Abstract
Changes in the quality of frozen mango cuboids were investigated during long-term glassy state storage with and without osmotic dehydration pretreatment. The mango cuboids were dehydrated in mixed solutions (sucrose: glucose: fructose in a ratio of 3.6:1:3) of different concentrations (30, 40, and 50% (wt/wt)) prior to freezing and then stored at −55 °C (in the glassy state) for 6 months. The results revealed that compared with the untreated samples, osmotic pretreatment decreased total color difference (reduced by 15.6–62.3%), drip loss (reduced by 8.2–29.5%) and titration acidity (reduced by 1.3–9.4%), while increasing hardness (increased by 48.8–82.3%), vitamin C content (increased by 72.5–120.6%) and total soluble solids (increased by 21.8–53.7%) of frozen mangoes after 6 months. Dehydration with a sugar concentration of 40% was considered as the optimal pretreatment condition. In addition, a storage temperature of −55 °C provided better retention of quality than rubbery state storage at −18 °C. With prolonged storage time, the quality of frozen mangoes continued to change, even in the glassy state. However, the changes in quality of the osmotic-dehydrated samples were less than those of the untreated samples. The current work indicates that osmotic pretreatment and glassy state storage significantly improved the quality of frozen mangoes during long-term storage.
Keywords: Frozen storage, Mango, Osmotic dehydration, Glass transition, Quality
Introduction
Mangoes (Mangifera indica L.) are one of the most important fruits worldwide and are a rich source of carotenoids, vitamin C, phenolic compounds and other nutrients (Pott et al. 2003; Suresh Kumar and Sagar 2014). The worldwide mango production was approximately 42.66 million tons, and China produced approximately 4.62 million tons, according to FAO data for 2013 (FAO 2014). However, due to their perishability, the post-harvest shelf life of mangoes is relatively short (Sriwimon and Boonsupthip 2011; Zhao et al. 2014a). At present, freezing is the most frequently used method to extend the shelf life of mangoes and to maintain their original quality attributes. However, mangoes may undergo quality degradation, such as texture damage, color degradation, and flavor loss, in the traditional freezing process. Osmotic dehydration, as a pretreatment before freezing (i.e., osmo-dehydrofreezing), is feasible for improving textural characteristics, as well as for the reduction of browning, structural collapse, and drip loss that occur after freezing fruits and vegetables (Zhao et al. 2014a). This can be attributed to the fact that the osmotic pretreatment not only reduces the amount of water to be frozen but can also preserve product texture and enhance flavor and other sensory properties (Dermesonlouoglou et al. 2008; Yadav and Singh 2014). In our previous work, we demonstrated that osmotic pretreatment could significantly improve mango quality during freezing for 24 h (Zhao et al. 2014a). Additionally, osmo-dehydrofreezing has been used for strawberries, apples, pears, tomatoes, kiwifruit, and green beans, among others (Dermesonlouoglou et al. 2007; Marani et al. 2007; Ramallo and Mascheroni 2010; Zhao et al. 2014a). However, few studies have been carried out on osmo-dehydrofrozen fruits under long-term frozen storage.
The storage temperature is expected to be one of the most important factors influencing the stability of the quality of frozen products. According to the glass transition concept, foods can be considered to be very stable in the glassy state (below the glass transition temperature) because molecular mobility is significantly reduced and therefore the rates of deterioration reactions are significantly inhibited below the characteristic glass transition temperature (T g ′) (Rahman 2012; Syamaladevi et al. 2011). T g ′ and the characteristic temperature of the end point of freezing (T m ′) of mango were identified as −54.6 and −33.0 °C, respectively, from our previous work (Zhao et al. 2015). The mango matrices consist of ice and glass at temperatures less than their T g ′ (<−54.6 °C), recommended for long-term storage (Syamaladevi et al. 2009; Rahman 2012). T m ′ is the end point of freezing with the maximal freeze-concentration-condition, and all possible freezable water is transformed into ice at this maximal freeze-concentration-condition (Rahman 2012). Therefore, at temperatures greater than T m ′ (>−33.0 °C), mango matrices may be plasticized by the melting of ice. This condition may result in a partial-freeze-concentration in the mango matrices and may not be suitable for long-term storage of mangoes (Syamaladevi et al. 2011; Sablani et al. 2010). The literature shows studies on the relationship between the stability of frozen food and its glass transition. Akkose and Aktas (2008) reported that the total volatile basic nitrogen (TVB-N) and the thiobarbituric acid-reactive substances (TBARS) in ground beef differed significantly during 6 months of storage when stored above and below the T g ′. However, no significant influence of the glass transition and storage temperature was reported on the degradation of anthocyanins in raspberries during long-term frozen storage (Syamaladevi et al. 2011). According to the literature, the influence of glassy state storage on the quality attributes of frozen mangoes has not been reported.
Therefore, the aim of the current work was to explore whether frozen storage in the glassy state (at −55 °C) can help improve the quality attributes of frozen mangoes compared with our previous work on storage in the rubbery state (at −18 °C) (Zhao et al. 2014b). In addition, the influence of osmotic dehydration pretreatments (different osmotic concentrations) on the physico-chemical properties of mangoes during frozen storage was evaluated.
Materials and methods
Samples
Fresh mangoes (Mangifera indica L.) of the Tainong No. 1 variety were obtained directly from a local market in Beijing, China. They were carefully picked so as to obtain homogeneous properties, i.e., visually similar color and size (Jha et al. 2013). The level of ripeness of the test samples was medium mature.
