Abstract
An attempt was made to hydrolyze proteins and lactose in whey to improve the nutritive value of this byproduct, and extend its application as an ingredient to healthy beverages. Flavourzyme in different concentrations was used at pH 7.0 to hydrolyze protein at 50 °C. pH stat method, SDS-PAGE and RP HPLC–MS were used to evaluate degree of protein hydrolysis, pattern of peptide formation and characterize smaller peptides in hydrolysate, respectively. Higher concentration of enzymes produced more number of small peptides. Protein hydrolysate was again hydrolyzed at 30 °C with β—galactosidase at pH 5.5 to hydrolyze lactose. HPLC analysis indicated the degree of lactose hydrolysis and number of tri/poly saccharides formed due to varied enzyme concentration. Results from the experiment can be utilized to formulate healthy whey beverages for specific purpose.
Keywords: Whey proteins, Lactose, Enzymatic hydrolysis, HPLC, Electrophoresis
Introduction
Whey is the byproduct of Cheese, Paneer and Chhana production units. Whey is considered as the gold mine of many nutritional, clinical, therapeutically and dietetically active ingredients which constitutes about 50% of total milk solids (Horton 1995). Lactose is one of the main milk solids present in whey which varies between 4.5 and 6.0% of bovine milk. α-lactalbumin, β-lactoglobulin, bovine serum albumin, immunoglobulins and lactoferrin with excellent functional and nutraceutical properties, are the main proteins in whey. Besides lactose and whey proteins, the whey also contains many vitamins and minerals which are very helpful for the physiological functions. The composition of sweet whey is: TS—6.0 to 6.5; Lactose—4.5 to 5.0; Proteins—0.8 to 1.0 and Ash—0.5 to 0.7% (Tsakali et al. 2010). Whey is used as animal feed, alcohol and beverages production, and fermentation medium. Whey, its concentrates and dried forms are being used in many food products formulations. The protein part of whey has lot of biological value which can be further improved by hydrolyzing these proteins into peptides (Madureira et al. 2010). These liberated peptides have several health promoting activities like Opioid (Antila et al. 1991; Pihlanto-Leppala et al. 1997), Antihypertensive (Pihlanto-Leppala et al. 2000; da Costa et al. 2007; Wang et al. 2012; Lim et al. 2012), Antimicrobial and Immunostimulatory activities (Biziulevicius et al. 2006). Overall nutritional value of whey can be improved by enzymatic hydrolysis of proteins and increase in the functional, technical, physiological and bioactive properties of its proteins (Cheison et al. 2009).
Upon enzymatic hydrolysis, peptide bonds in a protein are cleaved, new amino groups are formed and one amino group for each broken peptide bond is found. The amount of newly formed amino groups causes a linear increase in amino nitrogen. This amino nitrogen can be determined by formal titration. The increase of amino nitrogen, corrected for the total number of peptide bonds available for cleavage in the protein is calculated. Whey proteins hydrolysates (WPH) are considered to be ideal ingredients in the formulation of human milk substitutes due to their high nutritional value, low bitterness and low antigenicity. By employing appropriate proteinase, partially hydrolyzed whey proteins can play an important role in areas of preventive and therapeutic health approaches because of favourable combination of various biochemical and physiological features in the hydrolysates (Meisel 1998), production of bioactive peptides (Pihlanto-Leppala 2001) and tailored amount and sizes of peptides for special diets (Boza et al. 2000). Functional properties like gelation, foaming and emulsion can also be altered (Nielsen 1977). Sadat et al. (2011) observed small peptides with potential antioxidative properties in the hydrolysate of α lactoalbumin whereas antioxidant properties in whey protein hydrolysate were observed by Dryakova et al. (2010) and Peng et al. (2010). Several methods are available to monitor protein hydrolysis and to characterize the resulting peptides. Sodium dodecyl sulphate- polyacrylamide gel electrophoresis (SDS-PAGE) has been used to assess the proteolysis activity and liberation of other components due to hydrolysis of whey proteins (Schmidt et al. 1995).
High performance liquid chromotagram–mass spectrometry (HPLC–MS) has emerged as an effective technique for the characterization of peptides and proteins. It has been used in the identification of complex mixture of peptides formed upon hydrolysis of whey proteins (Otte et al. 1997, 2000).
Lactose, the major milk solids present in whey, has great therapeutic and nutritional value for the brain development of infants. However, lactose interolanace became the problem for the adults which resulted in health hazard. Hydrolysis of lactose in whey using β-galactosidase has been reported to form five or six oligosaccharides (Asp et al. 1980). HPLC analysis of these oligosaccharides has been studied by Jeon and Mantha (1985). Hence, reduction of lactose content either by fermentation or controlled enzymatic hydrolysis have been claimed to have beneficial effect like greater digestibility (Clemente 2000; Frokjaer 1994). Therefore, an attempt was made to hydrolyze lactose in protein hydrolyzed whey for the benefit of consumers.
