (RIF) has proven to be an important tool in the diagnosis of pulmonary TB and in the detection of multidrug-resistant tuberculosis (MDR-TB). Data on the utility of Xpert MTB/RIF in the diagnosis of ITB are scant. A test that can effectively diagnose ITB and exclude CD would benefit clinicians treating ulceroconstrictive diseases of the intestine in regions where TB is endemic.
Another crucial question after an ATT trial is whether the nonresponse is because the patient has CD or because of MDR. If a patient is categorized as having CD and steroids are started, it can have disastrous consequences if the patient has instead developed MDR-TB. The emergence of MTB resistant to multiple drugs poses a serious threat to the existing burden of TB. MDR-TB is TB due to organisms that show high-level resistance to both isoniazid and RIF, with or without resistance to other anti-TB drugs. In pulmonary TB, the prevalence of MDR-TB is reported to vary from 1.1% to 20.4% in naïve and previously treated cases.15,16,17 There are a paucity of data on drug resistance in ITB, with only three retrospective studies from Taiwan, South Korea, and India, respectively, published recently.18,19,20 We therefore undertook this study to evaluate the performance of the Xpert MTB/RIF assay in diagnosing intestinal TB. Based on this assay we also assessed the prevalence of MDR-TB in patients with ITB.
METHODS
1. Patient Population
Consecutive patients referred with a confirmed diagnosis of ITB or CD or with a diagnostic confusion between intestinal TB and CD who visited the IBD clinic at All India Institute of Medical Sciences, New Delhi, India, between June 2013 and December 2014 were included in the study. Patients were recruited from June 2013 until June 2014 and followed up until December 2014. All patients with ITB as well as IBD visiting the gastroenterology department in All India Institute of Medical Sciences are managed at the IBD clinic. Permission from the ethics committee of All India Institute of Medical Sciences, New Delhi, was obtained, and written consent was obtained from all study participants.
2. Study Design
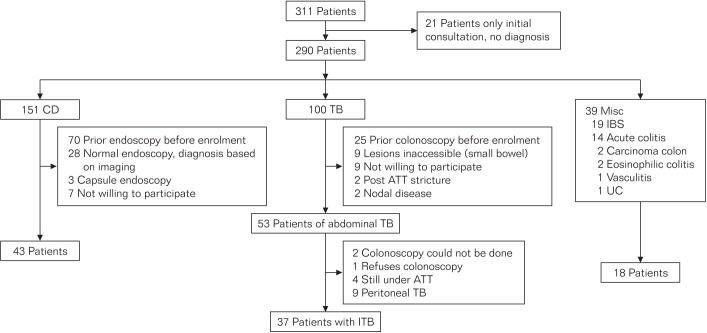
The study was conducted in a prospective fashion. Consecutive patients referred with a diagnosis of ITB or CD were recruited. The patients were investigated to confirm a diagnosis of ITB or CD. Some patients referred with ITB or CD were found on further investigation not to have either of these diagnoses (Fig. 1). The included patients were divided into three study groups: disease group, ITB; disease control group A, CD; disease control group B, IBS. Patients with peritoneal TB were excluded from the analysis.
Fig. 1. Flow chart showing patient recruitment. TB, tuberculosis; ATT, antitubercular therapy; Misc, miscellaneous; ITB, intestinal tuberculosis.
3. Definitions
The patients were diagnosed with CD on the basis of the European Crohn's and Colitis Organization guidelines, by a combination of clinical, endoscopic, and histological features.21 The diagnosis of ITB was made in an appropriate clinical setting (characteristic clinical features such as abdominal pain, constipation and/or diarrhea, constitutional symptoms, and intestinal obstruction, endoscopic features such as ileocecal area involvement, ulcerations, nodularity, and strictures) with the demonstration of necrotizing granulomas on histopathology or AFB on histopathology or intestinal tissue culture.22 Patients who did not fulfill the above definitions were administered a therapeutic trial of ATT. A diagnosis of ITB was made if the patient had a clinical and endoscopic response to ATT,23 and a diagnosis of CD was made if the patient showed no response, worsened, or worsened after initial improvement with standard ATT and subsequently showed a clinical and/or endoscopic response to oral steroids/CD specific therapy. A diagnosis of IBS was made on the basis of Rome III criteria.24
MDR-TB was defined as TB resistant to at least both isoniazid and RIF. Among the MDR-TB cases, XDR-TB (extensively drug resistant TB) was defined as MDR-TB with additional resistance to any fluoroquinolone and to at least one of the three following injectable drugs: kanamycin, capreomycin, and amikacin.25
A composite reference standard (CRS) comprised of either culture positivity or the presence of caseating granuloma or AFB positivity on biopsy, and the response to treatment was used as a reference standard for the diagnosis of ITB.26
4. Treatment and Follow-Up
ATT: an induction regimen of isoniazid 5 mg/kg, RIF 10 mg/kg, pyrazinamide 20 to 25 mg/kg, and ethambutol 15 to 20 mg/kg for 2 months followed by maintenance therapy of isoniazid and RIF was administered for 4 months. The total treatment duration was 6 months, after which a repeat endoscopy was performed to assess mucosal healing. Patients were routinely followed up at 1, 3, and 6 months and when required to assess symptom response and to monitor drug toxicity by assessment of liver function tests.
