Abstract
BACKGROUND: We constructed a genetically modified adenovirus vector incorporating an IgG Fc-binding motif from staphylococcal protein A, Z33 (Adv-FZ33). Adv-FZ33 allows an antibody to redirect the vector to a target molecule on the cell surface. We attempted to search for target antigen candidates and antibodies that allowed highly selective gene transduction into malignant tumors. METHODS: Hybridoma libraries producing monoclonal antibodies (mAbs) were screened that increased transduction efficiency in cancer cell lines after cross-linking with Adv-FZ33. Target antigens of the mAbs were identified by immunoprecipitation and mass spectrometry. Of these mAbs, we noted a clone, F2-27, that recognized the receptor tyrosine kinase EphA2. Next, we generated an adenovirus vector, Ax3CMTK-FZ33, that expressed a herpes simplex virus thymidine kinase (HSV-TK). The therapeutic efficacy of F2-27–mediated HSV-TK gene transduction, followed by ganciclovir (GCV) administration, was studied in vitro. The inhibitory effect of F2-27 on cancer cell invasion was investigated by a three-dimensional spheroid formation assay. RESULTS: In vitro reporter gene expression after Adv-FZ33 infection via F2-27 was 146 times higher than with control mAb in EphA2-expressing cancer cell lines. F2-27–mediated Ax3CMTK-FZ33 infection induced the HSV-TK gene in an F2-27–dependent manner and had a highly effective cytotoxic effect in a GCV-dependent manner. Additionally, F2-27 independently inhibited migration of EphA2-positive breast cancer cell lines in three-dimensional culture. CONCLUSION: Our modified adenovirus and hybridoma screening system is useful for the development of targeted cancer therapy, and F2-27 has the potential to be an antibody-based therapy for various EphA2-positive cancers.
Introduction
In 2012, the World Health Organization announced that cancer was a leading cause of morbidity and mortality worldwide, with approximately 14 million new cases and 8.2 million cancer-related deaths [1]. Within the next two decades, the number of new cases is expected to rise by about 70%. Additionally, in relation to metastatic cancer, mortality rates or prolonged survival times remain unsatisfactory. In terms of cancer treatment, by focusing on molecular and cellular changes specific to cancer, targeted cancer therapies may be more effective than other types of existing treatments, including chemotherapy and radiotherapy, and less harmful to normal cells.
We have previously reported on a genetically modified adenovirus vector, Adv-FZ33, with an incorporated IgG-binding domain inserted into the adenovirus serotype 5 (Ad5) virus having fiber protein [2]. Adv-FZ33 allows an antibody to redirect the vector to a target molecule at the cell surface. We subsequently established a screening method to search for antibody and cell surface target candidates that could provide highly selective gene transduction to malignant tumors. Hybridoma libraries producing monoclonal antibodies (mAbs) were screened against human cancer cell lines, and 20 different mAbs that increased the transduction efficiency of Adv-FZ33 for cancer cell lines were established. We selected a mAb (F2-27) that recognized the receptor tyrosine kinase EphA2.
The Eph family is comprised of EphA (EphA1-A10) or EphB (EphB1-B6) subclasses of receptors, classified as per their sequence homologies and their binding affinities for their ligands, ephrins [3], [4], [5]. Ephrins, in turn, are divided into two subclasses, ephrin-A (ephrin-A1-A6) and ephrin-B (ephrin-B1-B3) [6], [7]. Ephrin-A members are anchored to the plasma membrane by a glycosylphosphatidylinositol linkage, whereas ephrin-B members have a transmembrane and a cytoplasmic domain. In general, ephrin-A and ephrin-B ligands interact with EphA and EphB receptors, respectively. A unique property of ephrins, derived from their membrane localization, is their ability to transduce “reverse” signals into the cells on which they are expressed, in addition to eliciting “forward” signaling into Eph receptor–expressing cells. Ephs are expressed at the highest level during development and are found at low levels in normal adult tissue [8], [9], [10].
Increasing interest has arisen in recent years in Ephs and ephrins, particularly EphA2 and ephrin-A1, due to their documented or suspected involvement in mediating processes leading to the formation and progression of malignancy. The EphA2 receptor is a 130-kDa, 976–amino acid transmembrane glycoprotein that is abundantly overexpressed in several solid tumors [11]. Overexpression has been shown at both mRNA and protein levels in established cell lines and in human tumor tissue specimens.
In the present study, we established a novel antibody screening system based on the infectivity of modified adenovirus and examined whether F2-27 would be useful for targeted therapy against human cancer cells.
Materials and Methods
Materials
Recombinant human epidermal growth factor (rEGF; Cell Signaling Technology Inc., Danvers, MA), BSA (Fr V; Roche Applied Science, Mannheim, Germany), recombinant human ephrin-A1 Fc chimera (R&D Systems, Minneapolis, MN), DMEM and F12K medium (Sigma, St. Louis, MO), alpha-MEM and opti-MEM I (Invitrogen, Carlsbad, CA), ganciclovir (GCV) and 2-mercaptoethanol (Wako Pure Chemical Industries Ltd., Osaka, Japan), sulfo-NHS-biotin (Pierce, Rockford, IL), and Protein G sepharose beads (GE Healthcare, Buckinghamshire, UK) were purchased. Small interfering RNA (siRNA) oligonucleotides were obtained from Ambion Inc. (Austin, TX). Short hairpin RNA (shRNA) constructs were obtained from OriGene Technologies, Inc. (Rockville, MD).
