Abstract
The effect of variety and ripening stage on the distribution of phenolic compounds and in vitro antioxidant capacity of Gala, Fuji Suprema and Eva apples were evaluated. Hydroxycinnamic acids, flavonoids, flavanols, flavonols, dihydrochalcones and antioxidant activity (FRAP and DPPH) were assessed in the epicarp, mesocarp and endocarp of three varieties at three ripening stages (unripe, ripe and senescent). The Fuji Suprema variety distinguished by its content of flavonols at senescent stage, while Eva variety distinguished by its content of dihydrochalcones (unripe stage) and anthocyanins (ripe stage). In general, phenolic acids and flavonoids decreased with ripening in the epicarp and endocarp. However, in the mesocarp, the effect of ripening was related with the apple variety. Hierarchical cluster analysis confirmed the influence of ripening in the apple tissue. The evolution of these compounds during ripening occurred irregularly and it was influenced by the variety.
Keywords: Malus domestica Borkh, Eva, Gala, Fuji Suprema, Ripening, Phenolic profile
Introduction
Apples (Malus domestica Borkh) contain significant quantities of phenolic compounds, which are responsible for various sensory attributes of fruits and their products, such as color, bitterness and astringency (Khanizadeh et al. 2008; Alberti et al. 2016). Furthermore, these compounds have antioxidant activity and are related to health benefits, such as reducing the risk of cardiovascular disease, lung cancer, asthma and diabetes (Babu et al. 2013; Yao et al. 2004; Tsao et al. 2005).
Phenolic compounds are products of the secondary metabolism of plants and they play an important role in the growth and reproduction of plants (by attracting pollinators) and providing protection against pathogens (antimicrobial activities). They can be synthesized in response to adverse conditions such as infection, injury, and UV irradiation (Ignat et al. 2011; Karaman et al. 2010; Duda-Chodak et al. 2011). These compounds are stored in vacuoles and cell walls after polymerization or conjugation with sugars or organic acids (Bidel et al. 2011). In the fruit, the phenolic compounds are formed during growth until the full maturity stage (Zhang et al. 2010) and they are distributed in various tissues and fractions (epicarp, mesocarp, endocarp and seeds). However, the compounds and their concentration may vary depending on the type of fruit and the variety (Le Bourvellec et al. 2015; Guo et al. 2013).
Studies have demonstrated the distribution of phenolic compounds in ripe apples (Kalinowska et al 2014; Tsao et al. 2003). Usually, epicarp is richer in phenolic compounds than the other apple tissues. Some groups of flavonoids are almost exclusively found in epicarp such as anthocyanins and flavonols (glycosides of quercetin). In other hand, monomeric and polymeric flavan-3-ols are the major phenolics of epicarp and mesocarp and can represents about 60% of the total phenolic compounds in apples. Phloridzin (dihydrochalcone) that can be used as a biomarker of apple products, are distributed in epicarp and mesocarp, as well as the hydroxycinnamic acids.
However, knowledge about the distribution and evolution of phenolic compounds during ripening stages of apples can be helpful for consumers and the food industry in order to obtain high content of bioactive compounds. Thus, this study was intended to evaluate the effect of the variety and ripening stage on the distribution of phenolic compounds and the antioxidant capacity in apples.
Materials and methods
Materials
Apples from the Fuji Suprema variety were collected from the EPAGRI experimental station (Agricultural Research and Rural Extension of Santa Catarina), Caçador, Santa Catarina, Brazil (26°50′12″S, 50°58′23″O), while the Gala and Eva varieties were obtained from Boutin Agrícola, Porto Amazonas, Paraná, Brazil (25°32′08″S, 49°53′33″O). The fruits were collected (20 kg) in different cardinal points, at the top, middle and bottom from six trees at three ripening stages (unripe, ripe and senescent). The ripening index was determined by using the starch-iodine test (Blanpied and Silsby 1992). The iodine values for the fruits of all the varieties were 1 for unripe, 4–5 for ripe, and 8 for senescent.
Liquid nitrogen (99%) was produced with StirLIN-1 (Stirling Cryogenics, Dwarka, New Delhi, India). Folin-Ciocalteau reagent, TPTZ (2,4,6-Tri (2-pyridyl)-s-triazine), DPPH (2,2-diphenyl-2-picrylhydrazyl), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 5-caffeoylquinic acid, caffeic acid, gallic acid, p-coumaric acid, (+)-catechin, (–)-epicatechin, procyanidin B1, procyanidin B2, phloridzin, phloretin, quercetin, quercetin-3-d-galactoside, quercetin-3-β-d-glucoside and quercetin-3-rutinoside were purchased from Sigma–Aldrich (St. Louis, MO., USA). Acetonitrile (99.9%), acetic acid (99.9%), methanol (99.9%) and acetone (99.5%) were purchased from J. T. Baker (Phillipsburg, NJ, USA). Sodium nitrite and aluminum chloride were purchased from Vetec (Rio de Janeiro, RJ, Brazil) and Fluka (St. Louis, MO., USA), respectively.