5. Clinical Data
Demographic details, clinical manifestations, endoscopic and radiologic investigations, final diagnosis, medical treatments and their outcomes, and any surgical history were recorded on a predefined form.
6. Mucosal Biopsy Collection
Patients underwent ileocolonoscopy with a video-colonoscope (Olympus EVIS EXERA II 180 series; Olympus, Tokyo, Japan) after bowel preparation with a colonic lavage solution namely, polyethylene glycol. Upper GI endoscopy or double-balloon enteroscopy was performed in patients in whom the diseased segment was localized to the upper or mid GI tract. Multiple mucosal biopsies, including five to six fragments from lesional areas, were fixed in formalin and sent for histopathology. Histopathological evaluation was done for the presence of macro and micro granulomas, caseation necrosis, and AFB. Another five to six fragments of mucosal biopsies were taken in normal saline for culture in Lowenstein Jensen medium (LJ) and Xpert MTB/RIF for detection of MTB complex and drug resistance.
7. Molecular Diagnostic Methods
A molecular diagnostic technique was performed using the Xpert MTB/RIF assay, which is an automated real-time PCR. Xpert MTB/RIF integrates DNA extraction, genomic amplification, semiquantitative detection of MTB complex, and RIF resistance determination in a single cartridge. Briefly, the GX assay consisted of inactivation of the sample with sample reagent in a 1:2 ratio for 15 minutes, during which the closed tube was manually agitated twice before 2 mL of the inactivated sample reagent-sample mixture was transferred to the Xpert MTB/RIF test cartridge. For ascitic fluid samples, 1 mL of ascitic fluid was used and 2 mL of the sample buffer was added to the crude sample. For biopsy samples, the tissue sample was homogenized and phosphate buffer saline was added to the sample to make it 1 mL. This 1 mL was then added to 2 mL of sample buffer and processed further. The cartridge also included spores of Bacillus globigii as an internal control of the sample processing and the real-time PCR assay. Cartridges were inserted into the Xpert MTB/RIF device instrument for DNA extraction and amplification of a 192-bp segment of the rpoB gene, and the automatically generated results were read after 90 minutes. Detection consisted of the hybridization of the amplicon with five overlapping probes complementary to the rpoB “core” region (81 bp), determining RIF resistance.27 RIF resistance is considered to be a surrogate marker for MDR-TB, as <10% of RIF resistance has been reported to be monoresistance.28
8. Statistical Analysis
Continuous variables were expressed as mean±SD. Categorical variables were expressed as percentages. With respect to clinical features, continuous variables were compared by Student t-test or ANOVA test, categorical variables were compared by chi-square test, and P<0.05 was considered to be significant. All statistical analyses were performed using Stata version 11.0 (StataCorp, College Station, TX, USA).
RESULTS
A total of 311 patients referred with a diagnosis of ITB or CD were enrolled in the ITB/CD clinic from June 2013 until July 2014 and followed up until December 2014. Of these, 21 patients were excluded as they did not follow up subsequently and a diagnosis could not be established. One hundred patients were diagnosed with peritoneal or intestinal TB. The nine patients who had peritoneal TB were excluded from this analysis, and 37 patients who had ITB were included in the study. They were all HIV negative. One hundred and fifty-one patients were diagnosed with CD, and 43 of these were included. Thirty-nine patients were diagnosed with neither TB nor CD, and 18 of these patients diagnosed with IBS served as the second disease control group (Fig. 1).
