Abstract
Olive oil samples were obtained from six cultivars grown in different environments, and graded by chemical analyses as extra virgin (EVOOs). These were evaluated for flavors and off-flavors, and relative VOCs spectrum as determined by PTR–ToF–MS. A hierarchical clustering of Panel test data separated olive oil in three groups, one including the samples with perceived off-flavor (VOOs), regardless of cultivar and environment. The Pearson’s correlation coefficients between the mass data from PTR–ToF–MS and the sensory characteristics perceived by the Panel test were determined. A mass-to-sensory attributes correlation index was calculated. A color-coded card was built up based on the intensities (ncps) of five selected protonated mass data that was able to distinguish EVOOs from VOOs olive oil samples.
Keywords: Olea europaea L., Chemical analysis volatile organic compounds (VOCs), Panel test, Flavors off-flavors
Introduction
Traditionally, the attributes of flavor of horticultural products are measured by means of sensory analysis (SA) panels. A specific “Panel test” (PT) was developed by International Olive Oil Council (IOOC) to uniformly evaluate the commercial grade of VOOs and applied by EU (EU Reg. no. 2568/91 and its successive modifications) for trade.
Detected by SA, the presence of specific odors (flavors if pleasant and off-flavors if unpleasant) has discriminant power for quality: a sample can be defined EVOO only if it is without detectable off-flavors and has rich fruity flavor.
The SA currently utilized in all olive oil world trade presents some disadvantages (Tena et al. 2015), as it is subjective due to the lack of standardized common referenced oils for the different off-flavors; furthermore, it requires a large number of trained panelist (8–12) tasters/panel to allow the statistical validation of the results). The volatile organic compounds (VOCs) production in the olive at the mill and the relative aromatic imprint in the oil, are mostly related to oxidative reactions, following tissues injuries during the fruits crushing and malaxation processes; VOCs develop according to distinctive biosynthetic pathways and, among these, the “LipOXygenase (LOX) cascade” determines the enzymatic splitting of polyunsatured fatty acids (linoleic and linolenic) with the “controlled” production of aldehydes, ketones, alcohols, carboxylic acids, esters and other VOCs (Angerosa et al. 2004; Kalua et al. 2007).
The amount of VOCs in VOOs is related to the enzymatic background of the fruits, depending mainly on the genotype (cultivar) (Luna et al. 2006a), and, in turn, on the ripening stage (Angerosa et al. 1996a; Masi et al. 2015a), pedoclimatic conditions (Muzzalupo et al. 2012), fruit storage as well as the processing techniques and oil storage (Angerosa et al. 1996b; Morales et al. 2005; Kalua et al. 2007). C6 and C5 compounds are the most important VOCs contributing to the flavor (Angerosa 2002), and the only ones conferring positive attributes as green and fruity.
Unpleasant odors can be present, due to biogenic enzymatic activities in the fruits before the oil extraction process, or to alteration during the oil storage (Luna et al. 2006b).
Analytical methods based on the gas chromatography-mass spectrometry (GC–MS) are currently utilized for the VOCs identification and quantification, and with the adoption of official “Panel test”, studies on the relationship between SA and VOCs in defective oils were carried out to detect and identify the compounds and their distribution in different defective oils, including the standard proceeding from IOOC (Procida et al. 2005), or to point out an instrumental method for the evaluation of olive oil quality (Dierkes et al. 2012; Procida et al. 2015).
The utilized gas-chromatographic analytical methods, although valuable and, in many cases, essential, have some disadvantages such as the low time resolution, the laborious sample preparation and the long operation time; different alternative analytical methods have been proposed (Biasioli et al. 2011), and as faster alternative technique it can be considered the direct headspace proton transfer reaction, time of flight, mass spectrometry (PTR–ToF–MS) (Biasioli et al. 2011; Makhoul et al. 2014).
The PTR–MS coupled with a quadrupole detector was earlier utilized to study VOCs in the atmosphere (Lindinger et al. 1998). Its use was later extended to VOCs emission studies in biochemical and horticultural analysis, for the determination of VOCs spectra fingerprinting in olive oil samples for detection of oxidative alterations (Aprea et al. 2006), geographical origin classification (Araghipour et al. 2008), geographical origin, cultivar and harvesting time determination (Van Ruth et al. 2009), the relationships between harvesting time and special processing systems (Vezzaro et al. 2011), and for monovarietal EVOO identification and certification (Ruiz-Samblás et al. 2012).
Recently, the evaluation of VOCs emitted by food has improved by new version of the PTR–MS coupled with a time of flight mass analyzer (PTR–ToF–MS), which enables a rapid and precise analysis of the VOCs spectra (Taiti et al. 2014). This technique was reported to have several applications in the field of food aroma analysis of ham (Sánchez del Pulgar et al. 2011), saffron (Masi et al. 2015a, b), bakery yeast starters (Makhoul et al. 2014), and dried porcini mushrooms aroma (Aprea et al. 2015).