Hence, this study was conducted to know the hydrolysis pattern of both whey proteins and lactose with the variation in enzymes addition. Knowledge of peptides formation patterns and type of saccharides formation from lactose can help to formulate healthy whey beverages.
Materials and methods
Enzymatic hydrolysis of whey proteins
Fresh cheese whey of homogenised and pasteurized low fat (1.5% fat) milk was obtained for hydrolysis. Whey was further pasteurized at 65 °C for 20 min, cooled to 50 °C and pH was adjusted to 7.0 using diluted NaOH solution. Enzymatic hydrolysis of whey protein was performed using Flavourzyme (1000 LAPU/g, Novozymes Australia Ltd., produced by Aspergillus oryzae, containing both endo- and exopeptidase activities). Enzyme of 0.01, 0.02 and 0.05% of whey was added to the whey and incubated at 50 °C in water bath for 30 min. After 30 min of hydrolysis, the pH was re-adjusted to 7.0 using 0.5 N NaOH. The amount of base required to get back pH of 7.0 was noted. Thereafter, the hydrolysate was heated at 85 °C for 6–7 min to inactivate the enzyme and to arrest further hydrolysis. The cooled samples were kept in deep freeze (−20 °C) for further analysis.
The percent degree of hydrolysis (DH) which is defined as the percentage of peptide bonds cleaved by the enzyme was determined according to the method of base consumption as per pH stat technique of Adler-Nissen (1986). The calculation of DH was done as follows and expressed as per cent.
where B, base consumption in ml (NaOH); Nb, normality of the base (0.5 N); MP, total mass of protein in hydrolysate in g; htot, total number of peptide bonds in the protein substrate (meq/g protein for whey protein htot = 8.8); α, average degree of dissociation of the α-NH2 groups. These were computed by taking pKa values for different amino acids at various temperatures and pH and 1/α factor of 2.27 was considered as Calibration factor at pH 7.0 and 50 °C (Adler-Nissen 1986).
Protein content of the cheese whey was determined as per the method described by Lowry et al. (1951) using commercial Folin-Ciocalteu reagent.
SDS-PAG
The whey hydrolysate was diluted to contain 2 mg/ml protein. Protein samples were mixed in 1:1 ratio with 2× sample buffer, heated at 100 °C for 5 min and cooled to room temperature. The mixture was centrifuged at 10,000g after heating to ensure proper mixing. The whey protein hydrolysate was then analyzed by SDS-PAGE using bio-rad tris tricine precast ready 16.5% resolving gel and 4% stacking gel, 10 well, 30 µ, 8.6 × 6.8 cm (W × L), tricine sample buffer and running buffer as per the method of Schagger and Jagow (1987).
Reverse phase high performance liquid chromatography: mass spectrometer (RP-HPLC MS)
RP-HPLC MS is currently used in the separation and study of peptides. RP-LCMS was performed to objectively quantify the peptides present in the different whey hydrolysates. The equipment of LCMS—2010 EM from M/s. Shimadzu, Japan was equipped with column of Everest™ C18 of 250 × 4.6 mm, 300 A, Grace Division. Samples were fed using 100 μL injection loops. The peptides were eluted by a linear gradient from 100 to 0% solvent A (0.1% Trifluoroacetic acid, TFA) in deionized water in solvent B (0.1% TFA in 90% aqueous acetonitrile). Separation was conducted at room temperature (~20 °C) at flow rate of 0.75 mL min−1. Samples-eluted peptides were detected at 214 nm using Varian 9050 variables wave length UV/visible detector. Total run time was 90 min for each sample.
Sample preparation
Hydrolyzed whey sample was first diluted 0.50 to 5.0 mL with double distilled water followed by a 0.50 to 5.0 mL dilution with 70% Acetonitrile. After mixing, the sample was filtered using a syringe filter of pore size 0.45 micron (Cheleicher & Schuell GmbH, Germany).
Enzymatic hydrolysis of lactose
After termination of proteolysis in the whey, it was further hydrolyzed for lactose. Hot whey protein hydrolysate was cooled to 30 °C and pH was re adjusted from 6.5–7.0 to 5.5 for further hydrolysis of lactose. β-galactosidase (M/s. Sigma Aldrich chemicals Pvt. Ltd., from Aspergillus oryzae) of 0.01, 0.02 and 0.05% was used to hydrolyze lactose in whey. The enzyme mixture was incubated in water bath at optimum temperature of 30 °C as mentioned by the supplier. The sample was drawn after 30 min and heated immediately to 85 °C for 5–6 min to stop further hydrolysis. The heated and cooled samples were kept at −20 °C in deep freeze for further residual lactose analysis.
