Abstract
This study aimed to evaluate the impact of various oil extraction procedures on yield, color properties, fatty acid profile and oxidative stability of common Kilka (Clupeonella cultriventris caspia) oil. The supercritical fluid extraction (SFE) with carbon dioxide, than other techniques (wet reduction, ammonia and enzymatic extractions), showed the highest oil yield (96.94%), the best oxidative and color characteristics, with 0.68 mg KOH/g oil, 2.62 meq O2/kg oil, 6.5 and 5.36 in terms of acid value (AV), peroxide value (PV), yellowness and redness, respectively, and have the highest total of unsaturated fatty acids average (10.33), especially the omega-3 family. Furthermore, the oil obtained by SFE had the best oxidative parameters; AV, PV and thiobarbituric acid values, during the whole 90 days of storage time at 4 °C. Therefore, the SFE is the most effective procedure at obtaining the oil from common Kilka tissue than other methods studied in this research.
Keywords: Common Kilka oil, Oil extraction, Oil stability, Oxidative quality, Oil color quality
Introduction
Long chain polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA, C20:5n-3) and docosahexaenoic acid (DHA, C22:6n-3), naturally exist in large quantities in seafood (Barrento et al. 2010). The human body is unable to synthesize n-3 PUFAs, therefore, these compounds must be provided only through food consumption (Ghomi et al. 2012). Several studies have shown that the presence of PUFAs in the diet can greatly reduce the risk of diseases such as brain ageing, cardiovascular and Alzheimer’s diseases, prostate and breast cancers (Connor 2000; Pirestani et al. 2010). Therefore, in recent years, the attention of consumers to use those food sources that contain high amounts of PUFAs, such as fish oil, is growing.
Kilka is a common name used for the fish belonging to the clupeids in the Caspian Sea. Common kilka (Clupeonella cultriventris caspia), anchovy kilka (C. engrauli formis), and bigeye kilka (C. grimmi) are three species of Kilka that have been identified in the Caspian Sea (Fazli et al. 2009). It has been shown that the average value of omega-3 fatty acids and PUFAs in common Kilka oil can be more than 16 and 19%, respectively (Pirestani et al. 2010). One of the important parameters about the benefits of fish oil consumption on human health is that the ratio between omega-3 and omega-6 in this category of oils is from 1 to 5 (Osman et al. 2001), while, the value of this ratio about common Kilka oil can be more than 6 (Pirestani et al. 2010). Also, among the oils obtained from commercially important fish species from south Caspian Sea, the common Kilka oil has the maximum proportion of EPA + DHA to C16:0 (polyene index) (Pirestani et al. 2010).
The most common technique to extract the oil from the fish is wet reduction (Chantachum et al. 2000). Generally, in laboratory scale and to conduct scientific research, organic solvents are broadly used to extract oil from different sources, but, this method for fish oil extraction is not suitable on an industrial scale (Adeniyi and Bawa 2006). Enzymatic extraction, especially with the use of proteases, is another process to extract the fish oil (Linder et al. 2005). Although the costs associated with this process is high, but, several researches have shown that by controlling of the some factors, this technique is feasible (Nascimento et al. 2008; Soto et al. 2007; Zhang et al. 2012). In recent years, several studies have been done in the field of application of SFE technique to achieve the fish oil from by-products (Letisse et al. 2006; Rubio-Rodríguez et al. 2008). Despite the high yield of oil extraction in this technique, necessity of using high pressure equipment and freeze drying of raw materials are the most important constraints on the use of SFE on an industrial scale (Rubio-Rodríguez et al. 2012). The objective of the present investigation was to evaluate the impacts of various oil extraction techniques on the color and oxidative quality, fatty acid profile and oxidative stability of common Kilka oil.
Materials and methods
Materials
The experimental fish, common Kilka (Clupeonella cultriventris caspia) (weight: 60 ± 5 g, length: 10 ± 2 cm), were purchased in October 2015 from special Kilka fishing boats in Bandar-e Anzali harbor, Gilan Province, north of Iran. On boat, the fish were beheaded, eviscerated, washed and then transported to the laboratory in ice boxes [2:1 (w/w), ice to fish]. In the laboratory, the fish edible parts (muscle tissue) were cut into very small pieces (1–8 mm in diameter) which were used for further analysis. The enzyme (food grade neutral protease from Bacillus licheniformis) was obtained from Novozyme (Bagsvaerd, Denmark). All chemicals and solvents used in this study were of analytical grade and provided by Sigma (USA) and Merck (Germany).