This technique allowed to characterize cultivars and clones in apples (Cappellin et al. 2012) to evaluate VOCs emission from olive fruits at different ripening stage (Masi et al. 2015a), and to verify differences in storage of mango fruits harvested at different ripening stage (Taiti et al. 2015b).
The aim of this study was to understand the relationship between the sensory characteristics of samples of virgin olive oils, graded by the PT, and PTR–ToF–MS spectra. The mass data related to specific perceived odors.
Materials and method
Oil samples
The oil samples were obtained from six cultivars of Olea europaea subsp. europaea var. europaea Green (Arbequina, Arbosana, Koroneiki, Coratina, Maurino sel. Vittoria, Tosca), grown in eight orchards located in five countries of three different continents. The oils were produced from fruits obtained from 4 to 7 years-old trees grown from hedge-trained systems, with a density of around 1600 trees per hectare. For each cultivar, the harvesting time was determined from fruits color as it change from green to yellow-brown (Color Index = 3.0–4.0). Oils were obtained from processing systems with two phases, within 48 h of harvesting, were filtered and stored in steel containers.
All oil samples (250 ml), sealed in glass bottles, and stored in dark at 15 °C until chemical, organoleptic and VOCs analyses were carried out. The data referring to location and milling date are summarized in Table 1. All samples were subjected to chemical analyses: free acidity and peroxide index (PI) were determined by EU official methods (Table 2).
Table 1.
Oil samples cultivar, geographical location and harvesting time
| Sample number | Sample codea | Cultivar | Geographical zone of production | Time of milling |
|---|---|---|---|---|
| 1 | AqAR1 | Arbequina IRTA i-18® | Argentina | March 2013 |
| 2 | AqAR2 | Arbequina | Argentina | March 2013 |
| 3 | AqAR3 | Arbequina | Argentina | April 2013 |
| 4 | AsAR | Arbosana IRTA i-43® | Argentina | April 2013 |
| 5 | KrAR | Koroneiki IRTA i-38® | Argentina | April 2013 |
| 6 | CoAR | Coratina | Argentina | April 2013 |
| 7 | AqCL | Arbequina IRTA i-18® | Chile | May 2012 |
| 8 | AsCL | Arbosana IRTA i-43® | Chile | May 2012 |
| 9 | KrCL | Koroneiki IRTA i-38® | Chile | May 2012 |
| 10 | AqMA | Arbequina IRTA i-18® | Morocco | November 2012 |
| 11 | AsMA | Arbosana IRTA i-43® | Morocco | November 2012 |
| 12 | KrMA | Koroneiki IRTA i-38® | Morocco | November 2012 |
| 13 | AqTN | Arbequina IRTA i-18® | Tunisia | October 2012 |
| 14 | AsTN | Arbosana IRTA i-43® | Tunisia | December 2012 |
| 15 | KrTN | Koroneiki IRTA i-38® | Tunisia | December 2012 |
| 16 | AqIT1 | Arbequina IRTA i-18® | Italy (Lazio) | October 2012 |
| 17 | AsIT1 | Arbosana IRTA i-43® | Italy (Lazio) | November 2012 |
| 18 | KrIT1 | Koroneiki IRTA i-38® | Italy (Lazio) | November 2012 |
| 19 | AqIT2 | Arbequina IRTA i-18® | Italy (Tuscany) | November 2012 |
| 20 | AsIT2 | Arbosana IRTA i-43® | Italy (Tuscany) | November 2012 |
| 21 | KrIT2 | Koroneiki IRTA i-38® | Italy (Tuscany) | November 2012 |
| 22 | MVIT1 | Maurino sel. Vittoria | Italy (Tuscany) | October 2012 |
| 23 | MVIT2 | Maurino sel. Vittoria | Italy (Tuscany) | October 2012 |
| 24 | ToIT | Tosca® | Italy (Tuscany) | November 2012 |
aCode: Aq = Arbequina, As = Arbosana, Kr = Koroneiki, MV = Maurino sel. Vittoria, To = Tosca, Co = Coratina. AR = Argentina, CL = Chile, MA = Morocco, TN = Tunisia, IT = Italy (International Organization for Standardization, ISO 3166-1 alpha-2)
Table 2.