Lactose determination
Initial lactose in whey and lactose content in hydrolyzed whey was estimated by using HPLC. The amount of lactose was determined using HPLC (Waters, Milford, MA, USA) with the specification of solvent delivery pump operating at 0.5 ml/min (Model 515), 20 µl injection loop, waters model 2414 refractive index detector, Hi-Plex Ca coloumn, 300 × 7.7 mm (id) and 8 µm film (Agilent Technologies, USA). Column temperature was set up at 85 °C and injection volume was 20 µl. Isocratic elution with mobile phase of water (HPLC grade) at flow rate 0.5 ml/min was used. Detector temperature was set at 35 °C. Data obtained were collected and evaluated by software Empower Build (Waters, Milford, MA, USA).
Pure Lactose monohydrate was obtained from Sigma-Aldrich Chemical Co. A standard stock of lactose solution was prepared at 1.0 mg/mL in dilution with mobile phase (HPLC grade water) and was used as standard.
Sample preparation
Whey (1g) was added to 0.2 ml of acetic acid. The solution was diluted with distilled water (10 ml) in such a way that final concentration of lactose in the sample would yield 1 mg/ml. The diluted sample was heated to 85 °C for 10 min. in water bath and cooled to room temperature. The sample was filtered through whatman No 40 filter paper. To the filtrate, 2 ml of chloroform was added to de-fat the sample and mixed thoroughly. Tubes were centrifuged at 5000 rpm (1118×g) for 10 min. Top aqueous layer was collected and filtered through 0.22 µm filter (PVDF, Hemedia, Mumbai) and the filtrate was used for the analysis using HPLC.
Data obtained from the investigation was subjected to randomized one way ANOVA. The data on lactose hydrolysis was plotted against enzyme concentration with error bar by applying suitable computer packages (MS- excel 2007 version).
Results and discussion
Protein hydrolysis
Table 1 depicts the higher proteolysis with higher amount of enzyme addition. It was observed that the addition of 0.01% of flavourzyme hydrolyzed 4.13% protein whereas other two levels hydrolyzed 8.58 and 15.11%. Degree of hydrolysis was maximum (15.11%) and minimum (4.13%) when 0.05% and 0.01% of flavourzymes were added, respectively. The statistical analysis (Table 1) revealed that the higher amount enzyme addition resulted in a significantly (p < 0.05) higher degree of protein hydrolysis. There was significant (p < 0.05) differences in the increase of %DH among the three levels of enzyme concentration. Since the use of 0.01% concentrations did not yield higher hydrolysis, 0.02 and 0.05% enzyme concentration were used to produce hydrolysate for further analysis of peptide formation characteristics.
Table 1.
Effect of enzyme concentration on protein hydrolysis of cheese whey
| Sample | Enzyme conc. (%) | %DH | Mean |
|---|---|---|---|
| 1 | 0.01 | 3.51–4.98 | 4.13a ± 0.64 |
| 2 | 0.02 | 7.30–9.54 | 8.58b ± 0.93 |
| 3 | 0.05 | 13.92–16.46 | 15.11c ± 1.07 |
Different superscripts are significantly different (p < 0.05)
Electrophoretic study of protein hydrolysate
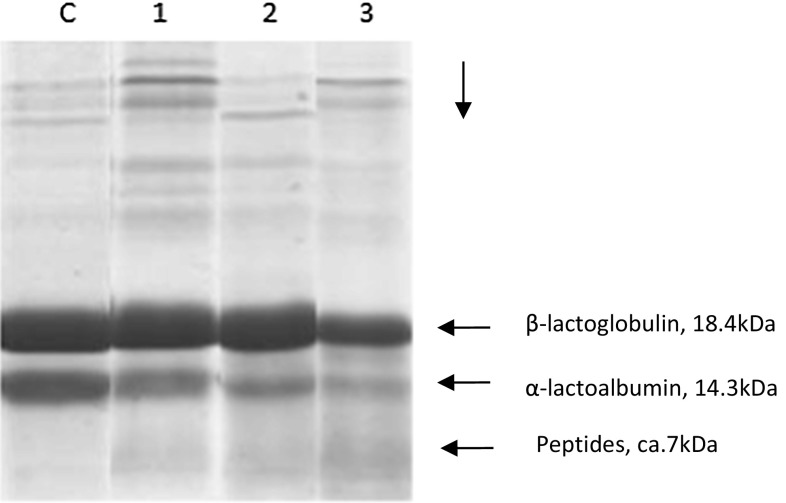
Tricine SDS-PAGE was carried out to find out the pattern of protein hydrolysis and peptide profiles among the hydrolysates. Figure 1 shows electrophoretic bands of hydrolysate with 0.01, 0.02, and 0.05% enzyme concentration. The electropherogram of whey and its hydrolysates showed two thicker bands i.e. β-lactoglobulin (18,400 Da) and α-lactalbumin (14,300).
Fig. 1.