Sample preparation
Two centimeters of the top and bottom sections of the mangoes were removed, and only the middle section was used for analysis due to the variations of hardness in the whole mangoes. Moreover, all samples were classified as belonging to either the sun-exposed side (softer) or the shaded side (firmer), based on varying degrees of ripeness/hardness determined using puncture tests. Mangoes with hardness ranging from 12 to 15 N were classified as the shaded side, while the hardness of the sun-exposed side (ranging from 10 to 13 N) was softer than that of the shaded side. The puncture test was performed by a TMS-Pro texture analyzer (Food Technology Corporation, Sterling, VA, USA) using a P/10-mm diameter cylinder probe, and 0.5 N initial force. In this test, the force was measured when the probe was penetrated into the samples at room temperature (25 °C). The penetration depth was held constant at 5 mm in all experiments. The speed of penetration was 20 mm/min, and the pre-speed was 50 mm/min. The instrument automatically recorded the force–displacement curve, and the hardness was expressed in terms of the maximum force (N) (Rahman and Sablani 2003). Before the experiment, the mangoes were manually peeled, deseeded, and cut with a stainless steel knife (Wang Mazi, China) into cuboid-shaped pieces (2 cm × 2 cm × 1 cm) for further processing.
Osmotic pretreatment
Mango cuboids in the dehydration group were subjected to osmotic dehydration in mixed sugars, with sucrose: glucose: fructose in a ratio of 3.6:1:3, according to the ratio of these three main sugar contents in Tainong No. 1 variety mangoes (Liu et al. 2013). The mixed sugars were used as osmotic agents for improving the quality and flavor of the mangoes due to the presence of sucrose, glucose, and fructose in the mangoes themselves (Sriwimon and Boonsupthip 2011). These mixed sugar solutions were prepared to have concentrations of 30, 40, and 50% (wt/wt). CaCl2 (1.5% wt/wt) was also added for tissue and texture preservation during processing. Osmotic dehydration was performed for 2 h at 30 °C, based on our previous work (Zhao et al. 2014a). The ratio of mango cuboids to the osmotic solution was 1:5 (weight to weight). The samples were gently blotted with a tissue paper to remove adhering water and then weighed.
Frozen storage studies
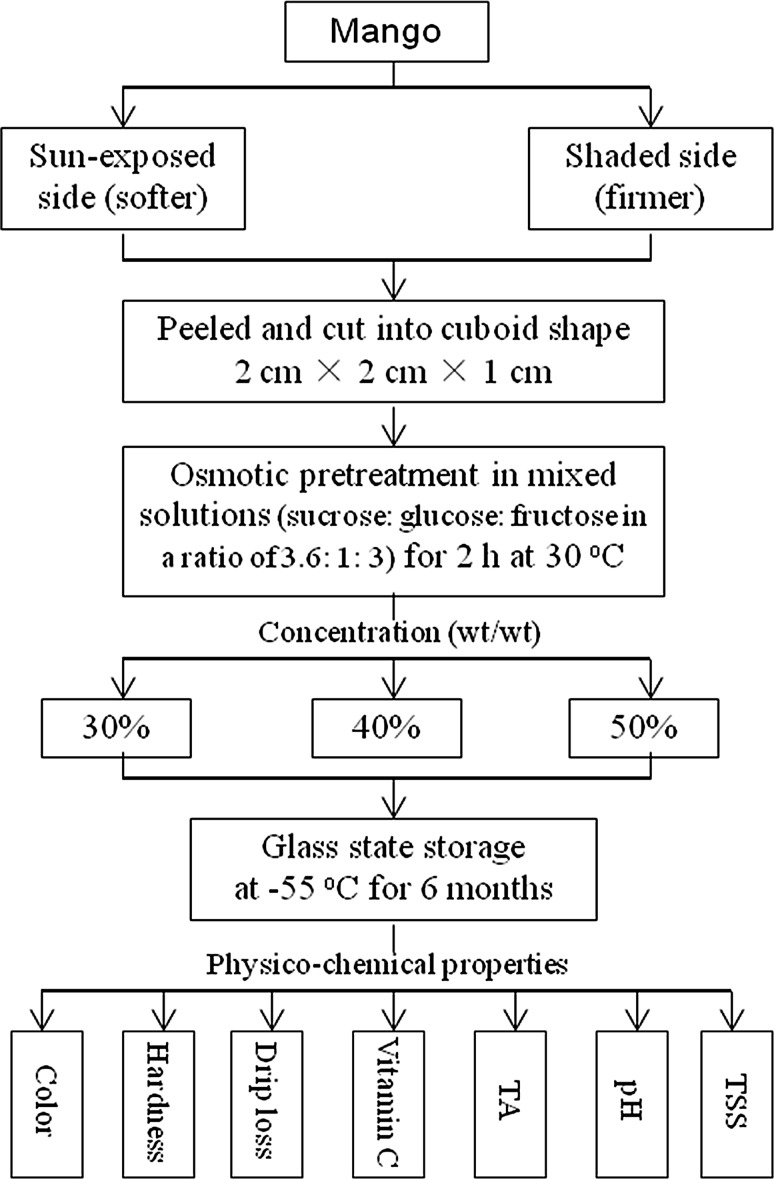
The dehydrated and fresh samples were packaged in polyethylene pouches (The thickness of polyethylene pouches is 0.08 mm.) and transferred to the freezer, where they were stored for 6 months. The temperature (−55 °C) was selected to be below the characteristic glass transition temperatures T g ′ (−54.6 °C) of mangoes (Zhao et al. 2015), and close to T g ′. Samples were retrieved each month and allowed to thaw in water for 2 h at ambient temperature (20 ± 2 °C), after which the quality was analyzed. A diagram of the treatment procedures is shown in Fig. 1.
