Abstract
Researchers have studied psychological disorders extensively from a common cause perspective, in which symptoms are treated as independent indicators of an underlying disease. In contrast, the causal systems perspective seeks to understand the importance of individual symptoms and symptom-to-symptom relations. In the current study, we used network analysis to examine the relationships between and among depression and anxiety symptoms from the causal systems perspective. We utilized data from a large psychiatric sample at admission and discharge from a partial hospital program (N = 1029, mean treatment duration = 8 days). We investigated features of the depression/anxiety network including topology, network centrality, stability of the network at admission and discharge as well as change in the network over the course of treatment. Results revealed that individual symptoms of depression and anxiety were more related to other symptoms within each disorder than to symptoms between disorders. Sad mood and worry were among the most central symptoms in the network. The network structure was stable both at admission and between admission and discharge, although the overall strength of symptom relationships increased as symptom severity decreased over the course of treatment. Examining depression and anxiety symptoms as dynamic systems may provide novel insights into the maintenance of these mental health problems.
Keywords: network analysis, causal systems, depression, anxiety, comorbidity
Traditional conceptualizations of psychopathology presume that symptoms of mental disorders are reflective of underlying diseases. In this conceptualization, the co-occurrence or non-random clustering of symptoms is due to an underlying common cause (see Borsboom, 2008; Schmittmann et al., 2013). Thus, an entity such as major depressive disorder (MDD) is hypothesized to cause sad mood, anhedonia, and insomnia in the same way that the smallpox virus causes pustules, fever, and headache (Fried, 2015). The models employed to investigate psychopathology have supported the common cause perspective of mental disorders. For example, reflective latent variable models of psychopathology, in which symptoms are indicators of an underlying latent variable, are consistent with a common cause perspective. Similarly, the use of sum-scores to describe psychopathology severity assumes that symptoms are interchangeable indicators of the same underlying condition and can thus be summed to create a total score (see Fried & Nesse, 2015).
Importantly, this approach has the potential to obscure important differences between specific symptoms, as well as relationships among symptoms. For example, symptoms are differentially associated with impairments (Fried & Nesse, 2014), predisposing risk (Fried et al., 2014) and neural substrates (e.g., Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Kapur, Phillips, & Insel, 2012). Further, the potential influence of symptoms on the development of other symptoms is not uniformly distributed. For example, animal and human models suggest that restricted sleep is followed by depression and anxiety symptoms (e.g., Neckelmann et al., 2007; Novati et al., 2009; Baglioni et al., 2011), and hopelessness prospectively predicts suicidal ideation (e.g., Beck et al., 1990; Fawcett et al., 1990). Similarly, the alleviation of one symptom may positively affect other symptoms. In individuals receiving treatment for depression, changes in one symptom have been found to predict changes in other symptoms the following week, independent of a general decrease in symptom severity (Bringmann et al., 2015). One interpretation of this finding is that effective therapies target some symptoms first, which leads to downstream effects on other symptoms (Kim & Ahn 2002; Cramer et al., 2010). This interpretation directly conflicts with the common cause perspective. If symptoms directly interact, the assumption that the covariance among symptoms results from a common cause is not fully equipped to elucidate the structure of psychopathology.
The causal systems perspective (Borsboom, 2008) describes the possibility that symptom co-occurrence is due to direct symptom-to-symptom relationships rather than a common cause. According to this perspective, “symptoms are constitutive of mental disorder, not reflective of it” (McNally et al., 2014, p. 2): in other words, “causal, meaningful relations between symptoms not only exist and should be acknowledged, but in fact are the very stuff of which mental disorders are made” (Borsboom & Cramer, 2013, p. 96). Thus, anhedonia, sad mood, and insomnia are not caused by an entity “depression” in the same way that a brain tumor causes a headache. Rather, these symptoms are likely to directly influence each other and have their own genetic, neural, and psychological underpinnings.
One approach to assessing these symptom-symptom interactions is network analysis (REF). Network analysis describes a set of procedures based on the modeling of dynamical systems (Barrat, Barthélemy, & Vespignani, 2012; Newman, Barabási, & Watts, 2006), and can operationalize the inter-symptom relationships posited by the causal systems perspective. This technique provides a visual depiction of the complex associations among symptoms, which can be understood as partial correlations. Network analysis also allows identification of “central” symptoms, defined by strong correlations with a large number of other symptoms. Similar to a domino effect, the presence of a central symptom is likely to have greater influence over the entire network of symptoms due to its high degree of interconnectedness. Interestingly, recent work suggests that DSM symptoms of depression (e.g., sad mood) are not more central than non-DSM symptoms (e.g., sympathetic arousal) (Fried et al., 2016) in a network of depression symptoms.
Network analysis has been particularly useful for understanding factors contributing to psychiatric comorbidity. For example, Cramer et al. (2010) examined the networks of Generalized Anxiety Disorder (GAD) and MDD symptoms using data from the National Comorbidity Survey-Replication (NCS-R; Kessler et al., 2004). They found that shared diagnostic symptoms (concentration difficulty, fatigue, sleep disturbance) contributed meaningfully to comorbidity, as individuals with these symptoms had more tightly connected symptom networks compared to those without these symptoms. Researchers have suggested that a tightly connected network is a riskier network because activation of one symptom can quickly spread to other symptoms. Supporting this hypothesis, van Borkolu and colleagues (2015) found that individuals with MDD were less likely to recover over time if their depression symptoms were more strongly correlated with one another.
The Present Study
In the current study, we sought to extend the work of Cramer and colleagues (2010) in several ways. First, the instrument used in the NCS-R contains “skip-out” criteria, so that failure to endorse core symptoms (e.g., sad mood or anhedonia for depression) leads to skipping all other symptoms of that disorder, resulting in large amounts of missing data. Some individuals who do not endorse screening items still experience other symptoms such as insomnia or concentration problems, and instruments in which participants respond to all items are better equipped to estimate associations between symptoms than those that use skip-out (Fried & Nesse, 2015). Additionally, it is important to determine whether symptom networks obtained from a general population sample will generalize to a psychiatric sample (Fried, van Borkulo, Epskamp, Schoevers, Tuerlinckx, & Borsboom, 2016). Finally, Cramer and colleagues (2010) examined the network structure at one time point. Thus, we were interested in whether the depression and anxiety symptom network changes over the course of treatment.