Methods
Wet reduction extraction
The wet reduction extraction technique was carried out according to the method described by Rubio-Rodríguez et al. (2012) with some modifications. A fish sample (100 g) was mixed with 150 mL of water in a 1000-mL glass bottle and cooked in a water bath at 70 °C for 2 h. During the cooking process, the sample was stirred every 15 min. Then, the sample was transferred to a cloth bag and pressed mechanically. The obtained liquid was filtrated with a Buchner funnel with filter paper. Ultimately, the oil phase was separated using a centrifuge (EBA21 Hettich, Germany) at 2772×g for 15 min at 15 °C.
Ammonia extraction
The ammonia extraction technique was carried out according to the method described by Hao et al. (2013) with some modifications. Initially, a fish sample (100 g) was mixed with water (150 ml) and heated in a water bath to 50 °C. Then, the mixture pH was set in the range of 8–9 by adding ammonia (12.5%) and heated to 90 °C for 40 min. In the following, the mixture was cooled to room temperature and then ammonium carbonate (6%) was added. After 15 min, the mixture was centrifuged to obtain the oil phase.
Enzymatic extraction
The enzymatic extraction technique was carried out according to the method described by Rubio-Rodríguez et al. (2012) with some modifications. A fish sample (100 g) was mixed with water (150 mL) and neutral protease (500 mg) in a 1000-mL glass bottle and then placed in a bain-marie shaker (OLS 200 Grant, UK) at 40 °C for 2 h. In the final step, different phases of the mixture was separated by centrifugation at 2772×g for 15 min at 15 °C in order to obtain the oil phase.
Supercritical fluid extraction (SFE)
The SFE extraction technique was carried out in a semi-pilot SFE plant according to the method described by Vaquero et al. (2006) with some modifications. Common components of an SFE plant with solvent recycling were set up, i.e. pump, extractor, separator, heating and cooling systems, pressure dampers, rupture disks, controlling and measuring equipments.
At first, a fish sample was frozen dried at −55 °C (Alpha 1–2 LD, Christ, Germany). In the following, 100 g of freeze dried fish sample was put into the extractor and then, carbon dioxide (Carburos Metálicos, liquid CO2 ≥ 99.9%) was circulated with a flow rate of 10 kg CO2/h under the pressure of 25 MPa at 40 °C for 60 min. In the separator, carbon dioxide was removed from the extracted section by reducing the pressure to 5.2 MPa.
Definition of the extraction yield
In all extraction techniques, the yield of oil extraction was determined based on the procedure reported by Sahena et al. (2010) and expressed as the percentage of extracted oil compared to the total oil content in the sample.
Measurement of oil color
The color of common Kilka oil samples were determined according to the AOCS Official Method Cc13e-92 (AOCS 2009). For this purpose, the colorimeter (Lovibond Tintometer, Model F, UK) was used and results were expressed on the basis of yellow and red color units.
Analysis of oil fatty acids composition
The common Kilka oil fatty acids profile were determined by gas chromatography after conversion of oil to their methyl esters (FAME) using the standard method of boron trifluoride–methanol (BF3) (Moss et al. 1974). A 1 µL of FAME sample was injected into a gas chromatograph (Hewlett‐Packard 6890 Series gas chromatograph, H.P. G1530A, USA) equipped with a flame ionization detector (FID) and a polar capillary column (SP 2560) with an internal diameter of 0.32 mm, length of 100 m and film thickness of 0.25 µm (Suppelco, USA). The carrier gas was helium, and the split ratio was 1:20. Injector and detector temperatures were set at 240 and 270 °C, respectively. The column temperature was programmed starting at a constant temperature of 160 °C for 5 min, heated to 180 °C at 20 °C/min, held at 180 °C for 10 min, heated again to 200 °C at 1 °C/min, held at 200 °C for 1 min, heated again to 230 °C at 30 °C/min and finally held at 230 °C for 5 min. 1 mL of hexane containing 0.5 mg of heptadecanoic acid was used as an internal standard. The FAME peaks were identified by comparing their retention time with those of chromatographic standards in order to identify and quantify the individual fatty acid. The Clarity™ Data Apex software (Prague, Czech Republic) was used to analyze peak position and area.