Electropherogram of hydrolysed whey proteins; C control whey, 1 hydrolysed with 0.01%, 2 hydrolysed with 0.02% and 3 hydrolysed with 0.05%
On hydrolysis, these bands became lighter. As the concentration of the enzyme increased from 0.01 to 0.05%, the bands became lighter in descending order i.e. lane1 > lane 2 > lane 3 resulting in higher hydrolysis. Thicker bands are indicative of lower hydrolysis. When more of peptides are formed, new bands have appeared in the lane 1, 2 and 3 below α-lactalbumin band. The protein bands in all the three samples became slightly lighter as compared to control. Appearance of smaller bands in lanes 1, 2 and 3 in addition to bands of β-lactoglobulin and α-lactalbumin indicated the formation of more peptides. The intensity of these bands gradually increased from lane 1 to lane 3 indicating more hydrolysis in sample 3. This electropherogram also indicated generation of newer peptides at the end of 30 min of hydrolysis. Tricine gel of 16.5% was used for the electrophoretic study. This gel did not allow high molecular weight proteins or peptides to pass through whereas lower molecular weight peptides/compounds passed through the gel which is visible as peptides on the lower part of lane 3 (Fig. 1). However, to know the characteristics of these peptides, the RP HPLC MS was carried out for further investigation.
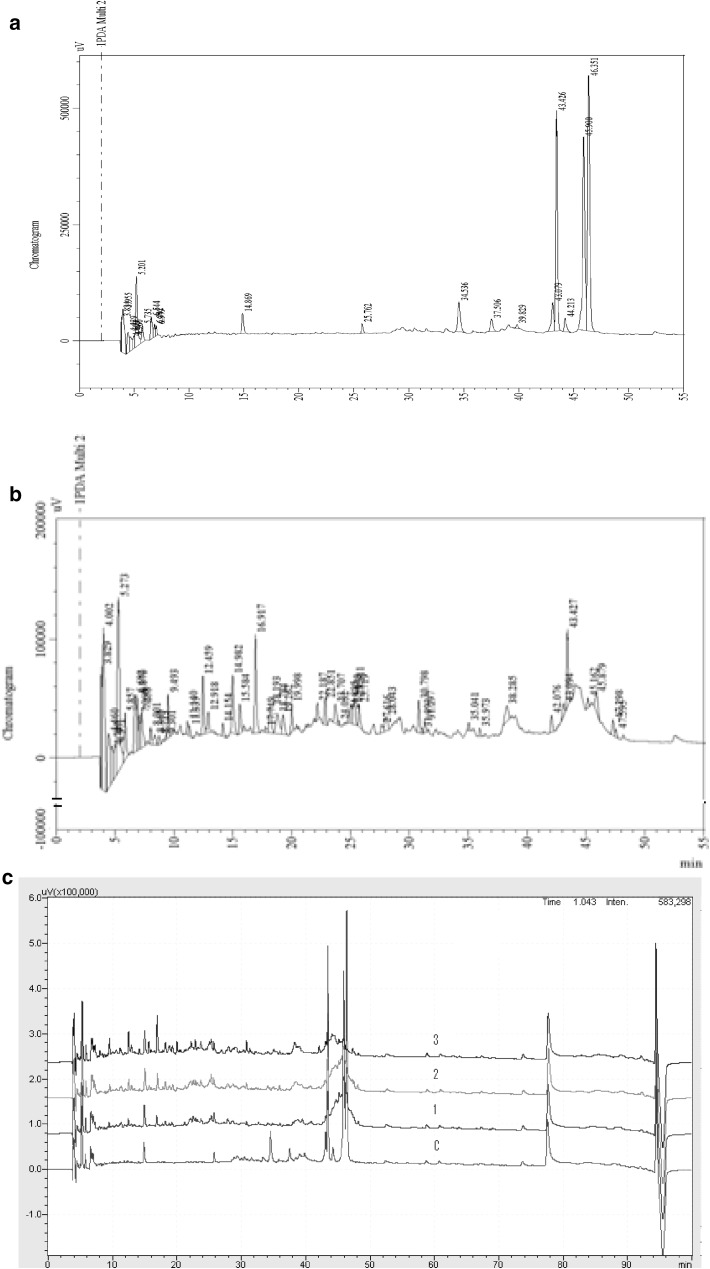
RP-HPLC/MS was performed to identify the peptides present in proteloyzed cheese whey. Figure 2a represents the presence of less number of lower molecular weight peptides in fresh pasteurized cheese whey as compared to the whey hydrolyzed for 30 min with 0.05% flavourzyme at 50 °C (Fig. 2b). The chromatogram of the hydrolyzed whey shows the presence of more number of lower molecular weight peptides during initial 30 min of retention time.
Fig. 2.