Methods
Extraction of phenolic compounds
The tissues of fruit epicarp (1–2 mm outside of the fruit), mesocarp and endocarp (cylinder of the central part of the fruit with 20 mm diameter, without seeds) of each variety were mechanically removed and sprayed with an aqueous solution of cysteine (2.0 mmol/L) to inhibit oxidation reactions (Zardo et al. 2013). The samples were freeze-dried (LS 3000, São Paulo, SP, Brazil), and homogenized in a mortar. Two grams of the samples (epicarp, mesocarp and endocarp) were extracted with 30 mL of 85% methanol for 15 min at 28 °C (twice), followed by 65% acetone for 20 min at 10 °C (twice). The mixture was centrifuged (8160×g, 20 min at 4 °C) (Hitache Koki Co., Hitachi, Ibaraki, Japan), concentrated by evaporation under vacuum (40 °C, −600 mmHg) (Tecnal TE-211, Piracicaba, SP, Brazil), and freeze-dried. A solution (2 mL) of 2.5% acetic acid and methanol (3:1, v/v) was used to reconstitute the samples.
Analysis
Total phenolic compounds, flavonoids and flavanols
The content of phenolic compounds was determined by colorimetric analysis using the Folin–Ciocalteau method (Singleton and Rossi 1965). The results were expressed as milligrams of 5-caffeoylquinic acid equivalents per kilogram of the tissue of apple using a calibration curve (phenolic compounds content = 1470.4 × absorbance; R2 = 0.997; p < 0.001). The total flavonoid content was determined according to the method described by Zhishen et al. (1999). The results were expressed as milligrams of catechin equivalents using a calibration curve (flavonoid concentration = 399.4 × absorbance; R2 = 0.999; p < 0.001). The vanillin method was performed to determine the flavanols (Broadhurst and Jones 1978). The results were expressed in catechin equivalents (flavanols concentration = 351.4 × absorbance; R2 = 0.7 p < 0.001).
HPLC analysis of phenolic compounds
The analysis of individual phenolic compounds was performed according to Alberti et al. (2014). The chromatographic system used was a 2695 Alliance (Waters, Milford, MA, USA), with photodiode array detector PDA 2998 (Waters), quaternary pump and auto sampler. Separation was performed on a Symmetry C18 column (4.6 × 150 mm, 3.5 µm; Waters) at 20 °C. The identification and quantification of the phenolic compounds was performed by comparison of the spectra and retention times of the standards. When commercial standards were not available, the quantification was performed from compounds belonging to the same class of phenolic compounds, as verified after the fractionation of the samples (Jaworski and Lee 1987; Alberti et al. 2016). The total peak area (obtained by HPLC analysis) was summed for each phenolic class and quantified as the phloridzin, 5-caffeoylquinic acid and quercetin 3-β-d-glucoside equivalents for dihydrochalcones, hydroxycinnamic acids and flavonols, respectively.
Antioxidant capacity
The total antioxidant capacity of the extracts was determined using the ferric reduction antioxidant power (FRAP) method as described by Benzie and Strain (1996) and by DPPH according to the Brand-Williams et al. (1995) method. Measurements were performed using a microplate reader (Epoch microplate spectrophotometer, Synergy-BIOTEK, Winooski, VT, USA) and the results were expressed as Trolox equivalents per kilogram of apple (μmol TE /kg).
Statistical analysis
The data were presented as mean and pooled standard deviation (PSD). Hierarchical cluster analysis (HCA) was performed to assess the similarities between the fruit tissues (epicarp, mesocarp and endocarp) according to the phenolic composition and antioxidant capacity. Hartley’s test was performed to check for homogeneity of variances, and one-way ANOVA and Fisher’s LSD were performed to verify the differences between the groups. The p values below 0.05 were used to reject the null hypothesis. All the statistical analyses were performed using Statistica 7.0 software (Statsoft Inc., Tulsa, OK, USA).
Results and Discussion
The content of phenolic compounds was different between the analyzed varieties. Eva had the lowest levels of phenolic compounds, however, the highest level of phenolics were found in both Gala and Fuji Suprema depending on the type of tissues and the ripening stage (Table 1). Other studies have reported the effect of the variety on the concentration of phenolics in apples (Guo et al. 2013; Huber and Rupasinghe 2009), mainly between apples for fresh consumption and industrial apples; the latter usually have higher concentrations of phenols and are classified as astringent and/or bitter (Sanoner et al. 1999; Tsao et al. 2005).
Table 1.