We designed the current study with three main goals: (1) characterize the GAD/MDD symptom network structure in a psychiatric sample, (2) determine the stability of the network, and (3) test whether the network changed over the course of treatment. For Aim 1, we investigated the connectedness between symptoms, the centrality of different symptoms, and potential bridge symptoms. Based on prior results (e.g., Bringmann et al., 2015; Fried et al., 2016), we hypothesized that sad mood and anhedonia would exhibit high centrality among depression symptoms and that worry symptoms would be most central among anxiety symptoms. Similar to Cramer et al. (2010), we predicted that symptoms appearing in the diagnostic criteria of both GAD and MDD would serve as bridge symptoms between anxiety and depression. In particular, we expected sleep (Fawcett et al., 1990; Durmer & Dinges, 2005; Ferentinos et al., 2009) and concentration (Davis & Nolen-Hoeksema, 2000; Joormann & Gotlib, 2008; Stefanopoulou et al., 2014) to link other symptoms of depression and anxiety symptoms. For Aim 2, given our sample size, we expected the network edges representing the magnitude of association between symptoms and centrality indices to be stable. Finally, for Aim 3, we hypothesized that symptom networks created from data collected at pre- and post-treatment would have a stable structure. For example, a prior network analysis study showed that even as symptoms decreased overall, the most central symptoms remained the same (e.g., Robinaugh, LeBlanc, Vuletich, & McNally, 2014). At the same time, a recent study showed that the correlations among depression symptoms increased strongly and consistently over time while patients improved in symptomatology (Fried et al., 2016). Thus, we hypothesized that the interconnectedness or global strength of symptom associations would increase over the course of treatment, even as the network structure (i.e., centrality of specific symptoms) remained stable.
Method
Participants and Treatment Setting
Participants were receiving treatment for mood, anxiety, personality, and psychotic disorders at the Behavioral Health Partial Hospital Program at McLean Hospital (for a review of the treatment, see Beard & Björgvinsson, 2013). Partial hospitals provide intensive treatment during the day with patients returning to their homes in the evening. The current study utilized self-report data collected in the routine clinical care of 1235 patients from July 2012 to July 2014 at admission and discharge. Missing data were handled with listwise deletion because typically participants were missing all items from one or both questionnaires. We excluded 206 subjects from admission data (final N=1029) and 465 from discharge data (final N=807; final N for both admission and discharge=742). Data were collected using Research Electronic Data Capture (REDCap) (Harris et al., 2009). Partners Healthcare Internal Review Board approved the study as exempt due to the use of a de-identified dataset.
Measures
The Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) was administered by doctoral practicum students and clinical psychology interns with weekly supervision by a postdoctoral fellow. The MINI is a structured interview to assess DSM-IV Axis I disorders. It has strong reliability and validity in relation to the Structured Clinical Interview for DSM-IV (kappas range from .89 to 1.0; Sheehan et al., 1998).
MDD and GAD symptoms were assessed via the Patient Health Questionnaire-9 (PHQ-9; Kroenke, Spitzer, & Williams, 2001) and the 7-item Generalized Anxiety Disorder Scale (GAD-7; Spitzer, Kroenke, Williams, & Löwe, 2006), self-report measures of depression and anxiety symptom severity, respectively, over the prior two weeks. Participants rated symptoms on a scale from 0 (not at all) to 3 (nearly every day). Both PHQ-9 and GAD-7 have demonstrated good psychometric properties (Kroenke, Spitzer, & Williams, 2001); Kroenke, Spitzer, Williams, Monohan, & Löwe, 2007; Löwe et al., 2008; Spitzer et al., 2006) and have been validated as severity measures in our partial hospital population (Beard & Björgvinsson, 2014; Beard, Hsu, Rifkin, Busch & Björgvinsson, 2016).
Analyses
Aim 1: Characterization of MDD/GAD symptoms network at admission
Edges
In network parlance, symptoms are “nodes,” and relationships between symptoms are “edges.” To calculate the edges, we computed polychoric correlations between all items. Polychoric correlations estimate the association between two variables that are theorized to be continuous and normally distributed but are measured on ordinal scales. We estimated the network via a Graphical Gaussian Model (GGM) (Lauritzen, 1996), in which edges represent conditional independence relationships among the nodes. These edges can be understood as partial correlations, representing the relationship between two nodes when controlling for all other relationships in the network. GGMs estimate a large number of parameters (i.e. 16 nodes requires the estimation of 136 parameters: 16 threshold parameters and 16*15/2=120 pairwise association parameters) that likely result in some false positive edges. Therefore, it is common to regularize GGMs via the graphical lasso (glasso; see Friedman, et al. 2008; Tibshirani, 1996 for details). This algorithm shrinks all edges in the network, and sets small edges exactly to zero, which leads to a sparse (i.e., parsimonious) network that explains the covariance among nodes with as few edges as necessary. We estimated the GGMs using the R-package qgraph (Epskamp, Cramer, Waldorp, Schmittmann, & Borsboom, 2012) that automatically implements the glasso regularization in combination with extended Bayesian Information Criterion (EBIC) model selection as described by Foygel & Drton (2010). First, 100 different network models with different degrees of sparsity are estimated. Second, the model with the lowest EBIC is selected, given a certain value on the hyperparameter γ, which controls the trade-off between including false positive edges and removing true edges. We set the starting value of γ to 0.5 as recommended by Foygel and Drton (2010). Detailed tutorials on network estimation, inference, stability, and regularization for psychopathological networks using the free statistical programming language R can be found elsewhere (Epskamp, Borsboom & Fried, 2016; Eskamp & Fried, 2016). For network visualization, the thickness of the edges represents the magnitude of the association. Node placement was determined by the Fruchterman-Reingold algorithm, which places nodes with stronger average associations closer to the center of the graph (Fruchterman & Reingold, 1991). The R (R Core Team; version 3.2.3) package qgraph (Version 1.3.3; Epskamp, et al., 2012, Friedman et al., 2014) was used to calculate and visualize the networks.
Centrality
We calculated several indices of node centrality to identify which symptoms are most central to the network (Opsahl, Agneessens, & Skvoretz, 2010). For each node, we calculated strength (absolute sum of edge weights connected to a node), closeness (average distance from the node to all other nodes in the network), and betweenness (the number of times that a node lies on the shortest path between two other nodes).
Aim 2: Stability of MDD/GAD Network
We used two approaches to determine network stability, explained in detail in the supplemental materials. First, we used a permutation-based approach in which we divided the full sample (separately for both admission and discharge) into two randomly selected sub-samples, estimated networks independently, correlated edge and centrality values from the independent networks, and repeated this process 10,000 times.
