Abstract
Sickle cell disease (SCD) is a life-threatening genetic condition. Patients suffer from chronic systemic and cerebral vascular disease that leads to early and cumulative neurological damage. Few studies have quantified the effects of this disease on brain morphometry and even fewer efforts have been devoted to older patients despite the progressive nature of the disease. This study quantifies global and regional brain volumes in adolescent and young adult patients with SCD and racially matched controls with the aim of distinguishing between age related changes associated with normal brain maturation and damage from sickle cell disease.
T1 weighted images were acquired on 33 clinically asymptomatic SCD patients (age = 21.3 ± 7.8; F = 18, M = 15) and 32 racially matched control subjects (age = 24.4 ± 7.5; F = 22, M = 10). Exclusion criteria included pregnancy, previous overt stroke, acute chest, or pain crisis hospitalization within one month. All brain volume comparisons were corrected for age and sex.
Globally, grey matter volume was not different but white matter volume was 8.1% lower (p = 0.0056) in the right hemisphere and 6.8% (p = 0.0068) in the left hemisphere in SCD patients compared with controls. Multivariate analysis retained hemoglobin (β = 0.33; p = 0.0036), sex (β = 0.35; p = 0.0017) and mean platelet volume (β = 0.27; p = 0.016) as significant factors in the final prediction model for white matter volume for a combined r2 of 0.37 (p < 0.0001). Lower white matter volume was confined to phylogenetically younger brain regions in the anterior and middle cerebral artery distributions.
Our findings suggest that there are diffuse white matter abnormalities in SCD patients, especially in the frontal, parietal and temporal lobes, that are associated with low hemoglobin levels and mean platelet volume. The pattern of brain loss suggests chronic microvascular insufficiency and tissue hypoxia as the causal mechanism. However, longitudinal studies of global and regional brain morphometry can help us give further insights on the pathophysiology of SCD in the brain.
Abbreviations: SCD, sickle cell disease; MRI, magnetic resonance imaging; WMHI, white matter hyperintensities; GM, grey matter; WM, white matter; ROI, region of interest; MPV, mean platelet volume; HgB, hemoglobin; MCA, middle cerebral artery; ACA, anterior cerebral artery; PCA, posterior cerebral artery
Keywords: Sickle cell disease, White matter, Hemoglobin, Mean platelet volume, Structural MRI
Graphical abstract
Highlights
-
•
Total white matter brain volume is decreased in sickle cell disease patients.
-
•
Global white matter decrease is found to be due to anemia.
-
•
Diffuse WM volume decrease is found especially in watershed areas.
-
•
Diffuse WM volume decrease spatially colocalize with silent stroke in SCD patients.
1. Introduction
Sickle cell disease (SCD) is an autosomal-recessive genetic blood disorder affecting over 100,000 people in the United States (Hassell, 2010). SCD is caused by a single base-pair replacement on the allele which encodes for the beta subunit of hemoglobin. SCD ultimately leads to chronic and progressive vascular disease complications which adversely affects the vital organ systems including the heart, lungs, kidney, liver and the brain (Adams et al., 2001, Chaturvedi and DeBaun, 2016).
Stroke is one of the leading causes of morbidity and mortality in SCD patients (Fitzhugh et al., 2010, Sandhu and Cohen, 2015, Strouse et al., 2009). Primary stroke events dramatically increase the risk of recurrence (Miller et al., 2001, Scothorn et al., 2002). Prior to routine transcranial Doppler screening and use of chronic transfusion therapy in high risk patients, 11% of patients had a major cerebral accident by the age of 20, with a lifetime risk of 40% (Bernaudin et al., 2011, Ohene-Frempong et al., 1998). However, even with the introduction of this meticulous screening, 39% of SCD patients still experience small white matter strokes by the age of 18 (Debaun and Kirkham, 2016, Hulbert et al., 2011). Although called “silent strokes”, these microstrokes are associated with neurocognitive decline as well as an increased risk for overt stroke (Armstrong et al., 1996, DeBaun et al., 2014, DeBaun et al., 2012a, DeBaun et al., 2012b, Powars et al., 1978, Schatz et al., 2002, van der Land et al., 2015, Van Der Land et al., 2013). Stroke rate is highest between the ages of two and ten(Bernaudin et al., 2011, Ohene-Frempong et al., 1998, Pegelow et al., 2002, Scothorn et al., 2002) when metabolic demands of the brain are highest (Chiron et al., 1992, Chugani, 1998). Therefore, the existing literature on SCD focuses mainly on infant and pediatric subjects, even though almost 95% of SCD patients live into adulthood (Chaturvedi and DeBaun, 2016, Quinn et al., 2010).
Few studies have explored the structure, function or pathophysiological elements of the neurological consequences of SCD (Chen et al., 2014, Kawadler et al., 2013, Schatz and Buzan, 2006, Steen et al., 2005). Even fewer studies have explored the SCD brain outside the neonatal and pediatric age group (Balci et al., 2012, Baldeweg et al., 2006, Sun et al., 2012). Disease progression and accumulation affecting cerebrovascular distribution in SCD patients occurs during the most dynamic periods of brain development. Perturbations during critical periods of neurodevelopment can lead to irreversible damage (Andersen, 2003, Fox et al., 2010, Rakic, 2002) and understanding the structural outcomes in these patients' brain can help us retrospectively understand injury progression during maturation.
In this current study, we examine measures of brain morphology of clinically asymptomatic SCD patients outside of the pediatric age range in comparison to a racially-matched control group using structural magnetic resonance imaging (MRI). We further use multivariate statistical modeling to find covariates between neuroanatomy, blood counts, hemoglobin subtypes, markers of intravascular hemolysis and vital signs.
2. Subjects and methods
2.1. Participants
A total of 65 subjects were recruited as part of a study on sickle cell disease and neurocognitive outcome at the Children's Hospital Los Angeles. The SCD group consisted of 33 clinically asymptomatic SCD patients (mean age = 22.4 ± 8.4; age range = 11.7–41.8; F = 15, M = 18). 27 subjects had hemoglobin SS genotype, three had Sβ0 genotype and three had SC genotype. All SCD patients received universal access to transcranial Doppler (TCD) screening and transfusions when appropriate. In accordance with current NIH guidelines (Yawn et al., 2014), the Sickle Cell team at Children's Hospital Los Angeles recommends hydroxyurea treatment for all children with SS and Sβ0-thalasemia after the age of 9 months unless they have been placed on chronic transfusion. The dose is advanced to the maximum tolerated dose according to standard protocol. 20 patients were on monthly transfusions and 13 of the non-transfused patients were prescribed hydroxyurea. No differences were found for total GM or WM volume (corrected for age and sex) in patients on transfusions against medication.
The control group consisted of 32 racially matched control subjects (mean age = 25.0 ± 7.6; age range = 12.3–41.8; F = 22, M = 10). Most control subjects were recruited from first degree relatives of patients. 18 control subjects had hemoglobin AA genotype and 14 had hemoglobin AS genotype (SCD trait). No differences were found for total GM or WM volume (corrected for age and sex) in subjects with hemoglobin AA against AS genotype.
MRI scans, vital signs and blood samples were obtained on the same day for each subject. Exclusion criteria included pregnancy, previous overt stroke, acute chest or pain crisis hospitalization within one month. All subjects were recruited with informed consent or assent; the study was approved by the Institutional Review Board at Children's Hospital Los Angeles (CCI#11-00083). Demographics are reported in Table 1.
Table 1.