RP- HPLC MS chromatogram of whey and hydrolysed whey proteins. Detection was done at 214 nm. Initial 45 min fractions refer as peptides. a peptides profile in control whey, b peptides profile of the hydrolysate with 0.05% enzymes, c Comparison of four chromatograms c control whey, 1 hydrolysed with 0.01%, 2 hydrolysed with 0.02% 3 hydrolysed with 0.05%
Comparison of chromatogram in Fig. 2c showed that small peptides in all the samples of cheese whey eluted predominantly in the hydrophilic region of chromatogram within the 35 min of RP HPLC separation. This observation suggested that the higher amount of flavourzyme produced more number smaller peptides as compared to the use of lesser amount of enzyme at same conditions.
Whey protein hydrolysis was carried out for 30 min and a controlled hydrolysis was performed to liberate more number of small peptides (<10 kDa). On further hydrolysis, the hydrolysate became very bitter due to the breakdown of small peptides and production of more amino acids. Therefore, it is very important to know the degree of hydrolysis, time, enzymes etc. to obtain more number of small peptides with less bitterness. Whey proteins hydrolysate with more number of low molecular weight bioactive peptides have physiological effects in the human body i.e. on the nervous system via their opiate and ileum- contracting activities (Pihlanto-Leppala et al. 1997), nutrition system via their improved digestibility, antihypertensive activities and hypocholesterolemic effect (Madureira et al. 2010). A product with low content of amino acids is absorbed more efficiently in the gut because of intestinal absorption differences between peptides (primarily, di- and tripeptides) and frees amino acids (Boza et al. 2000 and Clemente 2000). Therefore, controlled higher enzymatic hydrolysis is necessary in order to obtain least amount free amino acids in the final product. Higher amount of enzyme (0.05%) has produced more number of smaller peptides within 30 min of hydrolysis (Fig. 2b or c3). A comparison for the presence of smaller peptides (<10 kDa) among the three hydrolysates indicated most number of smaller peptides in the sample hydrolyzed with 0.05% enzyme. Small peptides formation from whey protein and their properties have been studied by Pescuma et al. (2010). Formation of more peptides and role of amino acids in the hydrolysate on extensive hydrolysis have also been reported (Schmidt and Poll 1991). Extensive hydrolysis of whey protein isolate by enzyme was shown to induce gelation mainly via hydrophobic interaction (Doucet et al. 2003). With the increase in degree of hydrolysis, the stability and emulsifying properties also decreases (Mahmoud 1994).
Lactose estimation
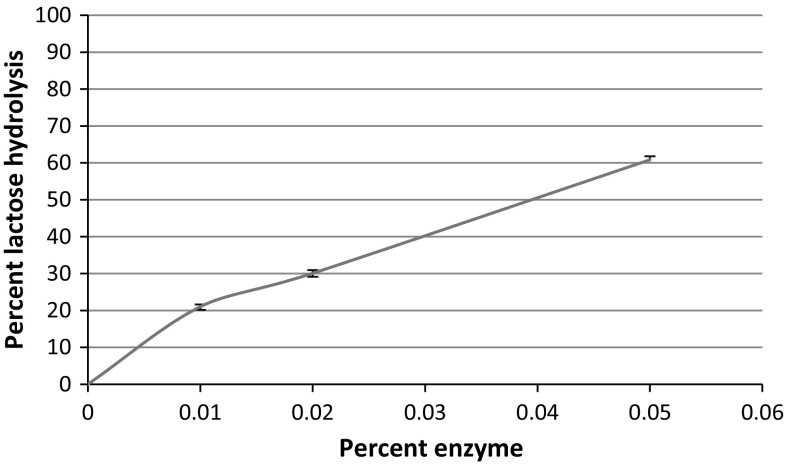
Lactose in proteolyzed whey was hydrolyzed using β-galactosidase enzyme. The addition of 0.01% enzyme resulted in hydrolysis of lactose to an extent of 21.18%, whereas 0.02 and 0.05% enzyme hydrolyzed 30.88 and 60.88% lactose respectively (Fig. 3).
Fig. 3.
