Abstract
BACKGROUND: FGFR2 amplification is associated with aggressive gastric cancer (GC), and targeted drugs have been developed for treatment of GC. We evaluated the antitumor activity of an FGFR inhibitor in FGFR2-amplified GC patients with peritoneal carcinomatosis. METHODS: Two GC patients with FGFR2 amplification confirmed by fluorescence in situ hybridization showed peritoneal seeding and malignant ascites. We used the patient-derived xenograft model; patient-derived cells (PDCs) from malignant ascites were used to assess FGFR2 expression and its downstream pathway using immunofluorescence analysis and immunoblot assay in vitro. Apoptosis and cell cycle arrest after treatment of FGFR inhibitor were analyzed by Annexin V-FITC assay and cell cycle analysis. RESULTS: FGFR2 amplification was verified in both PDC lines. AZD4547 as an FGFR inhibitor decreased proliferation of PDCs, and the IC50 value was estimated to be 250 nM in PDC#1 and 210 nM in PDC#2. FGFR inhibitor also significantly decreased levels of phosphorylated FGFR2 and downstream signaling molecules in FGFR2-amplified PDC lines. In cell cycle analysis, apoptosis was significantly increased in AZD4547-treated cells compared with nontreated cells. The proportion of cells in the sub-G1 stage was significantly higher in AZD4547-treated PDCs than in control cells. CONCLUSION: Our findings suggest that FGFR2 amplification is a relevant therapeutic target in GC with peritoneal carcinomatosis.
Introduction
Despite advances in targeted therapy for gastric cancer (GC) and an increase in early diagnoses, the prognosis of metastatic GC is still poor, and GC remains the second major cause of cancer death [1], [2]. A majority of GC patients are admitted with advanced, inoperable, or metastatic tumors, and the 5-year survival rate of patients with such advanced GC is only 3.1% [3], [4]. Peritoneal carcinomatosis (PC) accounts for 30% to 40% of the metastatic GC cases, and the peritoneum is the second most common tumor site after the liver [5], [6]. PC can cause bowel obstruction or malignant ascites and thus decrease the quality of life. Furthermore, as PC in GC is difficult to treat with conventional therapy, its prognosis is grave, and the median survival is estimated to be 1 to 3 months [7], [8].
Fibroblast growth factor receptors (FGFRs) are widely distributed transmembrane tyrosine kinase receptors that mediate development, differentiation, cell survival, migration and angiogenesis, as well as carcinogenesis [9], [10]. FGFR2 mutation, gene fusion, and gene amplification can cause progression of several types of cancers and resistance for drugs that target other oncogenic pathways [11], [12], [13]. According to recent study of FGFR aberration in 4835 solid tumors, FGFR2 mutations were found in 1.5% of cancers, and most aberrations were gene amplification [14]. These discoveries have spurred the development of anti-FGFR therapeutics for the treatment of various cancers [15].
In GC, multiple FGFR alterations also have been identified [16], [17]. Overexpression of the FGFR2 protein was demonstrated in the diffuse type of GC and correlated with aggressive behavior [18]. Many preclinical studies have suggested that FGFR2 amplification was associated with increased sensitivity to FGFR inhibitors both in vitro and in vivo, which led to an increased application of FGFR inhibitors for GC treatment [18], [19], [20]. A randomized phase II trial that compared the efficiency of AZD4547 and paclitaxel as second-line treatment methods for advanced GC and gastroesophageal junction cancer with FGFR2 amplification or polysomy has been completed (NCT01457846). However, the results of the interim analysis did not show any increase in survival benefit compared to paclitaxel [21], [22]. Recently, a monoclonal antibody against FGFR2b (FPA144) in FGFR2-amplified GC demonstrated a response rate of over 30% in salvage setting [23].
In this study, we established an FGFR2-amplified GC patient-derived cell (PDC) line and evaluated the antitumor efficacy of FGFR inhibitors.