Results of chemical and sensory analyses
| Sample number | Sample code | Chemical analyses | Sensory analysisa | Md | Mf | Trade category |
|---|---|---|---|---|---|---|
| Free acidity (%) | Peroxide index (meq O2 kg−1) | |||||
| 1 | AqAR1 | 0.15 | 7.30 | Md = 0.0 | Mf = 4.0 | EVOO |
| 2 | AqAR2 | 0.13 | 7.0 | Md = 0.0 | Mf = 3.8 | EVOO |
| 3 | AqAR3 | 0.16 | 7.4 | Md = 0.0 | Mf = 5.7 | EVOO |
| 4 | AsAR | 0.29 | 10.6 | Md = 0.0 | Mf = 5.0 | EVOO |
| 5 | KrAR | 0.29 | 10.7 | Md = 0.0 | Mf = 5.0 | EVOO |
| 6 | CoAR | 0.15 | 6.6 | Md = 0.0 | Mf = 4.9 | EVOO |
| 7 | AqCL | 0.20 | 10.3 | Md = 3.0 | Mf = 2.8 | VOO |
| 8 | AsCL | 0.31 | 13.9 | Md = 2.5 | Mf = 2.5 | VOO |
| 9 | KrCL | 0.28 | 10.1 | Md = 2.9 | Mf = 3.3 | VOO |
| 10 | AqMA | 0.13 | 8.10 | Md = 0.0 | Mf = 4.4 | EVOO |
| 11 | AsMA | 0.20 | 9.70 | Md = 0.0 | Mf = 4.2 | EVOO |
| 12 | KrMA | 0.22 | 6.50 | Md = 0.0 | Mf = 5.3 | EVOO |
| 13 | AqTN | 0.19 | 8.40 | Md = 0.0 | Mf = 3.3 | EVOO |
| 14 | AsTN | 0.18 | 13.7 | Md = 2.4 | Mf = 2.5 | VOO |
| 15 | KrTN | 0.18 | 9.80 | Md = 2.7 | Mf = 3.4 | VOO |
| 16 | AqIT1 | 0.10 | 8.60 | Md = 0.0 | Mf = 4.8 | EVOO |
| 17 | AsIT1 | 0.16 | 9.30 | Md = 0.0 | Mf = 4.6 | EVOO |
| 18 | KrIT1 | 0.10 | 5.80 | Md = 0.0 | Mf = 5.3 | EVOO |
| 19 | AqIT2 | 0.07 | 7.00 | Md = 0.0 | Mf = 4.8 | EVOO |
| 20 | AsIT2 | 0.10 | 5.30 | Md = 0.0 | Mf = 4.5 | EVOO |
| 21 | KrIT2 | 0.10 | 6.20 | Md = 0.0 | Mf = 5.3 | EVOO |
| 22 | MVIT1 | 0.12 | 6.6 | Md = 0.0 | Mf = 6.0 | EVOO |
| 23 | MVIT2 | 0.13 | 5.2 | Md = 0.0 | Mf = 5.8 | EVOO |
| 24 | ToIT | 0.14 | 7.1 | Md = 0.0 | Mf = 3.3 | EVOO |
EVOO Extra virgin olive oil, VOO virgin olive oil
aCommission Regulation (EU) no. 61/2011 of 24 January 2011 amending Regulation (EEC) no. 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis
Sensory analysis
The panel evaluating the oil samples was a specifically organized Tasters Commission composed of a panel head and eight trained members from the professional board (National Association for Olive Oil Tasters). The team was adequately informed about the objectives of the test. The procedures indicated in EU olive oil sensory analysis Regulation (no. 2568/91 and its successive modifications) were used for organoleptic assessment and grading, together with IOOC official method for organoleptic evaluation for granting designation of origin (D.O.) status (Organoleptic assessment of EVOO applying to use a designation of origin, referenced IOOC/T.20/Doc. no. 22). The oils were graded in line with the median of the ‘fruity’ (Mf) and the median of the defects (Md) (Table 2).
PTR–ToF–MS 8000
VOCs emitted were detected with a PTR–ToF–MS 8000 (Ionicon Analytik GmbH, Innsbruck, Austria) using H3O+ as reagent ion for the proton transfer reaction. The reaction takes place between H3O+ ions and all the biogenic or anthropogenic VOCs having a proton affinity higher than that of water (165.2 kcal mol−1). Separation of single ions happens accordingly to their mass to charge (m/z) ratio. The sampling time for each channel of ToF acquisition was 0.1 ns, for a mass spectrum ranging from m/z = 20 to m/z = 250. In this experiment the conditions in the drift tube were: drift voltage 600 V, temperature 110 °C, pressure 2.25 mbar, extraction voltage at the end of the tube (Udx) 32 V.
Twelve hours before starting the analysis, the oil samples were transferred in the climatic room (22–23 °C, RU 90%) where the VOCs analysis were performed. Subsequently the oil samples were equilibrated at 30 °C in a water bath to simulate the temperature in the glasses during the sensory evaluation tests. For each sample, 10 ml of oil were transferred in a 25 ml glass jar, specifically selected to expose a surface of approximately 8.0 cm2; the jar lid was fitted with inlet and outlet Teflon tubes, which were, respectively, connected to a zero-air generator and to the PTR–ToF–MS system. The VOCs in the headspace were measured by direct injection into the PTR–ToF–MS via a heated (60 °C) peek inlet tube with a flow rate of 100 sccm for 120 s, after 5 min of exposure; each sample was triplicated. Preliminary measurements on an empty jar were run and used for background subtraction. Internal calibration was performed off-line and was based on m/z = 29.997 m/z = 59.049 (C2H5O2 +) and m/z = 180.937 (C6H4Cl3 +).
Spectra raw data (ncps) were acquired with TofDaq software (Tofwerk AG, Switzerland) using a dead time of 20 ns for the Poisson correction. For each sample, the average data resulting from the last 20 consecutive seconds of the measurement were extracted after 5 min from the beginning of each experiment. All spectra were corrected for count losses due to the detector dead time, applying Poisson correction in the DAQ settings of TofDAQ configuration options.
