Abstract
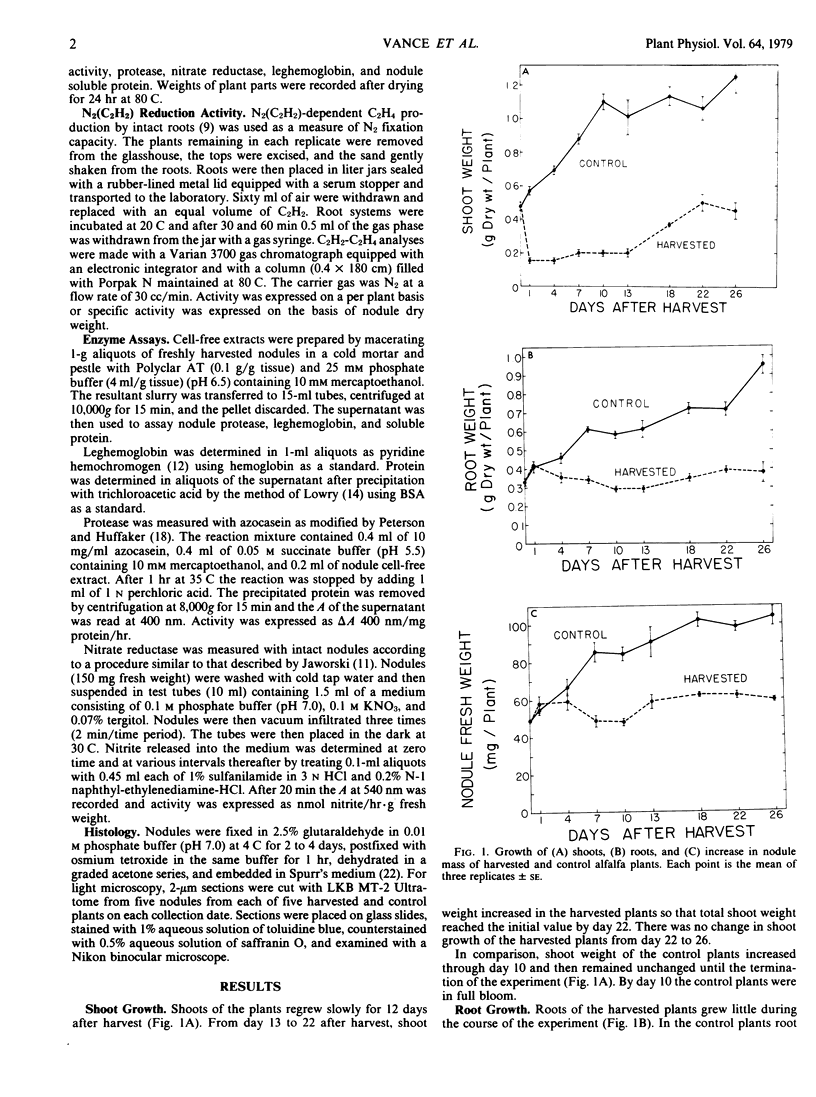
Nitrogenase-dependent acetylene reduction, nodule function, and nodule regrowth were studied during vegetative regrowth of harvested (detopped) alfalfa (Medicago sativa L.) seedlings grown in the glasshouse. Compared with controls, harvesting caused an 88% decline in acetylene reduction capacity of detached root systems within 24 hours. Acetylene reduction in harvested plants remained low for 13 days, then increased to a level comparable to the controls by day 18.