Material and Methods
Patients
Malignant ascites were collected from patients after they signed the informed consent form provided by the SMC Institutional Review Board. The collected effusions (1-5 l) were distributed into 50-ml tubes, centrifuged at 1500 rpm for 10 minutes, and washed twice with PBS, as previously described [18]. Cell pellets were resuspended in the culture medium and were grown in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 1% antibiotic-antimycotic (Gibco BRL, Paisley, UK), 0.5 g/ml of hydrocortisone (Sigma Aldrich), 5 mg/ml of insulin (PeproTech, Rocky Hill, NJ), and 5 ng of EGF (PeproTech). Cells were maintained at 37°C in a humidified 5% CO2 incubator, and the medium was changed twice a week. PDCs were passaged using TrypLE Express (Gibco BRL, Paisley, UK) to detach cells when the culture achieved 80% to 90% confluence.
Fluorescence In Situ Hybridization (FISH)
Tumor sections were cut to 1-μm thickness and deparaffinized by incubation for 30 minutes with the pretreatment reagent (Abbott, 30-801250) at 80°C. Protease digestion was achieved by treatment with the protease reagent (Abbott, 30-801255) for 20 minutes at 37°C. FGFR2 probes (LSI FGFR2 Spectrum Orange Probe, 08N42-020) and CEP 10 (Spectrum Green Probe, 06J37-020) from Vysis (Abbott Molecular, IL) were hybridized at 73°C for 5 minutes and then at 37°C for 20 hours. After hybridization, slides were washed in 2× saline-sodium citrate/0.3% NP-40 at 72°C for 5 minutes, air dried, and counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) I and DAPI II (Abbott Molecular). Slides were then examined under a fluorescence microscope equipped with Spectrum Texas Red with isothiocyanate and DAPI filters. The FGFR2/CEP 10 ratio was estimated after counting at least 50 tumor cell nuclei. For all samples evaluated with FISH, IHC-negative stained areas in the tumors were also evaluated to determine the specificity of the IHC test. An FGFR2/CEP 10 ratio higher than 2.0 was interpreted as gene amplification positive. If the FGFR2 copy number was greater than 4 in the absence of gene amplification, FGFR2 polysomy was assumed.
Immunofluorescence
Cells were grown on four-chamber slides in an appropriate growth medium supplemented with 10% FBS for 5 days and then fixed with 4% paraformaldehyde for 20 minutes at room temperature. After washing three times with PBS, cells were treated with 0.25% Triton X-100 in PBS for 15 minutes at room temperature, blocked with 5% BSA in PBS for 30 minutes, and incubated overnight at 4°C with specific primary antibodies FGFR2 (1:25) from Cell Signaling Technology (Beverly, MA) and Phalloidin (1:100) from Thermo Fisher Scientific (Paisley, UK). After this incubation, samples were incubated for 1 hour at room temperature with the corresponding secondary antibodies (Alexa Fluor 488 donkey anti-rabbit, Thermo Fisher Scientific) and mounted. DAPI images were acquired with a confocal laser scanning microscope (LSM 780, Zeiss, Jena, Germany).
Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Primers for amplifying FGFR2 were constructed based on the PubMed sequence NM_000141.2. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers obtained from Bioneer were used as the internal control. The reaction conditions were as follows: 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds, 56°C for 30 seconds, and 72°C for 30 seconds. Real-time PCR amplifications were performed using an ABI PRISM 7000 cycler (Applied Biosystems), and threshold cycle numbers were transformed using the ΔCT comparative method. Each sample was analyzed in triplicate. Gene-specific expression values were normalized to the expression value of GAPDH within each sample. The amount of target, normalized to an endogenous control and relative to a calibrator, was determined by the comparative CT method (ΔΔCT). In brief, the ΔCT value was determined by subtracting the average of GAPDH CT values from the average of the target gene values for each sample. The ΔΔCT calculation involves subtraction of the ΔCT calibrator value.
Cell Proliferation Inhibition Assay
Cells (7 × 103 cells in 100 μl/well) were seeded on 96-well plates, incubated for 24 hours at 37°C, and treated with AZD4547 for 5 days at 37°C. After this drug treatment, 100 μl of the CTG reagent (CellTiter-Glo, Promega, Madison, WI) was added to each well. The luminescence signal was recorded using the Mithras2 LB 943 Monochromator Multimode Reader (BERTHOLD TECHNOLOGIES GmbH & Co. KG, Germany).