Cell Lines
The murine myeloma cell line P3U1, human pancreatic cancer cell lines (KP-2, KP-3, SUIT-2, MIAPaCa-2), human prostate cancer cell line PC-3, human gastric cancer cell line MKN-1, and human malignant lymphoma cell line A3/KAW were purchased from the Japanese Collection of Research Bioresources Cell Bank (JCRB, Osaka, Japan). The human breast cancer cell line MDA-MB-231 and the colon cancer cell line Caco-2 were purchased from the American Type Culture Collection (Manassas, VT). Human dermal fibroblast (Fb) cells and Chinese hamster ovary (CHO) cells were purchased from DS Pharma Biomedical (Osaka, Japan). MDA-MB-231, PC-3, and CHO cell lines were maintained in DMEM, F12K, and alpha-MEM medium, respectively, containing 10% fetal bovine serum (FBS; Equitech-Bio Inc., Kerrville, TX) and antibiotics (100,000 U/l penicillin G potassium and 100 mg/l streptomycin sulfate; Meiji Seika Pharma Co. Ltd., Tokyo, Japan) in a humidified tissue culture incubator at 37°C and in 5% CO2. Fb cells were cultured in CS-C medium (DS Pharma Biomedical). Other cells were cultured in RPMI-1640 supplemented with 10% FBS and antibiotics as above. All cells were tested for mycoplasma and shown to be free of contamination. Stocks of cell lines were stored frozen in liquid nitrogen. Cells were thawed as needed and maintained for no longer than 3 months.
Adenoviral Vectors
The recombinant adenovirus vectors used were based on E1- and E3-deleted Ad5 with a modified fiber FZ33, harboring an IgG-binding Z33-motif, FNMQQQRRFYEALHDPNLNEEQRNAKIKSIRDD, within the HI loop of its knob protein (Adv-FZ33) according to a previously described procedure [2]. Ax3CAZ3-FZ33, a modified adenovirus, carried lacZ as a reporter gene. For a therapeutic approach, Ax3CMTK-FZ33, which carried a herpes simplex virus thymidine kinase (HSV-TK gene), conferred cytotoxic sensitivity to ganciclovir in tumor cells.
Immunization, Production, and Screening of Hybridomas
Balb⁄c mice were immunized by intraperitoneal injection of cancer cell lines (KP-2, KP-3, SUIT-2, MIAPaCa-2), and harvested splenocytes were fused with P3U1 myeloma cells using polyethylene glycol. After selection with HAT medium, hybridoma supernatants were added to cultured-MIAPaCa-2 cells in 96-well microplates, and cells were then infected with Ax3CAZ3-FZ33, a recombinant adenovirus expressing β-galactosidase, at 3 × 103 viral particles (VP)/cell. Three days after infection, chemiluminescent β-galactosidase (β-gal) reporter gene assays (Roche Diagnostics, Tokyo, Japan) were performed according to the company's recommendations. Hybridomas that showed high β-gal activity were isolated. Immunoglobulin subclasses were determined with an IsoStrip mouse monoclonal antibody isotyping kit (Roche Diagnostics) according to the manufacturer's instructions.
Detection of Antigens
The antigen recognized by F2-27 was identified by biotin labeling and immunoprecipitation, followed by avidin-mediated detection as follows: Human epithelial colorectal adenocarcinoma Caco-2 cells (3 × 106 cells) were solubilized on ice for 30 minutes in 1 ml of buffer containing 1% NP40, 50 mM Tris-HCl, pH 7.6, 150 mM NaCl, and a protease inhibitor cocktail (Roche Diagnostics). Samples were mixed with protein G sepharose beads and incubated for 2 hours at 4°C, after which beads were centrifuged to remove nonspecifically bound proteins. Five micrograms of either the F2-27 or a control mouse IgG1 (Ancell Corporation, Bayport, MN) was added to each sample. Immunocomplexes were precipitated by the addition of protein G sepharose beads to each sample and incubated for 2 hours at 4°C. The supernatant was discarded, and beads were washed six times with solubilization buffer. Immunocomplexes that had bound the beads were boiled for 5 minutes in 45 μl of SDS sample buffer containing 5% 2-mercaptethanol. Samples were separated using 5% to 20% gradient polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA), and proteins were transferred onto PVDF membranes (Bio-Rad). After blocking with 5% milk in PBS, membranes were incubated for 30 minutes at room temperature with avidin-horseradish peroxidase (dilution 1:25,000; Vector Laboratories, Inc., Burlingame, CA). Detection was carried out by ECL Western blotting detection reagents (GE Healthcare, Buckinghamshire, UK) according to the manufacturer's instructions. Immunoprecipitated antigen molecules were cut out from the SDS-PAGE gel. The resulting peptide was analyzed by liquid chromatography–tandem mass spectrometry conducted by APRO Science Institute (Tokushima, Japan). Binding specificity to the candidate antigen was examined by flow cytometry analysis of CHO cells transfected with human EphA2.
Flow Cytometry Analysis
The reactivity of F2-27 with various human cells, including pancreas, colon, gastric, breast, lymphoma, and prostate cancer cell lines, was examined. Cells (3-5 × 105) were first incubated with 10 μg/ml of antibody for 1 hour on ice. Subsequently, cells were washed in wash buffer (0.5% BSA/Dulbecco's PBS) and incubated with FITC-conjugated rabbit anti-mouse immunoglobulins (Dako, Glostrup, Denmark) for 30 minutes on ice in the dark. After washing in wash buffer, cells were resuspended in 500 μl of wash buffer. Flow cytometry analysis was performed on a FACS Calibur (BD Biosciences, San Jose, CA).