Analysis of oil stability
In this study, after oil extraction by any of the techniques, oil samples were stored in dark glass bottles at 4 °C for 90 days. Acid, peroxide and thiobarbituric acid values of oil samples were measured at different time intervals (on days 0, 15, 30, 60 and 90) to analyze the stability of oil during storage time.
Acid value (AV)
The AV was calculated according to the AOCS Official Method Cd 3d-63 (AOCS 2009). For this purpose, initially, a fish oil sample (5 g) was dissolved in toluene-isopropyl alcohol solution (1:1 v/v, 50 mL). Then, the obtained solution was titrated with standard solution of potassium hydroxide in isopropyl alcohol (0.1 M) in the presence of phenolphthalein as the indicator.
Peroxide value (PV)
The PV was calculated according to the AOCS Official Method Cd 8–53 (AOCS 2009). For this purpose, a fish oil sample (5 g) was dissolved in a solution of acetic acid–chloroform (3:2 v/v, 30 mL) and then saturated potassium iodide solution (0.5 mL) was added. The resulting solution was placed in the dark at room temperature for 1 min. Then, distilled water (30 mL) was added. In the next step, the reaction solution was titrated with sodium thiosulphate (0.1 N) to a pale yellow, then, the starch indictor (2 mL) was added and titration process was continued until reaching the endpoint of colorless.
Thiobarbituric acid (TBA) value
The TBA value was calculated according to the AOCS Official Method Cd 19–90 (AOCS 2009). For this purpose, a 5 mL aliquot of fish oil sample (200 mg) solution in 25 mL 1-butanol with 5 mL TBA reagent was heated in a water bath at 95 °C for 2 h and then cooled at room temperature. The TBA value was determined through reading the absorbance at 532 nm (UV 2100, UNICO, USA) against a blank and using reference cuvette.
Statistical analysis
All results of three independent experiments were expressed as the mean ± standard deviation (SD). The data were analyzed by analysis of variance (ANOVA) and the Duncan’s test for the 5% significance level. Each of which were done with the SPSS software, Version 21 (IBM, New York, USA).
Results and discussion
Oil extraction yield and oxidative quality
Fish protein matrix is linked with fatty tissue. Wet reduction technique was reported to be suitable for extracting oil from fish with high oil content (Rubio-Rodríguez et al. 2008). According to Table 1, ammonia and wet reduction techniques had a low efficiency in the extraction of oil from common Kilka muscle.
Table 1.
Common Kilka oil extraction yield in different extraction techniques
| Extraction techniques | Extraction yield (%) |
|---|---|
| Wet reduction extraction | 54.28 ± 2.35c |
| Ammonia extraction | 41.41 ± 2.26d |
| Enzymatic extraction | 85.63 ± 1.92b |
| Supercritical fluid extraction | 96.94 ± 1.65a |
Mean ± SD (standard deviation) within a column with different superscript letters are significantly different (p < 0.05)
It seems that although cooking stage was led to denaturation of the protein matrix, but, rearrangement and creation of special structures by denatured protein molecules in cooking stage and also, the effects of emulsifying properties of proteins in the centrifugation stage (Rubio-Rodríguez et al. 2012) hindered the release of oil phase in ammonia and wet reduction methods. In the enzymatic technique compared with the above two oil extraction techniques, the relative increase in the yield of oil extraction can be attributed to the hydrolysis of the protein matrix by proteases. The results of this study showed that the use of SFE technique for extracting oil from common Kilka not only had the highest yield (96.94%) but also the resulting oil had the highest oxidative quality [lowest AV(0.68 mg KOH/g oil) and PV(2.62 meq O2/kg oil)] (Table 2). These were in accordance with the results reported by Rubio-Rodríguez et al. (2008) on the extraction of oil from hake (Merluccius capensis–Merluccius paradoxus) by-products by SFE method. Sahena et al. (2010) had reached up to 98.5% yield on the extraction of oil from Indian mackerel skin by SFE technique. One of the reasons for the higher oil extraction yield in SFE technique may be a significant reduction in raw material moisture content during the freeze-drying process. King et al. (1989) reported that the yield of fat extraction from meat products in SFE method increased significantly by dehydration before the extraction process. Dunford et al. (1998) found that water content in the raw material had a considerable effect on oil solubility in supercritical CO2, so that, with reducing of moisture content, the solubility of the oil phase and therefore the yield of oil extraction was increased.