Immunoblot Analysis
Total cell extracts were obtained using the RIPA buffer (Thermo Fisher Scientific, Waltham, MA) containing a protease inhibitor cocktail (Roche, Mannheim, Germany) and a phosphatase inhibitor cocktail (Roche). Equal amounts (30 μg) of cell lysates were dissolved in 8% or 12% Bis-Tris gels with MOPS running buffer (Thermo Fisher Scientific), transferred onto nitrocellulose membranes, and incubated with antibodies against the following: pFGFR1/2 (Y463/Y466, cat # PA5-37816, ThermoFisher, USA), FGFR2 (cat # 11835, Cell Signaling, MA, USA), pAkt (Ser473, cat # 9018, Cell Signaling), Akt (cat # 9272, Cell Signaling), pMEK (cat # 2338, Cell Signaling), MEK (cat # 9122, Cell Signaling, pERK (Thr202/Tyr204, cat # 4370, Cell Signaling), ERK (Thr202/Tyr204, cat # 9102, Cell Signaling), and beta actin (Sigma Aldrich). Primary antibody diluted 1:1000 in 3% BSA and beta-actin diluted 1:5000 in 3% nonfat dry milk. Immune complexes were visualized by enhanced chemiluminescence using the ECL Western Blotting Substrate (Thermo Fisher Scientific).
Annexin V Assay
Cells (1 × 106 cells/well) were seeded in a 60-mm dish and incubated for 24 hours before treatment with 1 μM AZD4547 for 5 days. After washing twice with PBS, the cells were stained using the Annexin V-FITC/Propidium iodide apoptosis kit (BD Bioscience, San Jose, CA) according to the manufacturer's instructions. Stained cells were detected and analyzed using FACSverse (Becton Dickinson, San Jose, CA).
Cell Cycle Analysis
For cell cycle analysis, cultured cells were removed with trypsin, fixed with 70% ethanol, and incubated for 20 minutes on ice. They were then stained with propidium iodide (20 μg/ml of propidium iodide, 200 μg/ml of DNase-free RNase A, and 0.1% triton X-100, prepared fresh in PBS). Cellular DNA complement was analyzed using FACSverse.
Statistical Analysis
The statistical significance of differences in cell growth, apoptosis, and cell cycle between different groups was calculated using Student's t test. All P values less than .05 were considered statistically significant. All statistical tests were two-sided.