Effect of enzyme concentration on the lactose hydrolysis
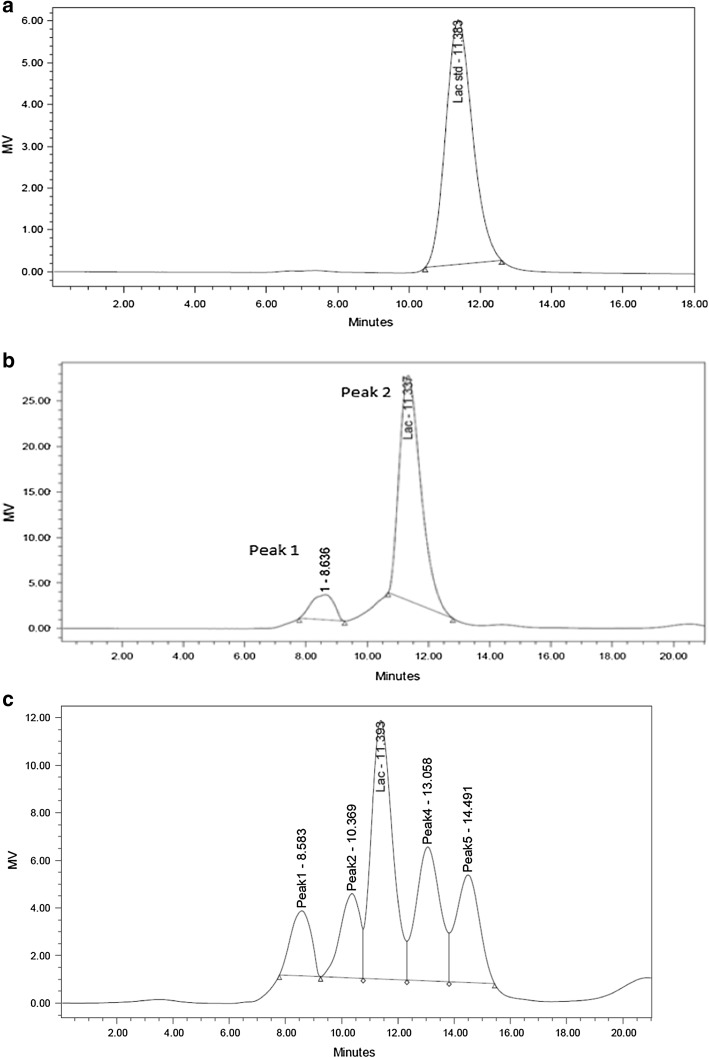
Typical chromatogram from pure lactose, whey and hydrolyzed whey are shown in Fig. 4a, b, c. The single peak in Fig. 4a of the chromatogram was the pure lactose peak containing 1 mg/ml lactose in it. The retention time was recorded for identification of lactose peak for subsequent chromatogram of whey and its hydrolysate. The area of lactose was measured using in- built software for quantification of lactose. The first peak of fresh whey chromatogram (peak 1 in Fig. 4b) was not identified. It may be an anion peak coming from salts in milk system (Richmond et al. 1982) or a trisaccharide (Bakken et al. 1992). Peak 2 indicates the presence of lactose in whey.
Fig. 4.
HPLC chromatogram of pure lactose, whey & hydrolysed whey. Y axis MV Response/Signal in milli Volt), a single peak for pure lactose, b two peaks for fresh cheese whey, c Five peaks for hydrolysed whey with 0.05% enzyme
As mentioned earlier, whey was treated with β-galactosidase and three additional peaks eluted on hydrolysis with 0.05% enzyme (Fig. 4c). Peak 2 was before lactose and peaks 4 and 5 was after the lactose peak. These are probably trisaccharide (peak 2) and disaccharides (peaks 4,5 glucose or galactose) obtained on hydrolysis of lactose (Jeon et al.1984). Decrease in peak area (peak 3) indicates the reduction of lactose and formation of glucose and galactose which was achieved between 12–16 min. It was also observed that lactose concentration reduced with the increase in enzyme concentration and height of both the peaks (4 and 5) increased. Appearance of five peaks on hydrolysis of lactose is in agreement with the observation of Asp et al. (1980). Our aim was to know the reduction in lactose content of whey due to controlled hydrolysis with different concentrations of enzyme and finally to establish rate of hydrolysis and pattern of other saccharide formation. With 0.05% of enzyme, lactose was rapidly hydrolyzed to 2.40% from an initial amount of 4.53% which is 53% lactose hydrolysis. Our observation indicated that rate of lactose hydrolysis is not truly proportional to the enzyme concentration. Hydrolysis of 22–30% lactose has been observed in several lactic fermented products (Alm 1982). Therefore, depending upon requirement, it is possible to calculate percent lactose hydrolysis to be obtained from the concentration of enzymes to develop health drinks. Enzymatic hydrolysis of lactose also is accompanied by galactosyl transfer to other sugars, thereby producing oligosaccharides. These are hydrolyzed slowly, both in vitro and in vivo. These are of low molecular weight, non-viscous and water-soluble dietary fiber. They are considered to be physiologically functional foods and promote the growth of bifidobacteria in the colon and a wide variety of health benefits has been claimed (Mahoney 1998).
Conclusion
Small peptides are known to provide physiological benefit and low lactose levels improve gastrointestinal characteristic in consumers. Hence, an attempt was made to enzymatically hydrolyze both proteins and lactose in whey, under controlled condition, to improve its biological value. This whey containing more number of smaller bioactive peptides and reduced lactose and its derivatives may be used for the preparation of health beverages with added sugar, flavour and colour to improve the palatability.
Acknowledgements
B.C.G. expresses sincere thanks to the Australian Government for awarding Australian Endeavour Executive Award during the stay at Melbourne, Australia to carry out the work.