Table 2.
Oxidation indices of common Kilka oil obtained by different extraction techniques
| Extraction techniques | AV (mg KOH/g oil) | PV (meq O2/kg oil) |
|---|---|---|
| Wet reduction extraction | 1.84 ± 0.14b | 3.62 ± 0.21a |
| Ammonia extraction | 2.28 ± 0.19a | 2.87 ± 0.07c |
| Enzymatic extraction | 1.24 ± 0.10c | 3.22 ± 0.13b |
| Supercritical fluid extraction | 0.68 ± 0.09d | 2.62 ± 0.17d |
AV acid value, PV peroxide value
Mean ± SD (standard deviation) within a column with different superscript letters are significantly different (p < 0.05)
Lack of oxygen and applying a relatively mild heat during the extraction of oil leads to a reduction in the rate of oxidation of the resulting oil by the SFE technique, meanwhile, in this technique, a high level of contact between carbon dioxide and the oil phase causes the lowering of the interaction of water and oil molecules relative to each other (Letisse et al. 2006). Consequently, these factors increased extraction yield and oxidative quality of the oil obtained by SFE than other three oil extraction techniques.
Oil color quality
Oils color is one of the most important parameters affecting the cost of manufacturing of these products. Because, in order to obtain the oil with acceptable color quality, high costs should be spent to increase the efficiency of the production process (Noriega-Rodríguez et al. 2010). In this study, Lovibond values for yellowness of oil samples were in the range of 6.37–6.94, whilst redness varied from 5.22 to 5.98 (p > 0.05). In terms of yellowness, the oil extracted from the SFE had lowest value and there was no significant difference (p > 0.05) among three other procedures (Fig. 1a), whereas the highest and lowest values of redness were obtained in the case of enzymatic and SFE methods, respectively, and there was no significant difference (p > 0.05) between wet reduction and ammonia extraction techniques (Fig. 1b). A higher percentage of hemoglobin degradation by enzymes and therefore, the release of greater amounts of haem pigments during oil extraction with enzymatic method may be the cause of this difference.
Fig. 1.
Color characteristics of common Kilka oil obtained by various extraction techniques; a yellowness, b redness (color figure online)
Fatty acids profile
The most important fatty acids detected in Kilka oil were C14:0, C16:0, C16:1n-7, C18:0, C18:1n-9, C20:5n-3 (EPA) and C22:6n-3 (DHA) (Table 3) and were in accordance with results reported by Pirestani et al. (2010).
Table 3.
Fatty acid (%) profile of common Kilka oil obtained by different extraction techniques
| Fatty acids | Wet-reduction extraction | Ammonia extraction | Enzymatic extraction | Supercritical fluid extraction |
|---|---|---|---|---|
| C14:0 (myristic acid) | 6.94 ± 0.13a | 6.62 ± 0.12b | 6.98 ± 0.17a | 6.03 ± 0.09c |
| C16:0 (palmitic acid) | 22.13 ± 0.19a | 22.10 ± 0.14a | 22.09 ± 0.35a | 19.05 ± 0.14b |
| C16:1n-7 (palmitoleic acid) | 10.89 ± 0.08a | 10.88 ± 0.05a | 10.91 ± 0.11a | 10.93 ± 0.19a |