Results
Case Presentation
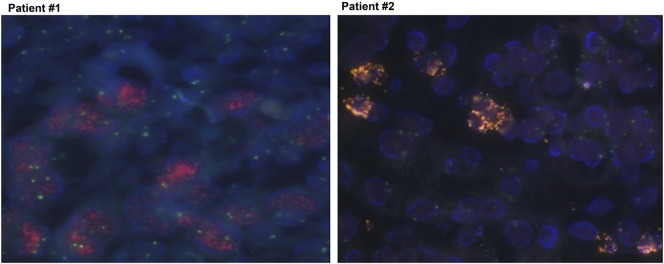
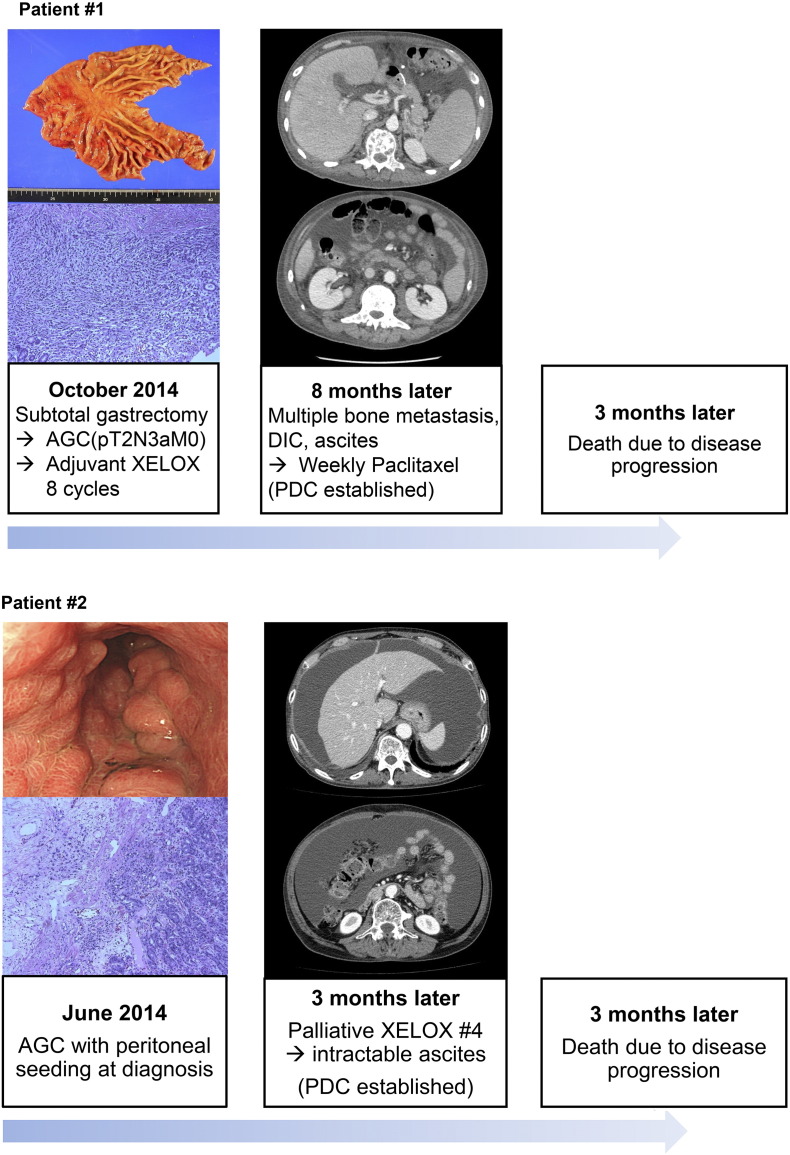
Two GC patients had confirmed FGFR2-amplification. Patient #1 was a 40-year-old male who complained of epigastric soreness and dyspepsia. He was diagnosed with advanced GC without metastasis and underwent curative subtotal gastrectomy. Pathological diagnosis was poorly differentiated tubular adenocarcinoma, and the TNM stage was IIIA (pT2N3aM0) according to AJCC seventh edition. Eight cycles of XELOX (capecitabine plus oxaliplatin) as adjuvant therapy were treated. Four months after the last cycle of adjuvant therapy, he complained of back pain. Computed tomographic scan and blood tests showed multiple bone metastases, further complicated by disseminated intravascular coagulopathy and malignant ascites. The cancer was refractory to weekly paclitaxel as well as irinotecan monotherapy. The FGFR2/CEP 10 ratio was 36, which was verified using FISH (Figure 1). A PDC line was established from the malignant ascites after obtaining informed consent (Figure 2).
Figure 1.
FGFR2 amplification in PDCs by FISH showing high-level amplification of target probe (red signal) to CEP-10 (green signal).
Figure 2.
Clinical features of two cases showing illustrations of clinical courses. Left panel demonstrated gross and microscopic images, and computed tomographic scan showed malignant ascites (middle panel).
Patient #2 was a 63-year-old male who presented with an abnormal finding in annual esophagogastroduodenoscopy screening. Endoscopic biopsy showed tubular adenocarcinoma, and peritoneal seeding was detected at the surgical field leading to open and closure. The disease progressed rapidly and was refractory to capecitabine/oxaliplatin chemotherapy. After four cycles of XELOX, the patient developed medically intractable malignant ascites and died of the disease. The patient's tumor was confirmed to have FGFR2 amplification by FISH with FGFR2/CEP 10 ratio of 42 (Figure 1). The PDC line was established from malignant ascites after obtaining informed consent (Figure 2).