Second, we used a bootstrap approach to calculate 95% confidence intervals (CIs) for the edge values (Epskamp, Borsboom & Fried, 2016). Because bootstrapped CIs could not be estimated for centrality values, we repeatedly correlated (a) centrality values calculated from the complete data set with (b) centrality values calculated from a subsample with a percentage (e.g. 20% or 50%) of nodes or participants missing. For the latter analysis, if correlation values decline substantially as nodes or participants are removed, we would consider this centrality metric to be unstable.
Aim 3: Comparing admission and discharge networks
We examined two characteristics of the network that could change from admission to discharge: global network strength (i.e. change in the sum of all edges from admission to discharge) and network structure (e.g. if several of the most connected nodes at admission become some of the least connected at discharge and vice versa, it would indicate large structural change). We used a permutation test called the Network Comparison Test (NCT) to test for change in global network strength (van Borkulo et al., 2015). We investigated whether the observed difference between the absolute sum of all edges in each network was more extreme than the 95th percentile (alpha = .05) on a null distribution. To make a distribution of NCT values under the null hypothesis that admission and discharge networks (i.e., dependent samples) are equal, we randomly switched, 50% of participants’ admission and discharge data, constructed networks, calculated a NCT score and repeated this process 10,000 times.
To test for change in network structure, we correlated (a) the values for edges from the admission and discharge networks, and (b) the values for each centrality index (with Spearman-rank-order correlations). We evaluated the stability of the network structure by examining the magnitude of the correlations rather than statistical significance. All analyses investigating changes of network global strength and structure included the 742 participants with complete data at both time points.
Results
Participants and overall treatment response
Patients were primarily single, White, and middle-age (see Table 1). Table 2 presents mean scores for each symptom on the PHQ9 and GAD-7. Paired-samples t-tests, Bonferroni corrected for 18 tests, revealed that individual symptoms and total scores significantly decreased from admission to discharge (ps < .001; mean treatment duration = 8.2 days (SD = 3.2)).
Table 1.
Demographic and Clinical Characteristics (n=1029)
| Variable | M (SD) or N (%) |
|---|---|
| Age | 35 (13.8) |
| Female | 533 (52%) |
| Education | |
| High School/GED or less | 82 (8%) |
| Some college | 401 (39%) |
| 4-year college graduate | 263 (26%) |
| Post-college education | 281 (27%) |
| Marital Status | |
| Never married/single | 637 (62%) |
| Separated/Divorced/Widowed | 136 (13%) |
| Married/living with partner | 251 (25%) |
| Race/ Ethnicity | |
| White | 866 (84%) |
| Asian | 36 (4%) |
| Multi-racial | 39 (4%) |
| Black/African American | 23 (2%) |
| American Indian or Alaskan Native | 1 (< 1%) |
| Native Hawaiian or Pacific Islander | 1 (< 1%) |
| Latino/a | 17 (2%) |
| Did not report | 46 (4%) |
| Primary Diagnosis from Medical Chart (n=1022) | |
| Schizophrenia | 15 (2%) |
| Schizoaffective Disorder | 29 (3%) |
| Delusional Disorder/Unspecified Psychosis | 45 (4%) |
| Major Depressive Disorder | |
| Without Psychotic Features | 469 (46%) |
| With Psychotic Features | 47 (5%) |
| Bipolar I Disorder | 105 (10%) |
| Bipolar I with Psychotic Features | 83 (8%) |
| Bipolar II Disorder | 44 (4%) |
| Mood Disorder NOS | 113 (11%) |
| Anxiety Disorder NOS | 7 (1%) |
| Depressive Disorder NOS | 8 (1%) |
| Panic Disorder | 9 (1%) |
| Generalized Anxiety Disorder | 11 (1%) |
| Social Anxiety Disorder | 1 (<1%) |
| Obsessive Compulsive Disorder | 18 (2%) |
| Post Traumatic Stress Disorder | 16 (2%) |
| Adjustment Disorder | 2 (<1%) |
| Current Clinical Episode from MINI (n=751)a | |
| Depressive Episode | 449 (60%) |
| Manic Episode | 8 (1%) |
| Hypomanic Episode | 4 (<1%) |
| Mood Disorder with Psychotic Features | 31 (4%) |
| Psychotic Disorder | 50 (7%) |
| Panic Disorder | 143 (22%) |
| Generalized Anxiety Disorder | 170 (23%) |
| Social Anxiety Disorder | 211 (28%) |
| Obsessive Compulsive Disorder | 84 (11%) |
| Post Traumatic Stress Disorder | 95 (13%) |
| Alcohol Dependence | 90 (12%) |
| Alcohol Abuse | 41 (6%) |
Percentages exceed 100% due to comorbidity. 279 patients did not complete a structured interview while attending the partial hospital.
Table 2.
Mean score for each symptom on the PHQ-9 and GAD-7 at T1 and T2
| Mean rating | ||
|---|---|---|
| T1 | T2 | |
| Depression symptoms (PHQ-9) | ||
| PHQ-1: Low interest or pleasure | 1.79 | 1.26 |
| PHQ-2: Feeling down, hopeless | 1.90 | 1.31 |
| PHQ-3: Trouble sleeping | 1.86 | 1.20 |
| PHQ-4: Tired or little energy | 1.91 | 1.44 |
| PHQ-5: Poor appetite/overeating | 1.51 | 0.99 |
| PHQ-6: Guilt | 2.02 | 1.38 |
| PHQ-7: Trouble concentrating | 1.72 | 1.20 |
| PHQ-8: Moving slowly/restless | 0.84 | 0.50 |
| PHQ-9: Suicidal thoughts | 0.83 | 0.44 |
| Anxiety symptoms (GAD-7) | ||
| GAD-1: Nervous, anxious, on edge | 1.97 | 1.44 |
| GAD-2: Uncontrollable worry | 1.77 | 1.17 |
| GAD-3: Worry about different things | 1.84 | 1.16 |
| GAD-4: Trouble relaxing | 1.81 | 1.12 |
| GAD-5: Restless | 1.08 | 0.71 |
| GAD-6: Irritable | 1.36 | 0.96 |
| GAD-7: Afraid something awful might happen | 1.26 | 0.73 |
Note. PHQ-9 = Patient Health Questionnaire-9 (Kroenke, Spitzer, & Williams, 2001); GAD-7 = 7-item Generalized Anxiety Disorder Scale (Spitzer, Kroenke, Williams, & Löwe, 2006).