| C17:0 (margaric acid) | 1.25 ± 0.10a | 1.22 ± 0.08a | 1.24 ± 0.05a | 1.28 ± 0.12a |
| C17:1n-7 (heptadecanoic acid) | 0.95 ± 0.02a | 0.97 ± 0.01a | 0.95 ± 0.06a | 0.98 ± 0.02a |
| C18:0 (stearic acid) | 5.05 ± 0.13a | 5.07 ± 0.06a | 5.08 ± 0.11a | 5.10 ± 0.02a |
| C18:1n-9 (oleic acid) | 29.09 ± 0.21b | 29.11 ± 0.29b | 29.06 ± 0.12b | 30.76 ± 0.52a |
| C18:1n-7 (vacenic acid) | 1.51 ± 0.01b | 1.49 ± 0.10b | 1.53 ± 0.14b | 1.98 ± 0.08a |
| C18:2n-6 (linoleic acid) | 2.11 ± 0.02b | 2.12 ± 0.09b | 2.14 ± 0.03b | 2.38 ± 0.12a |
| C18:3n-3 (linolenic acid) | 1.13 ± 0.09a | 1.11 ± 0.02a | 1.15 ± 0.11a | 1.16 ± 0.06a |
| C20:0 arashidic acid | 1.39 ± 0.07a | 1.36 ± 0.09a | 1.38 ± 0.03a | 1.40 ± 0.02a |
| C20:1n-9 (gadoleic acid) | 1.85 ± 0.01b | 1.82 ± 0.03b | 1.86 ± 0.12b | 2.14 ± 0.05a |
| C20:2n-6 (eicosadienoic acid) | 0.15 ± 0.05b | 0.13 ± 0.01b | 0.14 ± 0.04b | 0.15 ± 0.02a |
| C20:3n-3 (eicosatrienoic acid) | 0.38 ± 0.04a | 0.27 ± 0.02b | 0.28 ± 0.01b | 0.17 ± 0.01c |
| C20:4n-6 (arachidonic acid) | 0.28 ± 0.02b | 0.30 ± 0.04b | 0.31 ± 0.02b | 0.49 ± 0.03a |
| C20:5n-3 (EPA) | 5.44 ± 0.08b | 5.41 ± 0.01b | 5.43 ± 0.05b | 6.21 ± 0.12a |
| C22:0 (behenic acid) | 0.22 ± 0.04a | 0.22 ± 0.05a | 0.23 ± 0.01a | 0.24 ± 0.01a |
| C22:1n-9 (erucic acid) | 0.16 ± 0.01a | 0.18 ± 0.03a | 0.18 ± 0.01a | 0.17 ± 0.02a |
| C22:4n-6 (docosatetraenoic acid) | 0.20 ± 0.06a | 0.20 ± 0.01a | 0.21 ± 0.03a | 0.19 ± 0.05a |
| C22:5n-3 (docosapentaenoic acid) | 0.59 ± 0.03b | 0.61 ± 0.02b | 0.60 ± 0.07b | 0.92 ± 0.01a |
| C22:6n-3 (DHA) | 8.54 ± 0.04c | 8.91 ± 0.09b | 8.87 ± 0.11b | 10.93 ± 0.06a |
| Average of total saturated fatty acids (SFA) | 6.16 ± 0.12a | 6.10 ± 0.09a | 6.16 ± 0.16a | 5.51 ± 0.08b |
| Average of total monounsaturated fatty acids (MUFAs) | 7.40 ± 0.09b | 7.40 ± 0.12b | 7.41 ± 0.10b | 7.82 ± 0.22a |
| Average of total polyunsaturated fatty acids (PUFAs) | 2.09 ± 0.05b | 2.11 ± 0.04b | 2.12 ± 0.06b | 2.51 ± 0.06a |
| Total unsaturated fatty acids (UFAs) average | ~9.49 | ~9.51 | ~9.53 | ~10.33 |
Mean ± SD (standard deviation) within a row with different superscript letters are significantly different (p < 0.05)
The highest total of UFAs average (~10.33) was obtained for oil extracted by SFE compared to other extraction techniques. The major fatty acids cause such a difference were C20:5n-3 (EPA) and C22:6n-3 (DHA), considered as the main fatty acids found in fish tissue (Weber et al. 2008). Also, the oil obtained by SFE technique had the highest average of total monounsaturated fatty acids. Meanwhile, in other three oil extraction techniques, in terms of unsaturation degree, there was no significant difference (p < 0.05). Considering that consumption of unsaturated fatty acids are more important in terms of health than saturated fatty acids (Lawrence 2010), therefore, the common Kilka oil extracted by SFE method had a better nutritional quality compared to other methods of oil extraction.