Confirmation of FGFR2 Amplification in PDCs from Malignant Ascites of GC
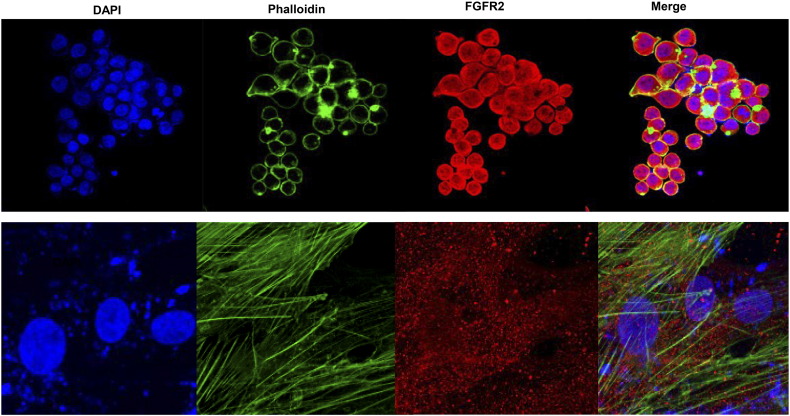
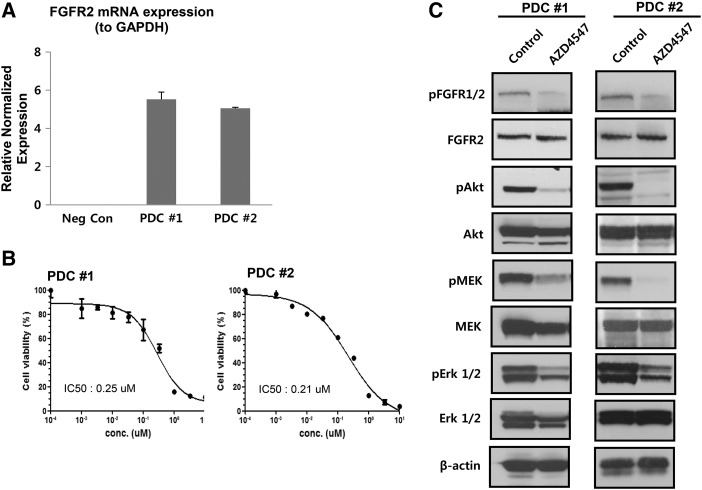
We performed immunofluorescence analysis for detection of FGFR2 expression in the cultured cell lines, as shown in Figure 3. Next, we tested the degree of FGFR2 gene amplification and protein expression in the established PDC lines. The FGFR2 expression values obtained from quantitative RT-PCR were normalized to GAPDH values within each sample (Figure 4A). We found that the FGFR2 gene was amplified in PDCs of GC in contrast to the negative control. To evaluate the antitumor effect of AZD4547, a novel selective FGFR inhibitor, FGFR2-amplified PDCs were treated for 5 days, after which cell viability was assessed using the CellTiter-Glo reagent. Figure 4B shows that AZD4547 inhibited the proliferation of PDCs, and the IC50 values were estimated to be 250 nM in PDC#1 and 210 nM in PDC#2.
Figure 3.
The blue color in the nucleus shows DAPI staining, the green color in the cytoskeleton shows phalloidin staining, and the red color is the FGFR signal. FGFR2 protein expression was observed in the cytoplasm.
Figure 4.
(A) Detection of FGFR2 gene amplification in FGFR2-amplified PDCs by quantitative RT-PCR analysis. (B) The viability of FGFR2-amplified PDCs was measured by CTG assay after treatment with various concentrations of AZD4547 for 5 days. Cell viability (%) represents the percentage of growth compared to the control (no treatment). IC50 values are 250 nM and 210 nM for PDC#1 and PDC#2, respectively. (C) Immunoblot assay for determining FGFR phosphorylation and targeted downstream pathways. Cells were treated with 1 μM AZD4547 for 5 days. Control cells were treated with DMSO.
To investigate the FGFR-signaling activity of AZD4547 in the PDCs, we examined the expression of FGFR2 and its downstream molecules in FGFR2-amplified PDCs using immunoblot assay. We performed the immunoblot assay after incubation with 1 μM of AZD4547. We evaluated levels of phosphorylated FGFR2 and its downstream signaling molecules including Akt, MEK, and ERK1/2. Treatment of the PDCs with AZD4547 attenuated the phosphorylation of downstream proteins, as levels in the treated cells were reduced compared with those in the control (Figure 4C).