Aim 1: Characterize MDD/GAD symptoms network at admission
Network structure
Figure 1 presents the network at admission, and Figure 2 presents the centrality indices. Approximately 38% of all network edges were set to zero. The two strongest edges were between “too much worry” and “unable to control worry” among anxiety items, and between “sad mood” and “anhedonia” among depression symptoms. Based on confidence intervals (see supplementary materials), both of these edges were significantly larger than all other edges. Within the anxiety items, “unable to control worry” had a strong connection with “being nervous,” which had a strong connection with “unable to relax.” Among the ten (8.3%) strongest edges, only one linked anxiety and depression symptoms: “motor” from depression scale and “restlessness” from the anxiety scale. Although this cross-diagnostic connection makes this edge a candidate for a bridge symptom, “motor” (which does not distinguish between motor agitation and retardation) was on average more strongly related to anxiety items (average edge weight=.051) than depression symptoms (average edge weight=.036). All other PHQ-9 and GAD-7 items displayed higher connections with other items from the same questionnaire (average edge weight range=.047–.149) than across questionnaires (average edge weight range=.003–.025). Finally, there were two other edges with CIs that did not contain zero and bridged anxiety and depression symptoms: “guilt”- “too much worry” and “sad mood”-”nervous”.
Figure 1.
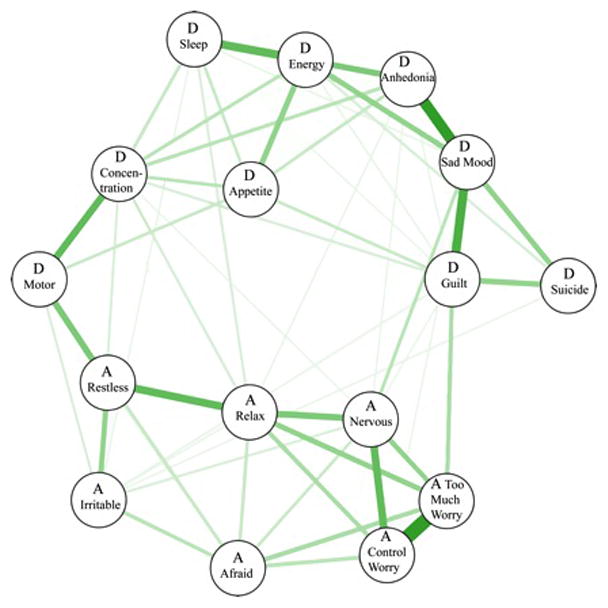
Figure 2.
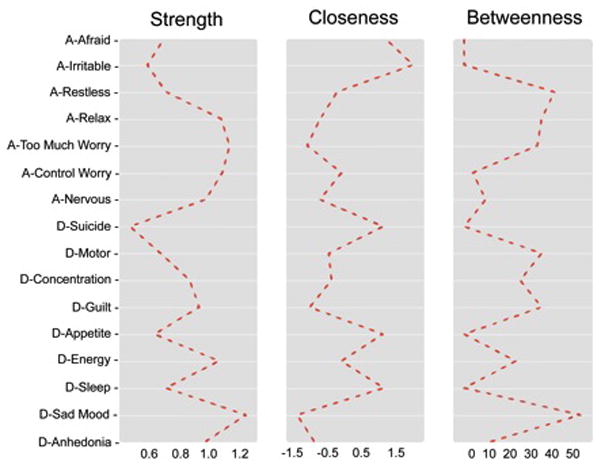
In the entire network, “sad mood” was the most central symptom across all centrality indices, followed by the anxiety symptoms: “too much worry,” “unable to control worry,” and “unable to relax.” Following these, the most central depression symptoms were “low energy,” “anhedonia,” and “guilt/worthlessness.” “Suicide” and “irritable” were the least central symptoms.
Aim 2: Stability of networks
For network edges, both the split-half permutation method (admission mean split-half rs=.75, inter-quartile range=.77–.72) and bootstrap 95% confidence intervals revealed high stability. Among centrality indices, strength was highly stable. Consistent with prior work (Epskamp, Borsboom & Fried, 2016), closeness and betweenness had relatively poor stability. These results were consistent across both admission and discharge (see supplemental materials).
Aim 3: Comparing admission and discharge networks
The repeated-measures NCT revealed that the global edge strength significantly increased from admission (NCT sum = 6.87) to discharge (NCT sum = 7.05; NCT difference = 18.51, p = .007). In other words, the sum of the absolute values of all edge weights was larger at discharge compared to admission. Regarding network structure, spearman correlations between admission and discharge were large for network edges (rs = .78) and centrality indices (strength (rs = 0.96), closeness, (rs = 0.60), betweenness (rs = 0.71)). In combination, these findings suggest that the global connectivity of the network increased over time, but the structure of the network remained roughly intact.
Discussion
This study is the first to characterize depression and anxiety symptom networks within a large psychiatric sample, and with instruments that did not include skip-out criteria. Overall, the findings suggest that some symptom associations are stronger than others and that individual depression and anxiety symptoms are not equally important in the network. In general, connections between symptoms within each disorder were higher than connections between disorders. Importantly, both network edges and the strength centrality metric were stable, increasing confidence in drawing conclusions from the cross-sectional networks. In terms of change over the course of partial hospitalization, while symptom severity decreased and the strength of symptom associations increased from admission to discharge, the structure of the network remained stable.
The edges between “too much worry” and “unable to control worry” and between “sad mood” and “anhedonia” were significantly stronger than all other edges in the network. The motor symptom from MDD and the restlessness symptom from GAD were the most strongly connected items across the two disorders. Interestingly, the motor symptom from MDD showed stronger connections with anxiety symptoms than with MDD symptoms. Contrary to expectations, there was no strong bridge pathway involving sleep or concentration symptoms; however, unexpected bridge pathways emerged. Edges between “guilt” and “too much worry” and between “sad mood” and “feeling nervous” had confidence intervals that did not include zero. While our cross-sectional design does not permit inferences regarding the directionality of these bridge pathways, prior longitudinal data supports the possibility of a bi-directional connections such that anxiety can lead to depression (Avenevoli et al., 2001; Kaufman & Charney, 2000; Wittchen et al., 2000) and that depression leads to anxiety (Cramer et al., 2010; ; Moffitt et al., 2007; Zavos, Rijsdijk, & Eley, 2012).