Oil stability
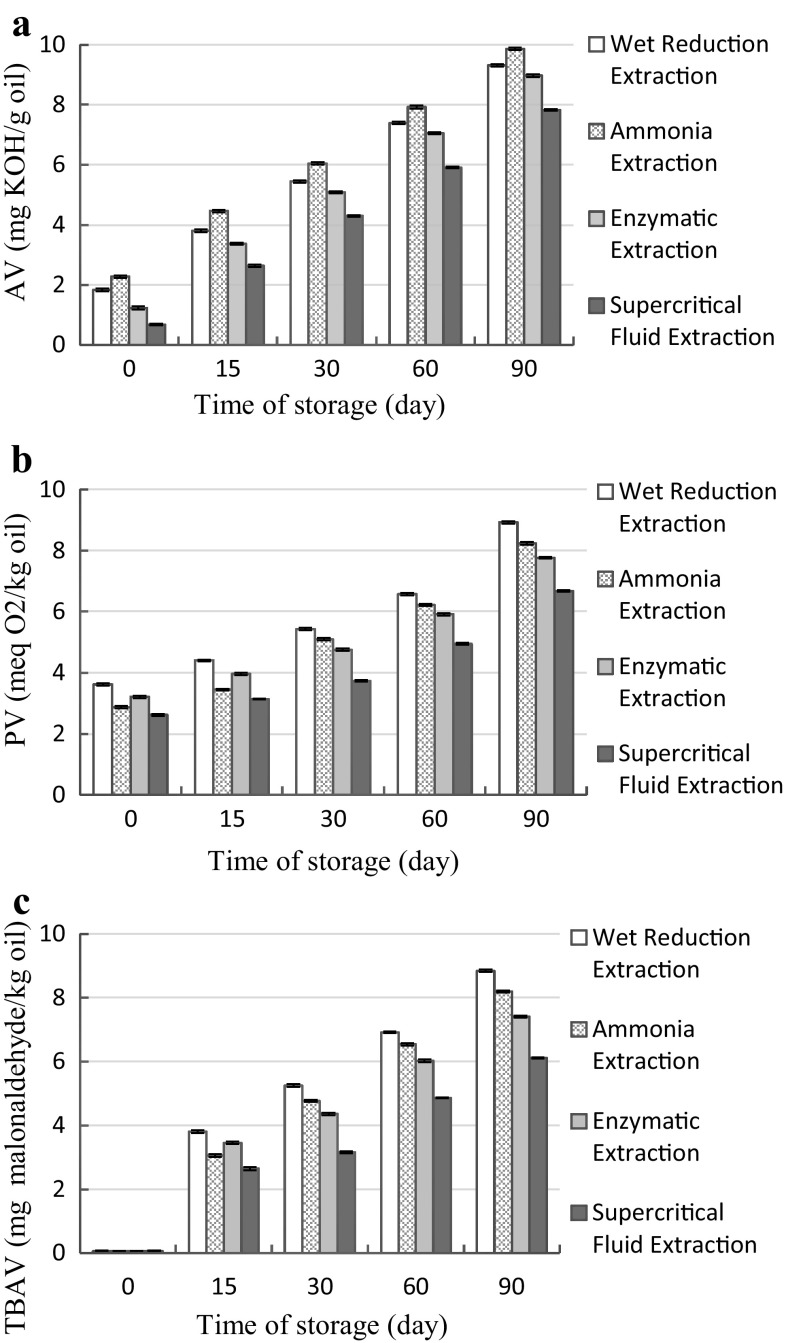
The quality of raw material, oil extraction technique and oil composition are the main parameters affecting the stability of oils during storage (Rubio-Rodríguez et al. 2012). In this study, in general, for all common Kilka oil samples obtained by different extraction techniques, AVs were enhanced with increase of storage time (Fig. 2a).
Fig. 2.
Effect of various extraction techniques on the AV (a), PV (b) and TBAV (c) of common Kilka oil stored at 4 °C for 90 days
The AV for oil extracted by SFE was minimum value compared to other techniques during the whole 90 days of storage. In the case of crude fish oils, the acceptable range of AV is 7–8 mg KOH/g oil (Boran et al. 2006). Our results showed that, after 60 days of storage at 4 °C, except for SFE technique, AVs for the common Kilka oil obtained by other three oil extraction procedures were reached to the above limit. Rubio-Rodríguez et al. (2012) found that in comparing the different techniques for extracting oil from various fish by-products, oil extracted by SFE technique had the lowest AV. On the other hand, oil samples related to ammonia extraction technique had the highest AVs during the whole storage time. It may suggest that the formation of free fatty acids resulting from triacylglycerides hydrolysis was more intense in this method.
In this research, PVs of oil samples were measured to assess their primary oxidation (Fig. 2b). PVs, in the case of all four techniques of oil extraction, like the AV, were significantly uptrend with increase of storage time. This indicates that the rate of hydroperoxides (ROOH) decomposition process was lower than their formation rates during the entire storage time. The lowest increase in PV was observed in oil samples extracted by SFE technique. The acceptable range of PV for crude fish oils is 7–8 meq O2/kg oil (Boran et al. 2006). The common Kilka oil obtained through SFE technique showed lower PV than the above limit compared to other oil extraction methods at the end of storage time. Meanwhile, in all over the storage period, the oil extracted by wet reduction extraction technique had the largest PV, probably due to more severe oxidizing conditions during the extraction of oil in this technique and thus highest level formation of ROOH than other methods.