Subject demographics. Group averages (Avg) and standard deviations (SD) are given. Group differences were assessed using Student's unpaired t-tests.
| Controls avg (SD) | SCD avg (SD) | p-Value | |
|---|---|---|---|
| N | 32 | 33 | |
| Age | 24.4(7.5) | 21.3(7.8) | 0.11 |
| Male:Female | 10:22 | 15:18 | 0.24 |
| Height | 166.2 (7.4) | 164.0 (9.2) | 0.029⁎ |
| Weight (kg) | 68.4 (18.7) | 64.9 (23.2) | 0.050 |
| Body mass index (kg/m2) | 24.7 (6.4) | 24.2 (8.7) | 0.77 |
| Body surface area (m2) | 1.8 (0.2) | 1.7 (0.3) | 0.33 |
| Heart rate (min− 1) | 75.2 (19.0) | 79.9 (12.2) | 0.24 |
| Systolic blood pressure (mm Hg) | 115.6 (9.6) | 110.8 (10.9) | 0.063 |
| Diastolic blood pressure (mm Hg) | 66.8 (9.2) | 61.2 (7.2) | 0.0087⁎ |
| O2 saturation (%) | 99.3 (0.9) | 97.6 (2.3) | < 0.0003⁎⁎⁎ |
| Hemoglobin (g/dL) | 13.5 (1.4) | 9.7 (1.6) | < 0.0001⁎⁎⁎ |
| Hematocrit (%) | 40.0 (3.8) | 28.2 (4.4) | < 0.0001⁎⁎⁎ |
| White blood cell count (× 103) | 5.7 (1.7) | 10.1 (5.0) | < 0.0001⁎⁎⁎ |
| Platelets | 242.5 (57.0) | 279.1 (115.3) | 0.11 |
| Mean platelet volume (fL) | 10.6 (0.9) | 10.0 (0.7) | 0.0029⁎⁎ |
| Reticulocytes (%) | 1.4 (0.6) | 9.9 (6.2) | 0.024⁎⁎ |
| Cell-free hemoglobin | 5.6 (3.8) | 17.9 (16.8) | < 0.0007⁎⁎⁎ |
| Lactose dehydrogenase | 517.0 (75.8) | 973.8 (554.7) | < 0.0001⁎⁎⁎ |
p < 0.05.
p < 0.01.
p < 0.001.
2.2. MRI acquisition and assessment
3D T1-weighted images (TE = 3.8 ms TR = 8.3 ms; resolution = 1 mm3) and T2-weighted FLAIR images (TE = 2.5 ms; TR = 4.8 ms; resolution = 1.3 × 1.0 × 1.0 mm) were acquired using an 8-channel head coil on a 3 T Philips Achieva (v.3.2.1).
T2-weighted FLAIR images were examined by a neuroradiologist to identify white matter hyperintensities (WMHI). Silent WMHI were classified as 3–5 mm lesions on T2 Flair, observed in two orthogonal planes, that had no known neurological sequelae (Helton et al., 2014). Since WMHI can be observed in normal children (Nelson et al., 2000) and the prevalence increases with age (Postma et al., 2014), more than one lesion per decade of age was required to be considered abnormal. 4 of 32 control subjects and 9 of 23 SCD patients were identified with WMHI greater than expected for age. The cumulative volume of tissue with WMHI accounted for < 0.1 cm3 in most individuals (< 0.01% of total brain volume) and were mostly observed in the parietal and frontal lobes. 1 SCD subject had abnormal MR angiography with severe, bilateral stenosis of the anterior cerebral arteries but all other subjects had normal MR angiograms. No differences were found for total GM or WM volume (corrected for age and sex) in subjects with normal versus abnormal neuroradiological interpretations.
2.3. Image preprocessing and analysis
T1-weighted images were processed using BrainSuite (brainsuite.org, v15a) in a semi-automated fashion to classify tissue types, extract and render the surfaces of the inner and pial cortices. Manual correction was performed on cortical boundaries and grey-white boundaries to minimize extraneous inclusion of meninges or exclusion of cortex and to correct occipito-cerebellar boundaries. The brainstem was cut at the base of the cerebellum as seen on the axial slice.
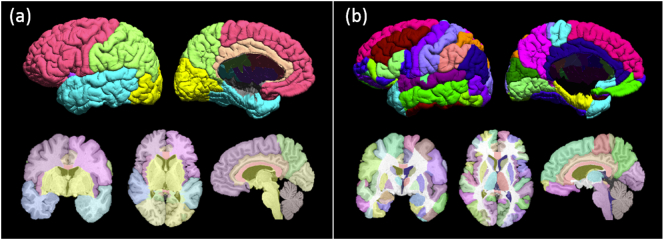
BrainSuite was used for atlas-based registration using the BCI-DNI Brain Atlas (http://brainsuite.org/svreg_atlas_description/) to label volumetric images and surfaces with two separate segmentation schemes. The first segmentation divided the cerebrum into four lobes and the second sub-divided the brain into 90 regions of interest (Fig. 1).
Fig. 1.
BCI-DNI Brain Atlas's labeled 3D surface and (from left to right) single coronal, axial and sagittal 2D image volume of 2 segmentation schemes: (a) lobes and (b) 90 regions of interest.
Gyral white matter boundaries were defined on the atlas by the two opposing sulcal edges of the gyrus from the coronal view. The sulcal and gyral boundaries were transferred from atlas to subject using BrainSuite (Joshi et al., 2012). BrainSuite performed cortically constrained volumetric brain registration using curvature based alignment of the cortical surface followed by coregistration of subcortical areas using intensity based elastic registration. This was subject to a cortical matching constraint. This resulted in atlas-to-subject matching that was consistently accurate throughout the brain, both at the cortical and subcortical regions.
Average cortical thickness, grey matter (GM) volume, white matter (WM) volume and cortical surface area were calculated for the whole brain and regions of interest (ROIs). Age and sex were controlled for by regressing out age (log-transformed to improve normality) and sex then adding the residuals to the population mean of each measurement. Student t-tests were performed on group comparisons and the Benjamini and Hochberg False Discovery Rate (q < 0.05) was applied to adjust p-values to account for multiple comparisons (SAS, Version 12.1.0, Cary, North Carolina; Version 8.6, Mathworks Inc., Natick, MA). Multivariate regression analysis was performed on the total population. All variables having a univariate p-value < 0.10 were included as candidates for the stepwise multivariate regression. Variables were only retained in the final model for p < 0.05.
3. Results
3.1. Sex differences in global brain volumes
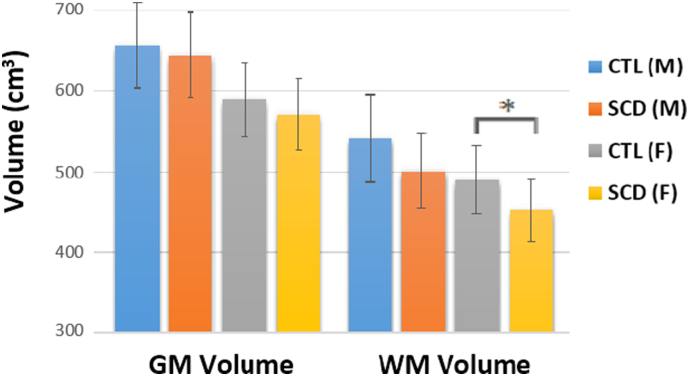
No difference in global or regional brain volume was observed between controls with AS (sickle cell trait) or AA hemoglobin, so these data were pooled for all comparisons. Fig. 2 shows age-corrected whole-brain WM volume and GM volume in males and females with SCD compared to racially matched controls. GM volume was slightly lower in both males (p = 0.56) and females (p = 0.27), 1.9% and 3.2% respectively, but this difference did not reach statistical significance. In contrast, WM volume was 7.6% lower in males (p = 0.11) and 8.0% in females (p = 0.020). Using two-way analysis of variance, both sex (p < 0.0001) and disease state (p = 0.0010) were associated with lower WM volume but the interaction term was not significant (p = 0.93).