Fig. 1.
Experimental process flow diagram
The osmo-dehydrofrozen mangoes of the sun-exposed side, pretreated in concentrations of 0, 30, 40, and 50% were labeled R0 (i.e., the untreated and the representative of the control sample), R30, R40, and R50, respectively. The osmo-dehydrofrozen mangoes of the shaded side, pretreated in concentrations of 0, 30, 40, and 50% were labeled Y0 (i.e., the untreated and the control sample), Y30, Y40, and Y50, respectively.
Qualitative analysis
Color
The sample color was measured at room temperature using a color difference meter (HunterLab ColorQuest XE, Hunter Associates Laboratory, Inc., Virginia, USA) in the reflectance mode, following the method described by Xiao et al. (2014b). The L* (lightness), a* (redness-greenness), and b* (yellowness-blueness) parameters of the CIELAB colorimetric system were used to assess the color change of the mango samples. The total color difference (ΔE) of the samples was calculated from the following equation (Zhao et al. 2014a), where L 0, a 0, and b 0 are the values for the fresh mango cubes:
| 1 |
Hardness
The sample hardness was analyzed using a TMS-Pro texture analyzer (Food Technology Corporation, Sterling, VA, USA) using a P/38-mm diameter cylinder probe in the TPA (texture profile analysis) tests. In the TPA tests, samples were compressed twice to 30% of their original height between flat plates at a test speed of 20 mm/min and an initial force of 0.5 N. A force–time curve was recorded by the instrument, and the hardness was expressed using the peak force of the first compression cycle in N (Zhao et al. 2014a).
Drip loss
After the samples had thawed, the formed water was allowed to drip off, and the samples were then weighted. The drip loss was calculated as follows (Wen et al. 2015):
| 2 |
Vitamin C content
The vitamin C content was measured by titration with 2, 6-dichloroindophenol, using the titrimetry method according to AOAC Method No. 967.21 (AOAC 2000), following the procedure described by Zhao et al. (2014a). The values of vitamin C content were expressed as mg ascorbic acid per 100 g of fresh sample.
Titration acidity (TA) and pH
The titration acidity (TA) was determined via titration with standardized 0.1 mol/L NaOH set to reach pH 8.1 by an automatic potentiometric titrator (751 GPD Titrino, Metrohm, Switzerland), according to the method of Liu et al. (2013). TA was expressed as gram malic acid equivalent per 100 g of fresh weight, considering that malic acid is the predominant acid found in mangoes. The pH value was determined using an Orion 868 pH meter (Thermo Orion, USA), with a combined pH electrode at 25 °C.
Total soluble solids (TSS)
TSS was determined as % using a WAY-2S digital Abbe Refraction meter (Shanghai Precision and Scientific Instrument Co., Ltd., Shanghai, China) at ambient temperature (20 ± 1 °C).
Statistical analysis
All experiments were performed in triplicate for each storage method. Data were expressed as the mean ± standard deviation (SD). ANOVA and Duncan’s multiple range tests were performed at a significance level of P < 0.05 using the SPSS 17.0 software (SPSS, Chicago, IL, USA).
Results and discussion
Color
Color is an important quality attribute that affects the appearance, presentation, and acceptability of many foods (Xiao et al. 2014a). Table 1 shows the changes in the L* value of mangoes of different osmotic concentrations during storage at −55 °C (in the glassy state) for 6 months. At the end of storage, L* values for all samples were significantly (P < 0.05) reduced to different levels, and untreated frozen mangoes (R0) changed the most (reduced by 7.9%) from 1 to 6 months of storage. The reduction in L* of frozen mangoes was mainly attributed to ice crystals formed during freezing and frozen storage, which may result in enzymatic oxidation due to the damaged tissue and consequently triggered chemical reactions between phenolic compounds, oxygen, and enzymes (Liang et al. 2015). Compared with untreated frozen mangoes from the shaded side (Y0), the most osmotic-dehydrated samples had significantly (P < 0.05) higher L* values at the same storage time (except in the first month of storage). The osmotic dehydration could improve the L* value of the osmotic-dehydrated mangoes. This phenomenon may be due to the faster freezing rate as a result of lower water content of osmotic-dehydrated samples (Zhao et al. 2014a; Li and Sun 2002). The faster freezing rate could result in lower structural damage and by thus lower rate of enzymatic browning reactions. In our previous work, we proved that osmotic pretreatment could enhance the freezing rate of mangoes (Zhao et al. 2014a). Among all osmotic-dehydrated mangoes, the L* values of samples pretreated in sugar concentration of 30% were highest during the whole duration of frozen storage, which implies that this pretreatment condition protects the sample color and reduces the occurrences of enzymatic browning reactions during frozen storage. At the same concentration and storage time, the L* value on the shaded side of most pretreated mangoes was significantly (P < 0.05) higher than that on the sun-exposed side (except in the fourth month of storage) in Table 1. This phenomenon may be due to the fact that the shaded side of the mangoes was exposed to less sunlight and resulted in immaturity of the sample with lighter color and a higher L* value. L* value for the inner surface of mango was decreased since the internal color turning white to yellow during ripening (Nambi et al. 2015).