1. Patient Characteristics
The demographic details and disease characteristics of patients in the ITB, CD, and IBS groups are shown in Table 1. The median age (range) of the patients in the ITB, CD, and IBS groups were 31 (24–42.5), 32 (23–40) and 30.5 (22–41.25) years, respectively (P=0.96). Men comprised 48%, 67%, and 83% of the ITB, CD, and IBS groups, respectively. Patients with CD had a median disease duration of 12 months (range, 6–36 months) compared to a disease duration of 14 months (range, 6–24 months) in patients with ITB (P<0.76). Hemoglobin was significantly lower in patients with ITB and CD when compared to patients with IBS (P=0.027). The median follow-up duration in the ITB and CD groups was 7 months (range, 6–9 months) and 7 months (range, 4–9.5 months), respectively (P=0.08). In the ITB group, five patients (15.2%) had already received ATT. The reasons for previous administration of ATT were as follows: three for ITB and two for pulmonary TB.
Table 1. Baseline Demographic and Clinical Characteristics of Patients.
| Characteristic | ITB (n=37) | CD (n=43) | IBS (n=18) |
P-value ITB vs. CD vs. IBS |
|---|---|---|---|---|
| Age (yr) | 31.0 (24.00–42.50) | 32.0 (23.00–40.00) | 30.5 (22.00–41.25) | 0.96 |
| Sex (male:female) | 18:19 | 29:14 | 15:3 | 0.03 |
| Hemoglobin (g/dL) | 11.3 (10.4–12.6) | 11.8 (9.7–13.2) | 13.4 (11.4–15.0) | 0.02 |
| Serum albumin (g/dL) | 4.3 (4.0–4.8) | 4.0 (3.6–4.6) | 4.2 (3.9–5.0) | 0.22 |
| Montreal classification | ||||
| Locationa | ||||
| L1 | 15 (40.5) | 17 (39.5) | ||
| L2 | 12 (32.4) | 11 (25.5) | ||
| L3 | 8 (21.6) | 13 (30.2) | ||
| L4 | 8 (21.6) | 3 (6.9) | ||
| Behavior | ||||
| B1 | 28 (75.6) | 30 (69.7) | ||
| B2 | 9 (24.3) | 13 (30.2) | ||
| B3 | 0 | 0 | ||
| P | 0 | 0 | ||
| History of prior antituberculosis therapy | 5 (13.5) | 18 (41.8) | ||
| Duration of follow-up (mo) | 7 (6.0–9.0) | 7 (4.0–9.5) | ||
| Duration of disease (mo) | 14 (6.0–24.0) | 12 (6.0–36.0) |
Values are presented as median (IQR) or number (%).
aIn the intestinal tuberculosis (ITB) group, three patients had both upper gastrointestinal (UGI) and distal ileal involvement, two had both UGI and colonic involvement, and one had both UGI and ileocolonic involvement.
P, perianal disease modifier.
2. Location and Phenotypic Expression of Diseased Segments in Patients with ITB and CD
The location of disease as per the Montreal classification in ITB and CD was the ileal (40.5% vs. 39.5%), colonic (32.4% vs. 25.5%), ileocolonic (21.6% vs. 30.2%), and upper GI (21.6% vs. 6.9%). The phenotypic expression in ITB was inflammatory in nature in 75.6% and stricturing in 24.3% of patients, whereas in CD, 69.7% were inflammatory and 30.2% had stricturing disease. None of the patients in either group had fistulizing disease (Table 1).
3. Histopathological Features
Granulomas were seen in 45.9% (17/37) of patients with ITB, out of which 16.2% (6/37) were caseating granulomas. One patient had AFB positivity on Ziehl-Neelsen staining. In patients with CD, the granuloma positivity rate was 11.6% (5/43).
4. Culture
Mycobacterial cultures were performed in all patients across all groups. In the ITB group, one out of 37 biopsy samples was culture positive (grew AFB on LJ culture medium).
5. Diagnostic Criteria in the TB Cohort
Patients were diagnosed to have ITB on the basis of caseating granulomas in six patients, which included AFB in one patient, LJ medium culture positivity in one, and symptomatic improvement and complete endoscopic healing in the remaining 30 patients after ATT (Table 2).