Transduction Efficiency
Cells were incubated in serum-free medium, with (F2-27 or control isotype mAb) or without antibody, at 37°C in 5% CO2 for 1 hour. After incubation, cells were cultured in serum-free medium containing Ax3CAZ3-FZ33 (104 VP/cell) at 37°C in 5% CO2 for 1 hour. After incubation, cells were washed and then cultured in RPMI-1640 with 10% FBS for 24 hours. LacZ activity was measured by a β-gal reporter gene assay (Roche Molecular Biochemicals, Indianapolis, IN).
Internalization of F2-27
PC-3 cells were plated at 104 cells/well in a 96-well plate and cultured overnight. The cell medium was exchanged for culture medium without FBS, and cells were cultured for a further 5 hours before immunostaining. The immunostaining procedure was as follows. The culture plate was placed on ice for 10 minutes, washed with ice-cold Kreb's Ringer buffer, and incubated with 5 μg/ml of F2-27 or isotype IgG1 for 30 minutes while still on ice. Five micrograms per milliliter of Cy5-conjugated anti-mouse goat IgG (Jackson Immuno-Research Laboratories, Inc., West Grove, PA) containing 2.5 μM Hoechst 33342 was added to each well, and cells were incubated at room temperature for 15 minutes, washed twice with cold Kreb's Ringer buffer, and incubated at 37°C for 30 minutes. Images were captured using a BZ-9000 fluorescence microscope from Keyence Corp. (Itasca, IL) using a 20× objective and BZ filter Cy5.
Ganciclovir Sensitivity
PC-3 cells were treated with (F2-27 or control isotype mAb) or without antibody and infected with Ax3CMTK-FZ33 at various concentrations of VP/cell. Infected cells were cultured in medium containing GCV (0.001-1000 μg/mL) for 5 days. Cell survival was evaluated by MTT cell proliferation assay (CellTiter 96 Aqueous One Cell Proliferation Assay, Promega, Madison, WI; Cell Counting kit-8, Dojindo Laboratories, Kumamoto, Japan).
ShRNA-Mediated Knockdown of EphA2
EphA2-targeting shRNA expression plasmids were obtained from OriGene Technologies (TR320327A-D and TR300012 retroviral plasmids were used as negative controls and were constructed with a pRS vector containing puromycin-N-acetyltransferase). The shRNA expression plasmids were transfected into MDA-MB-231 and PC-3 cell lines using jetPRIME transfection reagent (Polyplus-transfection SA, Illkirch, France) according to product manual instructions. For each well of a 24-well plate format, 0.8 μg DNA was diluted in 75 μl of jetPRIME buffer and mixed with 1.6 μl of jetPRIME reagent, and the mixture was added as drops to cells. After incubation overnight, the growth medium was replaced, and puromycin selection started on day 2 posttransfection.
Spheroid Formation Assay
A Cultrex 96-well three dimensional (3-D) spheroid BME cell invasion assay kit (Trevigen, Inc., Gaithersburg, MD) was employed and used according to kit instructions as described briefly: MDA-MB-231 (3000 cells/well) were seeded (day 0) and cultured for spheroid formation in the presence of extracellular matrix protein (50 μl/well) in a 3-D culture-qualified, 96-well spheroid formation plate. Three days later, invasion matrix (50 μl/well) was added, and plates were gently centrifuged to position each spheroid in the center of each well and incubated at 37°C for 1 hour for matrix gel formation. Cell culture medium (100 μl/well) containing chemoattractant and invasion modulating compounds was added, and cellular spheroids were cultured for a further 3 to 6 days. The spheroid in each well was photographed every 24 hours using a 4× objective. Images were analyzed to evaluate 3-D culture cell invasion using ImageJ.
Statistical Evaluation
Statistical values are presented as mean ± standard deviation (S.D.). The significance of differences between groups was assessed by a Student's t test. Statistical significance was defined as P < .01.
Results
Isolation of F2-27 and Identification of Target Antigen
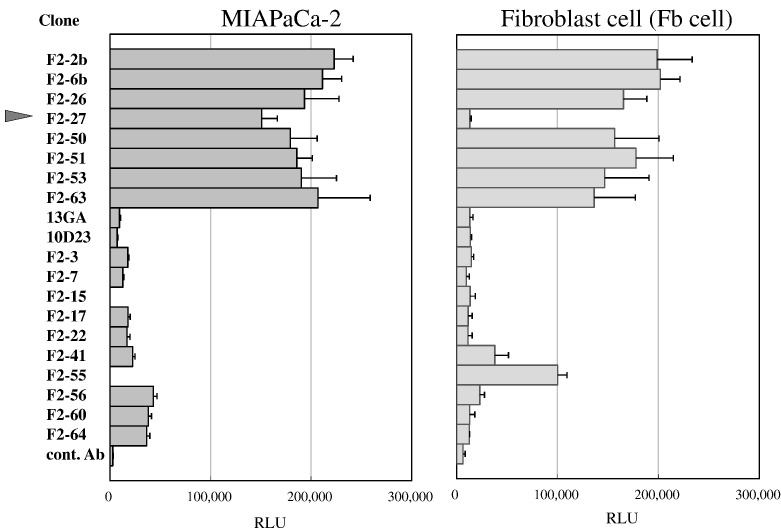
By cross-linking the Adv-FZ33 virus and hybridoma libraries that produce various antibodies, we searched for antibodies and cell surface target candidates with the potential to provide a highly selective gene delivery system for human cancer cell lines. After screening for the ability to enhance the gene transduction efficiency of Ax3CAZ3-FZ33 encoding lacZ, we obtained 20 hybridoma clones that recognized cancer-associated antigens. Of these clones, we selected F2-27, a mouse IgG1 antibody that had demonstrated strong β-gal activity in MIAPaCa-2 cells and no reactivity to Fb cells (Figure 1).