Table 1.
Effect of different osmotic concentrations on color (L* value and total color difference) of mangoes during frozen storage (−55 °C) in the glassy state
| Osmotic solutions | 0 month | 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
|---|---|---|---|---|---|---|---|---|
| L * | R0 | 49.22 ± 3.32Ad | 46.91 ± 0.86ABb | 46.11 ± 2.03ABCbc | 45.71 ± 1.12BCc | 44.57 ± 1.43BCb | 44.28 ± 1.58BCb | 43.22 ± 0.47Cc |
| R30 | 51.43 ± 3.36Acd | 49.56 ± 1.86ABab | 51.14 ± 3.43Aab | 50.16 ± 1.60ABab | 49.54 ± 1.43ABa | 47.05 ± 2.45ABab | 45.97 ± 1.84Babc | |
| R40 | 56.56 ± 1.95Aab | 46.54 ± 1.15Bb | 46.00 ± 0.68Bc | 45.32 ± 1.58Bc | 46.65 ± 3.26Bab | 45.18 ± 0.74Bb | 44.42 ± 0.61Bbc | |
| R50 | 59.20 ± 0.54Aa | 47.54 ± 3.36Bab | 45.72 ± 1.55Bc | 47.47 ± 1.90Bbc | 46.43 ± 1.50Bab | 46.37 ± 2.16Bab | 44.82 ± 2.70Bbc | |
| Y0 | 51.68 ± 2.65Abcd | 47.34 ± 2.85Bab | 46.24 ± 0.51Bbc | 45.94 ± 2.01Bc | 44.95 ± 1.49Bb | 44.65 ± 1.14Bb | 44.59 ± 0.54Bbc | |
| Y30 | 55.71 ± 3.00Aabc | 52.03 ± 1.75Ba | 52.20 ± 1.53Ba | 51.43 ± 1.71Ba | 48.75 ± 0.90Ba | 49.27 ± 1.58Ba | 48.54 ± 2.68Ba | |
| Y40 | 55.69 ± 2.94Aabc | 48.49 ± 0.74Bab | 49.09 ± 4.19Babc | 47.45 ± 1.09Bbc | 47.12 ± 1.93Bab | 46.57 ± 2.36Bab | 45.85 ± 0.61Babc | |
| Y50 | 59.53 ± 2.43Aa | 49.61 ± 4.56Bab | 48.56 ± 4.13Babc | 46.22 ± 1.37Bc | 48.59 ± 1.11Ba | 46.63 ± 1.55Bab | 46.85 ± 2.65Bab | |
| △E | R0 | 0.00 ± 0.00Ef | 8.20 ± 0.64Da | 8.43 ± 0.54Da | 11.02 ± 1.00Ca | 14.60 ± 1.88Ba | 14.96 ± 1.07Ba | 16.53 ± 0.18Aa |
| R30 | 8.27 ± 0.15Ac | 4.29 ± 0.21Cc | 6.03 ± 0.08Bcde | 3.88 ± 1.19Cd | 5.58 ± 0.30Bd | 6.50 ± 0.58Bef | 6.24 ± 1.37Bf | |
| R40 | 10.97 ± 0.67BCb | 6.02 ± 0.81Eb | 6.74 ± 0.19Eabcd | 10.78 ± 1.17CDa | 10.00 ± 0.00Db | 11.75 ± 0.45Bb | 13.18 ± 0.89Ab | |
| R50 | 16.57 ± 0.07Aa | 6.71 ± 2.52CDab | 6.16 ± 3.78Dbcde | 8.84 ± 0.04Cb | 8.08 ± 2.45CDc | 7.30 ± 0.35CDe | 12.26 ± 0.49Bbc | |
| Y0 | 0.00 ± 0.00Df | 6.20 ± 1.65Cb | 8.27 ± 0.74Bab | 9.48 ± 0.55ABab | 9.89 ± 0.14ABb | 9.90 ± 0.21ABc | 10.75 ± 3.09Acd | |
| Y30 | 4.20 ± 0.35BCe | 2.99 ± 0.55Cc | 4.26 ± 2.20BCe | 4.53 ± 1.27BCd | 5.31 ± 0.45Bd | 7.22 ± 0.28Ae | 5.09 ± 2.04Bf | |
| Y40 | 6.62 ± 1.88Bd | 6.02 ± 1.29Bb | 7.48 ± 0.47ABabc | 6.06 ± 2.44Bc | 8.30 ± 0.20Ac | 8.53 ± 0.49Ad | 8.75 ± 0.24Ae | |
| Y50 | 11.98 ± 1.77Ab | 3.11 ± 1.11Dc | 4.67 ± 0.69Cde | 8.01 ± 0.66Bb | 9.06 ± 0.29Bbc | 5.68 ± 1.41Cf | 9.07 ± 0.27Bde |
Different letters (a–f) within a same column represent significant difference (P < 0.05). Different letters (A–E) within a same row represent significant difference (P < 0.05)
The evolution of the color changes (ΔE) in mangoes after osmotic dehydration pretreatment during frozen storage is shown in Table 1. It can be seen that most osmotic-dehydrated samples had significantly (P < 0.05) lower ΔE values than untreated ones at the same storage time, mainly due to the infusion of sugars into the cellular tissue as a result of osmotic dehydration, and the protective effects of sugars on color (Chottanom and Srisa-ard 2011). Additionally, because of the lower freezing rate of the control (R0 and Y0), the size of the ice crystals may have been larger, resulting in more cellular and tissue damage and therefore, more color changes. In our previous work, we proved that osmotic pretreatment could enhance the freezing rate of mangoes (Zhao et al. 2014a). This result implies that osmotic pretreatment is a better way to conserve the original color of mangoes during frozen storage. At the end of storage (6 months), the ΔE value for all samples was significantly (P < 0.05) increased compared with that after 1 month of storage. In addition, the storage time had a significant effect on the color changes of frozen mangoes (P < 0.05). With the progression of storage time (from 1 to 6 months of storage), most osmotic-dehydrated samples obtained smaller changes in ΔE than the corresponding control (R0 and Y0) on both the sun-exposed side and shaded side. The ΔE value on the shaded side of most mangoes was lower than that on the sun-exposed side, mainly due to greater hardness of the shaded side and smaller tissue damage as a result of the ice crystals formed during frozen storage and therefore smaller color changes in the shaded side.