Table 2. Criteria on Which Diagnosis of Intestinal Tuberculosis Was Based (n=37).
| Diagnostic criteria | No. (%) |
|---|---|
| Caseating granuloma (one case also had AFB stain positive) | 6 (16.21) |
| LJ culture positivity | 1 (2.70) |
| Complete symptomatic and endoscopic healing at the end of ATT in patients without the above findings | 30 (81.08) |
LJ, Lowenstein Jensen medium; ATT, antitubercular therapy.
6. Xpert MTB/RIF Positivity in the Patients with ITB, CD and Control Groups
A total of 98 colonic biopsy samples underwent Xpert MTB/RIF analysis. Three of 37 samples (8.1%) from the ITB group were positive by Xpert MTB/RIF. None of the patients in the CD group or IBS group tested positive by Xpert MTB/RIF.
7. Performance of Xpert MTB/RIF Assay in Diagnosing Intestinal TB
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of Xpert MTB/RIF assay in comparison to CRS were 8.1%, 100%, 100%, and 64.2%, respectively (Table 3). The negative likelihood ratio of the Xpert MTB/RIF assay in comparison to CRS inclusive of clinical response was 0.92. If clinical and endoscopic responses were excluded from the CRS criteria, the sensitivity, specificity, PPV, and NPV were found to be 40%, 100%, 100%, and 92.4%, respectively. The negative likelihood ratio of the Xpert MTB/RIF test in comparison to CRS exclusive of clinical response was found to be 0.57.
Table 3. Diagnostic Accuracy of Xpert MTB/RIF for Intestinal Tuberculosis.
| Sensitivity | Specificity | PPV | NPV | Likelihood ratio | ||
|---|---|---|---|---|---|---|
| Positivea | Negative | |||||
| CRS (inclusive of clinical response) | 8.1 (2.1–23.0) | 100 (92.6–100) | 100 (31.0–100) | 64.2 (53.6–73.6) | - | 0.92 (0.83–1.01) |
| CRS (excluding clinical response) | 40.0 (12.0–80.0) | 100 (86.0–100) | 100 (31.0–100) | 92.4 (89.0–99.0) | - | 0.60 (0.30-10.80) |
Value are presented as % (95% CI).
aPositive likelihood ratios become infinite, as specificity is 100%.
MTB, Mycobacterium tuberculosis; RIF, rifampicin; PPV, positive predictive value; NPV, negative predictive value; CRS, composite reference standard.
8. Prevalence of MDR-TB Based on the Xpert MTB/RIF Assay
In the ITB group, 32 patients had no history of prior ATT and three (9.3%) tested positive by Xpert MTB/RIF. None of them showed resistance to RIF. Five patients with ITB had a prior history of ATT, but none of these patients had Xpert MTB/RIF positivity. Overall, no patient with ITB had MDR-TB. Of the 43 patients with CD, 18 (41.8%) were administered an ATT trial. At the end of 6 months of therapy, none of these patients had mucosal healing. Colonic biopsies taken at the end of the 6-month ATT trial were negative on Xpert MTB/RIF in all these patients.
DISCUSSION
We prospectively included patients with abdominal TB in this study, which assessed the diagnostic utility of the Xpert MTB/RIF assay. This is also the first study reporting the use of a molecular diagnostic method, i.e., Xpert MTB/RIF, to determine the prevalence of MDR-TB in abdominal TB.
The diagnosis of abdominal TB in a patient with ulceroconstrictive disease of the intestine can be perplexing for clinicians in a TB-endemic region, as the phenotypic features of both CD and TB are similar. A misdiagnosis of CD and treatment with immunosuppression in a patient with TB may have catastrophic results. The situation is further complicated by the fact that most tests used in the diagnosis of intestinal TB have a poor yield. The advent of molecular probes in the diagnosis of TB has been a great advancement in both the diagnosis and the detection of resistance in pulmonary TB. Molecular diagnostic testing data by Xpert MTB/RIF have been reported in EPTB in biopsy specimens, but it has still not been reported in intestinal TB.29 We found in our study that the Xpert MTB/RIF assay was positive in 8.1% (3/37) of ITB cases. The sensitivity, specificity, PPV, and NPV compared to CRS were 8.1%, 100%, 100%, and 64.2%, respectively. Compared to CRS with exclusion of the clinical response to ATT, it showed a sensitivity of 43%, a very high specificity and PPV of 100% each, and an NPV of 96%. Thus, the Xpert MTB/RIF assay has a high specificity for TB and can therefore effectively help in confirming the diagnosis of TB and greatly aid gastroenterologists in decision-making.