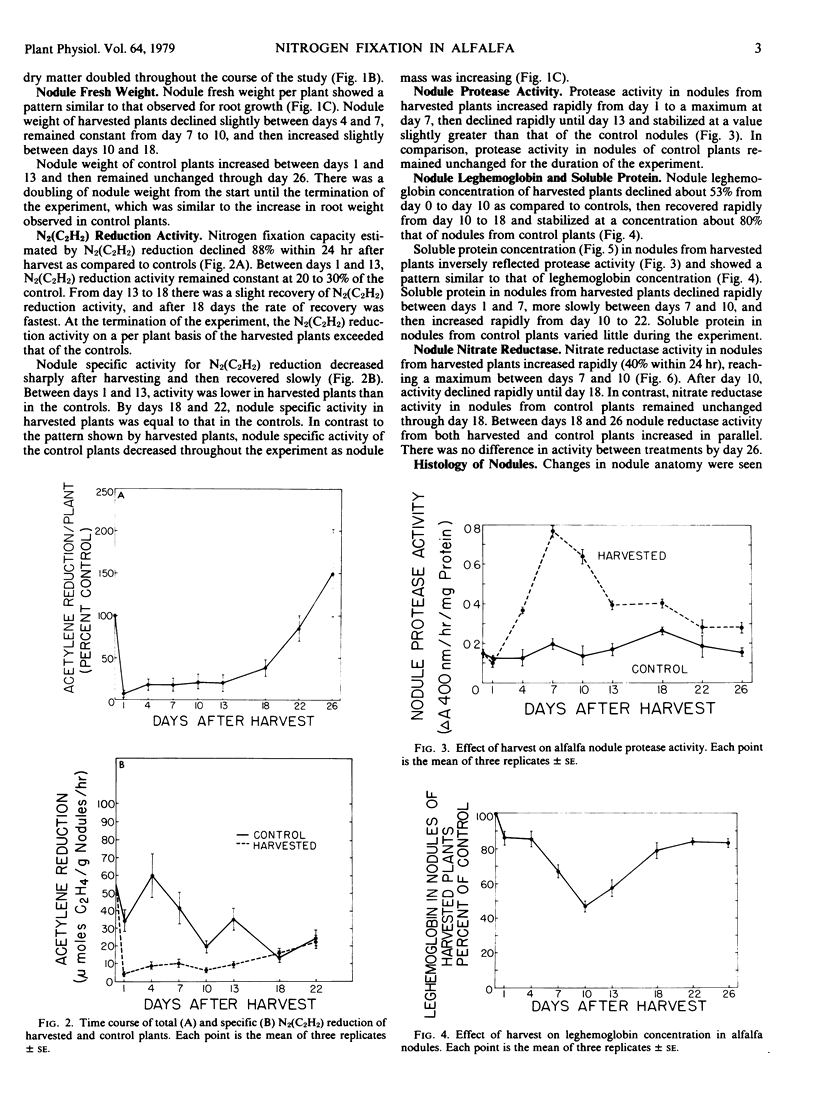
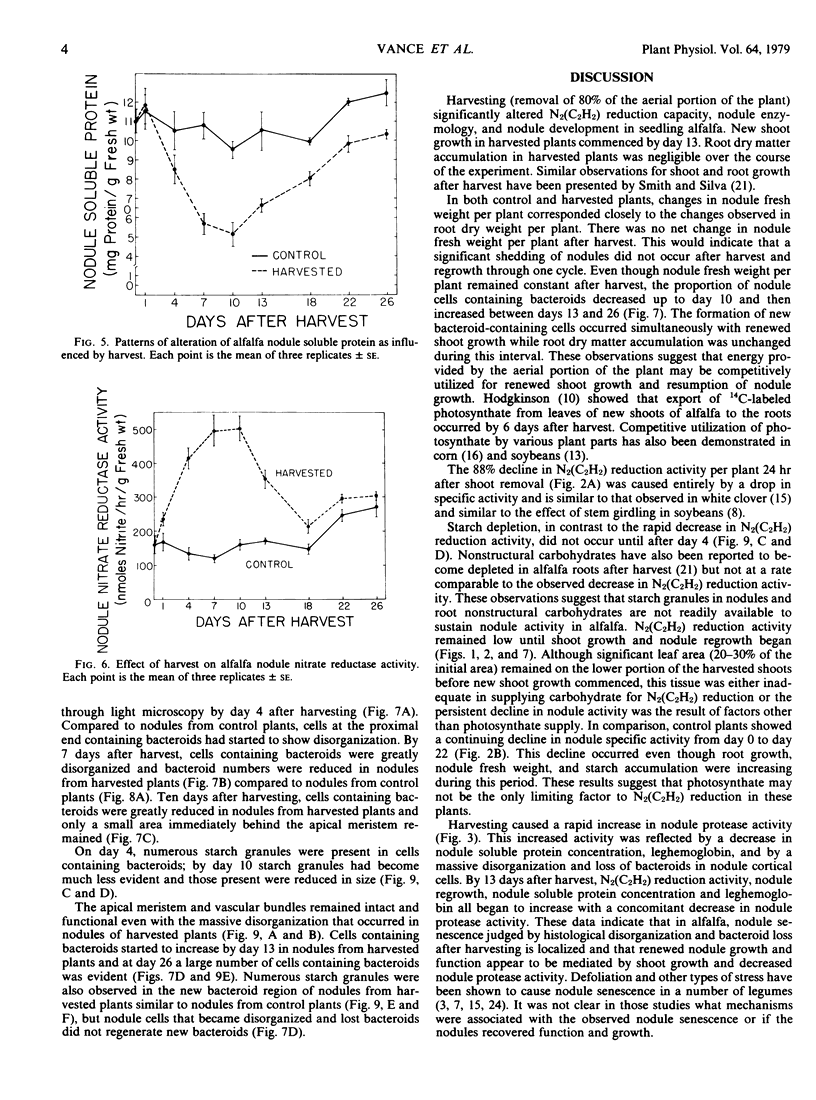
Protease activity increased in nodules from harvested plants, reached a maximum at day 7 after harvest, and then declined to a level almost equal to the control by day 22 after harvest. Soluble protein and leghemoglobin decreased in nodules from harvested plants in an inverse relationship to protease activity.
Nitrate reductase activity of nodules from harvested plants increased significantly within 24 hours and was inversely associated with acetylene reduction. The difference in nitrate reductase between nodules from harvested plants and control plants became