Our previous work reported the quality changes of frozen mangoes during storage at −18 °C (commercial frozen storage in the rubbery state, T > T m ′) for 6 months (Zhao et al. 2014b), and those with osmotic pretreatments were the same when stored at −55 °C. The changing trends in the L* and ΔE values of frozen mangoes at −55 °C, with time were similar to those at −18 °C. Compared with the color at −18 °C during storage (Zhao et al. 2014b), the L* values were significantly (P < 0.05) higher, and the ΔE values were significantly (P < 0.05) lower in the glassy state (at −55 °C). This result suggests that glassy state storage can improve the color of frozen mangoes. It has been reported in the literature that foods can be considered very stable in the glassy state because below the glass transition temperature, the rates of diffusion-controlled reactions (browning reaction) are significantly reduced (Guizani et al. 2010). However, there was a significant change of color (P < 0.05) with storage time in the glassy state. This indicates that changes in the quality of food can occur, even when it is stored at a temperature below T g ′, probably due to occurring enthalpy relaxation over time (Syamaladevi et al. 2012).
Hardness
The textural characteristics of frozen foods, which are mainly influenced by the freezing rate, play an essential role in determining the acceptability of these products to consumers (Zhang et al. 2007). Good-quality mango products exhibited a high degree of hardness of the pulp during frozen storage (Liang et al. 2015). Table 2 shows the changes in the hardness of untreated and osmotic-dehydrated mangoes in the glassy state during 6 months of frozen storage. Before frozen storage, the hardness values of most pretreated samples were significantly (P < 0.05) lower than those of fresh mangoes, probably due to osmotic dehydration causing lower water content and thus having a negative impact on the hardness. Similar results have been reported by Tedjo et al. (2002), and Rincon and Kerr (2010). Moreover, samples pretreated in 50% osmotic solutions should not be subsequently frozen because of the higher osmotic pressure, which can possibly result in tissue shrinkage, as per our previous work (Zhao et al. 2014a). During frozen storage, the hardness of the osmotic-dehydrated samples was significantly (P < 0.05) higher than that of the untreated ones in Table 2. This result could be attributed to the higher freezing rate of pretreated mangoes, which could reduce the damage to cell structure and result in firmer textures. Additionally, calcium chloride also contributed to the increased hardness due to its interaction with the cellular wall components of the plant cellular matrix (Zhao et al. 2014a). Among all osmotic-dehydrated samples, the hardness values of those in solutions of concentration 30% were lowest during the entire duration of frozen storage. And the samples pretreated in 50% osmotic solutions should not be subsequently frozen. Therefore, dehydration in the concentration of 40% was recommended as the most favorable pretreatment condition for mango hardness. Obviously, the hardness was lower in riper fruit. The decrease in hardness has been reported to be due to changes in structure of the pectin polymers of the cell wall during ripening (Jha et al. 2013). Therefore, the hardness value on the shaded side of mangoes was higher than that on the sun-exposed side in the same freezing conditions. In addition, there was a gradual decrease of hardness during the frozen storage. The changes of hardness for the osmotic-dehydrated samples on the shaded side (Y30 and Y40) were slower after 1 month of storage than those of the untreated one (Y0), which did not significantly change over time.
Table 2.