The lack of an optimal confirmatory test to differentiate between ITB and CD necessitates that clinicians resort to a therapeutic trial of ATT in a substantial proportion of patients suspected of having either of the diseases and then subsequently classify the patients as having ITB or CD based on their response to ATT, both clinical and endoscopic. This is a common practice in India and other Asian countries where both these diseases are commonly seen.30 However, at the end of an ATT trial, if a patient has not responded and mucosal inflammatory changes are still seen on colonoscopy, confusion arises as to whether the patient has CD or MDR-TB. This diagnostic confusion is unique to many Asian countries where TB is prevalent and the incidence of CD is increasing, unlike Western populations. Therefore, it is imperative that clinicians in these countries know the prevalence of MDR-TB. A very low prevalence of MDR-TB would shift the decision towards CD in a nonresponder to ATT.
Our study has evaluated the primary and secondary prevalence of MDR-TB in a prospective manner. In our study of 37 ITB patients, none had MDR-TB. Of 37 patients with ITB, primary and secondary MDR-TB prevalence was 0%. Our study therefore suggests a low prevalence of MDR-TB in patients with ITB based on Xpert assay.
Three studies from Asia have studied the prevalence of MDR-TB. In a series of 30 patients with colonic TB in Taiwan, four (13%) had MDR-TB.17 This high prevalence could be because of selection bias in this retrospective study. Among 30 cases of lower GI TB in the Taiwanese study, 22 (73%) had concomitant pulmonary TB, with type II diabetes mellitus in 23% and chronic alcoholism in 23%. The 1-year mortality rate was 20% but was 50% in patients with MDR-TB. This patient population seems to be vastly different from our patient population. In another study from Korea, of the 74 patients who tested positive for MTB strains from colonoscopic biopsy specimens, MDR-TB was confirmed in two patients (2.7%). The results of this Korean study were based on colonic biopsy cultures.18 In a recent study from Mumbai, of 61 patients with abdominal TB, the drug sensitivity pattern was analyzed in 18 patients; MDR-TB was detected in three patients (5.4% of all subjects and 16.6% of those in whom drug sensitivity was available). In the study from Mumbai, Samant et al.20 found that AFB culture positivity was 50.8% using the Bactec Mycobacterial Growth Indicator Tubes (MGIT) system. The detection rate of AFB organisms in nonsputum specimens is usually low.19 The technique used for culture was Bactec MGIT in the study from Mumbai, which has led to some concerns about false-positive rates for drug resistance observed using Bactec MGIT.29 In comparison to these studies, we used the Xpert MTB/RIF assay to determine the prevalence of MDR-TB.
We found a low prevalence of MDR-TB, and therefore our results suggest that the probability of having CD is much higher than the probability of having MDR-TB in a setting where the patient does not respond to ATT. We found that none of the patients with CD had a positive result for TB using the Xpert MTB/RIF assay. Hence, if a patient has undergone an ATT trial and has not shown an adequate response, then testing for MDR-TB is mandatory; however, the probability of the diagnosis being Crohn's rather than MDR-TB is high.
The limitations of our study include a small sample size and a low culture positivity rate. The culture positivity of intestinal biopsy specimens in the LJ medium in our study was 2.1% (1/37). Shah et al.31 reported a culture positivity rate of 6% (3/50), and Bhargava et al.32 reported an LJ culture positivity rate of 40% (6/15). The culture positivity of MTB using GI biopsy specimens can be low as this form of TB is paucibacillary, and culturing MTB can be extremely difficult, especially in a solid culture medium. Using a liquid culture medium such as MGIT could have given a better culture yield and subsequently better drug sensitivity testing. Furthermore, we found that five of 37 patients (13.5%) with ITB and 18 of 43 patients (41.8%) with CD had a prior history of ATT, which may have influenced our results. Even though the culture positivity rates were low, epithelioid cell granulomas were seen in 45.9% of cases (17/37), and caseating granulomas were seen in 16.3% of cases (6/37) on histopathological examination, which is comparable to the results of other studies.31,32,33,34 Furthermore, the prevalence we described is based only on the Xpert MTB/RIF assay, whose sensitivity we found to be low, which may underestimate the true prevalence of MDR-TB. Diagnostic methods with a better sensitivity and larger studies will be needed before we can truly define the prevalence of MDR-TB in India.