Figure 1.
Twenty different mAb clones that increased transduction efficiency.
MiaPaCa-2 and Fb cells were treated with mAbs derived from hybridoma libraries or a control mAb. After infection with Ax3CAZ-FZ33, β-gal activity was analyzed (n = 4). Values are expressed as mean ± SD. RLU, relative light units.
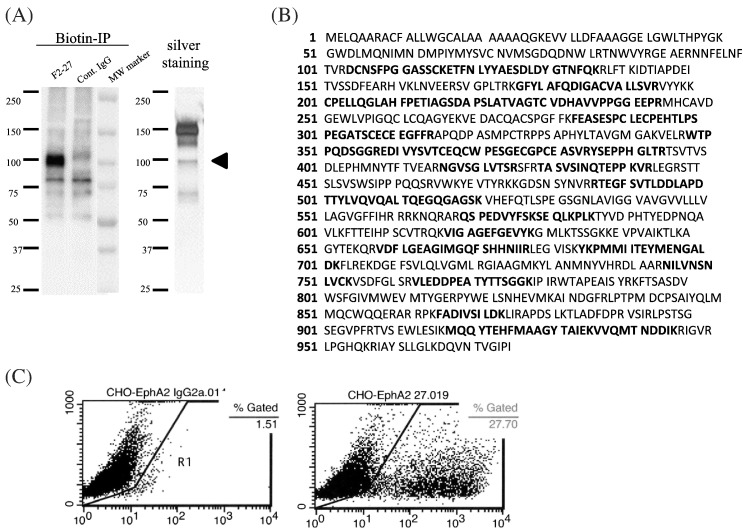
Biotinylated Caco-2 cell lysate was immunoprecipitated with F2-27, and an approximately 100-kDa band was detected by Western blot and silver staining (Figure 2A). The protein sequence of the putative F2-27 target was identified by mass spectrometry as EphA2 (Figure 2B). The specificity of the antibody was evaluated using CHO cells transfected with plasmid vectors encoding EphA2 cDNA as a positive control, as well as using negative controls. F2-27 was recognized by EphA2-expressing CHO cells only (Figure 2C).
Figure 2.
Establishment of F2-27 and its antigens.
(A) Biotinylated Caco-2 lysate was immunoprecipitated with F2-27 or control IgG1. Proteins were detected by Western blot and silver staining. (B) EphA2 amino acid sequence. Bold letters indicate the sequence of the detected peptide. (C) Flow cytometry of the reactivity of F2-27 mAb with CHO cells transfected with EphA2 cDNA. F2-27 reacted only with CHO cells transfected with EphA2 cDNA. The numbers in each panel indicate the percentage of cells expressing EphA2.
Reactivity of F2-27 to Cancer Cell Lines
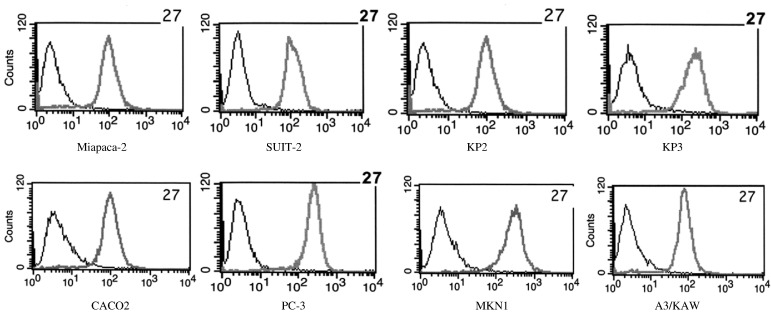
Flow cytometry analysis was performed to examine the expression of EphA2 in various tumor cell lines, including pancreatic, colon, prostate, and gastric cancer and lymphoma lines, using F2-27. F2-27 bound strongly to epithelial cancer cell lines (Figure 3), indicating these cells to express EphA2.
Figure 3.
Expression of EphA2 in cancer cells.
The reactivity of F2-27 with various cancer cell lines by flow cytometry. Gray histograms indicate stained cells; black histograms indicate unstained control cells.