Apoptosis and Cell Cycle Arrest Were Increased by AZD4547
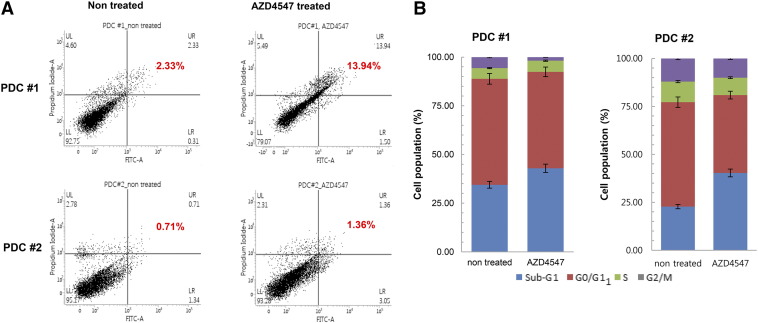
We also assessed the effect of AZD4547 on apoptosis of PDCs using Annexin V-FITC assay. Annexin V-FITC/PI labeling in cells was quantitatively measured by flow cytometry. Compared with nontreated cells, AZD4547-treated cells showed a consistent and statistically significant (P < .0001) increase in apoptosis (PDC#1: nontreated versus AZD4547-treated, mean apoptosis rate = 2.33 ± 0.41% and 13.94 ± 0.217%, respectively, P < .0001; PDC#2: nontreated versus AZD4547-treated, mean apoptosis rate = 0.71 ± 0.08% and 1.36 ± 0.12%, respectively, P < .0001) (Figure 5A). We then investigated the effect of AZD4547 on cell cycle progression of FGFR2-amplified PDCs. Cell cycle progression was analyzed using propidium iodide and FACSverse after treatment with AZD4547 for 5 days. As shown in Figure 5B, the proportion of cells in the sub-G1 stage was significantly higher in AZD4547-treated PDCs than in control cells (PDC#1: nontreated versus AZD4547-treated, mean proportion of cells in the sub-G1 stage =34.45 ± 0.52% and 42.94 ± 0.63%, P = .0002; PDC#2: nontreated versus AZD4547-treated, mean proportion of cells in the sub-G1 stage =22.73 ± 0.57% and 40.30 ± 0.72%, P < .0001).
Figure 5.
(A) Effect of AZD4547 on apoptosis of FGFR2-amplified PDCs. The AZD4547-treated PDC culture showed a higher apoptotic cell population (11.3%) than the control cell culture (0.65%) (P < .001). (B) Flow cytometry analysis of cell cycle of PDCs treated with 1 μM AZD4547 for 5 days. Cell cycle progression was analyzed using propidium iodide.
Discussion
Variations in the FGFR gene have been investigated in several solid tumors including breast cancer [24], non–small cell lung cancer [25], bladder cancer [26], and glioblastomas [27]. In particular, FGFR2 amplification has been correlated with lung cancer [28], colon cancer, and GC [11]. These findings have led to the development of FGFR inhibitors such as dovitinib, BGJ398, ponatinib, LY2874455, and AZD4547. Among these agents, AZD4547 has shown great promise in studies on FGFR-dysregulated cancers [29], [30]. AZD4547 also showed favorable therapeutic activity against GC with FGFR2 amplification in vitro and in vivo[20], and a phase II study on the efficacy of AZD4547 versus paclitaxel in advanced GC has been conducted (SHINE study, NCT01457846). However, the proportion of patients with PC was not provided in that study, and subgroup analysis of patients with PC was not performed [22]. Our results indicate that patients with advanced GC with PC might benefit from treatment with FGFR inhibitors.
Previous studies have reported that the prevalence of FGFR2 amplification in GC was 4% to 7% and that it was correlated with aggressive behavior [31], [32]. However, some intratumor heterogeneity of FGFR was reported in an exploratory biomarker analysis as part of the SHINE study [22]. In our study, FGFR was shown to be markedly overexpressed in PDCs from malignant ascites of GC. This finding suggests a possible correlation between FGFR2 amplification and peritoneal metastasis. FGFR2 amplification could be one of the key signals involved in peritoneal metastasis. In addition, a low concordance between elevated FGFR2 expression and FGFR2 amplification was reported in the SHINE study. Our study also showed a discordant result for FGFR2 expression when estimated using IHC and FISH. Tissue from PDC#2 was FGFR2 negative according to IHC but was found to be FGFR2 amplified according to FISH analysis.