In conclusion, this prospective cohort study showed that Xpert MTB/RIF had a poor sensitivity but a high specificity in diagnosing abdominal TB and therefore can serve as an important tool in clinical practice where a positive test for TB may confirm ITB and rule out CD. Furthermore, this assay may be helpful in guiding therapy for patients who have not responded to ATT in order to differentiate between CD and drug-resistant TB. Our data show a low prevalence of MDR-TB in ITB, but further large studies using more sensitive tests are warranted to determine the true prevalence of MDR-TB.
Footnotes
Financial support: None.
Conflict of interest: None.
References
- 1.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–353. [PubMed] [Google Scholar]
- 2.Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120:305–315. [PubMed] [Google Scholar]
- 3.Horvath KD, Whelan RL. Intestinal tuberculosis: return of an old disease. Am J Gastroenterol. 1998;93:692–696. doi: 10.1111/j.1572-0241.1998.207_a.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhansali SK. Abdominal tuberculosis: experiences with 300 cases. Am J Gastroenterol. 1977;67:324–337. [PubMed] [Google Scholar]
- 5.Sharma MP, Ahuja V. Abdominal (gastrointestinal tract) tuberculosis in adult. In: Schaaf HS, Zumla AI, Grange JM, editors. Tuberculosis: a comprehensive clinical reference. Edinburgh: Saunders/Elsevier; 2009. pp. 424–431. [Google Scholar]
- 6.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11:134–147. doi: 10.1111/j.1751-2980.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 7.Ahuja V, Tandon RK. Inflammatory bowel disease: the Indian augury. Indian J Gastroenterol. 2012;31:294–296. doi: 10.1007/s12664-012-0272-3. [DOI] [PubMed] [Google Scholar]
- 8.Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14:111–119. doi: 10.5217/ir.2016.14.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weng MT, Wei SC, Lin CC, et al. Seminar report from the 2014 Taiwan Society of Inflammatory Bowel Disease (TSIBD) Spring Forum (May 24th, 2014): Crohn's disease versus intestinal tuberculosis infection. Intest Res. 2015;13:6–10. doi: 10.5217/ir.2015.13.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makharia GK, Srivastava S, Das P, et al. Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol. 2010;105:642–651. doi: 10.1038/ajg.2009.585. [DOI] [PubMed] [Google Scholar]
- 11.Kedia S, Sharma R, Nagi B, et al. Computerized tomography-based predictive model for differentiation of Crohn's disease from intestinal tuberculosis. Indian J Gastroenterol. 2015;34:135–143. doi: 10.1007/s12664-015-0550-y. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja V, Subodh S, Tuteja A, et al. Genome-wide gene expression analysis for target genes to differentiate patients with intestinal tuberculosis and Crohn's disease and discriminative value of FOXP3 mRNA expression. Gastroenterol Rep (Oxf) 2016;4:59–67. doi: 10.1093/gastro/gov015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh B, Kedia S, Konijeti G, et al. Extraintestinal manifestations of inflammatory bowel disease and intestinal tuberculosis: frequency and relation with disease phenotype. Indian J Gastroenterol. 2015;34:43–50. doi: 10.1007/s12664-015-0538-7. [DOI] [PubMed] [Google Scholar]
- 14.Munot K, Ananthakrishnan AN, Singla V, et al. Response to trial of antitubercular therapy in patients with ulceroconstrictive intestinal disease and an eventual diagnosis of Crohn's disease. Gastroenterology. 2011;140(5 Suppl 1):S159. [Google Scholar]
- 15.Ormerod LP. Multidrug-resistant tuberculosis (MDR-TB): epidemiology, prevention and treatment. Br Med Bull. 2005;73-74:17–24. doi: 10.1093/bmb/ldh047. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SK, Kaushik G, Jha B, et al. Prevalence of multidrug-resistant tuberculosis among newly diagnosed cases of sputum-positive pulmonary tuberculosis. Indian J Med Res. 2011;133:308–311. [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma SK, Kumar S, Saha PK, et al. Prevalence of multidrug-resistant tuberculosis among category II pulmonary tuberculosis patients. Indian J Med Res. 2011;133:312–315. [PMC free article] [PubMed] [Google Scholar]