F2-27–Mediated Gene Transduction in Cancer Cells
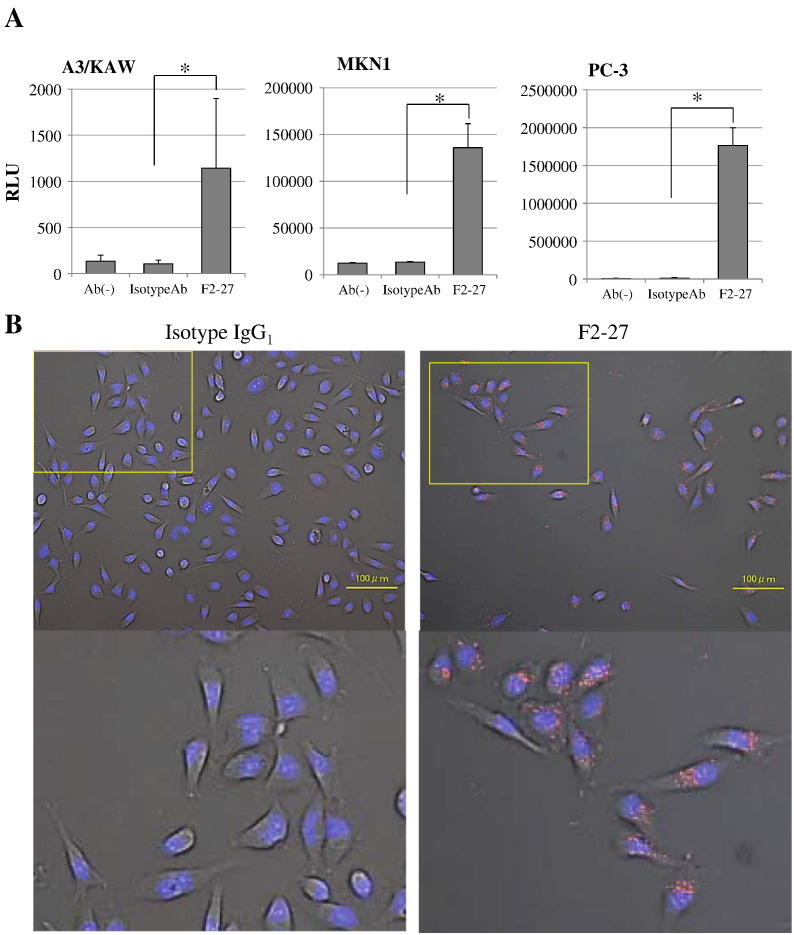
We quantitated the transduction efficiency mediated by Ax3CAZ3-FZ33 using F2-27, an isotype IgG1 control, or no antibodies in three EphA2-expressing cell lines (A3/KAW, MKN1, and PC-3) by chemiluminescent β-gal gene assay. As shown in Figure 4A, infection with Ax3CAZ3-FZ33 plus F2-27 significantly increased β-gal activities in these cell lines compared to infection with Ax3CAZ3-FZ33 plus isotype IgG1 control or with vector alone (n = 4, P < .01). In A3/KAW and MKN1 cell lines, F2-27–mediated vector infection showed a 10.7- and 10.1-fold enhancement, respectively, compared with isotype IgG1 control. In PC-3 cells, F2-27–mediated vector infection showed 145.9- and 236.1-fold enhancements compared with isotype IgG1 control or vector alone, respectively.
Figure 4.
(A) F2-27–mediated gene transduction in cancer cells.
EphA2-expressing cells (A3/KAW, MKN1, and PC-3) were treated with serum-free medium containing antibody (F2-27 or isotype IgG1 control) or no antibody (Ab−). After infection with Ax3CAZ-FZ33, β-gal activity was analyzed (n = 4). Chemiluminescent efficiencies according to β-gal expression levels were plotted at 104 VP/cell. Values are expressed as mean ± SD. *P < .01, Student's t test.
(B) Fluorescence microscopy images of the internalization and distribution of F2-27.
Immune complexes, composed of Cy5-conjugated isotype IgG1 (left) or F2-27 (right), were stained and visualized in red under a fluorescence microscope. The boxed area is shown at high magnification. Scale bars: 100 μm.
Internalization of F2-27 Analyzed by Fluorescence Microscopy
PC-3 cells were incubated with F2-27 or isotype IgG1 control. Antibody internalization was evaluated according to the Cy5 intensity within cells. F2-27 exhibited high internalization activity (Figure 4B).
F2-27–Mediated HSV-TK/GCV Suicide Gene Therapy
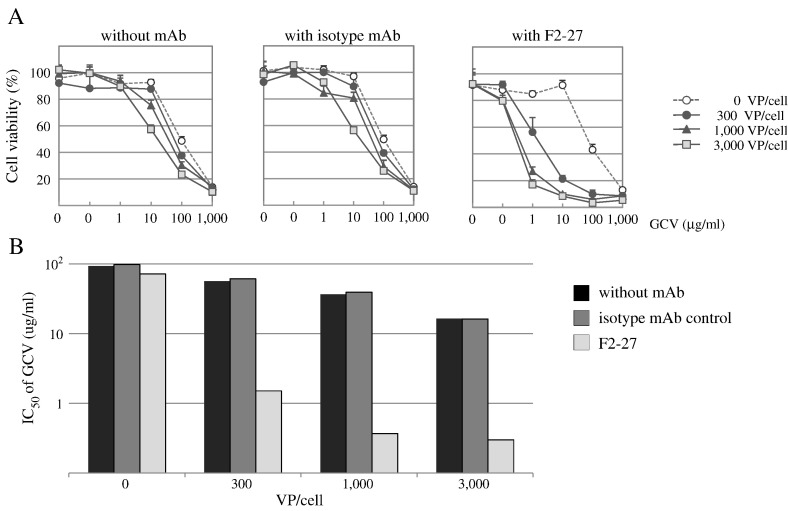
The retrovirus-mediated transfer of the HSV-TK gene has been used to confer cytotoxic sensitivity to the nucleoside analogue GCV to a variety of tumor cells [12]. HSV-TK converts GCV into a phosphorylated compound that acts as a chain terminator in DNA synthesis, killing HSV-TK–expressing cells [13]. After infection with Ax3CMTK-FZ33, PC-3 cells were treated with varying doses of GCV for 5 days, and the number of viable cells was determined by cell proliferation assay. Cytopathic effects by viral vectors were not evident when cells were infected at 3000 VP/cell. F2-27–mediated Ax3CMTK-FZ33 infection induced the HSV-TK gene in an F2-27–dependent manner and caused GCV to be highly cytotoxic to PC-3 cells, whereas it had less of an effect when an isotype control mAb was used (n = 4; Figure 5A). In particular, the IC50 of GCV in F2-27–treated cells was 0.37 μg/ml and that in isotype control mAb-treated cells was 39.28 μg/ml under the condition of a 1000-VP/cell infection, showing a significant 106-fold difference (Figure 5B).
Figure 5.
F2-27–mediated HSV-TK/GCV suicide gene therapy.