Fig. 2.
Mean whole brain GM and WM volume controlled for age grouped by disease status and sex. Error bars indicate 1 standard deviation above and below the mean. WM volume was found to be significantly lower in female SCD patients in comparison to control after adjusting for multiple comparisons (p = 0.020). *p < 0.05.
3.2. Global brain morphometric differences
Global brain morphometry was calculated for the left and right hemisphere then corrected for age and sex. The two groups were compared using Student t-tests and p-values were adjusted for multiple comparisons. SCD patients had significantly lower WM volume in comparison to control subjects by 8.1% (p = 0.0056) in the right hemisphere and 6.8% (p = 0.0068) in the left hemisphere. There were no statistically significant differences found for GM volume (Table 2).
Table 2.
Student t-test of the average GM volume, WM volume, cortical thickness and cortical surface area of CTL compared to SCD. All values were corrected by age and sex. p-Values were adjusted for multiple comparisons. WMV: white matter volume; GMV: grey matter volume; CTAvg: average cortical thickness; CSA: cortical surface area; CTL: control subjects; SCD: sickle cell disease patients; R: right; L: left; Avg: average; SD: standard deviation.
| Measurement | CTL avg (SD) |
SCD avg (SD) | % difference | Adjusted p-value |
|---|---|---|---|---|
| R. GMV (cm3) | 308.8 (22.5) | 300.8 (24) | 2.6 | 0.17 |
| L. GMV (cm3) | 307.7 (21.5) | 300 (23.7) | 2.5 | 0.17 |
| R. WMV (cm3) | 257.2 (23.6) | 237.2 (21.9) | 8.1 | 0.0056⁎⁎ |
| L. WMV (cm3) | 255.6 (21.7) | 238.9 (19.7) | 6.8 | 0.0068⁎⁎ |
| R. CTAvg (mm) | 3.96 (0.12) | 4.03 (0.18) | − 1.8 | 0.097 |
| L. CTAvg (mm) | 3.96 (0.13) | 4.03 (0.18) | − 1.8 | 0.097 |
| R. CSA (cm2) | 1013.9 (73) | 979.4 (68.4) | 3.5 | 0.097 |
| L. CSA (cm2) | 1010.8 (72.2) | 979.9 (66.9) | 3.1 | 0.10 |
p < 0.01.
3.3. Covariates of global volumetric data
Stepwise multivariate regression analysis was run on GM and WM volume against vital signs and laboratory data of our sample.
53% of the variability in GM volume could be accounted for by 3 different factors (F(3,57) = 21.38; p < 0.0001); r2 = 0.53). As expected, age (β = − 0.43; p < 0.0001) and sex (β = 0.52; p < 0.0001) were the strongest predictors for GM volume. MPV (β = 0.29; p = 0.0026) was also retained in the model.
For WM volume, age was not a significant predictor. Instead, hemoglobin (β = 0.33; p = 0.0036), sex (β = 0.35; p = 0.0017) and MPV (β = 0.27; p = 0.016) were included in the final model accounting for 37% of the variability of WM volume (F(3,58) = 11.30; p < 0.0001); r2 = 0.37).
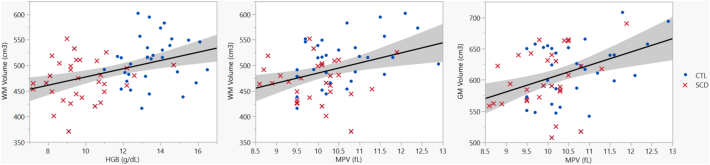
Fig. 3 shows age and sex corrected GM volume as a function of MPV (r2 = 17.1, p = 0.0008) and sex corrected WM volume as a function of hemoglobin (r2 = 17.1; p = 0.0006) and MPV (r2 = 13.4; p = 0.0035), demonstrating that the predictive value of hemoglobin and MPV are not simply secondary to a group effect.
Fig. 3.
WM volume after controlling for sex as a function of hemoglobin (left) and MPV (middle). GM volume after controlling for age and sex as a function of MPV (right). Solid line shows the linear regression of the data and shaded area delimits the 95% confidence interval. WM: white matter; GM: grey matter; HGB: hemoglobin; MPV: mean platelet volume; CTL: control subjects; SCD: sickle cell disease patients.
3.4. Regional distribution of brain volume changes
Brain lobe, subcortical and extra-cerebral WM volume controlled for age and sex are shown in Table 3 (see Fig. 1a for reference atlas). WM volume was found to be lower in the right and left frontal (p = 0.035; p = 0.034), parietal (p = 0.035; p = 0.034) and temporal (p = 0.018; p = 0.035) lobes in SCD patients in comparison to controls. The corpus callosum (p = 0.042), right brainstem (p = 0.035) and right cerebellum (p = 0.049) also showed significantly lower WM volume.
Table 3.
Brain lobe, subcortical and extra-cerebral WM volume controlled for age and sex.
R: right; L: left; Avg: average; SD: standard deviation.
| Region | CTL (cm3) Avg (SD) |
SCD (cm3) Avg (SD) |
% difference | Adjusted p-value |
|---|---|---|---|---|
| R frontal | 74.6 (7.0) | 68.9 (9.1) | 8.0 | 0.035⁎ |
| L frontal | 72.8 (7.2) | 67.1 (7.8) | 8.3 | 0.034⁎ |
| R parietal | 41.0 (5.0) | 37.6 (4.4) | 8.6 | 0.035⁎ |
| L parietal | 47.3 (4.7) | 43.6 (4.8) | 8.2 | 0.034⁎ |
| R temporal | 40.2 (4.2) | 36.5 (3.9) | 9.7 | 0.018⁎ |
| L temporal | 35.0 (3.4) | 32.6 (3.3) | 7.1 | 0.035⁎ |
| R occipital | 21.2 (2.6) | 20.1 (2.8) | 5.1 | 0.23 |
| L occipital | 22.1 (2.4) | 21.1 (2.9) | 4.8 | 0.23 |
| R subcortex | 31.2 (3.6) | 29.1 (3) | 7.1 | 0.087 |
| L subcortex | 29.2 (3.4) | 27.8 (2.8) | 5.1 | 0.25 |
| Corpus callosum | 6.0 (0.7) | 5.5 (0.7) | 8.70 | 0.042⁎ |
| R brainstem | 12.7 (1.5) | 11.8 (0.9) | 7.3 | 0.035⁎ |
| L brainstem | 12.6 (1.4) | 11.9 (1.1) | 6.3 | 0.074 |
| R cerebellum | 18.3 (4.4) | 16 (2.2) | 13.3 | 0.049⁎ |
| L cerebellum | 18.3 (4.0) | 17 (3.1) | 7.2 | 0.25 |
p < 0.05.
No significant regional differences were found for GM volume, average cortical thickness and cortical surface area.