- 18.Lin PY, Wang JY, Hsueh PR, et al. Lower gastrointestinal tract tuberculosis: an important but neglected disease. Int J Colorectal Dis. 2009;24:1175–1180. doi: 10.1007/s00384-009-0721-3. [DOI] [PubMed] [Google Scholar]
- 19.Ye BD, Yang SK, Kim D, et al. Diagnostic sensitivity of culture and drug resistance patterns in Korean patients with intestinal tuberculosis. Int J Tuberc Lung Dis. 2012;16:799–804. doi: 10.5588/ijtld.11.0252. [DOI] [PubMed] [Google Scholar]
- 20.Samant H, Desai D, Abraham P, et al. Acid-fast bacilli culture positivity and drug resistance in abdominal tuberculosis in Mumbai, India. Indian J Gastroenterol. 2014;33:414–419. doi: 10.1007/s12664-014-0467-x. [DOI] [PubMed] [Google Scholar]
- 21.Travis SP, Stange EF, Lémann M, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: current management. Gut. 2006;55(Suppl 1):i16–i35. doi: 10.1136/gut.2005.081950b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faustian FF. Tuberculosis of the intestine. In: Haubrich WS, Schaffner F, Berk JE, editors. Bockus gastroenterology. Volume 4. 5th ed. Philadelphia: Saunders; 1995. p. 3304. [Google Scholar]
- 23.Lee YJ, Yang SK, Byeon JS, et al. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn's disease. Endoscopy. 2006;38:592–597. doi: 10.1055/s-2006-924996. [DOI] [PubMed] [Google Scholar]
- 24.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functonal bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Report of the meeting of the WHO Global Task Force on XDR-TB. Geneva: World Health Organization; 2006. [Google Scholar]
- 26.Sharma SK, Kohli M, Chaubey J, et al. Evaluation of Xpert MTB/RIF assay performance in diagnosing extrapulmonary tuberculosis among adults in a tertiary care centre in India. Eur Respir J. 2014;44:1090–1093. doi: 10.1183/09031936.00059014. [DOI] [PubMed] [Google Scholar]
- 27.Moure R, Muñoz L, Torres M, Santin M, Martín R, Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol. 2011;49:1137–1139. doi: 10.1128/JCM.01831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drobniewski FA, Pozniak AL. Molecular diagnosis, detection of drug resistance and epidemiology of tuberculosis. Br J Hosp Med. 1996;56:204–208. [PubMed] [Google Scholar]
- 29.Piersimoni C, Mustazzolu A, Giannoni F, Bornigia S, Gherardi G, Fattorini L. Prevention of false resistance results obtained in testing the susceptibility of Mycobacterium tuberculosis to pyrazinamide with the Bactec MGIT 960 system using a reduced inoculum. J Clin Microbiol. 2013;51:291–294. doi: 10.1128/JCM.01838-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tandon R, Ahuja V. Differentiating intestinal tuberculosis and Crohn's disease. In: Jewell DP, Tandon R, Ahuja V, editors. Inflammatory bowel disease. Delhi: Macmillan Medical Communications; 2014. pp. 41–61. [Google Scholar]
- 31.Shah S, Thomas V, Mathan M, et al. Colonoscopic study of 50 patients with colonic tuberculosis. Gut. 1992;33:347–351. doi: 10.1136/gut.33.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhargava DK, Kushwaha AK, Dasarathy S, Shriniwas, Chopra P. Endoscopic diagnosis of segmental colonic tuberculosis. Gastrointest Endosc. 1992;38:571–574. doi: 10.1016/s0016-5107(92)70519-7. [DOI] [PubMed] [Google Scholar]
- 33.Gan HT, Chen YQ, Ouyang Q, Bu H, Yang XY. Differentiation between intestinal tuberculosis and Crohn's disease in endoscopic biopsy specimens by polymerase chain reaction. Am J Gastroenterol. 2002;97:1446–1451. doi: 10.1111/j.1572-0241.2002.05686.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim KM, Lee A, Choi KY, Lee KY, Kwak JJ. Intestinal tuberculosis: clinicopathologic analysis and diagnosis by endoscopic biopsy. Am J Gastroenterol. 1998;93:606–609. doi: 10.1111/j.1572-0241.1998.173_b.x. [DOI] [PubMed] [Google Scholar]