Peritoneal dissemination is a common cause (30%-40%) of GC recurrence after curative resection, and more than 10% of patients with de novo GC exhibit PC at diagnosis [33], [34], [35]. The survival period of GC patients with PC is less than 6 months because effective therapy for these patients has not yet been developed [8]. To understand the therapeutic activity of FGFR inhibitors against GC with PC, we treated FGFR2-amplified PDCs from GC with AZD4547. Patient-derived xenograft model was used in our study; this model is preferable than traditional cell lines. Patient-derived xenograft model has more similarities to the parental tumors and shows improved predictive value for preclinical studies [36], [37]. We demonstrated that AZD4547 effectively blocked the phosphorylation of FGFR and its subsequent downstream proteins, induced cell-cycle arrest at the G1-S transition phase, and induced apoptosis in PDCs from malignant ascites of GC. These results imply that AZD4547 could be part of a novel therapeutic strategy for GC patients with PC.
In conclusion, our results suggest that FGFR2 amplification is a key signal in patients with PC in GC and that these patients could benefit from treatment with FGFR inhibitors such as AZD4547.
Acknowledgements
Funding: This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C3418).
Footnotes
Conflicts of interest: none.
Contributor Information
Jeeyun Lee, Email: jyunlee@skku.edu.
Kyoung-Mee Kim, Email: kkmkys@skku.edu.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Xu W, Yang Z, Lu N. Molecular targeted therapy for the treatment of gastric cancer. J Exp Clin Cancer Res. 2016;35:1. doi: 10.1186/s13046-015-0276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 5.Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE, de Hingh IH. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622–628. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 6.Riihimaki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7(32):52307–52316. doi: 10.18632/oncotarget.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yonemura Y, Elnemr A, Endou Y, Hirano M, Mizumoto A, Takao N, Ichinose M, Miura M, Li Y. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol. 2010;2:85–97. doi: 10.4251/wjgo.v2.i2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Brooks AN, Kilgour E, Smith PD. Molecular pathways: fibroblast growth factor signaling: a new therapeutic opportunity in cancer. Clin Cancer Res. 2012;18:1855–1862. doi: 10.1158/1078-0432.CCR-11-0699. [DOI] [PubMed] [Google Scholar]
- 10.Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34:280–300. doi: 10.1002/med.21288. [DOI] [PubMed] [Google Scholar]
- 11.Jang JH, Shin KH, Park JG. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001;61:3541–3543. [PubMed] [Google Scholar]
- 12.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 13.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22:259–267. doi: 10.1158/1078-0432.CCR-14-3212. [DOI] [PubMed] [Google Scholar]
- 15.Katoh M. Therapeutics targeting FGF signaling network in human diseases. Trends Pharmacol Sci. 2016;37:1081–1096. doi: 10.1016/j.tips.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Dienstmann R, Rodon J, Prat A, Perez-Garcia J, Adamo B, Felip E, Cortes J, Iafrate AJ, Nuciforo P, Tabernero J. Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors. Ann Oncol. 2014;25:552–563. doi: 10.1093/annonc/mdt419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin EY, Lee BH, Yang JH, Shin KS, Lee GK, Yun HY, Song YJ, Park SC, Kim EG. Up-regulation and co-expression of fibroblast growth factor receptors in human gastric cancer. J Cancer Res Clin Oncol. 2000;126:519–528. doi: 10.1007/s004320000128. [DOI] [PubMed] [Google Scholar]
- 18.Toyokawa T, Yashiro M, Hirakawa K. Co-expression of keratinocyte growth factor and K-sam is an independent prognostic factor in gastric carcinoma. Oncol Rep. 2009;21:875–880. doi: 10.3892/or_00000297. [DOI] [PubMed] [Google Scholar]
- 19.Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, Elbi C, Lutterbach B. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008;68:2340–2348. doi: 10.1158/0008-5472.CAN-07-5229. [DOI] [PubMed] [Google Scholar]