The strength centrality index (i.e., absolute sum of edge weights connected to a node) demonstrated excellent reliability; thus, we focus our discussion of symptom centrality on strength. The symptoms of “sad mood” and “too much worry” were the most central to the network. These findings are consistent with their current status as hallmark symptoms required for a diagnosis of MDD and GAD and with prior studies (Fried, Epskamp, Nesse, Tuerlinckx & Borsboom, 2016; Fried & Nesse, 2014). Low-energy was another highly central depression symptom; a finding that deviates from common conceptualizations of depression, but converges with another recent study that found that low energy was the most central depression symptom (Fried et al., 2016). Finally, the least central symptom was suicidal ideation. Prior network analyses have yielded mixed findings regarding the centrality of suicidal ideation; although others have also found that is has low centrality (Fried et al., 2016), other work suggested high centrality (e.g., Bringmann et al., 2015). In the current study, suicidal ideation had the lowest mean and standard deviation, which may have artificially lowered its centrality. It will be important for future studies to investigate how the frequency of specific symptoms affects their centrality.
We found that the strength of the relationships between the symptoms significantly increased from admission to discharge. This is consistent with recent work showing that the correlations among depression symptoms increase over the course of treatment (Fried et al., 2016). These authors explored several possible explanations for changes in relationships across time, including spurious effects due to measurement flaws, but found no likely causes. For the current study, both admission and discharge sum scores are relatively normally distributed without any apparent strong floor or ceiling effects. There was a large shift from admission (50% of all responses were a 2 or 3 on the 0 to 3 scale) to discharge (28% of all responses were a 2 or 3), but we could find no relationship between this change in overall item endorsement and the increased correlations between the items at discharge. While the replication of this effect is intriguing, its cause it unclear. Additionally, as this study did not include a control group, it is not clear whether the increased global edge strength is due to treatment, repeated assessment, or some other factor. Importantly, while the global edge strength increased from admission to discharge, the overall network structure remained stable. Specifically, both edge and centrality values showed strong correlations between admission and discharge, even as symptom severity decreased.
It is worthwhile to note that the main difference between examining MDD and GAD from a causal systems perspective versus a common cause perspective is conceptual rather than statistical in nature. Latent variable models can be transformed into network models and vice versa (Epskamp, Maris, Waldorp, & Borsboom, in press; Molenaar, 2010; van der Maas et al., 2006). Instead, the two perspectives lead to different inquiries. If symptoms are indicators of an underlying cause, there is no theoretical basis to examine bridge symptoms between disorders; and, we know of no study using a common cause framework that has included such an analysis. Similarly, high factor loadings would suggest that some items are better indicators of the common cause than others; whereas, within a causal systems perspective, high centrality nodes in a network are interpreted as crucial in the etiology and maintenance of the network. The two perspectives also lead to divergent future directions. From the common cause perspective, future studies should explore the biological correlates of latent factors, such as the p-factor (Caspi, et al. 2014). From the casual systems perspective, important next steps are testing whether, compared with lower centrality nodes, nodes with higher centrality are better prospective predictors of overall network activation (Robinaugh et al, in press) and whether targeting more central nodes in treatment is more efficient and effective at reducing overall network activation compared with targeting peripheral nodes. Finally, it should be emphasized that these two conceptual frameworks are not mutually exclusive. There are likely to be some symptoms within a network that covary due to a common cause, which may itself be causally related to other symptoms.
There are several clinical implications of the current findings. As mentioned, interventions would likely be more efficient if they target central symptoms. Targeting the depression symptoms of sad mood, low energy, and anhedonia may therefore be most influential in reducing overall symptom severity. Cognitive Behavioral Therapy targets most of these symptoms directly via behavioral activation and cognitive restructuring, which may explain the ability of very brief CBT to improve depression symptoms (e.g., Björgvinsson, Kertz, Bigda-Peyton, Rosmarin, Aderka, & Neuhaus, 2014). Regarding anxiety symptoms, our findings suggest that treatments that first target worry should be most effective. However, CBT manuals (i.e., Craske & Barlow, 2006) for GAD do not target worry via problem solving and worry exposures until the end of treatment (Chapters 8 and 10 respectively). The current findings suggest that targeting worry earlier should improve efficiency.
The current study had several strengths. First, unlike prior studies that used instruments with skip-out criteria (Cramer et al., 2010), all participants rated each symptom, resulting in more accurate network estimates. Second, data were collected as part of standard clinical care and therefore obtained from individuals who may not typically participate in research. Third, the sample had a range of DSM diagnoses and severity levels. Given that comorbidity is more common than not, this sample provided a more realistic depiction of psychopathology than studies that screen out people with comorbid disorders. Additionally, a diverse diagnostic clinical sample likely provides increased endorsement and variability among all symptoms, including symptoms outside of participants’ diagnosed disorder(s), as compared to a general population sample, which may have a restricted range due to a large number of healthy individuals that endorse few or no symptoms.
The current study also had several limitations. First, the edges were calculated with cross-sectional data, precluding estimations of important network characteristics, such as the direction of edges or cyclical, self-reinforcing edges. Furthermore, cross-sectional edges represent both within- and between-subjects effects that cannot be disentangled (Hamaker, 2012). Experimental and prospective designs are required to test the assumptions underlying the causal systems perspective. Second, we do not report any goodness-of-fit metrics for networks, although the qgraph glasso and EBIC procedure conducts model comparison that maximizes fit. Third, we relied on self-report measures available from an existing database, and some items aggregated symptoms (e.g., combining insomnia and hypersomnia). Fourth, we used single-items to measure each symptom. This approach is crude (Fried & Nesse, 2015) given that there are entire research areas on some nodes included here (e.g., anhedonia, Treadway & Zald, 2011). Furthermore, some nodes may actually be measuring overlapping constructs (e.g. “too much worry” and “unable to control worry,”), which could artificially inflate edge weights and centrality. Currently, there is no canonical approach within networks to determine topological overlap (Oldham et al., 2008; Zhang and Horvath, 2005) and combine overlapping items. Fifth, the sample was limited in ethno-racial diversity. Finally, a high degree of comorbidity in our sample rendered subgroup analyses of “pure” diagnostic categories (e.g. those with just MDD) impossible, though this limitation is offset by the greater ecological validity of a highly comorbid sample.
In conclusion, consistent with the DSM, we found that anhedonia, sad mood, and worry were the most central symptoms of depression and anxiety. Although we found that anxiety and depression symptoms were more connected within-disorder than between-disorders, we identified a few potential edges bridging anxiety and depression. We also identified highly central items within each that would be prime candidates for future longitudinal and experimental research efforts to confirm their causal role and to identify their genetic, neurological, and cognitive underpinnings.