Statistical data analyses
A hierarchical clustering (unsupervised method) was firstly performed on the data of the Panel test, to organize the samples in categories. The complete linkage (farthest neighbor) method with Euclidean distance was selected to calculate the distance among clusters, since their exact number was unknown a priori. The cophenetic correlation of the dendrogram was calculated. Computations were performed by the SYN-TAX 2000 program package. A heat map of sensory attributes and the one dimensional dendrogram of the 24 olive oil samples was built up by R 3.2.2. (R Foundation for Statistical Computing, Vienna, Austria). The optimal number of clusters in the dendrogram was estimated computing connectivity, dunn and silhouette scores by R package clValid; gap statistic by R package NbClust. The hartigan index also validated the two subset of the EVOOs group by R package NbClust.
In order to relate the intensity of the protonated m/z from PTR–ToF–MS to the intensity of flavors and off-flavors as perceived by the Panel test, a correlation matrix containing the coefficients of correlation between each pair of variables was calculated by the Pearson product moment. p values below 0.01 indicate statistically significant non-zero correlations at the 99.0% confidence level. Computations were performed by Statgraphics Centurion XV v. 15.0.04.
Finally, a color-codified heat map of the 24 olive oil samples (average of replicates) vs the most relevant m/z characterized by high Pearson’s coefficients with the off-flavors, was obtained. Computations were performed by R 3.2.2.
The tentative (protonated) masses identification of the emerged VOCs was verified comparing the available data in the literature of studies conducted with the PTR–ToF–MS, regardless of the type of samples; subsequently, it was determined whether the identified VOCs had previously been found in olive oils through other several methods.
Results and discussion
Chemical and sensory analyses
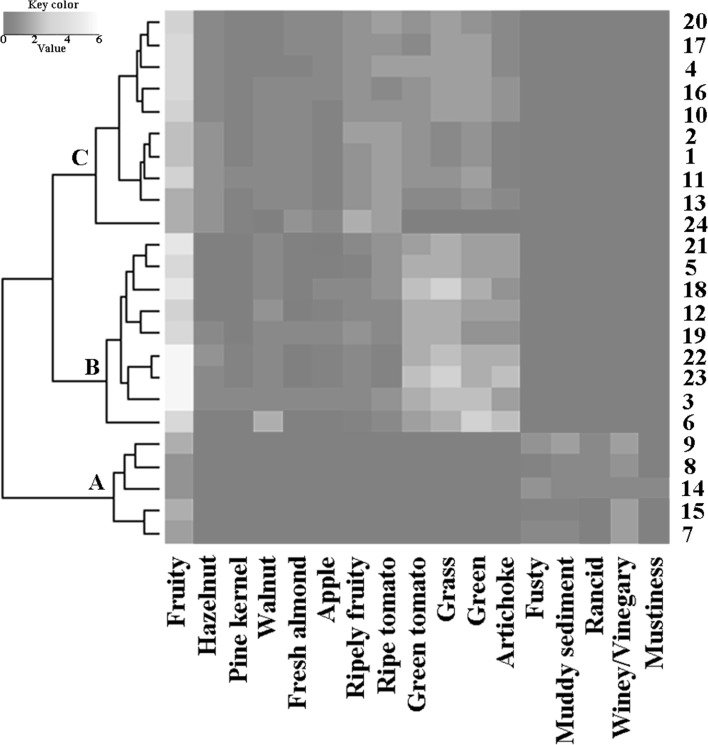
Based on the chemical parameters, all samples belonged to the EVOOs category. The free acidity and peroxide values are shown in Table 2. The evaluation of the Panel test data highlighted some discrepancies between chemical and sensory analysis for some samples which would be placed in the EVOOs category based only on chemical analyses (Table 2), but which were downgraded to VOOs based on sensory data. Since the consumer appreciates pleasant odors as a first impact in the choice of an olive oil, a more suitable assessment of organoleptic characteristics is needed, as resulted by the panel evaluations. To have a broader overview of the organoleptic profile, a hierarchical cluster analysis was applied on Sensory analysis results. The entire data set was organized in a dendrogram combined with a heat map (Fig. 1).
Fig. 1.
Heat map of sensory attributes and one dimensional dendrogram of the 24 olive oil samples. Sensory characteristics are clustered by columns, samples are clustered by rows. Numbers from 1 to 24 are associated to the samples as reported in Table 1; letters A, B, and C individuate the clusters. None autoscaling procedure was utilized for the color-coding
The dendrogram represents samples clustering based on the sensory characteristics with olfactory impact (fruity, flavors, off-flavors), where different colors were related to different intensities of each detected odor. The heat map shows that the samples can be grouped in two main clusters, clearly separating the EVOOs (groups B and C) from the VOOs (group A); in the EVOOs category, two groups emerged (B and C), differentiated by the presence and intensity of positive smells. This statistical elaboration highlights the strong effect of off-flavors, separating the defected oil samples (A), independently on the cultivar and on the geographical zone of cultivation. According to recent researches,40EVOOs resulted divided in two sub-groups (B and C), related to the intensity of differently perceived pleasant flavors.