Phenolic classes (mg/kg) and antioxidant capacity (μmol TE/kg) of apple (epicarp, mesocarp e endocarp) at different ripening stages
| Cv. | Ripening stage | Tissue | TPCA | Phenolic classes | Antioxidant capacity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCAB | Flavonoids | Flavanols | Flavonols | DHCC | Anthocyanins | FRAP | DPPH | ||||
| Eva | Unripe | Epicarp | 2252.71h | 47.05q | 1673.71h | 700.70h | 279.58i | 576.22a | 0.00 | 4593.81l | 5425.10i |
| Mesocarp | 232.79x | 12.58y | 102.03t | 13.12y | 5.39l | 6.47v | 0.00 | 895.78y | 805.63v | ||
| Endocarp | 675.69r | 73.77o | 416.58n | 128.51q | 0.00 | 187.89e | 0.00 | 1783.69v | 2044.65r | ||
| Ripe | Epicarp | 2880.93f | 28.34v | 1794.47g | 970.89g | 388.48f | 364.00c | 114.83a | 7555.46g | 6684.55f | |
| Mesocarp | 644.28r | 24.19w | 313.94q | 95.09t | 9.64k | 13.94s | 0.00 | 2245.74s | 1608.13t | ||
| Endocarp | 595.89s | 71.67o | 330.58q | 78.58u | 0.00 | 84.14n | 0.00 | 521.64α | 2168.04q | ||
| Senescent | Epicarp | 1960.41i | 33.59u | 1588.63i | 571.29i | 351.79h | 531.51b | 92.94b | 4633.27k | 5460.28i | |
| Mesocarp | 390.68v | 17.31x | 182.27r | 16.37x | 9.32k | 18.59r | 0.00 | 612.95z | 1034.92u | ||
| Endocarp | 723.03q | 77.75n | 319.52q | 42.16v | 0.00 | 45.86q | 0.00 | 2094.02t | 3177.85n | ||
| Gala | Unripe | Epicarp | 4440.80c | 170.30d | 3068.71d | 1430.40e | 477.68e | 212.45d | 11.13h | 16,021.03b | 8266.02c |
| Mesocarp | 1069.32k | 135.98f | 848.04j | 337.92k | 0.00 | 18.58r | 0.00 | 3383.03n | 3231.06m | ||
| Endocarp | 1540.59j | 268.22a | 729.58 k | 443.89j | 0.00 | 177.30f | 0.00 | 6374.95h | 5646.35h | ||
| Ripe | Epicarp | 4648.80b | 158.59e | 3893.57b | 1942.91b | 645.64d | 152.96j | 51.68d | 14,263.08c | 7267.95e | |
| Mesocarp | 600.57s | 110.04h | 446.15m | 193.53o | 0.00 | 17.23r | 0.00 | 2321.73r | 2887.32o | ||
| Endocarp | 1149.71k | 240.44b | 383.37p | 218.64l | 0.00 | 158.71h | 0.00 | 5130.94i | 4399.81j | ||
| Senescent | Epicarp | 3287.95e | 102.28j | 3117.28c | 1823.60c | 353.92g | 154.71i | 55.70c | 11,430.95d | 7548.54d | |
| Mesocarp | 759.36p | 80.00m | 330.21q | 201.38n | 0.00 | 11.46t | 0.00 | 2467.45q | 2889.28o | ||
| Endocarp | 513.89t | 105.03i | 392.67op | 108.67s | 0.00 | 68.19p | 0.00 | 1191.05w | 1801.12s | ||
| Fuji Suprema | Unripe | Epicarp | 5194.08a | 157.39e | 4742.57a | 2670.57a | 750.37c | 159.00h | 33.55g | 18,882.17a | 9034.43a |
| Mesocarp | 453.85u | 54.06p | 151.72s | 26.53w | 0.00 | 5.34u | 0.00 | 1149.52x | 1600.57t | ||
| Endocarp | 978.84m | 203.84c | 459.72m | 166.81p | 0.00 | 170.65g | 0.00 | 3301.79o | 3966.63k | ||
| Ripe | Epicarp | 2538.97g | 96.65k | 2452.56f | 1173.27f | 810.32b | 80.97o | 36.16f | 8677.43f | 6538.05g | |
| Mesocarp | 672.55r | 54.55p | 319.71q | 108.69s | 0.00 | 13.32s | 0.00 | 2002.22u | 2452.36p | ||
| Endocarp | 909.46n | 121.20g | 601.64l | 200.63n | 0.00 | 93.63l | 0.00 | 5055.57j | 2848.30o | ||
| Senescent | Epicarp | 3919.90d | 112.03h | 3008.86e | 1526.51d | 1071.92a | 113.06k | 40.98e | 10,324.17e | 8396.32b | |
| Mesocarp | 804.87o | 84.28l | 456.63m | 210.47m | 26.42j | 19.38r | 0.00 | 2688.97p | 3461.68l | ||
| Endocarp | 1035.62l | 123.45g | 410.39no | 118.40r | 0.00 | 93.07m | 0.00 | 3549.57m | 3470.80l | ||
| PSD1 | 1445,67 | 65,11 | 1294.13 | 708.04 | 292.51 | 146.65 | 23.47 | 486.27 | 2418.48 | ||
| p (Hartley)2 | 1,0 | 1,0 | 0.71 | 1.0 | 1.0 | 0.79 | 0.70 | 1.0 | 1.0 | ||
| p (ANOVA)3 | <0,001 | <0,001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
Values expressed as mean (n = 3) in wet basis
A TPC Total Phenolic compounds, B HCA hydroxycinnamic acids, C DHC dihydrochalcones, D PSD pooled standard deviation, E probability values obtained by Hartley test (F max) for homogeneity of variances, F probability values obtained by One-way ANOVA
Different letters in the same column represent statistical different results according to the Fischer LSD test (p ≤ 0.05)
The epicarp of the apples showed higher concentration of phenolic compounds (up to 11 times) followed by the endocarp and mesocarp (Table 1). The same occurred for total flavonoids and, consequently, for the antioxidant capacity. Flavanols were found mostly in the epicarp of fruits; they include catechin, epicatechin and procyanidins (Table 2). Catechin was identified in the three ripening stages only in the Gala variety, where a reduction in senescence (about 50% in epicarp and 33% in mesocarp) occurred. For the Eva and Fuji Suprema apples, catechin was only found in the epicarp of senescent and ripe fruits, respectively. Epicatechin was only found in the epicarp of Gala and Eva apples, and a reduction concomitant with ripening (27 and 63%, respectively) was observed. In the Fuji Suprema variety, the reduction in epicatechin was less significant (7%), and an increase of 400% in the content of this compound in the mesocarp was observed (Table 2). Procyanidin B2 showed the same behavior as epicatechin in relation to the varieties; however, procyanidin B1 was only quantified in the epicarp of senescent fruits, especially in the Eva apples, with a content of 400 mg/kg. The procyanidin B1 was only found in senescent fruits, possibly due to the disruption of the interflavan linkages of the procyanidins at a higher degree of polymerization due to the ripening process (Alonso-Salces et al. 2004). In addition to the high antioxidant activity, procyanidins are related to the sensory characteristics of fruits, such as bitterness and astringency. The higher the degree of polymerization of procyanidins the greater its contribution to an astringent taste (Vidal et al. 2003).