Acknowledgments
We are grateful to Denny Borsboom, Richard J. McNally, and Alexandra Silverman for their comments on a draft of the manuscript and to the patients and staff of the Behavioral Health Partial Hospital at McLean Hospital. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
References
- Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–1068. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: 2013. [Google Scholar]
- Andrews G, Slade T, Issakidis C. Deconstructing current comorbidity: data from the Australian National Survey of Mental Health and Well-Being. British Journal of Psychiatry. 2002;181(4):304–314. doi: 10.1192/bjp.181.4.306. [DOI] [PubMed] [Google Scholar]
- Avenevoli S, Stolar M, Li J, Dierker L, Ries Merikangas K. Comorbidity of depression in children and adolescents: models and evidence from a prospective high-risk family study. Biol Psychiatry. 2001;49(12):1071–81. doi: 10.1016/s0006-3223(01)01142-8. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, … Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of affective disorders. 2011;135(1):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Riemann D. Is chronic insomnia a precursor to major depression Epidemiological and biological findings. Current Psychiatry Reports. 2012;14(5):511–518. doi: 10.1007/s11920-012-0308-5. [DOI] [PubMed] [Google Scholar]
- Barrat A, Barthélemy M, Vespignani A. Dynamical processes on complex networks. Cambridge: Cambridge University Press; 2012. (Reprint edition) [Google Scholar]
- Beard C, Björgvinsson T. Commentary on Psychological Vulnerability: An Integrative Approach. Journal of Integrative Psychotherapy. 2013;23(3):281–283. [Google Scholar]
- Beard C, Björgvinsson T. Beyond generalized anxiety disorder: Psychometric properties of the GAD-7 in a heterogeneous psychiatric sample. Journal of Anxiety Disorders. 2014;28(6):547–552. doi: 10.1016/j.janxdis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Beard C, Hsu KJ, Rifkin LS, Busch A, Björgvinsson T. Validation of the PHQ-9 in a psychiatric sample. Journal of Affective Disorders. 2016;193:267–273. doi: 10.1016/j.jad.2015.12.075. [DOI] [PubMed] [Google Scholar]
- Björgvinsson T, Kertz SJ, Bigda-Peyton J, Rosmarin D, Aderka I, Neuhaus E. Effectiveness of cognitive behavior therapy for severe mood disorders in an acute naturalistic setting: A benchmarking study. Cognitive Behaviour Therapy. 2014;43:209–220. doi: 10.1080/16506073.2014.901988. [DOI] [PubMed] [Google Scholar]
- Blanco C, Rubio JM, Wall M, Secades-Villa R, Beesdo-Baum K, Wang S. The latent structure and comorbidity patterns of generalized anxiety disorder and major depressive disorder: a national study. Depress Anxiety. 2014;31(3):214–222. doi: 10.1002/da.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D. Psychometric perspectives on diagnostic systems. Journal of Clinical Psychology. 2008;64(9):1089–1108. doi: 10.1002/jclp.20503. [DOI] [PubMed] [Google Scholar]
- Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology. 2013;9:91–121. doi: 10.1146/annurev-clinpsy-050212-185608. [DOI] [PubMed] [Google Scholar]
- Borsboom D, Epskamp S, Kievit RA, Cramer AO, Schmittmann VD. Transdiagnostic Networks Commentary on Nolen-Hoeksema and Watkins 2011. Perspectives on Psychological Science. 2011;6(6):610–614. doi: 10.1177/1745691611425012. [DOI] [PubMed] [Google Scholar]
- Borsboom D, Mellenbergh GJ, Van Heerden J. The theoretical status of latent variables. Psychological Review. 2003;110(2):203. doi: 10.1037/0033-295X.110.2.203. [DOI] [PubMed] [Google Scholar]
- Bringmann LF, Lemmens LH, Huibers MJ, Borsboom D, Tuerlinckx F. Revealing the dynamic network structure of the Beck Depression Inventory-II. Psychol Med. 2015;45(4):747–757. doi: 10.1017/S0033291714001809. [DOI] [PubMed] [Google Scholar]
- Brown TA, Marten PA, Barlow DH. Discriminant validity of the symptoms constituting the DSM-III-R and DSM-IV associated symptom criterion of generalized anxiety disorder. Journal of Anxiety Disorders. 1995;9:317–328. [Google Scholar]
- Courrieu P, Brand-D’abrescia M, Peereman R, Spieler D, Rey A. Validated intraclass correlation statistics to test item performance models. Behavior Research Methods. 2010;43(1):37–55. doi: 10.3758/s13428-010-0020-5. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Meier MH, Ramrakha S, Shalev I, Poulton R, Moffitt TE. The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clin Psychol Sci. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen Z. Extended Bayesian information criteria for model selection with large model spaces. Biometrika. 2008;95(3):759–771. [Google Scholar]
- Cramer AO, Waldorp LJ, van der Maas HL, Borsboom D. Comorbidity: A network perspective. Behavioral and Brain Sciences. 2010;33(2–3):137–150. doi: 10.1017/S0140525X09991567. [DOI] [PubMed] [Google Scholar]
- Cramer AO, Borsboom D, Aggen SH, Kendler KS. The pathoplasticity of dysphoric episodes: differential impact of stressful life events on the pattern of depressive symptom inter-correlations. Psychological Medicine. 2012;42(5):957–965. doi: 10.1017/S003329171100211X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Barlow DH. Mastery of Your Anxiety and Worry: Workbook (Treatments That Work) Oxford University Press; New York: NY: 2006. [Google Scholar]
- Davis RN, Nolen-Hoeksema S. Cognitive inflexibility among ruminators and nonruminators. Cognitive Therapy and Research. 2000;24(6):699–711. [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Epskamp S, Cramer AO, Waldorp LJ, Schmittmann VD, Borsboom D. Qgraph: Network visualizations of relationships in psychometric data. Journal of Statistical Software. 2012;48(4):1–18. [Google Scholar]
- Epskamp S, Borsboom D, Fried EI. Estimating Psychological Networks and their Stability: a Tutorial Paper. 2016 Apr 28; doi: 10.3758/s13428-017-0862-1. ArXiv E-Prints, 1604, arXiv:1604.08462. Retrieved June 14, 2016. from the arXiv database. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S, Fried EI. A Primer on Estimating Regularized Psychological Networks. 2016 Jul 5; arXiv:1607.01367 Retrieved from http://arxiv.org/abs/1607.01367 Retrieved July 6, 2016, from the arXiv database.