F2-27–directed therapeutic gene transduction of GCV sensitivity was performed. PC-3 cells were treated with an antibody (F2-27 or isotype mAb control) or no antibody and infected with AxCMTK-FZ33 at 3000 VP/cell. Infected cells were cultured in medium containing GCV (0.01-1000 μg/mL) for 5 days. (A) Cell survival was evaluated by MTT assay (n = 4). Values are expressed as mean ± S.D. (B) IC50 (μg/mL) of GCV in Ax3CMTK-FZ33–infected PC-3 cells.
Inhibition of EphA2 Decreases Spheroid Formation
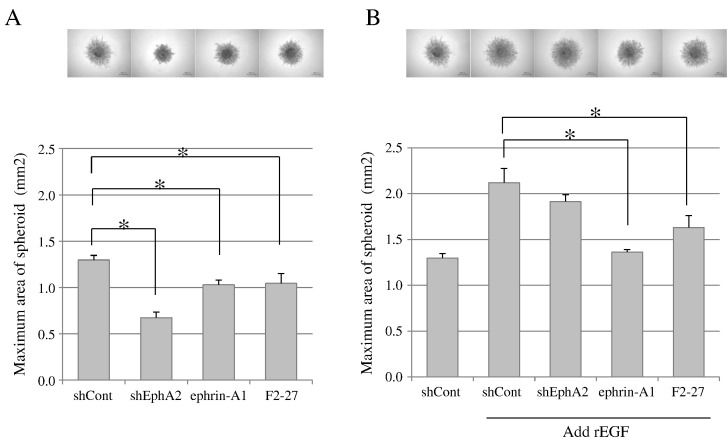
To investigate the role of EphA2 in cancer cell invasion in 3-D culture, a spheroid formation assay was performed. The downregulation of EphA2 in MDA-MB-231 cell lines was achieved by shRNA. Reverse transcription polymerase chain reaction and immunoblotting analyses confirmed that expressing shEphA2 markedly reduced the expression level of EphA2 mRNA and protein (data not shown). ShEphA2 stable cells grew as tight spheroids and showed a significant decrease in spheroid area compared with shControl cells (Figure 6A, n = 4, P < .01). In a similar fashion, ephrin-A1 or F2-27 also significantly suppressed spheroid invasion in the absence of rEGF (Figure 6A, n = 4, P < .01). Although it was not possible to suppress spheroid expansion during the treatment of shEphA2 cells in the presence of rEGF, ephrin-A1 or F2-27 significantly suppressed rEGF-induced spheroid invasion (Figure 6B, n = 4, P < .01). These findings demonstrated that not only silencing EphA2 but treatment with ephrin-A1 or F2-27 also suppressed the invasive properties of cancer cells.
Figure 6.
Inhibition of EphA2 decreased spheroid formation.
The effect of the inhibition of EphA2 on cell spheroid formation in MDA-MB-231 cells in the absence (A) or presence (B) of rEGF. Cells were treated with ephrin-A1 or F2-27 in the absence or presence of rEGF. EphA2 was downregulated in MDA-MB-231 cell lines by shRNA (shEphA2 cells); shControl cells are also shown. Photographs show spheroid formation (×40 magnification). Quantification of spheroid areas was measured using ImageJ at day 7, and sizes were compared among experimental groups. Values are expressed as mean ± S.D. *P < .01, Student's t test.
Discussion
Rituximab and trastuzumab were launched in the 1990s and soon became the antibodies of choice for many patients with cancer because of their beneficial effects and perceived safety. Further advances in research and development have led to the approval and use of many other therapeutic antibodies in clinical practice. These antibodies exert their therapeutic effects primarily through mechanisms of action such as antibody-dependent cell-mediated cytotoxicity and complement-dependent cell-mediated cytotoxicity. However, their efficacies are still rather limited. To exert sufficient therapeutic effects against a cancer cell, a therapeutic antibody should induce cancer cell death by selectively binding to key molecules required for cell survival, in addition to antibody-dependent cell-mediated cytotoxicity and complement-dependent cell-mediated cytotoxicity activities. Unfortunately, not all therapeutic antibodies possess such properties.
Fluorescence antibody methods or enzyme-linked immunosorbent assays have generally been used in the screening of mAbs for a therapeutic endpoint. In contrast, mAbs can also be screened from hybridoma libraries using Adv-FZ33, a modified adenovirus with an IgG-binding domain, which is able to transfect genes into cells in combination with a mAb in our screening system. The advantage of this method is that it allowed us to quantify gene transduction via an antibody selected from an antibody library, allowing the identification of antibodies with high gene transfer efficiency (Figure 1). Furthermore, using the selected mAb, we comprehensively searched for the recognized antigen on the surface of tumor cells (Figure 2). Although we predominantly aimed to establish a systematic screening method to search for antibodies and cell surface target candidates that would provide highly selective gene transduction into malignant tumors, any selected mAbs were also expected to show additional biological functions by themselves. In the present study, a clone, F2-27, was established that showed inhibitory activity against spheroid cell invasion of tumor cells in 3-D culture assays, as also observed when EphA2 was knocked down by shRNA. Interestingly, F2-27 treatment inhibited rEGF-induced spheroid cell invasion, whereas EphA2 knockdown did not (Figure 6). EphA2 overexpressed on the surface of tumor cells receives signals not only from extracellular ligands but also from intracellular growth factor receptors such as the EGFR, and activates motility and invasion of tumor cells in an EphA2 ligand-independent manner [14], [15], [16]. In these interactions, F2-27 has the ability to inhibit cross talk between EphA2 and the EGFR.