3.5. Group differences in cortical gyri
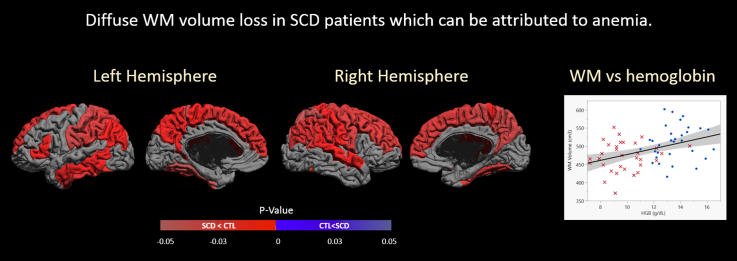
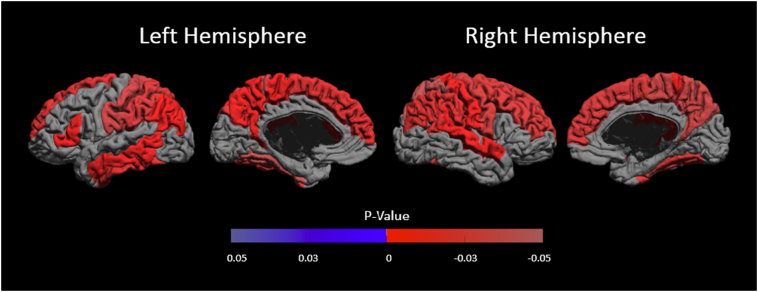
We compared regional WM volume, GM volume, average cortical thickness and cortical surface area group differences for cortical gyri using Student t-tests. Fig. 4 show spatial maps of adjusted p-values, controlling for multiple comparisons, on the cortical surface for WM volume (see Fig. 1b for reference atlas).
Fig. 4.
White matter volume group comparison results, where significant (p < 0.05), are shown as signed adjusted p-values on the mid-cortical surface. Positive p-values (in blue) indicate CTL < SCD and negative p-values (in red) indicate SCD < CTL.
Gyral WM volume was lower in SCD patients in comparison to control subjects diffusely throughout the brain. The bilateral superior frontal gyrus, postcentral gyrus, supramarginal gyrus, angular gyrus, superior parietal gyrus, fusiform gyrus, paracentral lobule, and precuneus were found to have statistically significant lower WM volume. Lateral differences were found in the right middle frontal gyrus, right precentral gyrus, right superior temporal gyrus, left pars triangularis, left middle temporal gyrus and left inferior temporal gyrus where again we saw lower WM volume.
After controlling for multiple comparisons, group differences were not observed in GM volume, average cortical thickness and cortical surface area in cortical gyral measurements.
3.6. Group differences in subcortical structures
Total volume, GM volume and WM volume were controlled for age and sex for subcortical structures. Group differences were not found in the subcortex after multiple comparisons.
4. Discussion
4.1. WM loss in SCD patients
Our most striking finding was the profound global white matter volume loss (6.8–8.1%) in clinically asymptomatic SCD patients in comparison to healthy, racially matched controls (Table 2). Regionally, effects of the disease were greatest in the frontal, parietal and temporal lobes bilaterally, as well as the corpus callosum, right brainstem and right cerebellum. Interestingly the occipital lobe was relatively spared (Table 3).
Effects of SCD on WM tissue has been reported using voxel based morphometry and diffusion tensor imaging (DTI) analysis (Balci et al., 2012, Baldeweg et al., 2006, Sun et al., 2012). Although numerous studies have reported grey matter loss, only one prior study documented total WM volume loss in SCD patients in comparison to controls (Steen et al., 2005). Our study is novel in showing regional gyral WM volume loss (Fig. 4) as well as associations with hemoglobin level and mean platelet volume (Fig. 3). While most of the brain is affected by the disease, specific regions appear to be spared, revealing a pattern of differential vulnerability.
4.2. WM loss explained by vascular distribution
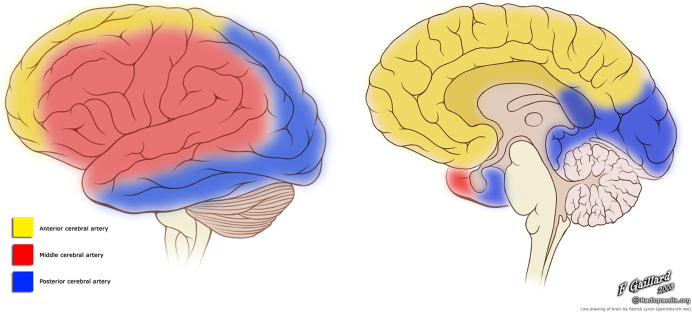
The pattern of WM loss (Fig. 4) generally follows vascular territories and its border zones, with relative sparing of the posterior circulation. The regional WM volume loss in the frontal, parietal and temporal lobes was consistent with patterns of the arterial territories of the middle cerebral artery (MCA) and anterior cerebral artery (ACA). The occipital lobe, which exhibited less shrinkage, is typically supplied by the posterior cerebral artery (PCA) (Tatu et al., 1998) (Fig. 5). In a study examining 266 patients with SCD from ages 6–19, stroke was reported to primarily involve the frontal lobe, followed by the parietal lobe, subcortical nuclei and temporal lobe, with very few lesions reported in the occipital lobe or cerebellum (Pegelow et al., 2002). The pattern of WM loss in SCD patients we found in our current study parallels the pattern of regional vulnerability to stroke in SCD patients in previous literature (Moser et al., 1996, Pegelow et al., 2002, Vichinsky et al., 2010).
Fig. 5.
Illustration depicting the vascular territories of the MCA (red), ACA (yellow), and PCA (blue) on a lateral (left) and midline (right) view of the cortical surface. Case courtesy of A.Prof Frank Gaillard, Radiopaedia.org, rID: 36099. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Vascular insufficiency as a stimulus for WM loss is further supported by the dominant effect of hemoglobin on whole brain WM volume. Anemic patients raise their cerebral blood flow to maintain oxygen delivery at rest (Borzage et al., 2016, Bush et al., 2016) but this leaves them with a blunted cerebrovascular reserve (Prohovnik et al., 2009). Although GM is more selectively sensitive than WM to complete occlusion because of its higher metabolic demand (Arakawa et al., 2006, Bristow et al., 2005), WM is more sensitive to chronic, hypoxia (Dewar et al., 1999). Focal ischemic events, caused by insults like fever, transient hypoxia, or sudden reductions in hemoglobin, can rapidly cause injury to oligodendrocytes (Pantoni et al., 1996) and present clinically as white matter hyperintensities (WMH) detected through in-vivo MR imaging (Bernaudin et al., 2015, DeBaun et al., 2012a, DeBaun et al., 2012b, Quinn et al., 2013). Similar findings have been found in murine models whose cerebral blood flow has been altered to induce moderate chronic hypoperfusion through narrowing of the carotid arteries. Differential WM injury and microstrokes were observed along with cognitive decline (Liu et al., 2013, Shibata et al., 2004).
The differential volume loss observed in our study is concordant with the described locations of silent strokes in SCD, as well as WMH found in other disorders (hypertension, sleep apnea) (Debette and Markus, 2010, Jeerakathil et al., 2004, Nagy et al., 2003). Recently, decreased measures of O2 saturation have been shown to correlate with compromised WM structure in SCD patients, supporting a role for acute-on-chronic hypoxia in white matter damage (Kawadler et al., 2015).