Secondary oxidation of oil samples were evaluated by determining their TBA values in this study (Fig. 2c). Aldehydes and ketones are the most important secondary oxidation products, which form from ROOH break down. Thiobarbituric acid reactive substances, especially malonaldehyde, will be spectrophotometrically quantifiable through the creation of colored compounds with TBA. According to Fig. 2c, like AV and PV, for all oil samples, TBA values were increased in the case of all oil extraction procedures. In entire length of storage time, oil samples obtained by wet reduction extraction and SFE techniques showed the largest and smallest TBA values, respectively, which were in agreement with the results of the evaluation of PVs.
Conclusion
With the aim of achieving the highest quality of fish oil using various extraction procedures, the SFE demonstrated to be a very efficient method for common Kilka oil. This method showed the highest extraction yield and resulted into oil with best oxidative stability, color quality and storage stability with the highest of UFAs. Furthermore, the SFE can be an effective method to increase the nutritional quality of fish oil by enhancing the amount of PUFAs content, specially EPA and DHA.
The future usage of this technique (SFE) could help in real time, the routine extraction of edible oils in research laboratories and in the food industry.
Abbreiviations
- SFE
Supercritical fluid extraction
- AV
Acid value
- PV
Peroxide value
- TBA
Thiobarbituric acid
Compliance with ethical standards
Conflict of interest
Authors have no conflict of interest.
References
- Adeniyi OD, Bawa AA. Mackerel (Scomberscrombrus) oil extraction and evaluation as raw materials for industrial utilization. Leonardo J Sci. 2006;8:33–42. [Google Scholar]
- American Oil Chemist’s Society . Official methods and recommended practices of the American oil chemists society. 6. Champaign: AOCS Press; 2009. [Google Scholar]
- Barrento S, Marques A, Teixeira B, Mendes R, Bandarra N, Vaz-Pires P, Nunes ML. Chemical composition, cholesterol, fatty acid and amino acid in two populations of brown crab (Cancer pagurus): ecological and human health implications. J Food Compos Anal. 2010;23:716–725. doi: 10.1016/j.jfca.2010.03.019. [DOI] [Google Scholar]
- Boran G, Karac H, Boran M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006;98:693–698. doi: 10.1016/j.foodchem.2005.06.041. [DOI] [Google Scholar]
- Chantachum S, Benjakul S, Sriwirat N. Separation and quality of fish oil from precooked and non-precooked tuna heads. Food Chem. 2000;69:289–294. doi: 10.1016/S0308-8146(99)00266-6. [DOI] [Google Scholar]
- Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71:171–175. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- Dunford NT, Goto M, Temelli F. Modelling of oil extraction with supercritical CO2 from Atlantic mackerel (Scomber scombrus) at different moisture contents. J Supercrit Fluids. 1998;13:303–309. doi: 10.1016/S0896-8446(98)00064-3. [DOI] [Google Scholar]
- Fazli H, Zhang IKC, Hay DE, Lee CW. Stock assessment and management implications of anchovy kilka (Clupeonella engrauliformis) in Iranian waters of the Caspian Sea. Fish Res. 2009;100:103–108. doi: 10.1016/j.fishres.2009.06.018. [DOI] [Google Scholar]
- Ghomi MR, Nikoo M, Babaei Z. Fatty acid composition in farmed great sturgeon (Huso huso) Comp Clin Pathol. 2012;21:111–114. doi: 10.1007/s00580-011-1228-1. [DOI] [Google Scholar]
- Hao SX, Huang H, Li LH, Yang XQ, Cen JW, Lin WL, Wei Y. Extraction of fish oil from the muscle of sturgeon using supercritical fluids. Adv Mater Res. 2013;657:1975–1981. doi: 10.4028/www.scientific.net/AMR.655-657.1975. [DOI] [Google Scholar]