less evident as shoot regrowth occurred and as acetylene reduction increased in the harvested plants.
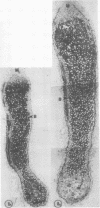
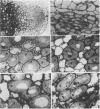
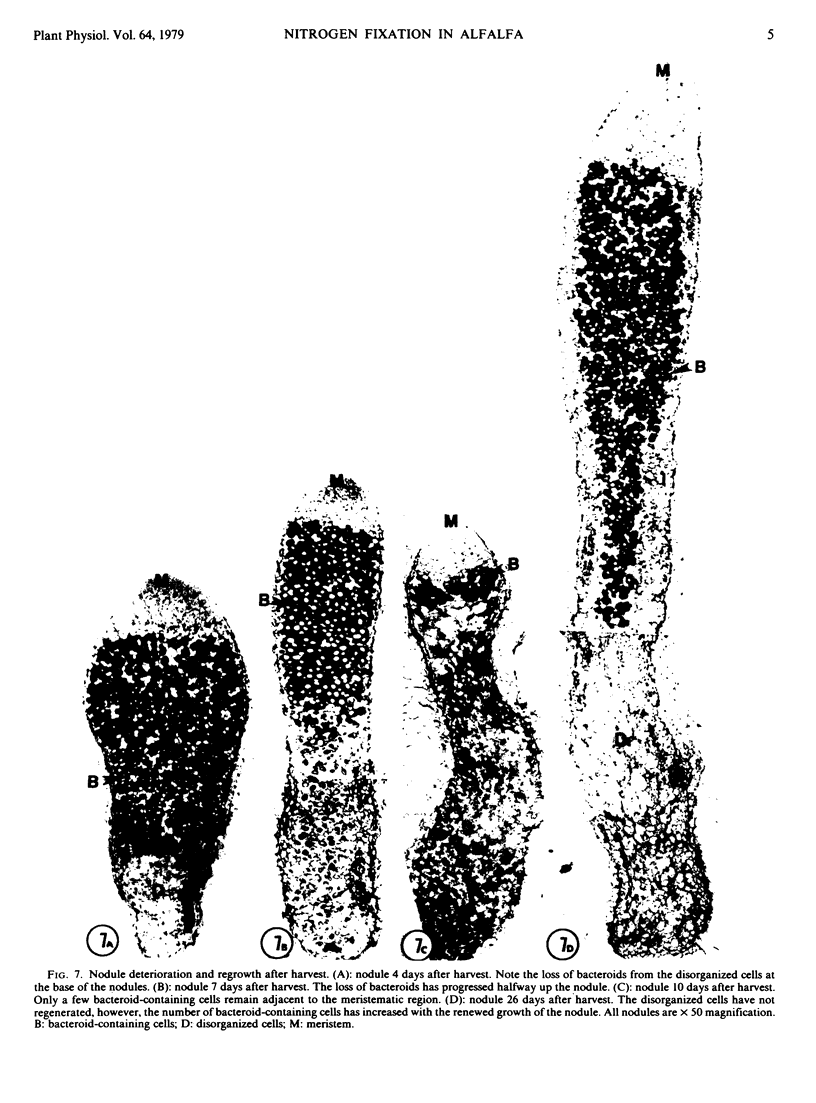
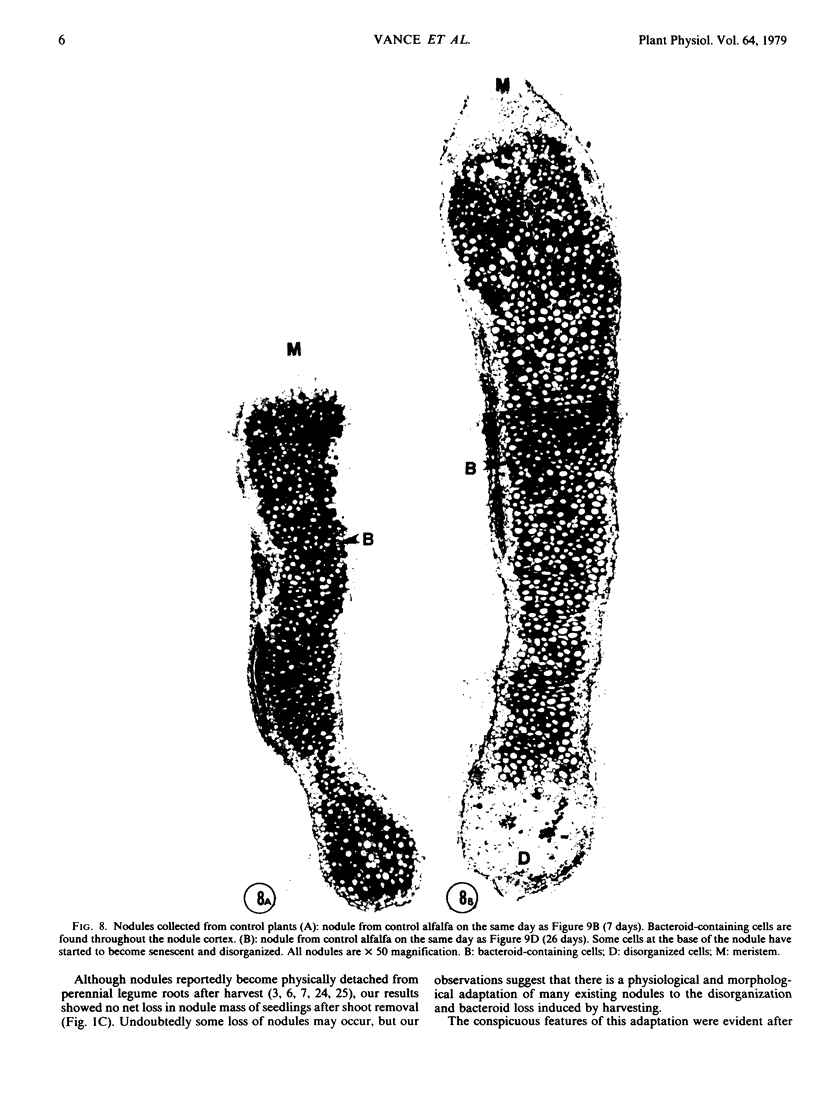
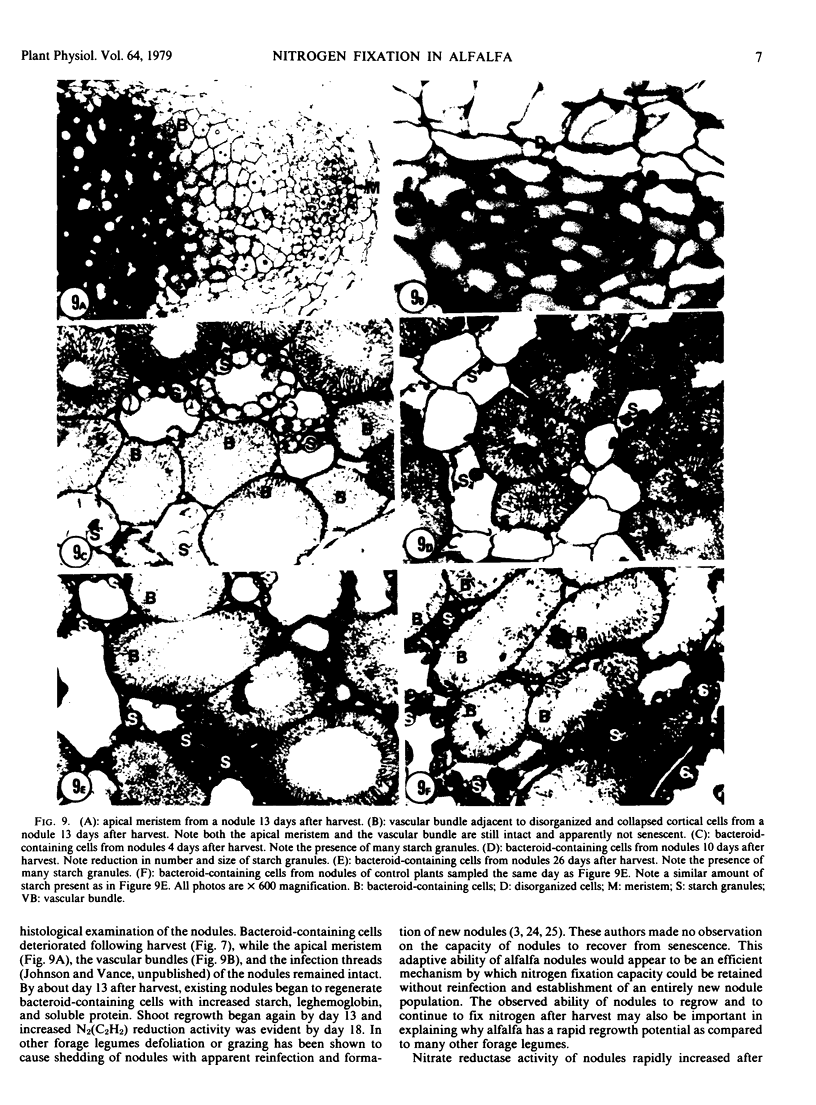
No massive loss of nodules occurred after harvest as evidenced by little net change in nodule fresh weight. There was, however, a rapid localized senescence which occurred in nodules of harvested plants. Histology of nodules from harvested plants showed that they degenerated at the proximal end after harvest. Starch in the nodule was depleted by 10 days after harvest. The meristem and vascular bundles of nodules from harvested plants remained intact. The senescent nodules began to regrow and fix nitrogen after shoot growth resumed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen P. C., Phillips D. A. Induction of Root Nodule Senescence by Combined Nitrogen in Pisum sativum L. Plant Physiol. 1977 Mar;59(3):440–442. doi: 10.1104/pp.59.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G., Evans H. J. Physiological Studies on Nodule-Nitrate Reductase. Plant Physiol. 1960 Jul;35(4):454–462. doi: 10.1104/pp.35.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski E. G. Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1274–1279. doi: 10.1016/s0006-291x(71)80010-4. [DOI] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Purification of horse-radish peroxidase and comparison of its properties with those of catalase and methaemoglobin. Biochem J. 1951 Jun;49(1):88–104. doi: 10.1042/bj0490088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurkovich G. V., Gol'dberg A. B., Soboleva I. P., Ignat'ev V. A., Ved'mina E. A. Primenenie dipasfena pri nekotorykh zabolevaniiakh verkhnikh dykhatel'nykh putei i ukha. Antibiotiki. 1969 May;14(5):464–467. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]