4.3. Phylogenetic predictors of vulnerability
In utero and the first few weeks of life, the fetus is protected from the damaging effects of SCD by fetal hemoglobin. After birth, hemoglobin production is gradually switched to producing abnormal hemoglobin (HbS) over a span of several months (Epstein and Bunn, 1997, Rees et al., 2010). Red blood cells comprised solely of HbS are rigid, oxidatively stressed and have a much shorter lifespan (8 days) than red cells with normal hemoglobin (100 days). Because SCD patients are functionally asplenic after the age of two, damaged sickle red cells are not cleared and often rupture within the vascular system. Over time, vascular endothelia suffer progressive damage because of abnormal mechanical forces and circulating free hemoglobin/heme (Epstein and Bunn, 1997, Rees et al., 2010, Schatz and McClellan, 2006, Verduzco and Nathan, 2009). SCD patients' disease worsens from accumulation of injury including vascular endothelium damage and hemolysis, ultimately affecting oxygen delivery to the brain (Baker et al., 2015, Powars et al., 1999, Scothorn et al., 2002, Voskaridou et al., 2007). Patients' cerebral blood flow will rise to compensate for the affected oxygen delivery (Adams et al., 1992, Adams et al., 1997, Helton et al., 2014) to the brain but the anemia and the vascular complications will tend to worsen with time (Moser et al., 1996, Wang et al., 1998).
The developing brain has a high metabolic demand. Cerebrovascular oxygen consumption peaks around 6–10 years of age before it sharply declines where resting cerebral blood flow drops and lowers cerebral vascular reserve (Chiron et al., 1992, Chugani, 1998). Coincidentally, stroke risks peak around this time for young patients with SCD (Bernaudin et al., 2011, Pegelow et al., 2002, Scothorn et al., 2002), where presumably, late developing regions would be more plastic and vulnerable to lasting injury caused by acute on chronic ischemia.
Neurodevelopment is asynchronous, where local developmental trajectories are distinct (Lenroot and Giedd, 2006, Shaw et al., 2008, Sowell et al., 2004), and the timing of perturbations will differentially affect outcome (Hensch, 2005, Thompson et al., 2009). Injury in areas during critical periods of peak development will cause permanent damage while those regions outside its developmental critical period may successfully recover. On a granular level, the white matter areas consistent with sparing in Fig. 4 are primarily located in more evolutionarily conserved brain regions. The few regions spared in our patients were mostly primary sensory cortices—bilateral primary visual cortex, Heschl's gyrus and the left precentral gyrus—along with the limbic cortices. These regions are phylogenetically older and may have a more robust blood supply as a result. These regions also develop the earliest and most rapidly (Gogtay et al., 2004, Shaw et al., 2008, Sowell et al., 2004, Wierenga et al., 2014).
Thus, we postulate that structures that are latest to develop are the first to suffer in response to repeated chronic vascular/hypoxic insults in SCD. Similar neurodegenerative patterns are observed in, Alzheimer's and in normal aging. Even some psychiatric disorders, such as schizophrenia, autism and attention deficit disorders, have shown that late-developing brain regions are the first to degenerate later in life (Douaud et al., 2014, Nour and Howes, 2015, Toga et al., 2006). While our patient population is only in their teens and early adult years, their progressive disease may simply reflect accelerated vascular aging and neurodegeneration. Similar patterns of WM loss and silent stroke have also been observed in hypertension and sleep apnea (Debette and Markus, 2010, Jeerakathil et al., 2004, Yaggi et al., 2005).
4.4. GM loss in SCD patients
Previous volumetric SCD studies showed diffuse grey matter volume and cortical thickness deficits (Baldeweg et al., 2006, Chen et al., 2014, Kawadler et al., 2013, Kirk et al., 2009, Mackin et al., 2014, Steen et al., 2005). However, our study was not able to show GM differences (which includes volume, cortical surface area and cortical thickness).
We believe this is because we examined an older patient population than many previous studies. While most other studies included school age children (Baldeweg et al., 2006, Chen et al., 2014, Kawadler et al., 2013, Kirk et al., 2009, Steen et al., 2005), our study's cohort had an average age of 22 years and did not include subjects under the age of 12. One possible explanation is that GM maturation occurs at a different rate in SCD patients, because of stress or other factors associated with their illness, compared with typically developing children (Chen et al., 2014, Steen et al., 2005). GM volume falls dramatically from childhood to early adulthood as a natural process of synaptic pruning (Sowell et al., 2004, Toga et al., 2006, Wierenga et al., 2014). The lower GM volume previously found in children with SCD could be attributed to delayed cortical development (Chen et al., 2014, Steen et al., 2005).
Still, there may be microstructural differences in the cortex that we are not able to detect using our current method. While we only observed lower WM volume, we did see a trend towards higher cortical thickness in SCD patients compared to controls (Table 2). During development, GM volume decreases while WM increases due to myelination. We start to see thickening of gyral white matter into the adjacent GM tissue which leads to decreases in GM volume and cortical thickness (Lenroot and Giedd, 2006, Sowell et al., 2004). So while our results showed no GM differences, we are hesitant to believe that GM tissue is unaffected in SCD patients.
While age differences are the most likely explanation for our discrepant grey matter findings, differences in our experimental design and methodology could have contributed. Out of the 6 studies showing GM differences in patients with SCD in comparison to controls, only 1 study reported having race-matched controls (Mackin et al., 2014). All studies had different scanner manufacturer and field strength, resolution, and preprocessing pipelines. The current study was well powered to detect WM volume differences so we do not believe we were simply underpowered to detect GM volume differences. Furthermore, our sample size was also larger than 3 of the 6 previous studies mentioned (Chen et al., 2014, Kawadler et al., 2013, Kirk et al., 2009).
4.5. Why is brain volume correlated with mean platelet volume?
We found a strong positive correlation between MPV and GM and WM volume accounting for 13.4% and 17.1% of the variance respectively, independent of the disease state. Scattergrams (Fig. 3) do not suggest an outlier or group effect. Elevated MPV is a marker of increased platelet metabolic and enzymatic activity which has been found to be indicative of vascular injury, cardiovascular disease, cerebrovascular disease and inflammatory processes (Bath et al., 2004, Chu et al., 2010, Vizioli et al., 2009). Our study shows positive correlations between MPV and brain volume, however, elevated MPV has been shown to be predictive of negative outcome (Chu et al., 2010, Vizioli et al., 2009). Our current ability to explain our results are limited and further analysis will be required for clarification.
4.6. Limitations
Much of our discussion on the relationship between WM volume loss, affected vascular distribution and the timing of perturbations during neurodevelopment in sickle cell disease cannot be confirmed without longitudinal data.
Our current imaging technique and resolution allows us to quantitate only macrostructural brain attributes and limits our ability to investigate the microstructural properties of the brain architecture. While we did not identify systematic changes in GM tissue, there may be subtler disease effects that we were not currently able to detect.
5. Conclusions
In this study, asymptomatic, young-adult SCD patients showed diffuse WM volume differences correlated with low hemoglobin and mean platelet volume, suggesting potential contributions of chronic hypoxia and inflammation. Limited disease effects were found in GM tissue and GM volume negatively correlated with MPV. The pattern of differential WM volume loss suggesting sparing of phylogenetically older brain structures as well as brain regions supplied from the posterior circulation. Longitudinal studies of cerebral abnormalities in SCD patients will provide further insight into disease mechanisms and progression.