References
- Adler-Nissen J. A review of food protein hydrolysis – specific areas. Enzymatic hydrolysis of food proteins. Amsterdam: Elsevier; 1986. p. 56. [Google Scholar]
- Alm L. Effect of fermentation on L(+) and D(−) lactic acid in milk. J Dairy Science. 1982;65:515–520. doi: 10.3168/jds.S0022-0302(82)82228-5. [DOI] [PubMed] [Google Scholar]
- Antila JH, Paakkari I, Jarvinen A, Mattila MJ, Laukkane M, Pihlanto-Leppala A, Mantsala P, Hellman J. Opioid peptides derived from in vitro proteolysis of bovine whey proteins. Int Dairy J. 1991;1:215–229. doi: 10.1016/0958-6946(91)90015-Z. [DOI] [Google Scholar]
- Asp NG, Burvall A, Dahlqvist A, Hallgren P, Lundblad A. Oligosaccharide formation during hydrolysis of lactose with Sacchromyces lactis lactase (Maxilact®): Part 2—Oligosaccharide structures. Food Chem. 1980;5(2):147–153. doi: 10.1016/0308-8146(80)90037-0. [DOI] [Google Scholar]
- Bakken AP, Hill CG, Jr, Amundson CH. Hydrolysis of lactose in skim milk by Immobilized β—Galactosidase (Bacillus circulans) Biotechnol Bioeng. 1992;39:408–417. doi: 10.1002/bit.260390407. [DOI] [PubMed] [Google Scholar]
- Biziulevicius GA, Kislukhina OV, Kazlauskaite J, Zukaite V. Food protein enzymatic hydrolsates possess bothantimicrobialand immunostimulatory activities: a “cause and effect”theory of bifunctionality. FEMS Immunol Med Microbiol. 2006;46:131–138. doi: 10.1111/j.1574-695X.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- Boza JJ, Moennoz D, Vuichoud J, Jarret AR, Gaudardde-Week D, Ballevre O. Protein hydrolysates vs free amino acid based diets on the nutritional recovery of te starved rat. Eur J Nutr. 2000;39:237–243. doi: 10.1007/s003940070001. [DOI] [PubMed] [Google Scholar]
- Cheison SC, Zhang SB, Wang Z, Xu SY. Comparison of a modified spectrophotometric and the pH-stat methods for determination of the degree of hydrolysis of whey proteins hydrolysed in a tangential-flow filter membrane reactor. Food Res Int. 2009;42:91–97. doi: 10.1016/j.foodres.2008.09.003. [DOI] [Google Scholar]
- Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci Technol. 2000;11:254–262. doi: 10.1016/S0924-2244(01)00007-3. [DOI] [Google Scholar]
- da Costa ELEL, Gontijob JAR, Nettoa FM. Effect of heat and enzymatic treatment on the antihypertensive activity of whey protein hydrolysate. Int Dairy J. 2007;17:632–640. doi: 10.1016/j.idairyj.2006.09.003. [DOI] [Google Scholar]
- Doucet D, Otter DF, Gauther SF, Foegeding EA. Enzyme based gelation of extensively hydrolysed whey proteins by alcalase: peptide identification and determination of enzyme specificity. J Agric Food Chem. 2003;51:6300–6308. doi: 10.1021/jf026242v. [DOI] [PubMed] [Google Scholar]
- Dryakova A, Pihlanto A, Marnila P, Curda L, Korhonen HJT. Antioxidant properties of whey protein hydrolysates as the measured by three methods. Eur Food Res Technol. 2010;230(6):865–874. doi: 10.1007/s00217-010-1231-9. [DOI] [Google Scholar]
- Frokjaer S. Use of hydrolysates for protein supplementation. Food Technol. 1994;10:86–88. [Google Scholar]
- Horton BS. Whey processing and utilization. Bull Int Dairy Fed. 1995;308:2–6. [Google Scholar]
- Jeon IJ, Mantha VR. High performance liquid chromatography analysis of oligosaccharides formed during β-galactosidase action on lactose. J Dairy Sci. 1985;68:581–588. doi: 10.3168/jds.S0022-0302(85)80861-4. [DOI] [Google Scholar]
- Jeon IJ, Galitzer SJ, Hennes KJ. Rapid determination of lactose and its hydrolysates in whey and whey permeate by high performance liquid chromatography. J Dairy Sci. 1984;67:884–887. doi: 10.3168/jds.S0022-0302(84)81382-X. [DOI] [Google Scholar]
- Lim SM, Jo MN, Lee NK, Yoon YC, Paik H. Antihypertensive effects and physiochemical characteristics onf non fat milk fortified with whey protein hydrolysates. Millchwissenchaft. 2012;67(3):304–307. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Madureira AR, Tavares AMP, Pintado ME, Malcata FX. Physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci. 2010;93(2):437–455. doi: 10.3168/jds.2009-2566. [DOI] [PubMed] [Google Scholar]