Table 2.
Phenolic composition (mg/kg) of apple (epicarp, mesocarp e endocarp) at different ripening stages
| Cv. | Ripening stage | Tissue | Phenolic compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CQA | PLZ | EPI | CAT | PB1 | PB2 | QGA | QGL | QRH | QRU | |||
| Eva | Unripe | Epicarp | 0.0 | 375.6a | 191.2g | 0.0 | 0.0 | 140.2e | 12.9g | 10.3i | 38.5g | 1.4g |
| Mesocarp | 0.0 | 4.5s | 0.0 | 0.0 | 3.5e | 2.9l | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Endocarp | 27.3l | 130.8d | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Ripe | Epicarp | 0.0 | 276.0c | 0.0 | 0.0 | 0.0 | 0.0 | 90.4d | 59.9e | 68.8e | 23.2b | |
| Mesocarp | 3.6r | 10.5p | 0.5m | 0.0 | 1.9f | 0.0 | 0.7i | 0.0 | 1.1f | 0.0 | ||
| Endocarp | 22.4m | 60.7j | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Senescent | Epicarp | 5.5q | 348.4b | 70.5h | 6.7g | 401.0a | 57.8f | 65.1f | 42.3h | 35.0h | 8.7d | |
| Mesocarp | 4.0qr | 7.0q | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Endocarp | 3.8r | 32.5n | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Gala | Unripe | Epicarp | 65.5e | 81.3 h | 327.1a | 38.5a | 0.0 | 248.7a | 82.8e | 51.9f | 107.6c | 7.1e |
| Mesocarp | 48.1h | 7.4q | 10.2k | 15.8e | 0.0 | 29.2j | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Endocarp | 90.9a | 112.3f | 0.0 | 0.0 | 0.0 | 36.8i | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Ripe | Epicarp | 78.6c | 57.3k | 268.6b | 29.7b | 0.0 | 222.4c | 122.4b | 88.1b | 114.5b | 16.8c | |
| Mesocarp | 34.4k | 5.3r | 0.0 | 10.6f | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Endocarp | 84.3b | 98.9g | 0.0 | 0.0 | 0.0 | 30.8i | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Senescent | Epicarp | 63.2e | 57.3k | 236.9e | 19.4d | 69.8c | 200.1d | 66.1f | 44.0g | 59.9f | 5.4f | |
| Mesocarp | 18.9n | 3.6t | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Endocarp | 38.2j | 35.6m | 13.5j | 0.0 | 0.0 | 25.9j | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Fuji Suprema | Unripe | Epicarp | 69.3d | 119.8e | 261.6c | 0.0 | 0.0 | 247.3a | 137.7a | 92.2a | 144.9a | 16.9c |
| Mesocarp | 3.6r | 4.1s | 4.5l | 0.0 | 0.0 | 12.4k | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Endocarp | 44.9i | 129.7d | 14.7j | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Ripe | Epicarp | 83.5b | 55.4l | 226.2f | 23.1c | 0.0 | 201.4d | 124.3b | 79.1c | 103.5d | 15.1c | |
| Mesocarp | 11.0p | 9.3p | 13.3j | 0.0 | 0.0 | 35.7i | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Endocarp | 58.7f | 82.9h | 0.0 | 0.0 | 0.0 | 41.3h | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Senescent | Epicarp | 85.4b | 98.1g | 243.5d | 0.0 | 161.8b | 240.0b | 118.9c | 64.0d | 68.2e | 25.0a | |
| Mesocarp | 15.2o | 12.3o | 18.5i | 0.0 | 0.0 | 37.9i | 4.9h | 1.5j | 6.1i | 0.0 | ||
| Endocarp | 52.3g | 69.4i | 0.0 | 2.7h | 11.0d | 45.7g | 0.0 | 0.0 | 0.0 | 0.0 | ||
| PSDA | 33.2 | 98.7 | 110.0 | 10.4 | 89.0 | 90.1 | 48.2 | 31.3 | 43.9 | 9.7 | ||
| p (Hartley)B | 1.0 | 0.8 | 0.9 | 1.0 | 1.00 | 0.80 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| p (ANOVA)C | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
CQA 5-caffeoylquinic acid, PLZ phloridzin, EPI epicatechin, CAT catechin, PB1 procyanidin B1, PB2 procyanidin B2, QGA quercetin-3-d-galactoside, QGL quercetin-3-β-d-glucoside, QRH quercetin-3-o-rhamnoside, QRU quercetin-3-rutinoside, A PSD pooled standard deviation, B probability values obtained by Hartley test (F max) for homogeneity of variances, C probability values obtained by one-way ANOVA
Different letters in the same column represent statistical different results according to the Fischer LSD test (p ≤ 0.05)