Effect of different osmotic concentrations on hardness of mangoes during frozen storage (−55 °C) in the glassy state
| Osmotic solutions | 0 month | 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
|---|---|---|---|---|---|---|---|---|
| Hardness (N) | R0 | 3.81 ± 0.23Acd | 1.15 ± 0.09Bd | 1.07 ± 0.09Bd | 1.02 ± 0.10Bd | 1.14 ± 0.13Bc | 1.18 ± 0.10Be | 1.13 ± 0.13Bb |
| R30 | 3.45 ± 0.25Ade | 2.31 ± 0.30Bb | 1.84 ± 0.40Cbc | 1.91 ± 0.20BCbc | 1.80 ± 0.10Cab | 1.73 ± 0.04Ccd | 1.79 ± 0.11Ca | |
| R40 | 3.66 ± 0.36Acd | 2.32 ± 0.16Bb | 2.17 ± 0.02BCb | 2.13 ± 0.19BCb | 1.84 ± 0.30Cab | 2.13 ± 0.15BCab | 1.83 ± 0.31Ca | |
| R50 | 3.01 ± 0.43Ae | 2.34 ± 0.12Bb | 2.41 ± 0.14Bab | 2.22 ± 0.10Bb | 2.04 ± 0.19BCa | 1.74 ± 0.30Ccd | 2.06 ± 0.24BCa | |
| Y0 | 5.30 ± 0.06Aa | 1.57 ± 0.12Bc | 1.44 ± 0.06BCcd | 1.57 ± 0.12Bc | 1.54 ± 0.17Bb | 1.43 ± 0.15BCde | 1.27 ± 0.20Cb | |
| Y30 | 4.62 ± 0.46Ab | 2.21 ± 0.27Bb | 2.27 ± 0.51Bab | 2.13 ± 0.41Bb | 1.76 ± 0.02Bab | 1.83 ± 0.12Bbc | 1.89 ± 0.23Ba | |
| Y40 | 5.26 ± 0.46Aa | 2.41 ± 0.43Bb | 2.37 ± 0.21Bab | 2.28 ± 0.36Bb | 2.02 ± 0.30Ba | 1.94 ± 0.10Bbc | 2.00 ± 0.17Ba | |
| Y50 | 4.22 ± 0.02Abc | 3.21 ± 0.19Ba | 2.92 ± 0.70BCa | 2.68 ± 0.08BCDa | 2.09 ± 0.04Da | 2.39 ± 0.28CDa | 2.08 ± 0.32Da |
Different letters (a–e) within a same column represent significant difference (P < 0.05). Different letters (A–D) within a same row represent significant difference (P < 0.05)
In general, decreasing trends with some fluctuation in the hardness were observed during storage, both at −18 °C (Zhao et al. 2014b) and −55 °C. Moreover, an increase of hardness was seen in the frozen mangoes at −55 °C after 6 months of storage compared with the hardness at −18 °C (Zhao et al. 2014b). The storage temperature exhibited significant influence on the hardness values (P < 0.05) when the selected temperatures were greater than and less than T g ′. Although the mangoes were in the glassy state during frozen storage, the hardness continued to decrease. This indicates that diffusion-limited reactions do not completely cease in the glassy state. Enthalpy relaxations may occur in frozen mangoes in the glassy state, resulting in molecular rearrangements and the diffusion of unfrozen water (Syamaladevi et al. 2012). This may cause the growth of ice crystals through recrystallization during storage and further stress the fragile cellular structures of mangoes.
Drip loss
As seen in Fig. 2, compared with the control groups (R0 and Y0), the samples with osmotic-dehydrated pretreatment showed significant reductions in drip loss (P < 0.05) during long-term frozen storage. This is because sugars were transferred into the cellular tissue of mangoes through osmotic dehydration and these are excellent cryoprotectants of a high water-holding capacity (Aleksandar et al. 2007). Moreover, the volume of released liquid reduced with increasing osmotic concentration at the same storage period, mainly due to the decrease of freezable water with increasing concentration after osmotic dehydration. In addition, according to our previous work (Zhao et al. 2014a), the samples pretreated in 50% osmotic solutions should not be subsequently frozen because of possible tissue shrinkage. Therefore, dehydration in a solution of concentration 40% was most favorable for mango drip loss. The drip loss increased gradually in both untreated and osmotic-pretreated samples with increasing storage time in Fig. 2. This result may be because the quantity of unfrozen water present may be enough for recrystallization to occur by diffusion, even with limited molecular motion, as shown by enthalpy relaxation below the glass transition temperature (Hagiwara et al. 2005). The recrystallization could result in ice crystals of increased size, causing more tissue damage and consequently, increasing the drip loss with storage time. The drip loss on the shaded side of most mangoes was lower than that on the sun-exposed side, in accordance with the above results of the hardness values reported in Table 2.
Fig. 2.
Changes of drip loss in frozen mangoes with different osmotic pretreatments during long-term storage (−55 °C) in the glassy state
It was found that the increasing trend in the drip loss of frozen mangoes at −55 °C was similar to that at −18 °C (Zhao et al. 2014b) during storage. A significant reduction of drip loss (P < 0.05) was observed in the glassy state of frozen mangoes compared with the drip loss during rubbery state storage (at −18 °C), mainly due to the smaller ice crystals in the glassy state, which could reduce cell structure damage and result in lower drip loss during storage.
Vitamin C content
Changes in the nutritional quality of frozen mangoes could be determined by comparing key quality indices. This study analyzed vitamin C to determine the effect of different osmotic pretreatments on nutrient preservation in frozen mangoes during long-term storage. Table 3 shows the changes of vitamin C content in mangoes during frozen storage in the glassy state. Before frozen storage, the vitamin C content of fresh mangoes was significant higher than that of the osmotic-pretreated samples on both sun-exposed and shaded sides (P < 0.05). The osmotic pretreatment significantly decreased the vitamin C content. Similar results were reported by Forni et al. (1990) and Rincon and Kerr (2010). In addition, vitamin C contents for all samples significantly (P < 0.05) reduced to different levels during frozen storage, and most of the reduction occurred during the first 3 months of storage. After 3 months, a drastic loss (89.8–90.2%) of vitamin C was detected in the control groups (R0 and Y0), likely due to rapid leaching via drip loss, whereas lower loss (56.1–74.9%) occurred in the osmotic-dehydrated samples. This result may be due to the lower freezing rate of the control groups and higher structural damage caused by larger ice crystals, resulting in the dissolution of more vitamins and minerals in the liquid released from the fruits compared with the osmotic-dehydrated samples. This result implies that osmotic pretreatment could prevent vitamin C losses during long-term frozen storage. The vitamin C contents of frozen mangoes pretreated in 30 and 40% osmotic solutions were significantly higher than those in other conditions (P < 0.05) at the same storage time in Table 3. In addition, the vitamin C contents on the shaded side in most pretreated mangoes were significantly (P < 0.05) higher than those on the sun-exposed side (except in the first and fifth months of storage), which was related to the degree of ripeness. It has been reported that the vitamin C content decreases as ripening progress in mangoes (Tovar et al. 2001).