PTR–ToF–MS analysis
The eight peaks from the PTR–ToF–MS were related to the sensory characteristics perceived by the Panel test and Pearson’s correlation coefficients between each pair of variables are reported in Table 3.
Table 3.
Peaks from the PTR–ToF–MS related to the sensory characteristics perceived by the Panel test with Pearson’s correlation coefficients statistically significant at p < 0.01
| Protonated measured m/z | Protonated chemical formula | Protonated theoretical m/z | Tentative identification | References | Flavor | Off-flavor | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apple | Ripely fruity | Green tomato | Grass | Green | Fusty | Muddy sediment | Winey/vinegary | |||||
| 47.049 | C2H7O+ | 47.0491 | Ethanol | Mancuso et al. (2015), Morales et al. (2000) | n.s. | n.s. | −0.64 | −0.69 | −0.77 | 0.64 | 0.65 | 0.67 |
| 61.028 | C2H5O2 + | 61.0284 | Acetic acid/acetates | Taiti et al. (2015a), Morales et al. (2000) | n.s. | n.s. | −0.67 | −0.70 | −0.66 | n.s. | n.s. | 0.70 |
| 75.044 | C3H7O2 + | 75.0441 | Propanoic acid | Aprea et al. (2015), Angerosa et al. (1996b) | n.s. | n.s. | n.s. | −0.73 | −0.71 | 0.61 | 0.61 | 0.64 |
| 81.070 | C6H9 + | 81.0699 | Fragment/terpenes fragment | Sánchez-Ortiz et al. (2012), Angerosa et al. (2004) | 0.71 | 0.67 | 0.60 | 0.70 | 0.58 | n.s. | n.s. | n.s. |
| 83.086 | C6H11 + | 83.0855 | Hexanal, hexenols | Park et al. (2013) | 0.65 | 0.68 | 0.58 | 0.58 | n.s. | −0.68 | −0.67 | −0.65 |
| 89.059 | C4H9O2 + | 89.0597 | Butanoic acid | Aprea et al. (2015), Morales et al. (2013) | n.s. | n.s. | −0.67 | −0.67 | −0.70 | −0.66 | 0.60 | 0.66 |
| 99.080 | C6H11O+ | 99.0804 | cis-3-hexenal | Taiti et al. (2014), Angerosa et al. (2004) | 0.60 | 0.60 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| 127.110 | C8H15O+ | 127.1077 | Octanal | Masi et al. (2015a, b), Morales et al. (2013) | n.s. | n.s. | n.s. | n.s. | n.s. | 0.62 | 0.61 | n.s. |
The positive correlation between VOCs and flavor or off-flavor are highlighted in bold
The high positive correlation between pleasant attributes (flavors) of EVOOs (apple, ripely fruity, green tomato, grass, and green) and the masses m/z = 81.070, m/z = 83.086, and m/z = 99.080. The flavour fusty, muddy sediment, and winey/vinegary olfactory sensations (off-flavors) were related to evaluate mass data.
The VOOs group is characterized by the presence of five protonated masses: m/z = 47.049, m/z = 61.028, m/z = 75.044, m/z = 89.059, m/z = 127.110. The protonated m/z = 47.049, TI as ethanol (Mancuso et al. 2015; Cappellin et al. 2014; Taiti et al. 2015a), and the protonated m/z = 61.028, TI as acetic acid/acetates (Mancuso et al. 2015; Taiti et al. 2015a), are both generally considered in olive oils as compounds deriving from alteration due to a long time of olive storage before processing (Morales et al. 2000; Angerosa et al. 1996a) the protonated m/z = 75.044, TI as propanoic acid (Aprea et al. 2015), is considered a defective compound that can be explained by fermentation processes induced by Clostridium spp. in olive fruits after a long time of storage (Angerosa et al. 1996b); the protonated m/z = 89.059, TI as butanoic acid (Aprea et al. 2015), seems related to the sugar fermentation (Morales et al. 2013); the protonated m/z = 127.110, TI as octanal (Masi et al. 2015a, b), is found in oxidized olive oil (Morales et al. 2013).
EVOOs groups are characterized by three protonated masses. The measured protonated mass m/z = 81.070, TI as trans-2-hexenal or terpenes fragment (Infantino et al. 2015), derives from LOX cascade by splitting of linolenic acid (Angerosa et al. 2004); the measured protonated mass m/z = 83.086, TI as a C6 fragment was related to the hexanal family (Park et al. 2013); the measured mass m/z = 99.080, TI as cis-3-hexenal (Taiti et al. 2014), has been found in olive oils as one of the most important VOCs derived from fatty acid degradation by the LOX pathway (Angerosa et al. 2004). It should be noted that the compounds maximizing the separation of the different samples belonging to the VOOs group were related to negative olfactory notes perceived in defective olive oils, and attributed to hexogen to fruits biological activity or generated by chemical oxidation. The analysis shows also that two EVOOs sub-groups (the B and C clusters highlighted by the hierarchical clustering) are separated from group A (VOOs) by a relevant presence of VOCs generated by LOX pathway and generally related to positive sensory attributes.