Flavonols were found almost exclusively in the epicarp of the fruits, and are related to the adaptation process, which exists in fruit in order to avoid damage caused by UV-B irradiation (Khanizadeh et al. 2008; Solovchenko and Merzlyak 2008). The Fuji Suprema apples had a high content of total flavonols, especially glycosides of quercetin. In this variety, the total flavonols and quercetin-3-rutinoside content increased with ripening; however, the levels of quercetin 3-β-d-glucoside, quercetin-3-d-galactoside and quercetin 3-O-rhamnoside decreased. In Gala and Eva varieties, the quercetin glycosides, as well as the total flavonols content, increased from the unripe to the ripe stages and decreased with senescence.
There was a reduction in the concentration of the dihydrochalcones in the epicarp and endocarp of the three varieties in line with the senescence of the fruits. However, an increase was observed in the mesocarp of the Fuji Suprema and Eva varieties. Phloridzin, which is a dihydrochalcone, is found in large quantities only in apples (Gosch et al. 2010). This compound is related to the prevention of diseases such as diabetes (Masumoto et al. 2009) and neurological diseases, as well as being used as an additive in foods and beverages (Guyot et al. 2007). The Eva variety had high levels of phloridzin in the epicarp, which decreased (7%) with ripening. In the Gala and Fuji Suprema varieties, this compound was predominant in the endocarp in the unripe and ripe stages. In the senescent fruits, due to the reduction in the levels in the endocarp (70 and 48%, respectively), the highest concentration was in the epicarp (Table 2).
Anthocyanins were found exclusively in the epicarp of the fruits and their behavior in relation to maturation varied according to the variety. Ripe Eva apples showed the highest content of anthocyanins with a reduction in senescence. This was also observed in the Gala variety, although this compound was identified in the unripe fruit. In the Fuji Suprema apples, the highest content was found in the unripe samples (Table 1), probably because this variety displayed a red color from the fruit growth phase (Zielinski et al. 2014).
Unlike flavonoids, hydroxycinnamic acids were found predominantly in the endocarp of the fruit. In the Gala and Fuji Suprema varieties, the phenolic acid content decreased with ripening, while in the Eva variety it remained stable from the unripe to ripe stages and then increased with senescence. 5-caffeoylquinic acid, which is the main phenolic acid in apples (Tsao et al. 2005), was found in the highest concentrations in the endocarp of the unripe and ripe Eva and Gala varieties. At senescence, the levels decreased (86 and 58%, respectively) in the endocarp, and thus there were higher concentrations of 5-caffeoylquinic acid in the epicarp. In the Fuji Suprema variety, hydroxycinnamic acid was mainly present in the epicarp and its contents, as well as in other tissues; the levels increased with the ripening of the fruit (Table 2).
The antioxidant capacity are strong correlated with total flavonoids (FRAP: r = 0.95; DPPH, r = 0.91) of apple tissues. The highest positive correlation of epicatechin (FRAP: r = 0.87; DPPH, r = 0.84), procyanidin B2 (FRAP: r = 0.89; DPPH, r = 0.86) and quercetin-3-o-rhamnoside (FRAP: r = 0.92; DPPH, r = 0.87) suggests that they are major contributors to the antioxidant activity of the apple epicarp. According Firuzi et al. (2005), the o-dihydroxy structure in the B ring, the 2,3-double bond and the 3-hydroxy group in the C ring, contribute to antioxidant activity. Although phloridzin was found in all apple tissues at all ripening stages, there was weak correlation of DPPH (r = 0.42) and no significant correlation (p > 0.05) with FRAP method, as previously reported by Tsao et al. (2005). Differences in antioxidant activity results occurs due to the different mechanisms of FRAP and DPPH assays. DPPH is a stable free radical that when mixed with the antioxidant compound that can transfer a hydrogen atom or eletrons, is converted in its reduced form. On the other hand, FRAP is characterized by electron transfer ability, that result in the reduction of Fe (III) to Fe (II) by antioxidants (APAK et al. 2016).