- Epskamp S, Maris G, Waldorp LJ, Borsboom D. Network Psychometrics. In: Irwing P, Hughes D, Booth T, editors. Handbook of Psychometrics. New York: Wiley; (in press) [Google Scholar]
- Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, Gibbons R. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147(9):1189–1194. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- Ferentinos PP, Kontaxakis VP, Havaki-Kontaxaki BJ, Dikeos DG, Papadimitriou GN. Fatigue and somatic anxiety in patients with major depression. Psychiatriki. 2009;20(4):312–318. [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Foygel R, Drton M. Extended Bayesian Information Criteria for Gaussian Graphical Models. Advances in Neural Information Processing Systems. 2010;23:604–612. arXiv:1011.6640. [Google Scholar]
- Fried EI, Nesse RM. The impact of individual depressive symptoms on impairment of psychosocial functioning. PLoS One. 2014;9(2):e90311. doi: 10.1371/journal.pone.0090311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, Nesse RM. Depression sum scores don’t add up: Why analyzing individual depression symptoms is essential. BMC Medicine. 2015 doi: 10.1186/s12916-015-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI. Problematic assumptions have slowed down depression research: why symptoms, not syndromes are the way forward. Frontiers in Psychology. 2015;6:309. doi: 10.3389/fpsyg.2015.00309. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, Nesse RM. Depression is not a consistent syndrome: an investigation of unique symptom patterns in the STAR* D study. Journal of affective disorders. 2015;172:96–102. doi: 10.1016/j.jad.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, Bockting C, Arjadi R, Borsboom D, Amshoff M, Cramer AO, Epskamp S, Tuerlinckx F, Carr D, Stroebe M. From Loss to Loneliness: The Relationship Between Bereavement and Depressive Symptoms. Journal of Abnormal Psychology. 2015 doi: 10.1037/abn0000028. http://doi.org/10.1037/abn0000028. [DOI] [PubMed]
- Fried EI, Epskamp S, Nesse RM, Tuerlinckx F, Borsboom D. What are “Good” Depression Symptoms? Comparing the Centrality of DSM and non-DSM symptoms of depression in a network analysis. Journal of Affective Disorders. 2016;189:314–320. doi: 10.1016/j.jad.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Fried EI, van Borkulo CD, Epskamp S, Schoevers RA, Tuerlinckx F, Borsboom F. Measuring Depression over Time … or not? Lack of Unidimensionality and Longitudinal Measurement Invariance in Four Common Rating Scales of Depression. Psychological Assessment. 2016 doi: 10.1037/pas0000275. http://dx.doi.org/10.1037/pas0000275. [DOI] [PubMed]
- Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008;9(3):432–441. doi: 10.1093/biostatistics/kxm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. glasso: Graphical lasso- estimation of Gaussian graphical models (Version 1.8) 2014. [Google Scholar]
- Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Software: Practice and Experience. 1991;21(11):1129–1164. [Google Scholar]
- Grömping U. Relative importance for linear regression in R: the package relaimpo. Journal of Statistical Software. 2006;17(1):1–27. [Google Scholar]
- Hamaker EL. Why researchers should think within-person: A paradigmatic view. Handbook of research methods for studying daily life. 2012:43–61. [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez J, Conde D. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. The Diagnosis of Mental Disorders: The Problem of Reification. Annual Review of Clinical Psychology. 2010;6(1):155–179. doi: 10.1146/annurev.clinpsy.3.022806.091532. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jacobi F, Wittchen HU, Holting C, Höfler M, Pfister H, Müller N, Lieb R. Prevalence, co-morbidity and correlates of mental disorders in the general population: results from the German Health Interview and Examination Survey (GHS) Psychol Med. 2004;34(4):597–611. doi: 10.1017/S0033291703001399. [DOI] [PubMed] [Google Scholar]
- Jansson-Fröjmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. Journal of Psychosomatic Research. 2008;64(4):443–449. doi: 10.1016/j.jpsychores.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Johnson JW, LeBreton JM. History and Use of Relative Importance Indices in Organizational Research. Organizational Research Methods. 2004;7(3):238–257. [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117(1):182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17(12):1174–9. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J Nerv Ment Dis. 1998;186(11):661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Toward a philosophical structure for psychiatry. American Journal of Psychiatry. 2005;162(3):433–440. doi: 10.1176/appi.ajp.162.3.433. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Gatz M, Pedersen NL. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol Med. 2007;37(3):453–62. doi: 10.1017/S0033291706009135. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Explanatory models for psychiatric illness. The American Journal of Psychiatry. 2008;165(6):695–702. doi: 10.1176/appi.ajp.2008.07071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder: Same genes, (partly) different environments? Archives of General Psychiatry. 1992;49(9):716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- Kertz SJ, Bigda-Peyton JS, Rosmarin DH, Björgvinsson T. The importance of worry across diagnostic presentations: Prevalence, severity, and associated symptoms in a partial hospital setting. Journal of Anxiety Disorders. 2012;26:126–133. doi: 10.1016/j.janxdis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the united states: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, DuPont RL, Berglund P, Wittchen HU. Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. Am J Psychiatry. 1999;156(12):1915–1923. doi: 10.1176/ajp.156.12.1915. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Chiu WT, Demler O, Heeringa S, Hiripi E, Jin R, Pennell BE, Walters EE, Zaslavsky A, Zheng H. The US National Comorbidity Survey Replication (NCS-R): design and field procedures. Int J Methods Psychiatr Res. 2004;13(2):69–92. doi: 10.1002/mpr.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Merikangas KR, Wang PS. Prevalence, Comorbidity, and Service Utilization for Mood Disorders in the United States at the Beginning of the Twenty-first Century. Annual Review of Clinical Psychology. 2007;3(1):137–158. doi: 10.1146/annurev.clinpsy.3.022806.091444. [DOI] [PubMed] [Google Scholar]
- Kolaczyk ED, Csárdi G. Statistical Analysis of Network Data with R. Vol. 65. New York, NY: Springer New York; 2014. [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;9:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, Monohan PO, Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317–25. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA. The structure and stability of common mental disorders (DSM-III-R): A longitudinal-epidemiological study. Journal of Abnormal Psychology. 1998;107(2):216–227. doi: 10.1037//0021-843x.107.2.216. http://doi.org/10.1037/0021-843X.107.2.216. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56(10):921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Understanding Psychopathology: Melding Behavior Genetics, Personality, and Quantitative Psychology to Develop an Empirically Based Model. Current Directions in Psychological Science. 2006;15(3):113–117. doi: 10.1111/j.0963-7214.2006.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen SL. Graphical Models. Clarendon Press; 1996. [Google Scholar]