Finally, to obtain a recognized grading marker for VOO/EVOO, a color-coded map was built (Table 4), reporting the intensity of the protonated masses m/z = 47.049, m/z = 61.028, m/z = 75.044, m/z = 89.059, m/z = 127.110, were able to clearly distinguish VOOs from EVOOs among the samples, characterized by high Pearson’s coefficients with the off-flavors. The color scale was defined by the sequential palette YlOrRd (multi-hue sequential color scheme) with nine different color values (RColorBrewer package, R 3.2.2.) autoscaled (zero mean and unitary variance) by column (m/z). The threshold between the two classes of EVOOs and VOOs is defined as follow: EVOOs were characterized by color index always <4; VOOs were characterized by at least one cell with color index ≥4. The lower limit of the class four corresponded to the lowest value of the whole matrix added of 1.5 s2, while the upper limit corresponded to the lowest value of the whole matrix added of 2 s2. Moreover it should be underlined that some compounds that contribute to generate a defect in the olive oil, are not perceived by the human olfactory and thus are incorrectly assessed as EVOO. Therefore, the results obtained in this study using a PTR-ToF-MS approach, show a real possibility to use this method in routinely operations for quality control at consumer level.
Table 4.
Color-codified heat map concerning relative level of intensity (ncps) for four defective masses in each sample. Numbers from 1 to 18 are associated to the samples as reported in Table 1. Colors from www.ColorBrewer.org by Cynthia A. Brewer, Geography, Pennsylvania State University
Conclusion
EVOO and VOO are the only vegetable oils directly edible without any refinement and EVOO is more valued for his superior organoleptic qualities. Through a legal Panel test is assigned the commercial category mainly on the basis of the odors (flavors and off-flavors) present in the evaluated oil samples.
In this study, the PTR-ToF-MS spectral data, explored by statistical analyses, were used to highlight the role of the single VOCs potentially responsible of odours (flavor and off flavor) and compared with the Panel Test results. By Pearson’s correlation coefficients, eight protonated masses, tentatively identified according to the current literature, can be used to clearly distinguish EVOOs from VOOs, as the Panel test did. Moreover, a correlation matrix demonstrates the linkage mass-to-odor and, moreover, a color card joining the intensity of the signal of 5 "defective" protonated masses with each oil sample, permits to correctly identify the qualitative categories as defined by the Panel test. It is clear the need of deeper researches, but this preliminary study of classification of different oil samples by the PTR-ToF-MS has provided clear and promising results, showing a real possibility to use this method in routinely operations for quality control at consumer level.
Acknowledgements
The Authors would like to thank: A. Ottanelli (DISPAA) for his invaluable technical help; A. Mersi for the collection of some oil samples and the organizing of the Sensory Evaluation; all the professional participants of the Taster Commission; A. Parenti, R. Galli, L. Ronca, R. Cioni, M. Migliorni, A. Mersi, A. Ottanelli; all the companies that kindly provided the oil samples. The authors acknowledge Ente Cassa di Risparmio di Firenze (Project VOLATOM) and the Regione Toscana “PRAF 2012-2015 MISURA 1.2″ (Project VOLATOSCA).
References
- Angerosa F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur J Lipid Sci Technol. 2002;104(9–10):639–660. doi: 10.1002/1438-9312(200210)104:9/10<639::AID-EJLT639>3.0.CO;2-U. [DOI] [Google Scholar]
- Angerosa F, Lanza B, Marsilio V, Cumitini S. Olive oil off-odour compounds produced by Aspergillus and Penicillium. Acta Hortic. 1996;474:695–699. [Google Scholar]
- Angerosa F, Lanza B, Marsilio V. Biogenesis of “fusty” defect in virgin olive oils. Grasas Aceites. 1996;47:142–150. doi: 10.3989/gya.1996.v47.i3.854. [DOI] [Google Scholar]
- Angerosa F, Servili M, Selvaggini R, Taticchi A, Esposto S, Montedoro GF. Review: volatile compounds in virgin olive oil: occurrence and their relationship with the quality. J Chromatogr. 2004;1054:17–31. doi: 10.1016/S0021-9673(04)01298-1. [DOI] [PubMed] [Google Scholar]