Kato previously reported intriguing results concerning antigen-antibody reactions and cellular internalization [17]. In that study, the responsiveness of nine different anti–carcinoembryonic antigen (CEA) mAbs to CEA-positive cells was tested; flow cytometry revealed that these mAbs differed only slightly in their reactive patterns. However, gene transfer assay using Ax3CAZ3-FZ33 encoding a lacZ gene revealed that anti-CEA mAb dependent-transduction efficiency differed greatly among the nine clones. This variation could not be exclusively explained only by differences in binding affinities among the antibody clones. Thus, the involvement of specific biological properties of antibodies, such as their ability to be internalized, or differences in recognizing epitopes were suggested as explanations for the observed variation in transduction efficiency. These findings became essential in developing specific strategies to target cancer cells. Our results suggest that the selection of the most suitable antibody from an antibody library recognizing antigens equally, and understanding of properties about the relationship between individual antibodies and their antigens are the most important steps in the identification of target antigens. Conversely, an Adv-FZ33 system allows the selection of suitable antibodies with a highly effective drug delivery system and the ability for cellular internalization. Volpers et al. [18] reported that an antibody-modified adenovirus could be used for gene transfer into antigen-positive cells; this was dependent on the specificity and affinity of the antibody. They demonstrated increased gene transfer efficacy using an adenovirus and anti-EGFR antibody or anti–neuronal cell adhesion molecule antibody compared with that achieved using conventional adenovirus-mediated methods (several tens of times higher than control). In the present study, reporter gene expression in PC-3 cells infected with Ax3CAZ3-FZ33 via the F2-27 was 145.9- and 236.1-fold enhanced compared with isotype control or vector alone, respectively (Figure 4). A clearly high transfer efficacy was obtained with our method.
The ultimate goal of cancer therapy is to only destroy cancer cells without affecting normal cells. This is the basic concept of targeted therapy. Thus, the identification of cancer cell–specific and novel molecular markers is a key component of the development of new drugs. Like the other members of the Eph receptor family, EphA2 is composed of both a tyrosine kinase domain and a sterile α motif. Stimulation of EphA2 by its ligand, ephrin-A1, triggers a series of cellular events such as intracellular RhoG-induced autophosphorylation, downstream signal transduction, repulsive cell-cell interaction, and cell growth inhibition [19], [20], [21]. Although EphA2 is expressed on normal adult cells, higher levels of expression have been reported in tumor cell lines and excised specimens from patients with advanced cancer [22], [23], [24], [25], [26]. In the present study, a very high level of EphA2 expression was confirmed in several tumor cell lines by flow cytometry analysis (Figure 3). EphA2 receptors also regulate a variety of oncogenesis-related events such as cell survival, cytoskeleton modulation, cell adhesion, tumor angiogenesis, and metastasis. Therefore, EphA2 is a reasonable candidate as a target molecule in cancer therapy. Another important factor for using EphA2 in targeted therapy is that EphA2 expression levels differ widely between normal and cancer tissues. Thus, EphA2 is a very appealing target molecule. Because the Adv-FZ33 system is capable not only of identifying suitable target molecules like EphA2 but also of determining therapeutic antibodies in parallel, this bioassay is beneficial for cancer therapy for these aspects alone.
To date, several attempts have been made to deregulate EphA2 signaling using agonistic antibodies, low–molecular weight inhibitors, viral vectors, or RNA interference. Carles-Kinch et al. [27] and Landen et al. [28] determined that treatment with EphA2 agonistic antibodies induced phosphorylation and internalization followed by degradation and inhibition of the Ras/MAP kinase pathway and Akt activation. These results suggested that EphA2-specific mAbs inhibited the oncogenic potential of tumor cells via mechanisms similar to those of ephrin-A1. Noblitt et al. [29] demonstrated that the activation of EphA2 is induced by expressing ephrin-A1 on the surface of tumor cells using an Ad5 vector, thereby increasing turnover and inducing a tumor inhibitory effect. However, it is difficult to transfer therapeutic genes into certain types of cancer cells, such as pancreatic cancer, melanoma, and hematological malignancies, which express low levels of adenovirus receptor. To resolve this issue, in pancreatic cancer cells, EphA2 tropism of the adenovirus was increased by incorporating a peptide Tyr-Ser-Ala (YSA) into the HI loop of the virus fiber knob [30]. The method described in the present study may seem similar to that described in the aforementioned report. However, we used a tumor-specific, high-affinity antibody to the EphA2 surface receptor expressed on tumor cells. Therefore, more highly effective therapeutic benefits can be expected with this system compared to conventional EphA2-targeted therapeutic methods. When a therapeutic HSV-TK gene was transfected using F2-27 into PC-3 cells that were subsequently treated with GCV (Figure 5), cytotoxicity of PC-3 cells into which the HSV-TK gene was transfected using F2-27 was induced by using 1/100th of the concentration of GCV used for the control antibody. Another important advantage of our strategy is that the targeting of another cancer antigen can be easily achieved by replacing the antibody. Because the virus vector itself can be used as a common tool for other applications, the production cost of a tailored vector for each carcinoma can be reduced. Additionally, the tumor selectivity of the vector may be improved by combining several antibodies against different target molecules.
In summary, we have succeeded in establishing a human EphA2-specific mAb, F2-27, using an Adv-FZ33 screening system. F2-27 not only was useful for strong gene transfer into EphA2-positive cancer cells when combined with Adv-FZ33 but also showed inhibitory effects on in vitro cancer metastasis. When the HSV-TK gene was transferred to PC-3 cells after mediation by Adv-FZ33 with F2-27, the efficacy of the GCV treatment increased approximately 100-fold. The present study indicates that our screening system is beneficial for the development of therapeutic antibodies. More importantly, F2-27 has the potential to be a successful antibody-based therapy for various EphA2-positive cancers.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no potential conflicts of interest to disclose.