- Lindeman R, Merenda P, Gold R. Introduction to Bivariate and Multivariate Analysis. Glenview, IL: Scott, Foresman and Company; 1980. [Google Scholar]
- Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, Herzberg PY. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Robinaugh DJ, Wu GW, Wang L, Deserno MK, Borsboom D. Mental disorders as causal systems: A network approach to Posttraumatic Stress Disorder. Clinical Psychological Science. 2014:1–14. [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and Generalized Anxiety Disorder: cumulative and sequential comorbidity in a birth chort followed prospectively to age 32 years. Archives of General Psychiatry. 2007;64:651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- Molenaar PCM. Latent variable models are network models. Behavioral and Brain Sciences. 2010;33(2–3):166–166. doi: 10.1017/S0140525X10000798. http://doi.org/10.1017/S0140525X10000798. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide. Los Angeles: Muthen & Muthen; 1998. [Google Scholar]
- Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30(7):873–880. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M, Barabási A-L, Watts DJ. The structure and dynamics of networks. 1. Princeton: Princeton University Press; 2006. [Google Scholar]
- Newman ME, Girvan M. Finding and evaluating community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69(2 Pt 2):026113. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic modelsof psychopathology explaining multifinality and divergent trajectories. Perspectives on Psychological Science. 2011;6(6):589–609. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- Novati A, Roman V, Cetin T, Hagewoud R, den Boer JA, Luiten PG, Meerlo P. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31(11):1579–1585. doi: 10.1093/sleep/31.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Statistical Methods in Medical Research. 2003;12(5):419–446. doi: 10.1191/0962280203sm341ra. http://doi.org/10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. URL http://www.R-project.org/ [Google Scholar]
- Rhebergen D, van der Steenstraten IM, Sunderland M, de Graaf R, Ten Have M, Lamers F, Penninx BW, Andrews G. An examination of generalized anxiety disorder and dysthymic disorder by latent class analysis. Psychol Med. 2014;44(8):1701–1712. doi: 10.1017/S0033291713002225. [DOI] [PubMed] [Google Scholar]
- Robinaugh DJ, LeBlanc NJ, Vuletich HA, McNally RJ. Network analysis of persistent complex bereavement disorder in conjugally bereaved adults. Journal of Abnormal Psychology. 2014;123(3):510–522. doi: 10.1037/abn0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinaugh DJ, Millner AJ, McNally RJ. Identifying Highly Influential Nodes in the Complicated Grief Network. Journal of Abnormal Psychology. doi: 10.1037/abn0000181. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MA, Neale MC, Pedersen NL, Mathé AA, Kendler KS. A twin study of generalized anxiety disorder and major depression. Psychological Medicine. 1995;25(5):1037–1049. doi: 10.1017/s0033291700037533. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Cole JO. Benzodiazepines in depressive disorders. Arch Gen Psychiatry. 1978;35(11):1359–1365. doi: 10.1001/archpsyc.1978.01770350085008. [DOI] [PubMed] [Google Scholar]
- Schmittmann VD, Cramer AO, Waldorp LJ, Epskamp S, Kievit RA, Borsboom D. Deconstructing the construct: A network perspective on psychological phenomena. New Ideas in Psychology. 2013;31(1):43–53. [Google Scholar]
- Schoevers RA, Deeg DJ, van Tilburg W, Beekman AT. Depression and generalized anxiety disorder: co-occurrence and longitudinal patterns in elderly patients. Am J Geriatr Psychiatry. 2005;13(1):31–39. doi: 10.1176/appi.ajgp.13.1.31. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan HK, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Sivertsen B, Salo P, Mykletun A, Hysing M, Pallesen S, Krokstad S, … Øverland S. The bidirectional association between depression and insomnia: the HUNT study. Psychosomatic Medicine. 2012;74(7):758–765. doi: 10.1097/PSY.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Stefanopoulou E, Hirsch CR, Hayes S, Adlam A, Coker S. Are attentional control resources reduced by worry in generalized anxiety disorder? Journal of Abnormal Psychology. 2014;123(2):330–335. doi: 10.1037/a0036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society. Series B (Methodological) 1996;58(1):267–288. [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience & Biobehavioral Reviews. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. http://doi.org/10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick GJ, Snowden L, Hastings J. Heterogeneity in comorbidity between major depressive disorder and generalized anxiety disorder and its clinical consequences. J Nerv Ment Dis. 2009;197(4):215–224. doi: 10.1097/NMD.0b013e31819d954f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Balkom AJ, van Boeijen CA, Boeke AJ, van Oppen P, Kempe PT, van Dyck R. Comorbid depression, but not comorbid anxiety disorders, predicts poor outcome in anxiety disorders. Depress Anxiety. 2008;25(5):408–415. doi: 10.1002/da.20386. [DOI] [PubMed] [Google Scholar]
- van Borkulo C, Boschloo L, Borsboom D, Penninx BH, Waldorp LJ, Schoevers RA. Association of symptom network structure with the course of longitudinal depression. JAMA Psychiatry. 2015:1219–1226. doi: 10.1001/jamapsychiatry.2015.2079. http://doi.org/10.1001/jamapsychiatry.2015.2079. [DOI] [PubMed]
- Wadsworth ME, Hudziak JJ, Heath AC, Achenbach TM. Latent class analysis of child behavior checklist anxiety/depression in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2001;40(1):106–114. doi: 10.1097/00004583-200101000-00023. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Kessler RC, Pfister H, Lieb M. Why do people with anxiety disorders become depressed? A prospective-longitudinal community study. Acta Psychiatr Scand Suppl. 2000;406:14–23. [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Kirov R, Rothenberger A. Comorbidity in the context of neural network properties. Behavioral and Brain Sciences. 2010;33(2–3):176–177. doi: 10.1017/S0140525X1000083X. http://doi.org/10.1017/S0140525X1000083X. [DOI] [PubMed] [Google Scholar]
- Zavos HMS, Rijsdijk FV, Eley TC. A longitudinal, genetically informative, study of associations between anxiety sensitivity, anxiety, and depression. Behavior Genetics. 2012;42(4):592–602. doi: 10.1007/s10519-012-9535-0. [DOI] [PubMed] [Google Scholar]
- Zbozinek TD, Rose RD, Wolitzky-Taylor KB, Sherbourne C, Sullivan G, Stein MB, Roy-Byrne PP, Craske MG. Diagnostic overlap of generalized anxiety disorder and major depressive disorder in a primary care sample. Depress Anxiety. 2012;29(12):1065–1071. doi: 10.1002/da.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]