Table 3.
Effect of different osmotic concentrations on vitamin C of mangoes during frozen storage (−55 °C) in the glassy state
| Osmotic solutions | 0 month | 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
|---|---|---|---|---|---|---|---|---|
| Vitamin C/(mg/100 g) | R0 | 18.77 ± 0.08Ab | 2.16 ± 0.01Bh | 2.00 ± 0.01Cg | 1.84 ± 0.09Dh | 1.27 ± 0.25Ef | 1.15 ± 0.14EFf | 1.02 ± 0.06Fd |
| R30 | 16.84 ± 0.01Ac | 8.10 ± 0.29Ba | 5.66 ± 0.26Dc | 6.02 ± 0.01Cb | 3.26 ± 0.23Fc | 3.88 ± 0.08Eb | 2.25 ± 0.23Ga | |
| R40 | 14.50 ± 0.18Ae | 6.97 ± 0.08Bd | 5.77 ± 0.07Cc | 5.31 ± 0.01Dc | 3.32 ± 0.01Ec | 2.93 ± 0.01Fc | 1.77 ± 0.15Gc | |
| R50 | 11.51 ± 0.06Ag | 5.87 ± 0.08Be | 4.38 ± 0.08Ce | 3.64 ± 0.10Df | 2.61 ± 0.18Fd | 2.87 ± 0.24Ec | 1.97 ± 0.08Gbc | |
| Y0 | 19.74 ± 0.32Aa | 3.15 ± 0.12Bg | 2.63 ± 0.15Cf | 2.01 ± 0.08Dg | 1.68 ± 0.01Ee | 1.24 ± 0.18Ff | 1.15 ± 0.16Fd | |
| Y30 | 16.78 ± 0.01Ac | 7.79 ± 0.01Bb | 7.72 ± 0.16Ba | 7.37 ± 0.08Ca | 3.98 ± 0.01Db | 2.69 ± 0.13Ed | 2.46 ± 0.26Fa | |
| Y40 | 15.72 ± 0.14Ad | 7.26 ± 0.01Bc | 6.02 ± 0.15Cb | 3.94 ± 0.15Ed | 5.65 ± 0.08Da | 4.08 ± 0.08Ea | 2.44 ± 0.25Fa | |
| Y50 | 13.21 ± 0.20Af | 4.88 ± 0.16Bf | 4.64 ± 0.07Cd | 3.81 ± 0.01De | 3.42 ± 0.13Ec | 2.49 ± 0.07Fe | 2.02 ± 0.22Gb |
Different letters (a–h) within a same column represent significant difference (P < 0.05). Different letters (A–G) within a same row represent significant difference (P < 0.05)
In general, decreasing trends were seen for the content of vitamin C both at –18 °C (Zhao et al. 2014b) and –55 °C with storage time. This result indicates that vitamin C losses continued in the glassy state, which was attributed to the occurrence of enthalpy relaxation during storage. In addition, the vitamin C content of frozen mangoes in the glassy state was significantly higher than that of mangoes in the rubbery state (at –18 °C) during storage (P < 0.05). This implies that glassy state storage of frozen mangoes was able to reduce the rates of vitamin C losses because the molecular mobility was greatly reduced below the glass transition temperature.
Titration acidity (TA) and pH
TA is related to the concentration of organic acids present in a food, and it is used as a quality parameter. Table 4 shows the effects of different osmotic pretreatments and storage times on the changes of titration acidity in mangoes during frozen storage. Initially, osmotic-dehydrated mangoes had a significantly (P < 0.05) lower TA than untreated samples before freezing, probably due to the loss of organic acids into the surrounding solution during osmotic dehydration. There was a reduction of the TA with prolonged storage time, which indicated that some diffusion of organic acids was still possible in the glassy state (–55 °C). The changes in the titration acidity during frozen storage could also be due to the modifications in the permeability of the cell membrane (Martínez et al. 2013). In addition, TA on the shaded side of most mangoes was significantly (P < 0.05) higher than that on the sun-exposed side during frozen storage, mainly due to the lower acidity of ripened fruit (sun-exposed side).
Table 4.