- 20.Xie L, Su X, Zhang L, Yin X, Tang L, Zhang X, Xu Y, Gao Z, Liu K, Zhou M. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res. 2013;19:2572–2583. doi: 10.1158/1078-0432.CCR-12-3898. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Kim KM, Kang WK, Ou SH. Innovative personalized medicine in gastric cancer: time to move forward. Clin Genet. 2014;86:37–43. doi: 10.1111/cge.12408. [DOI] [PubMed] [Google Scholar]
- 22.Yung-Jue Bang EVC, Mansoor Wasat, Petty Russell D, Chao Yee, Cunningham David, Ferry David, Landers Donal, Stockman Paul, Smith Neil R, Geh Catherine. A randomized, open-label phase II study of AZD4547 (AZD) versus paclitaxel (P) in previously treated patients with advanced gastric cancer (AGC) with fibroblast growth factor receptor 2 (FGFR2) polysomy or gene amplification (amp): SHINE study. J Clin Oncol. 2015;33:2015. [suppl; abstr 4014] [Google Scholar]
- 23.Jeeyun Lee JCB, Rha Sun Young, Bang Yung-Jue, Lee Clark, Xiang Hong, Pierce Kristen L, Krishnan Kartik, Sikorski Robert S, Rasco Drew W. Antitumor activity and safety of FPA144, an ADCC-enhanced, FGFR2b isoform-selective monoclonal antibody, in patients with FGFR2b+ gastric cancer and advanced solid tumors. J Clin Oncol. 2016;34:2016. (suppl; abstr 2502) [Google Scholar]
- 24.Reis-Filho JS, Simpson PT, Turner NC, Lambros MB, Jones C, Mackay A, Grigoriadis A, Sarrio D, Savage K, Dexter T. FGFR1 emerges as a potential therapeutic target for lobular breast carcinomas. Clin Cancer Res. 2006;12:6652–6662. doi: 10.1158/1078-0432.CCR-06-1164. [DOI] [PubMed] [Google Scholar]
- 25.Weiss J, Sos ML, Seidel D, Peifer M, Zander T, Heuckmann JM, Ullrich RT, Menon R, Maier S, Soltermann A. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62ra93. doi: 10.1126/scitranslmed.3001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rhijn BW, Montironi R, Zwarthoff EC, Jobsis AC, van der Kwast TH. Frequent FGFR3 mutations in urothelial papilloma. J Pathol. 2002;198:245–251. doi: 10.1002/path.1202. [DOI] [PubMed] [Google Scholar]
- 27.Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, Liu EM, Reichel J, Porrati P, Pellegatta S. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337:1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies H, Hunter C, Smith R, Stephens P, Greenman C, Bignell G, Teague J, Butler A, Edkins S, Stevens C. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res. 2005;65:7591–7595. doi: 10.1158/0008-5472.CAN-05-1855. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Zhang L, Su X, Li M, Xie L, Malchers F, Fan S, Yin X, Xu Y, Liu K. Translating the therapeutic potential of AZD4547 in FGFR1-amplified non–small cell lung cancer through the use of patient-derived tumor xenograft models. Clin Cancer Res. 2012;18:6658–6667. doi: 10.1158/1078-0432.CCR-12-2694. [DOI] [PubMed] [Google Scholar]
- 30.Gavine PR, Mooney L, Kilgour E, Thomas AP, Al-Kadhimi K, Beck S, Rooney C, Coleman T, Baker D, Mellor MJ. AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012;72:2045–2056. doi: 10.1158/0008-5472.CAN-11-3034. [DOI] [PubMed] [Google Scholar]
- 31.Su X, Zhan P, Gavine PR, Morgan S, Womack C, Ni X, Shen D, Bang YJ, Im SA, Ho Kim W. FGFR2 amplification has prognostic significance in gastric cancer: results from a large international multicentre study. Br J Cancer. 2014;110:967–975. doi: 10.1038/bjc.2013.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung EJ, Jung EJ, Min SY, Kim MA, Kim WH. Fibroblast growth factor receptor 2 gene amplification status and its clinicopathologic significance in gastric carcinoma. Hum Pathol. 2012;43:1559–1566. doi: 10.1016/j.humpath.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Moriguchi S, Maehara Y, Korenaga D, Sugimachi K, Nose Y. Risk factors which predict pattern of recurrence after curative surgery for patients with advanced gastric cancer. Surg Oncol. 1992;1:341–346. doi: 10.1016/0960-7404(92)90034-i. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol. 2002;9:394–400. doi: 10.1007/BF02573875. [DOI] [PubMed] [Google Scholar]
- 35.Tsujitani S, Oka S, Suzuki K, Saito H, Kondo A, Ikeguchi M, Maeta M, Kaibara N. Prognostic factors in patients with advanced gastric cancer treated by noncurative resection: a multivariate analysis. Hepatogastroenterology. 2001;48:1504–1508. [PubMed] [Google Scholar]
- 36.Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 2013;73:5315–5319. doi: 10.1158/0008-5472.CAN-13-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, Arcaroli JJ, Messersmith WA, Eckhardt SG. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]