Funding
This work was supported by National Heart, Lung, and Blood Institute (grant 1U01-HL-117718-01 and the Minority Supplement to grant 1U01-HL-117718-01), the National Center for Research (5UL1 TR000130-05) through the Clinical Translational Science Institute at Children’s Hospital Los Angeles, the National Institutes of Health (grant R01 NS074980), the National Institutes of Health Predoctoral Training in Interdisciplinary Neurosciences (1 T32 MH 111360-1 A1) and National Institute of Health grant (R01 ES024936). Philips Healthcare provided support for protocol development and applications engineering on a support-in-kind basis.
Contributor Information
Soyoung Choi, Email: choisoyo@usc.edu.
Adam M. Bush, Email: adbush@chla.usc.edu.
Matthew T. Borzage, Email: borzage@usc.edu.
Anand A. Joshi, Email: ajoshi@usc.edu.
William J. Mack, Email: William.Mack@med.usc.edu.
Thomas D. Coates, Email: TCoates@chla.usc.edu.
Richard M. Leahy, Email: leahy@sipi.usc.edu.
John C. Wood, Email: jwood@chla.usc.edu.
References
- Adams R., McKie V., Nichols F., Carl E., Zhang D.L., McKie K., Figueroa R., Litaker M., Thompson W., Hess D. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N. Engl. J. Med. 1992;326:605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- Adams R.J., McKie V.C., Carl E.M., Nichols F.T., Perry R., Brock K., McKie K., Figueroa R., Litaker M., Weiner S., Brambilla D. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann. Neurol. 1997;42:699–704. doi: 10.1002/ana.410420505. [DOI] [PubMed] [Google Scholar]
- Adams R.J., Ohene-Frempong K., Wang W. Sickle cell and the brain. Hematology Am. Soc. Hematol. Educ. Program. 2001;2001:31–46. doi: 10.1182/asheducation-2001.1.31. [DOI] [PubMed] [Google Scholar]
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arakawa S., Wright P.M., Koga M., Phan T.G., Reutens D.C., Lim I., Gunawan M.R., Ma H., Perera N., Ly J., Zavala J., Fitt G., Donnan G.A. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke. 2006;37:1211–1216. doi: 10.1161/01.STR.0000217258.63925.6b. [DOI] [PubMed] [Google Scholar]
- Armstrong F.D., Thompson R.J., Wang W., Zimmerman R., Pegelow C.H., Miller S., Moser F., Bello J., Hurtig A., Vass K. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Pediatrics. 1996;97:864–870. [PubMed] [Google Scholar]
- Baker C., Grant A.M., George M.G., Grosse S.D., Adamkiewicz T.V. Contribution of sickle cell disease to the pediatric stroke burden among hospital discharges of African-Americans-United States, 1997–2012. Pediatr. Blood Cancer. 2015 doi: 10.1002/pbc.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci A., Karazincir S., Beyoglu Y., Cingiz C., Davran R., Gali E., Okuyucu E., Egilmez E. Quantitative brain diffusion-tensor MRI findings in patients with sickle cell disease. AJR Am. J. Roentgenol. 2012;198:1167–1174. doi: 10.2214/AJR.11.7404. [DOI] [PubMed] [Google Scholar]
- Baldeweg T., Hogan A.M., Saunders D.E., Telfer P., Gadian D.G., Vargha-Khadem F., Kirkham F.J. Detecting white matter injury in sickle cell disease using voxel-based morphometry. Ann. Neurol. 2006;59:662–672. doi: 10.1002/ana.20790. [DOI] [PubMed] [Google Scholar]
- Bath P., Algert C., Chapman N., Neal B. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35:622–626. doi: 10.1161/01.STR.0000116105.26237.EC. [DOI] [PubMed] [Google Scholar]
- Bernaudin F., Verlhac S., Arnaud C., Kamdem A., Chevret S., Hau I., Coïc L., Leveillé E., Lemarchand E., Lesprit E., Abadie I., Medejel N., Madhi F., Lemerle S., Biscardi S., Bardakdjian J., Galactéros F., Torres M., Kuentz M., Ferry C., Socié G., Reinert P., Delacourt C. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011;117:1130–1140. doi: 10.1182/blood-2010-06-293514. 10.1182/blood-2010-06-293514 quiz 1436. [DOI] [PubMed] [Google Scholar]
- Bernaudin F., Verlhac S., Arnaud C., Kamdem A., Vasile M., Kasbi F., Hau I., Madhi F., Fourmaux C., Biscardi S., Epaud R., Pondarré C. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125:1653–1661. doi: 10.1182/blood-2014-09-599852. [DOI] [PubMed] [Google Scholar]
- Borzage M.T., Bush A.M., Choi S., Nederveen A.J., Václavů L., Coates T.D., Wood J.C. Predictors of cerebral blood flow in patients with and without anemia. J. Appl. Physiol. 2016;120:976–981. doi: 10.1152/japplphysiol.00994.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow M.S., Simon J.E., Brown R.A., Eliasziw M., Hill M.D., Coutts S.B., Frayne R., Demchuk A.M., Mitchell J.R. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J. Cereb. Blood Flow Metab. 2005;25:1280–1287. doi: 10.1038/sj.jcbfm.9600135. [DOI] [PubMed] [Google Scholar]
- Bush A.M., Borzage M.T., Choi S., Václavů L., Tamrazi B., Nederveen A.J., Coates T.D., Wood J.C. Determinants of resting cerebral blood flow in sickle cell disease. Am. J. Hematol. 2016;91:912–917. doi: 10.1002/ajh.24441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S., DeBaun M.R. Evolution of sickle cell disease from a life-threatening disease of children to a chronic disease of adults: the last 40 years. Am. J. Hematol. 2016;91:5–14. doi: 10.1002/ajh.24235. [DOI] [PubMed] [Google Scholar]
- Chen R., Arkuszewski M., Krejza J., Zimmerman R.A., Herskovits E.H., Melhem E.R. A prospective longitudinal brain morphometry study of children with sickle cell disease. AJNR Am. J. Neuroradiol. 2014;36:403–410. doi: 10.3174/ajnr.A4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C., Raynaud C., Mazière B., Zilbovicius M., Laflamme L., Masure M.C., Dulac O., Bourguignon M., Syrota A. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J. Nucl. Med. 1992;33:696–703. [PubMed] [Google Scholar]
- Chu S.G., Becker R.C., Berger P.B., Bhatt D.L., Eikelboom J.W., Konkle B., Mohler E.R., Reilly M.P., Berger J.S. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J. Thromb. Haemost. 2010;8:148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani H.T. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev. Med. (Baltim). 1998;27:184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Debaun M.R., Kirkham F.J. Central nervous system complications and management in sickle cell disease. Blood. 2016 doi: 10.1182/blood-2015-09-618579. [DOI] [PubMed] [Google Scholar]