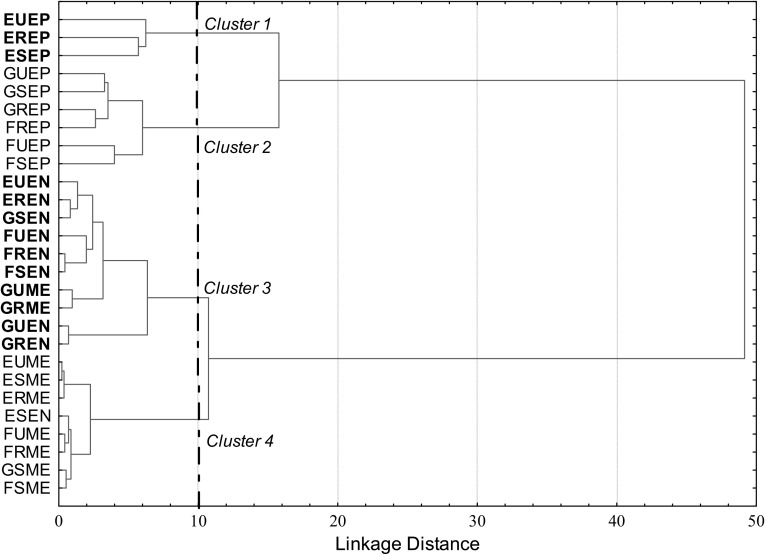
Hierarchical cluster analysis (HCA) was applied to analyze all the data simultaneously and to evaluate the distribution of phenolic compounds in the apples, as well as the influence of variety and the ripening stages. Consequently, it was possible to separate the samples into four clusters, as shown in Fig. 1.
Fig. 1.
Dendrogram for apple tissues at different ripening stages obtained from hierarchical cluster analysis. EUEP Epicarp of unripe Eva apple, EREP epicarp of ripe Eva apple, ESEP epicarp of senescent Eva apple, GUEP epicarp of unripe Gala apple, GREP epicarp of ripe Gala apple, GSEP epicarp of senescent Gala apple, FUEP epicarp of unripe Fuji Suprema apple, FREP epicarp of ripe Fuji Suprema apple, FSEP epicarp of senescent Fuji Suprema apple, EUEN endocarp of unripe Eva apple, EREN endocarp of ripe Eva apple, ESEN endocarp of senescent Eva apple, GUEN endocarp of unripe Gala apple, GREN endocarp of ripe Gala apple, GSEN endocarp of senescent Gala apple, FUEN endocarp of unripe Fuji Suprema apple, FREN endocarp of ripe Fuji Suprema apple, FSEN endocarp of senescent Fuji Suprema apple, EUME mesocarp of unripe Eva apple, ERME mesocarp of ripe Eva apple, ESME mesocarp of senescent Eva apple, GUME mesocarp of unripe Gala apple, GRME mesocarp of ripe Gala apple, GSME mesocarp of senescent Gala apple, FUME mesocarp of unripe Fuji Suprema apple, FRME mesocarp of ripe Fuji Suprema apple, FSME mesocarp of senescent Fuji Suprema apple
Cluster 1 consisted of the samples of Eva epicarp at the three analyzed ripening stages. The higher content of procyanidin B1, phloridzin, total dihydrochalcones and anthocyanins distinguished Cluster 1 from Cluster 2, which was formed by the epicarp of the Gala and Fuji Suprema varieties (Table 3).
Table 3.
Phenolic compounds (mg/kg) of the clusters of apple tissues according to HCA
| Phenolic compounds | Cluster 1 (n = 3) | Cluster 2 (n = 6) | Cluster 3 (n = 10) | Cluster 4 (n = 8) | PSDA | p (ANOVA)B |
|---|---|---|---|---|---|---|
| 5-Caffeoylquinic acid | 1.8c | 74.3a | 50.2b | 7.5c | 21.7 | <0.001 |
| Total hydroxycinnamic acid | 36.3b | 132.9a | 145.4a | 50.6b | 65.7 | <0.001 |
| Catechin | 2.2b | 18.4a | 2.9b | 0.0b | 8.5 | <0.01 |
| Epicatechin | 87.2b | 260.7a | 3.8c | 4.6c | 81.4 | <0.001 |
| Procyanidin B1 | 133.7a | 38.6ab | 1.1b | 0.7b | 82.4 | 0.05 |
| Procyanidin B2 | 66.0b | 226.6a | 21.0c | 11.1c | 91.2 | <0.001 |
| Total Flavanols | 747.6b | 1761.2a | 199.5c | 89.2c | 719.1 | <0.001 |
| Quercetin-3-d-galactoside | 56.1b | 108.7a | 0.0c | 0.7c | 38.9 | <0.001 |
| Quercetin-3-β-d-glucoside | 37.5b | 59.9a | 0.0c | 0.2c | 31.7 | <0.001 |
| Quercetin-3-o-rhamnoside | 47.4b | 99.8a | 0.0c | 0.9c | 34.5 | <0.001 |
| Quercetin-3-rutinoside | 11.1a | 14.4a | 0.0b | 0.0b | 4.4 | <0.001 |
| Total Flavonols | 339.9b | 685.0a | 0.0c | 6.3c | 310.0 | <0.001 |
| Phloridzin | 333.3a | 78.2b | 73.3b | 10.5c | 70.0 | <0.001 |
| Total Dihydrochalcones | 490.6a | 145.5b | 106.9b | 16.8c | 148.3 | <0.001 |
| Total Anthocyanins | 69.2a | 38.2b | 0.0c | 0.0c | 0.0 | <0.001 |
| Total Flavonoids | 1685.6b | 3380.6a | 501.9c | 272.0c | 1310.6 | <0.001 |
| Total Phenolic compounds | 2364.7b | 4005.1a | 907.0c | 585.2c | 1464.0 | <0.001 |
A PSD Pooled standard deviation, B probability values obtained by One-way ANOVA
Different letters in the same line represent statistical different results according to the Fischer LSD test (p ≤ 0.05)
Cluster 3 contained the samples of endocarp of the three varieties and the mesocarp of unripe and ripe Gala, while Cluster 4 consisted of the mesocarp of the three varieties and the endocarp of senescent Eva apples. The levels of 5-caffeoylquinic acid, hydroxycinnamic acid, phloridzin and total dihydrochalcones, which were mainly located in the endocarp, differentiated these two groups (Table 3). The presence of the samples of Gala mesocarp in the cluster formed mainly by fruit endocarp, and the endocarp of senescent Eva in the cluster formed by mesocarps shows the influence of the ripening stage in the content of phenolic compounds in apples.