- King JW, Johnson JH, Friedrich JP. Extraction of fat tissue from meat products with supercritical carbon dioxide. J Agric Food Chem. 1989;37:951–954. doi: 10.1021/jf00088a027. [DOI] [Google Scholar]
- Lawrence GD. The fats of life: essential fatty acids in health and disease. 18. Canada: Rutgers University Press; 2010. [Google Scholar]
- Letisse M, Rozieres M, Hiol A, Sergent M, Comeau L. Enrichment of EPA and DHA from sardine by supercritical fluid extraction without organic modifier: I. Optimization of extraction conditions. J Supercrit Fluids. 2006;38:27–36. doi: 10.1016/j.supflu.2005.11.013. [DOI] [Google Scholar]
- Linder M, Fanni J, Parmentier M. Proteolytic extraction of salmon oil and PUFA concentration by lipases. Mar Biotechnol. 2005;7:70–76. doi: 10.1007/s10126-004-0149-2. [DOI] [PubMed] [Google Scholar]
- Moss CW, Lambert MA, Mervin WH. Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol. 1974;28:80–85. doi: 10.1128/am.28.1.80-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento RJS, Couri S, Antoniassi R, Freitas SP. Fatty acids composition of açaí pulp oil obtained by enzymatic technology and hexane. Rev Bras Frutic. 2008;30:498–502. doi: 10.1590/S0100-29452008000200040. [DOI] [Google Scholar]
- Noriega-Rodríguez JA, Ortega-García J, Angulo-Guerrero O, García HS, Medina-Juárez LA, Gámez-Meza N. Oil production from sardine (Sardinops sagax caerulea) Producción de aceite a partir de sardina (Sardinops sagax caerulea) CyTA J Food. 2010;7:173–179. doi: 10.1080/19476330903010243. [DOI] [Google Scholar]
- Osman H, Suriah AR, Law EC. Fatty acid composition and cholesterol content of selected marine fish in Malaysian water. Food Chem. 2001;73:55–60. doi: 10.1016/S0308-8146(00)00277-6. [DOI] [Google Scholar]
- Pirestani S, Sahari MA, Barzegar M, Nikoopour H. Lipid, cholesterol and fatty acid profile of some commercially important fish species from south Caspian Sea. J Food Biochem. 2010;34:886–895. [Google Scholar]
- Rubio-Rodríguez N, de Diego-Rupérez S, Beltrán S, Jaime I, Sanz MT, Rovira J. Supercritical fluid extraction of the omega-3 rich oil contained in hake (Merluccius capensis–Merluccius paradoxus) byproducts: study of the influence of process parameters on the extraction yield and oil quality. J Supercrit Fluids. 2008;47:215–226. doi: 10.1016/j.supflu.2008.07.007. [DOI] [Google Scholar]
- Rubio-Rodríguez N, de Diego SM, Beltrán S, Jaime I, Sanz MT, Rovira J. Supercritical fluid extraction of fish oil from fish by-products: a comparison with other extraction methods. J Food Eng. 2012;109:238–248. doi: 10.1016/j.jfoodeng.2011.10.011. [DOI] [Google Scholar]
- Sahena F, Zaidul ISM, Jinap S, Yazid AM, Khatib A, Norulaini NAN. Fatty acid compositions of fish oil extracted from different parts of Indian mackerel (Rastrelliger kanagurta) using various techniques of supercritical CO2 extraction. Food Chem. 2010;120:879–885. doi: 10.1016/j.foodchem.2009.10.055. [DOI] [Google Scholar]
- Soto C, Chamy R, Zúniga ME. Enzymatic hydrolysis and pressing conditions effect on borage oil extraction by cold pressing. Food Chem. 2007;102:834–840. doi: 10.1016/j.foodchem.2006.06.014. [DOI] [Google Scholar]
- Vaquero EM, Beltran S, Sanz MT. Extraction of fat from pigskin with supercritical carbon dioxide. J Supercrit Fluids. 2006;37:142–150. doi: 10.1016/j.supflu.2005.11.003. [DOI] [Google Scholar]
- Weber J, Bochi VC, Ribeiro CP, Victório A, Emanuelli T. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 2008;106:140–146. doi: 10.1016/j.foodchem.2007.05.052. [DOI] [Google Scholar]
- Zhang Y, Li S, Yin C, Jiang D, Yan F, Xu T. Response surface optimization of aqueous enzymatic oil extraction from bayberry (Myrica rubra) kernels. Food Chem. 2012;135:304–308. doi: 10.1016/j.foodchem.2012.04.111. [DOI] [Google Scholar]