- DeBaun M.R., Armstrong F.D., McKinstry R.C., Ware R.E., Vichinsky E., Kirkham F.J. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119:4587–4596. doi: 10.1182/blood-2011-02-272682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun M.R., Sarnaik S.A., Rodeghier M.J., Minniti C.P., Howard T.H., Iyer R.V., Inusa B., Telfer P.T., Kirby-Allen M., Quinn C.T., Bernaudin F., Airewele G., Woods G.M., Panepinto J.A., Fuh B., Kwiatkowski J.K., King A.A., Rhodes M.M., Thompson A.A., Heiny M.E., Redding-Lallinger R.C., Kirkham F.J., Sabio H., Gonzalez C.E., Saccente S.L., Kalinyak K.A., Strouse J.J., Fixler J.M., Gordon M.O., Miller J.P., Noetzel M.J., Ichord R.N., Casella J.F. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119:3684–3690. doi: 10.1182/blood-2011-05-349621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun M.R., Gordon M., McKinstry R.C., Noetzel M.J., White D.A., Sarnaik S.A., Meier E.R., Howard T.H., Majumdar S., Inusa B.P.D., Telfer P.T., Kirby-Allen M., McCavit T.L., Kamdem A., Airewele G., Woods G.M., Berman B., Panepinto J.A., Fuh B.R., Kwiatkowski J.L., King A.A., Fixler J.M., Rhodes M.M., Thompson A.A., Heiny M.E., Redding-Lallinger R.C., Kirkham F.J., Dixon N., Gonzalez C.E., Kalinyak K.A., Quinn C.T., Strouse J.J., Miller J.P., Lehmann H., Kraut M.A., Ball W.S., Hirtz D., Casella J.F. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N. Engl. J. Med. 2014;371:699–710. doi: 10.1056/NEJMoa1401731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S., Markus H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341 doi: 10.1136/bmj.c3666. 10.1136/bmj.c3666 (c3666-c3666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar D., Yam P., McCulloch J. Drug development for stroke: importance of protecting cerebral white matter. Eur. J. Pharmacol. 1999;375:41–50. doi: 10.1016/s0014-2999(99)00280-0. [DOI] [PubMed] [Google Scholar]
- Douaud G., Groves A.R., Tamnes C.K., Westlye L.T., Duff E.P., Engvig A., Walhovd K.B., James A., Gass A., Monsch A.U., Matthews P.M., Fjell A.M., Smith S.M., Johansen-Berg H. A common brain network links development, aging, and vulnerability to disease. Proc. Natl. Acad. Sci. 2014;201410378 doi: 10.1073/pnas.1410378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein F., Bunn H. Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- Fitzhugh C.D., Lauder N., Jonassaint J.C., Telen M.J., Zhao X., Wright E.C., Gilliam F.R., De Castro L.M. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am. J. Hematol. 2010;85:36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.E., Levitt P., Nelson C.A. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell K.L. Population estimates of sickle cell disease in the U.S. Am. J. Prev. Med. 2010;38:S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Helton K.J., Adams R.J., Kesler K.L., Lockhart A., Aygun B., Driscoll C., Heeney M.M., Jackson S.M., Krishnamurti L., Miller S.T., Sarnaik S.A., Schultz W.H., Ware R.E. Magnetic resonance imaging/angiography and transcranial Doppler velocities in sickle cell anemia: results from the SWiTCH trial. Blood. 2014 doi: 10.1182/blood-2013-12-545186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hulbert M.L., McKinstry R.C., Lacey J.L., Moran C.J., Panepinto J.A., Thompson A.A., Sarnaik S.A., Woods G.M., Casella J.F., Inusa B., Howard J., Kirkham F.J., Anie K.A., Mullin J.E., Ichord R., Noetzel M., Yan Y., Rodeghier M., Debaun M.R. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117:772–779. doi: 10.1182/blood-2010-01-261123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T., Wolf P.A., Beiser A., Massaro J., Seshadri S., D'Agostino R.B., DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: the Framingham study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- Joshi A.A., Shattuck D.W., Leahy R.M. Biomedical Image Registration. 2012. A method for automated cortical surface registration and labeling; pp. 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawadler J.M., Clayden J.D., Kirkham F.J., Cox T.C., Saunders D.E., Clark C.a. Subcortical and cerebellar volumetric deficits in paediatric sickle cell anaemia. Br. J. Haematol. 2013;163:373–376. doi: 10.1111/bjh.12496. [DOI] [PubMed] [Google Scholar]
- Kawadler J.M., Kirkham F.J., Clayden J.D., Hollocks M.J., Seymour E.L., Edey R., Telfer P., Robins A., Wilkey O., Barker S., Cox T.C.S., Clark C.A. White matter damage relates to oxygen saturation in children with sickle cell anemia without silent cerebral infarcts. Stroke. 2015;46:1793–1799. doi: 10.1161/STROKEAHA.115.008721. [DOI] [PubMed] [Google Scholar]
- Kirk G.R., Haynes M.R., Palasis S., Brown C., Burns T.G., McCormick M., Jones R.A. Regionally specific cortical thinning in children with sickle cell disease. Cereb. Cortex. 2009;19:1549-56. doi: 10.1093/cercor/bhn193. [DOI] [PubMed] [Google Scholar]
- van der Land V., Hijmans C.T., de Ruiter M., Mutsaerts H.J.M.M., Cnossen M.H., Engelen M., Majoie C.B.L.M., Nederveen A.J., Grootenhuis M.a., Fijnvandraat K. Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br. J. Haematol. 2015;168:553–556. doi: 10.1111/bjh.13179. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Liu Q., He S., Groysman L., Shaked D., Russin J., Cen S., Mack W.J. White matter injury due to experimental chronic cerebral hypoperfusion is associated with C5 deposition. PLoS One. 2013;8:2–10. doi: 10.1371/journal.pone.0084802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin R.S., Insel P., Truran D., Vichinsky E.P., Neumayr L.D., Armstrong F.D., Gold J.I., Kesler K., Brewer J., Weiner M.W. Neuroimaging abnormalities in adults with sickle cell anemia: associations with cognition. Neurology. 2014;82:835–841. doi: 10.1212/WNL.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.T., Macklin E.A., Pegelow C.H., Kinney T.R., Sleeper L.A., Bello J.A., DeWitt L.D., Gallagher D.M., Guarini L., Moser F.G., Ohene-Frempong K., Sanchez N., Vichinsky E.P., Wang W.C., Wethers D.L., Younkin D.P., Zimmerman R.A., DeBaun M.R. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: a report from the Cooperative Study of Sickle Cell Disease. J. Pediatr. 2001;139:385–390. doi: 10.1067/mpd.2001.117580. [DOI] [PubMed] [Google Scholar]
- Moser F.G., Miller S.T., Bello J.A., Pegelow C.H., Zimmerman R.A., Wang W.C., Ohene-Frempong K., Schwartz A., Vichinsky E.P., Gallagher D., Kinney T.R. The spectrum of brain MR abnormalities in sickle-cell disease: a report from the Cooperative Study of Sickle Cell Disease. AJNR Am. J. Neuroradiol. 1996;17:965–972. [PMC free article] [PubMed] [Google Scholar]
- Nagy Z., Westerberg H., Skare S., Andersson J.L., Lilja A., Flodmark O., Fernell E., Holmberg K., Bohm B., Forssberg H., Lagercrantz H., Klingberg T. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr. Res. 2003;54:672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- Nelson M.D., Jr., Wilson D.A., Kisker C.T., Evatt B.L., Fenstermacher M.J., Lynn H.S., Donfield S.M., Maeder M.A. Incidence of focal white matter lesions in a population of hemophiliac children and their normal siblings. Hemophilia Growth and Development Study. Pediatr. Radiol. 2000;30:705–709. doi: 10.1007/s002470000290. [DOI] [PubMed] [Google Scholar]