Authors' Contributions
Conception and design: T. Tanaka.
Development of methodology: T. Tanaka, H. Yamada.
Acquisition of data: T. Tanaka, H. Yamada.
Analysis and interpretation of data: T. Tanaka.
Writing of the manuscript: T. Tanaka.
Administrative and material support: M. Kuroki, K. Tamura, Y. Takamatsu.
Study supervision: T. Tanaka.
Acknowledgements
I especially would like to express my deepest appreciation to my supervisor, former professor Hirofumi Hamada at the Tokyo University of Pharmacy and Life Sciences for his guidance that makes our research of great achievement and my study life unforgettable.
Footnotes
Grant support: This work was supported in part by a Grant-in-Aid for Scientific Research (grant 22501054) from Japan Society for the Promotion of Science (Toshihiro Tanaka).
References
- 1.David F, Jacques F. The global and regional burden of cancer. In: Stewart BW, Wild CP, editors. World Cancer Report 2014. International Agency for Research on Cancer; Lyon, France: 2014. pp. 16–53. [Google Scholar]
- 2.Tanaka T, Huang J, Hirai S, Kuroki Mo, Kuroki Ma, Watanabe N, Tomihara K, Kato K, Hamada H. Carcinoembryonic antigen-targeted selective gene therapy for gastric cancer through FZ33 fiber-modified adenovirus vectors. Clin Cancer Res. 2006;12:3803–3813. doi: 10.1158/1078-0432.CCR-06-0024. [DOI] [PubMed] [Google Scholar]
- 3.Gale NW, Yancopoulos GD. Ephrins and their receptors: a repulsive topic? Cell Tissue Res. 1997;290:227–241. doi: 10.1007/s004410050927. [DOI] [PubMed] [Google Scholar]
- 4.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 5.Murai KK, Pasquale EB. 'Eph'ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116(Pt 14):2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- 6.Pasquale EB. The Eph family of receptors. Curr Opin Cell Biol. 1997;9:608–615. doi: 10.1016/s0955-0674(97)80113-5. [DOI] [PubMed] [Google Scholar]
- 7.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi Y, Pasquale EB. Eph receptors in the adult brain. Curr Opin Neurobiol. 2004;14:288–296. doi: 10.1016/j.conb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–499. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- 11.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–1456. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 12.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 13.St Clair MH, Lambe CU, Furman PA. Inhibition by ganciclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Antimicrob Agents Chemother. 1987;31:844–849. doi: 10.1128/aac.31.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen MW, Pedersen N, Damstrup L, Villingshøj M, Sønder SU, Rieneck K, Bovin LF, Spang-Thomsen M, Poulsen HS. Analysis of the epidermal growth factor receptor specific transcriptome: effect of receptor expression level and an activating mutation. J Cell Biochem. 2005;96:412–427. doi: 10.1002/jcb.20554. [DOI] [PubMed] [Google Scholar]
- 15.Larsen AB, Pedersen MW, Stockhausen MT, Grandal MV, van Deurs B, Poulsen HS. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor–induced cancer cell motility. Mol Cancer Res. 2007;5:283–293. doi: 10.1158/1541-7786.MCR-06-0321. [DOI] [PubMed] [Google Scholar]
- 16.Larsen AB, Stockhausen MT, Poulsen HS. Cell adhesion and EGFR activation regulate EphA2 expression in cancer. Cell Signal. 2010;22:636–644. doi: 10.1016/j.cellsig.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Kato K. A novel screening method to establish tumor-targeting antibodies reliable for drug delivery system. Yakugaku Zasshi. 2013;133:931–938. doi: 10.1248/yakushi.13-00190-2. [DOI] [PubMed] [Google Scholar]
- 18.Volpers C, Thirion C, Biermann V, Hussmann S, Kewes H, Dunant P, von der Mark H, Herrmann A, Kochanek S, Lochmüller H. Antibody-mediated targeting of an adenovirus vector modified to contain a synthetic immunoglobulin G–binding domain in the capsid. J Virol. 2003;77:2093–2104. doi: 10.1128/JVI.77.3.2093-2104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 20.Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, Coffman K, Jackson D, Bruckheimer E, Muraoka-Cook RS. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 23.Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor alpha 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14:199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657–663. doi: 10.1002/ijc.10860. [DOI] [PubMed] [Google Scholar]
- 25.Kinch MS, Moore MB, Harpole DH., Jr. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–618. [PubMed] [Google Scholar]
- 26.Nasreen N, Mohammed KA, Antony VB. Silencing the receptor EphA2 suppresses the growth and haptotaxis of malignant mesothelioma cells. Cancer. 2006;107:2425–2435. doi: 10.1002/cncr.22254. [DOI] [PubMed] [Google Scholar]
- 27.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–2847. [PubMed] [Google Scholar]
- 28.Landen CN, Jr., Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, Mangala LS, Merritt WM, Lin YG, Gao C. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 29.Noblitt LW, Bangari DS, Shukla S, Knapp DW, Mohammed S, Kinch MS, Mittal SK. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–766. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- 30.Van Geer MA, Brevoord D, Kuhlmann KF, Bakker CT, Mizuguchi H, Wesseling JG, Ten Kate FJ, Gouma DJ, Oude Elferink RP, Bosma PJ. A fiber modified adenovirus vector that targets to the EphrinA2 receptor reveals enhanced gene transfer to ex vivo pancreatic cancer. Int J Oncol. 2010;36:233–244. [PubMed] [Google Scholar]