- Mahmoud MI. Physicochemical and functional properties of protein hydrolsates in nutritional products. Food Technol. 1994;48:89–99. [Google Scholar]
- Mahoney RR. Galactosyl-oligosaccharide formation during lactose hydrolysis: a review. Food Chem. 1998;63:147–154. doi: 10.1016/S0308-8146(98)00020-X. [DOI] [Google Scholar]
- Meisel H. Overview on milk protein-derived peptides. Int Dairy J. 1998;8:363–373. doi: 10.1016/S0958-6946(98)00059-4. [DOI] [Google Scholar]
- Nielsen PM. Functionality of proteins hydrolysates in Food proteins and their applications S. Damodaran and A. Paraf (eds) New York: Marcel Dekker; 1977. pp. 443–472. [Google Scholar]
- Otte J, Zakora M, Qvist KB, Olsen CE, Barkholt V. Hydroplysis of bovine β- lactoglobulin by various proteases and identification of selected peptides. Int Dairy J. 1997;7:835–848. doi: 10.1016/S0958-6946(98)00003-X. [DOI] [Google Scholar]
- Otte J, Lobolt SB, Halkier T, Qvist KB. Identification of peptides in aggregates formed during hydrolysis of β- lactoglobulin Bwith a Glu and Asp specific microbial protease. J Agric Food Chem. 2000;48:2443–2447. doi: 10.1021/jf990947o. [DOI] [PubMed] [Google Scholar]
- Peng X, Kong B, Xia X, Liu Q. Reducing and radical-scavenging activities of whey protein hydrolysates prepared with Alcalase. Int Dairy J. 2010;20:360–365. doi: 10.1016/j.idairyj.2009.11.019. [DOI] [Google Scholar]
- Pescuma M, Hebert EM, Mozzi F, de Valdez GF. Functional fermented whey—based beverage using lactic acid bacteria. Int J Food Micro. 2010;14:73–81. doi: 10.1016/j.ijfoodmicro.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Pihlanto-Leppala A. Bioactve peptides derived from bovine whey proteins: opioid and ace- inhibitory peptides. Trends Food Sci Technol. 2001;11:347–356. doi: 10.1016/S0924-2244(01)00003-6. [DOI] [Google Scholar]
- Pihlanto-Leppala A, Paakkari M, Rinnta-Koski M, Antila P. Bioactive peptide derived from in vitro proteolysis of bovine β-lactoglobulin and its effect on smooth muscle. J Dairy Res. 1997;64:149–155. doi: 10.1017/S0022029996001926. [DOI] [PubMed] [Google Scholar]
- Pihlanto-Leppala A, Koskinen P, Piilola K, Tupasela T, Korhonen H. Angiotension I—converting enzyme inhibitory properties of whey protein digest: concentration and characterization of active peptides. J Dairy Res. 2000;67:53–64. doi: 10.1017/S0022029999003982. [DOI] [PubMed] [Google Scholar]
- Richmond ML, Brafuss DL, Harte BR, Gray JI, Stine CM. Separation of carbohydrates in dairy products by high performance liquid chromatography. J Dairy Sci. 1982;65:1394–1400. doi: 10.3168/jds.S0022-0302(82)82360-6. [DOI] [Google Scholar]
- Sadat L, Cakir-Kiefer C, Andree N’Negue M, Luc Gailard J, Girardet JM, Miclo L. Isolation and Identification of antioxidative peptides from bovine α- lactalbumin. Int Dairy J. 2011;21:214–221. doi: 10.1016/j.idairyj.2010.11.011. [DOI] [Google Scholar]
- Schagger H, Jagow GV. Tricine –sodium dodecyl sulfate polyacrylamide gel electrophoresisfor the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–375. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schmidt DG, Poll JK. Enzymatic hydrolysis of whey proteins. Hydrolysis of α -lactalbumin and β- lactoglobulin in buffer solution by proteolytic enzymes. Neth Milk Dairy J. 1991;45:225–240. [Google Scholar]
- Schmidt DG, Meijer RJ, Slangen CJ, van Beresteijn EC. Raising the pH of the pepsin-catalysed hydrolysis of bovine whey proteins increases the antigenicity of the hydrolysates. Clin Exp Allergy. 1995;25(10):1007–1017. doi: 10.1111/j.1365-2222.1995.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Tsakali E, Petrotos KD, Alessandro AG, Goulas P (2010) A review on whey composition and the methods used for its utilization for food and pharmaceutical products. In: 6th International conference on simulation and modelling in the food and bio-industry FOODSIM 2010, CIMO Research Centre, Braganca, Portugal, 24–26 June. www.dairyforall.com
- Wang X, Wang L, Cheng X, Zhou J, Tang X, Mao XY. Hypertension –attenuating effect of whey protein hydrolysate on spontaneously hypertensive rats. Food Chem. 2012;134:122–126. doi: 10.1016/j.foodchem.2012.02.074. [DOI] [Google Scholar]