- Nour M.M., Howes O.D. Interpreting the neurodevelopmental hypothesis of schizophrenia in the context of normal brain development and ageing. Proc. Natl. Acad. Sci. 2015;112:E2745. doi: 10.1073/pnas.1502170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohene-Frempong K., Weiner S.J., Sleeper L.A., Miller S.T., Embury S., Moohr J.W., Wethers D.L., Pegelow C.H., Gill F.M. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- Pantoni L., Garcia J.H., Gutierrez J.A., Rosenblum W.I. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1647. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- Pegelow C.H., Macklin E.A., Moser F.G., Wang W.C., Bello J.A., Miller S.T., Vichinsky E.P., DeBaun M.R., Guarini L., Zimmerman R.A., Younkin D.P., Gallagher D.M., Kinney T.R. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99:3014–3018. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]
- Postma I.R., De Groot J.C., Aukes A.M., Aarnoudse J.G., Zeeman G.G. Cerebral white matter lesions and perceived cognitive dysfunction: the role of pregnancy. Am. J. Obstet. Gynecol. 2014;211 doi: 10.1016/j.ajog.2014.02.031. 10.1016/j.ajog.2014.02.031 (257.e1-257.e5) [DOI] [PubMed] [Google Scholar]
- Powars D., Wilson B., Imbus C., Pegelow C., Allen J. The natural history of stroke in sickle cell disease. Am. J. Med. 1978;65:461–471. doi: 10.1016/0002-9343(78)90772-6. [DOI] [PubMed] [Google Scholar]
- Powars D.R., Conti P.S., Wong W.Y., Groncy P., Hyman C., Smith E., Ewing N., Keenan R.N., Zee C.S., Harold Y., Hiti A.L., Teng E.L., Chan L.S. Cerebral vasculopathy in sickle cell anemia: diagnostic contribution of positron emission tomography. Blood. 1999;93:71–79. [PubMed] [Google Scholar]
- Prohovnik I., Hurlet-jensen A., Adams R., Vivo D. De, Pavlakis S.G. 2009. Hemodynamic Etiology of Elevated Flow Velocity and Stroke in Sickle-Cell Disease; pp. 803–810. [DOI] [PubMed] [Google Scholar]
- Quinn C.T., Rogers Z.R., McCavit T.L., Buchanan G.R. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C.T., McKinstry R.C., Dowling M.M., Ball W.S., Kraut M.A., Casella J.F., Dlamini N., Ichord R.N., Jordan L.C., Kirkham F.J., Noetzel M.J., Roach E.S., Strouse J.J., Kwiatkowski J.L., Hirtz D., DeBaun M.R. Acute silent cerebral ischemic events in children with sickle cell anemia. JAMA Neurol. 2013;70:58–65. doi: 10.1001/jamaneurol.2013.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat. Rev. Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- Rees D.C., Williams T.N., Gladwin M.T. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Sandhu M.K., Cohen A. Aging in sickle cell disease: co-morbidities and new issues in management. Hemoglobin. 2015;39:221–224. doi: 10.3109/03630269.2015.1040493. [DOI] [PubMed] [Google Scholar]
- Schatz J., Buzan R. Decreased corpus callosum size in sickle cell disease: relationship with cerebral infarcts and cognitive functioning. J. Int. Neuropsychol. Soc. 2006;12:24–33. doi: 10.1017/S1355617706060085. [DOI] [PubMed] [Google Scholar]
- Schatz J., McClellan C.B. Sickle cell disease as a neurodevelopmental disorder. Ment. Retard. Dev. Disabil. Res. Rev. 2006;12:200–207. doi: 10.1002/mrdd.20115. [DOI] [PubMed] [Google Scholar]
- Schatz J., White D.A., Moinuddin A., Armstrong M., DeBaun M.R. Lesion burden and cognitive morbidity in children with sickle cell disease. J. Child Neurol. 2002;17:891–895. [PubMed] [Google Scholar]
- Scothorn D.J., Price C., Schwartz D., Terrill C., Buchanan G.R., Shurney W., Sarniak I., Fallon R., Chu J.-Y., Pegelow C.H., Wang W., Casella J.F., Resar L.S., Berman B., Adamkiewicz T., Hsu L.L., Ohene-Frempong K., Smith-Whitley K., Mahoney D., Scott J.P., Woods G.M., Watanabe M., Debaun M.R. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J. Pediatr. 2002;140:348–354. doi: 10.1067/mpd.2002.122498. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Ohtani R., Ihara M., Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen R.G., Emudianughe T., Hunte M., Glass J., Wu S., Xiong X., Reddick W.E. Brain volume in pediatric patients with sickle cell disease: evidence of volumetric growth delay? AJNR Am. J. Neuroradiol. 2005;26:455–462. [PMC free article] [PubMed] [Google Scholar]
- Strouse J.J., Jordan L.C., Lanzkron S., Casella J.F. The excess burden of stroke in hospitalized adults with sickle cell disease. Am. J. Hematol. 2009;84:548–552. doi: 10.1002/ajh.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Brown R.C., Hayes L., Burns T.G., Huamani J., Bearden D.J., Jones R.A. White matter damage in asymptomatic patients with sickle cell anemia: screening with diffusion tensor imaging. AJNR Am. J. Neuroradiol. 2012;33:2043–2049. doi: 10.3174/ajnr.A3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatu L., Moulin T., Bogousslavsky J., Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- Thompson B.L., Levitt P., Stanwood G.D. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat. Rev. Neurosci. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Land V., Peters M., Biemond B.J., Heijboer H., Harteveld C.L., Fijnvandraat K. Markers of endothelial dysfunction differ between subphenotypes in children with sickle cell disease. Thromb. Res. 2013;132:712–717. doi: 10.1016/j.thromres.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Verduzco L.A., Nathan D.G. Review article sickle cell disease and stroke. Stroke. 2009;114:5117–5125. doi: 10.1182/blood-2009-05-220921. [DOI] [PubMed] [Google Scholar]
- Vichinsky E.P., Neumayr L.D., Gold J.I., Weiner M.W., Rule R.R., Truran D., Kasten J., Eggleston B., Kesler K., McMahon L., Orringer E.P., Harrington T., Kalinyak K., De Castro L.M., Kutlar A., Rutherford C.J., Johnson C., Bessman J.D., Jordan L.B., Armstrong F.D. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA. 2010;303:1823–1831. doi: 10.1001/jama.2010.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizioli L., Muscari S., Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int. J. Clin. Pract. 2009;63:1509–1515. doi: 10.1111/j.1742-1241.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- Voskaridou E., Tsetsos G., Tsoutsias A., Spyropoulou E., Christoulas D., Terpos E. Pulmonary hypertension in patients with sickle cell/thalassemia: incidence and correlation with serum N-terminal pro-brain natriuretic peptide concentrations. Haematologica. 2007;92:738–743. doi: 10.3324/haematol.11136. [DOI] [PubMed] [Google Scholar]
- Wang W.C., Langston J.W., Steen R.G., Wynn L.W., Mulhern R.K., Wilimas J.A., Kim F.M., Figueroa R.E. Abnormalities of the central nervous system in very young children with sickle cell anemia. J. Pediatr. 1998;132:994–998. doi: 10.1016/s0022-3476(98)70397-x. [DOI] [PubMed] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Yaggi H.K., Concato J., Kernan W.N., Lichtman J.H., Brass L.M., Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- Yawn B.P., Buchanan G.R., Afenyi-Annan A.N., Ballas S.K., Hassell K.L., James A.H., Jordan L., Lanzkron S.M., Lottenberg R., Savage W.J., Tanabe P.J., Ware R.E., Murad M.H., Goldsmith J.C., Ortiz E., Fulwood R., Horton A., John-Sowah J. Management of sickle cell disease. JAMA. 2014;312:1033. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]