The epicarp, mesocarp and endocarp differed qualitatively and quantitatively in terms of their phenolic composition. The epicarp had a higher content of phenolic compounds in all the analyzed classes (approximately 55%), except for phenolic acids, which were concentrated in the endocarp and mesocarp (around 42%). According to Zardo et al. (2013), the epicarp and mesocarp correspond to 7–10% and 73–83% of the weight of apples, respectively. However, compounds such as flavonols and anthocyanins are only present in the epicarp, indicating that consumption of apple skin is beneficial from a functional point of view.
Conclusion
The distribution of phenolic compounds and antioxidant capacity in apple depending on the variety, type of tissues and the ripening stage. The Fuji Suprema variety had the highest content of flavonols, while epicarp of Eva had the highest content of dihydrochalcones and anthocyanins (at ripe and senescent stage). The epicarp had a higher content of phenolic compounds in all the analyzed classes, except for phenolic acids, which were concentrated in the endocarp and mesocarp. The mesocarp contained lower content of phenols, however, if the percentage that corresponded to the fruit is analyzed, it provided greater quantity in the intake. Phloridzin was found in higher amounts in the endocarp of unripe fruits that decreased with ripening and consequently, in the senescent apples, the epicarp showed higher contents. In general, phenolic acids and flavonoids decrease with ripening in the epicarp and endocarp. However, in the mesocarp, the effect of the ripening is related with the apple variety. We would like to emphasize that the apple, in many countries it is one of the main sources of phenolic compounds. Therefore, future studies associated the distribution of phenols during the ripening process must be performed in others varieties in order to obtain an utilization with a higher functionality.
Acknowledgements
The authors are grateful to the National Council for Scientific and Technological Development (CNPq; Grant No. 310425/2013-1), the Araucaria Foundation (FA; Grant No. 227/2014), and the Coordination for the Improvement of Personnel in Higher Level (CAPES) for financial support and scholarships (CAPES/PNPD).
References
- Alberti A, Zielinski AAF, Zardo DM, Demiate IM, Nogueira A, Mafra LI. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014;149:151–158. doi: 10.1016/j.foodchem.2013.10.086. [DOI] [PubMed] [Google Scholar]
- Alberti A, Santos TPM, Zielinski AAF, Santos CME, Braga CM, Demiate IM, Nogueira A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT Food Sci Technol. 2016;65:436–443. doi: 10.1016/j.lwt.2015.08.045. [DOI] [Google Scholar]
- Alonso-Salces RM, Barranco A, Abad B, Berrueta LA, Gallo B, Vicente F. Polyphenolic profiles of basque cider apple cultivars and their technological properties. J Agric Food Chem. 2004;52(10):2938–2952. doi: 10.1021/jf035416l. [DOI] [PubMed] [Google Scholar]
- Apak R, Özyürek M, Güçlü K, Çapanoğlu E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J Agric Food Chem. 2016;64(5):997–1027. doi: 10.1021/acs.jafc.5b04739. [DOI] [PubMed] [Google Scholar]
- Babu PVA, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013;24:1777–1789. doi: 10.1016/j.jnutbio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bidel LPR, Coumans M, Baissac Y, Doumas P, Jay-Allemand C. Biological activity of phenolics in plant Cells. In: Santos-Buelga C, Escribano-Bailon MT, Lattanzio V, editors. Recent advances in polyphenol research. New York: Wiley; 2011. pp. 163–205. [Google Scholar]
- Blanpied GD, Silsby KJ (1992) Predicting harvest date windows for apples. Cornell cooperative extension information bulletin 221, Ithaca. http://www.plant.uoguelph.ca/treefruit/documents/PredictingHarvestDate.pdf. Accessed 25 Nov 2012
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. J Sci Food Agric. 1978;29:788–794. doi: 10.1002/jsfa.2740290908. [DOI] [Google Scholar]
- Duda-Chodak A, Tarko T, Tuszyński T. Antioxidant activity of apples—an impact of maturity stage and fruit part. Acta Sci Pol Technol Aliment. 2011;10:443–454. [PubMed] [Google Scholar]
- Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta. 2005;1721(1–3):174–184. doi: 10.1016/j.bbagen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Gosch C, Halbwirth H, Stich K. Phloridzin: biosynthesis, distribution and physiological relevance in plants. Phytochemistry. 2010;71:838–843. doi: 10.1016/j.phytochem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Guo J, Yue T, Yuan Y, Wang Y. Chemometric classification of apple juices according to variety and geographical origin based on polyphenolic profiles. J Agric Food Chem. 2013;61(28):6949–6963. doi: 10.1021/jf4011774. [DOI] [PubMed] [Google Scholar]
- Guyot S, Serrand S, Le Quéré JM, Sanoner P, Renard CMGC. Enzymatic synthesis and physicochemical characterisation of phloridzin oxidation products (POP), a new water-soluble yellow dye deriving from apple. Innov Food Sci Emerg Technol. 2007;8(3):443–450. doi: 10.1016/j.ifset.2007.03.021. [DOI] [Google Scholar]
- Huber GM, Rupasinghe HPV. Phenolic profiles and antioxidant properties of apple skin extracts. J Food Sci. 2009;74(9):C693–C700. doi: 10.1111/j.1750-3841.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Jaworski AW, Lee CY. Fractionation and HPLC determination of grape phenolics. J Agric Food Chem. 1987;35:257–259. doi: 10.1021/jf00074a022. [DOI] [Google Scholar]
- Kalinowska M, Bielawska A, Lewandowska-Siwkiewicz H, Priebe W, Lewandowski W. Apples: content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol Bioch. 2014;84:169–188. doi: 10.1016/j.plaphy.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Karaman S, Tütem E, Sözgen Baskan K, Apak R. Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC-CUPRAC assay. Food Chem. 2010;120(4):1201–1209. doi: 10.1016/j.foodchem.2009.11.065. [DOI] [Google Scholar]
- Khanizadeh S, Tsao R, Rekika D, Yang R, Charles MT, Rupasinghe HPV. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. J Food Compos Anal. 2008;21(5):396–401. doi: 10.1016/j.jfca.2008.03.004. [DOI] [Google Scholar]
- Le Bourvellec C, Bureau S, Renard CMGC, Plenet D, Gautier H, Touloumet L, Girard T, Simon S. Cultivar and year rather than agricultural practices affect primary and secondary metabolites in apple fruit. PLoS ONE. 2015;10(11):1–23. doi: 10.1371/journal.pone.0141916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto S, Akimoto Y, Oike H, Kobori M. Dietary phloridzin reduces blood glucose levels and reverses sglt1 expression in the small intestine in streptozotocin-induced diabetic mice. J Agric Food Chem. 2009;57(11):4651–4656. doi: 10.1021/jf9008197. [DOI] [PubMed] [Google Scholar]
- Sanoner P, Guyot S, Marnet N, Molle D, Drilleau J-F. Polyphenol profiles of french cider apple varieties (Malus domestica sp.) J Agric Food Chem. 1999;47(12):4847–4853. doi: 10.1021/jf990563y. [DOI] [PubMed] [Google Scholar]
- Singleton V, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Solovchenko AE, Merzlyak MN. Screening of visible and UV radiation as a photoprotective mechanism in plants. Russ J Plant Physiol. 2008;55(6):719–737. doi: 10.1134/S1021443708060010. [DOI] [Google Scholar]
- Tsao R, Yang R, Young JC, Zhu H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC) J Agric Food Chem. 2003;51(21):6347–6353. doi: 10.1021/jf0346298. [DOI] [PubMed] [Google Scholar]
- Tsao R, Yang R, Xie S, Sockovie E, Khanizadeh S. Which polyphenolic compounds contribute to the total antioxidant activities of apple? J Agric Food Chem. 2005;53(12):4989–4995. doi: 10.1021/jf048289h. [DOI] [PubMed] [Google Scholar]
- Vidal S, Francis L, Guyot S, Marnet N, Kwiatkowski M, Gawel R, Cheynier V, Waters EJ. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J Sci Food Agric. 2003;83(6):564–573. doi: 10.1002/jsfa.1394. [DOI] [Google Scholar]
- Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, Chen SS. Flavonoids in food and their health benefits. Plant Food Hum Nutr. 2004;59:113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- Zardo DM, Silva KM, Guyot S, Nogueira A. Phenolic profile and antioxidant capacity of the principal apples produced in Brazil. Int J Food Sci Nutr. 2013;64(5):611–620. doi: 10.3109/09637486.2013.763909. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li P, Cheng L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010;123(4):1013–1018. doi: 10.1016/j.foodchem.2010.05.053. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Zielinski AAF, Alberti A, Braga CM, Silva KM, Canteri MHG, Mafra LI, Granato D, Nogueira A, Wosiacki G. Effect of mash maceration and ripening stage of apples on phenolic compounds and antioxidant power of cloudy juices: a study using chemometrics. LWT Food Sci Technol. 2014;57(1):223–229. doi: 10.1016/j.lwt.2014.01.029. [DOI] [Google Scholar]