- Aprea E, Biasioli F, Sani G, Cantini C, Märk TD, Gasperi F. Proton transfer reaction–mass spectrometry (PTR–MS) headspace analysis for rapid detection of oxidative alteration of olive oil. J Agric Food Chem. 2006;54:7635–7640. doi: 10.1021/jf060970r. [DOI] [PubMed] [Google Scholar]
- Aprea E, Romano A, Betta E, Biasioli F, Cappellin L, Fanti M, Gasperi F. Volatile compound changes during shelf life of dried Boletus edulis: comparison between SPME-GC-MS and PTR–ToF–MS analysis. J Mass Spectrom. 2015;50:56–64. doi: 10.1002/jms.3469. [DOI] [PubMed] [Google Scholar]
- Araghipour N, Colineau J, Koot A, Akkermans W, Moreno Rojas JM, Beauchamp J, Wisthaler A, Märk TD, Downey G, Guillou C, Mannina L, Van Ruth S. analytical methods: geographical origin classification of olive oils by PTR–MS. Food Chem. 2008;108:374–383. doi: 10.1016/j.foodchem.2007.10.056. [DOI] [Google Scholar]
- Biasioli F, Yeretzian C, Märk TD, Dewulf J, Van Langenhove H. Direct-injection mass spectrometry adds the time dimension to VOC analysis. Trends Anal Chem. 2011;30(7):1003–1017. doi: 10.1016/j.trac.2011.04.005. [DOI] [Google Scholar]
- Cappellin L, Soukoulis C, Aprea E, Granitto P, Dallabetta N, Costa F, Viola R, Märk TD, Gasperi F, Biasioli F. PTR–ToF–MS and data mining methods: a new tool for fruit metabolomics. Metabolomics. 2012;8:761–770. doi: 10.1007/s11306-012-0405-9. [DOI] [Google Scholar]
- Cappellin L, Farneti B, Di Guardo M, Busatto N, Khomenko I, Romano A, Velasco R, Costa G, Biasioli F, Costa F. QTL analysis coupled with PTR–ToF–MS and candidate gene-based association mapping validate the role of Md-AAT1 as a major gene in the control of flavor in apple fruit. Plant Mol Biol Rep. 2014 [Google Scholar]
- Dierkes G, Bongartz A, Guth H, Hayen H. Quality evaluation of olive oil by statistical analysis of multicomponent stable isotope dilution assay data of aroma active compounds. J Agric Food Chem. 2012;60(1):394–401. doi: 10.1021/jf203406s. [DOI] [PubMed] [Google Scholar]
- Infantino A, Aureli G, Costa C, Taiti C, Antonucci F, Menesatti P, Pallottino F, De Felice S, D’Egidio M, Mancuso S. Potential application of PTR–TOFMS for the detection of deoxynivalenol (DON) in durum wheat. Food Control. 2015 [Google Scholar]
- Kalua CM, Allen MS, Bedgood DR, Jr, Bishop AG, Prenzler PD, Robards K. Olive oil volatile compounds, flavor development and quality: a critical review. Food Chem. 2007;100:273–286. doi: 10.1016/j.foodchem.2005.09.059. [DOI] [Google Scholar]
- Lindinger W, Hansel A, Jordan A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR–MS). Medical applications, food control and environmental research. Int J Mass Spectrom. 1998;173:191–241. doi: 10.1016/S0168-1176(97)00281-4. [DOI] [Google Scholar]
- Luna G, Morales MT, Aparicio R. Characterization of 39 varietal virgin olive oils by their volatile compositions. Food Chem. 2006;98:243–252. doi: 10.1016/j.foodchem.2005.05.069. [DOI] [Google Scholar]
- Luna G, Morales MT, Aparicio R. Changes induced by UV radiation during virgin olive oil storage. J Agric Food Chem. 2006;54(13):4790–4794. doi: 10.1021/jf0529262. [DOI] [PubMed] [Google Scholar]
- Makhoul S, Romano A, Cappellin L, Spano G, Capozzi V, Benozzi E, Märk TD, Aprea E, Gasperi F, El-Nakat H, Guzzoc J, Biasioli F. Proton-transfer-reaction mass spectrometry for the study of the production of volatile compounds by bakery yeast starters. J Mass Spectrom. 2014;49:850–859. doi: 10.1002/jms.3421. [DOI] [PubMed] [Google Scholar]
- Mancuso S, Taiti C, Bazihizina N, Costa C, Menesatti P, Giagnoni L, Arenella M, Nannipieri P, Renella G. Soil volatile analysis by proton transfer reaction-time of flight mass spectrometry (PTR–TOF–MS) Appl Soil Ecol. 2015;86:182–191. doi: 10.1016/j.apsoil.2014.10.018. [DOI] [Google Scholar]
- Masi E, Romani A, Pandolfi C, Heimler D, Mancuso S. PTR–TOF–MS analysis of volatile compounds in olive fruits. J Sci Food Agric. 2015 doi: 10.1002/jsfa.6837. [DOI] [PubMed] [Google Scholar]
- Masi E, Taiti C, Heimler D, Vignolini P, Romani A, Mancuso S. PTR–TOF–MS and HPLC analysis in the characterization of saffron (Crocus sativus L.) from Italy and Iran. Food Chem. 2015;192:75–81. doi: 10.1016/j.foodchem.2015.06.090. [DOI] [PubMed] [Google Scholar]
- Morales MT, Luna G, Aparicio R. Sensory and chemical evaluation of winey-vinegary defect in virgin olive oils. Eur Food Res Technol. 2000;211:222–228. doi: 10.1007/s002170050028. [DOI] [Google Scholar]
- Morales MT, Luna G, Aparicio L. Comparative study of virgin olive oil sensory defects. Food Chem. 2005;91(2):293–301. doi: 10.1016/j.foodchem.2004.06.011. [DOI] [Google Scholar]
- Morales MT, Aparicio-Ruiz R, Aparicio R (2013) Chromatographic methodologies: compounds for olive oil odor issues. In: Handbook of olive oil: analysis and properties, 2nd edn. Springer, New York, pp 261–310
- Muzzalupo I, Macchione B, Bucci C, Stefanizzi F, Perri E, Chiappetta A, Tagarelli A, Sindona G. LOX gene transcript accumulation in olive (Olea europaea L.) fruits at different stages of maturation: relationship between volatile compounds, environmental factors and technological treatments for oil extraction. Sci World. 2012 doi: 10.1100/2012/532179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Goldstein AH, Timkovsky J, Fares S, Weber R, Karlik J, Holzinger R. Eddy covariance emission and deposition flux measurements using proton transfer reaction–time of flight–mass spectrometry (PTR–TOF–MS): comparison with PTR–MS measured vertical gradients and fluxes. Atmos Chem Phys. 2013;13:1439–1456. doi: 10.5194/acp-13-1439-2013. [DOI] [Google Scholar]
- Procida G, Giomo A, Cichelli A, Conte LS. Study of volatile compounds of defective virgin olive oils and sensory evaluation: a chemometric approach. J Sci Food Agric. 2005;85:2175–2183. doi: 10.1002/jsfa.2122. [DOI] [Google Scholar]
- Procida G, Cichelli A, Lagazio C, Conte LS. Relationships between volatile compounds and sensory characteristics in virgin olive oil by analytical and chemometric approaches. J Sci Food Agric. 2015 doi: 10.1002/jsfa.7096. [DOI] [PubMed] [Google Scholar]
- Ruiz-Samblás C, Tres A, Koot A, Van Ruth SM, González-Casado A, Cuadros-Rodríguez L. Proton transfer reaction-mass spectrometry volatile organic compound fingerprinting for monovarietal extra virgin olive oil identification. Food Chem. 2012;134:589–596. doi: 10.1016/j.foodchem.2012.02.135. [DOI] [Google Scholar]
- Sánchez del Pulgar J, Soukoulis C, Biasioli F, Cappellin L, García C, Gasperi F, Granitto P, Märk TD, Piasentier E, Schuhfried E. Rapid characterization of dry cured ham produced following different PDOs by proton transfer reaction time of flight mass spectrometry (PTR–ToF–MS) Talanta. 2011;85:386–393. doi: 10.1016/j.talanta.2011.03.077. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ortiz A, Romero-Segura C, Sanz C, Pérez AG. Synthesis of volatile compounds of virgin olive oil is limited by the lipoxygenase activity load during the oil extraction process. J Agric Food Chem. 2012;60:812–822. doi: 10.1021/jf204241e. [DOI] [PubMed] [Google Scholar]
- Taiti C, Costa C, Menesatti P, Comparini D, Bazihizina N, Azzarello E, Masi E, Mancuso S. Class-modeling approach to PTR–TOFMS data: a peppers case study. J Sci Food Agric. 2014 doi: 10.1002/jsfa.6761. [DOI] [PubMed] [Google Scholar]
- Taiti C, Costa C, Menesatti P, Caparrotta S. Use of volatile organic compounds and physicochemical parameters for monitoring the post-harvest ripening of imported tropical fruits. Eur Food Res Technol. 2015 [Google Scholar]
- Taiti C, Marone E, Bazihizina N, Caparrotta S, Azzarello E, Petrucci AW, Pandolfi C, Giordani E. Sometimes a little mango goes a long way: a rapid approach to assess how different shipping systems affect fruit commercial quality. Food Anal Methods. 2015 [Google Scholar]
- Tena N, Wang SC, Aparicio-Ruiz R, García-González DL, Aparicio R. In-depth assessment of analytical methods for olive oil purity, safety, and quality characterization. J Agric Food Chem. 2015;63:4509–4526. doi: 10.1021/jf5062265. [DOI] [PubMed] [Google Scholar]
- Van Ruth S, Kiers J, Akkermans W, Perez R, Koot A, Perri E, Pellegrino M, Moreno Rojas JM, Guillou C, Rossignol-Castera A (2009) Geographical origin, cultivar and harvesting year verification of european and non-european olive oils using proton transfer reaction mass spectrometry with multivariate data analysis. In: Proceedings of the 5th CIGR section VI international symposium on food processing, monitoring technology in bioprocesses and food quality management, Germany, pp 791–799
- Vezzaro A, Boschetti A, Dell’Anna R, Canteri R, Dimauro M, Ramina A, Ferasin M, Giulivo C, Ruperti B. Influence of olive (cv Grignano) fruit ripening and oil extraction under different nitrogen regimes on volatile organic compound emission studied by PTR–MS technique. Anal Bioanal Chem. 2011;399:571–2582. doi: 10.1007/s00216-010-4636-1. [DOI] [PubMed] [Google Scholar]