Effect of different osmotic concentrations on titratable acidity of mangoes during frozen storage (−55 °C) in the glassy state
| Osmotic solutions | 0 month | 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
|---|---|---|---|---|---|---|---|---|
| Titratable acidity/(g malic acid/100 g) | R0 | 0.735 ± 0.003Ac | 0.439 ± 0.005Be | 0.427 ± 0.003Cd | 0.337 ± 0.002Ff | 0.356 ± 0.007Df | 0.351 ± 0.003Ee | 0.308 ± 0.001Gf |
| R30 | 0.650 ± 0.002Ad | 0.407 ± 0.002Ch | 0.334 ± 0.003Eh | 0.353 ± 0.003Dd | 0.474 ± 0.002Ba | 0.357 ± 0.005Dd | 0.296 ± 0.007Fg | |
| R40 | 0.496 ± 0.001Af | 0.411 ± 0.002Bg | 0.389 ± 0.001Cf | 0.408 ± 0.007Bb | 0.295 ± 0.008Fg | 0.375 ± 0.002Dbc | 0.304 ± 0.001Ef | |
| R50 | 0.461 ± 0.002Bg | 0.508 ± 0.002Ab | 0.433 ± 0.003Cc | 0.392 ± 0.003Dc | 0.377 ± 0.001Ee | 0.358 ± 0.006Fd | 0.358 ± 0.003Fb | |
| Y0 | 0.824 ± 0.002Aa | 0.533 ± 0.001Ba | 0.454 ± 0.003Ca | 0.426 ± 0.002 Da | 0.394 ± 0.001Ec | 0.370 ± 0.005Fc | 0.350 ± 0.003Gc | |
| Y30 | 0.589 ± 0.002Ae | 0.454 ± 0.002Bd | 0.360 ± 0.002Dg | 0.345 ± 0.001Ee | 0.454 ± 0.003Bb | 0.393 ± 0.003Ca | 0.332 ± 0.008Fd | |
| Y40 | 0.594 ± 0.011Ae | 0.431 ± 0.003Bf | 0.437 ± 0.001Bb | 0.423 ± 0.005Ca | 0.392 ± 0.002Dcd | 0.380 ± 0.008Eb | 0.378 ± 0.006Ea | |
| Y50 | 0.780 ± 0.012Ab | 0.479 ± 0.002Bc | 0.404 ± 0.006De | 0.412 ± 0.001Cb | 0.387 ± 0.002Ed | 0.316 ± 0.001Ff | 0.317 ± 0.001Fe |
Different letters (a–h) within a same column represent significant difference (P < 0.05). Different letters (A–G) within a same row represent significant difference (P < 0.05)
The changes in pH values of untreated and osmotically pretreated mangoes during frozen storage are shown in Table 5. The pH values of osmotic-dehydrated samples were significantly (P < 0.05) lower than those of the untreated groups during storage. In addition, the pH value on the shaded side of most mangoes was lower than that on the sun-exposed side at the same storage period, which was related to the degree of ripeness. With prolonged storage time, there was a general reduction of pH values (with the exception of R40), and the storage time had a significant effect on the pH changes of frozen mangoes (P < 0.05). It was found that the changes of pH values did not always mirror the changes of TA. The changes of pH values may result due to the regulation of cytosolic pH values by some cells. Additionally, the changes of pH values during storage may also result from crystallization and/or precipitation of various solutes, which occurs during freeze concentration of the unfrozen phase. Therefore, both ionic strength and pH values can change due to the changing ratios of buffer components (Rincon and Kerr 2010; Zhao et al. 2014a).
Table 5.
Effect of different osmotic concentrations on pH values of mangoes during frozen storage (−55 °C) in the glassy state
| Osmotic solutions | 0 month | 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
|---|---|---|---|---|---|---|---|---|
| pH | R0 | 4.42 ± 0.01Aa | 4.38 ± 0.00Ba | 4.24 ± 0.01Ca | 4.21 ± 0.01Da | 4.08 ± 0.01Ea | 4.06 ± 0.01Fa | 4.06 ± 0.00Fa |
| R30 | 4.02 ± 0.01Ad | 3.95 ± 0.00Ce | 4.00 ± 0.01Bc | 4.02 ± 0.00Ac | 3.82 ± 0.00Ee | 3.87 ± 0.01Dd | 3.88 ± 0.01Dc | |
| R40 | 3.80 ± 0.01Eg | 3.92 ± 0.00Bg | 3.95 ± 0.01Ad | 3.89 ± 0.01Ce | 3.81 ± 0.01Ef | 3.96 ± 0.02Ab | 3.86 ± 0.01Dde | |
| R50 | 4.11 ± 0.01Ac | 4.04 ± 0.00Bc | 3.93 ± 0.00Ce | 3.93 ± 0.02Cd | 3.94 ± 0.01Cd | 3.77 ± 0.01De | 3.78 ± 0.01Df | |
| Y0 | 4.26 ± 0.01Ab | 4.18 ± 0.00Bb | 4.12 ± 0.02Cb | 4.12 ± 0.00Cb | 4.06 ± 0.01Db | 4.06 ± 0.00Da | 3.99 ± 0.01Eb | |
| Y30 | 3.99 ± 0.01Ae | 3.80 ± 0.00Ch | 3.80 ± 0.01Cg | 3.80 ± 0.01Cf | 3.75 ± 0.00Eg | 3.89 ± 0.01Bc | 3.77 ± 0.00Df | |
| Y40 | 3.90 ± 0.01Bf | 4.02 ± 0.00Ad | 3.88 ± 0.01Cf | 3.89 ± 0.01BCe | 3.83 ± 0.01Ee | 3.72 ± 0.00Ff | 3.85 ± 0.01De | |
| Y50 | 4.00 ± 0.01Ae | 3.94 ± 0.01Cf | 3.93 ± 0.01Ce | 3.88 ± 0.00De | 3.97 ± 0.00Bc | 3.86 ± 0.01Ed | 3.86 ± 0.01Ed |
Different letters (a–h) within a same column represent significant difference (P < 0.05). Different letters (A–F) within a same row represent significant difference (P < 0.05)
The trends in the TA and pH values of frozen mangoes at −55 °C were similar to those at −18 °C with time (Zhao et al. 2014b). Compared with rubbery state storage (−18 °C), the TA levels of frozen mangoes in the glassy state were lower during storage, and the pH values in the glassy state were higher.
Total soluble solids (TSS)
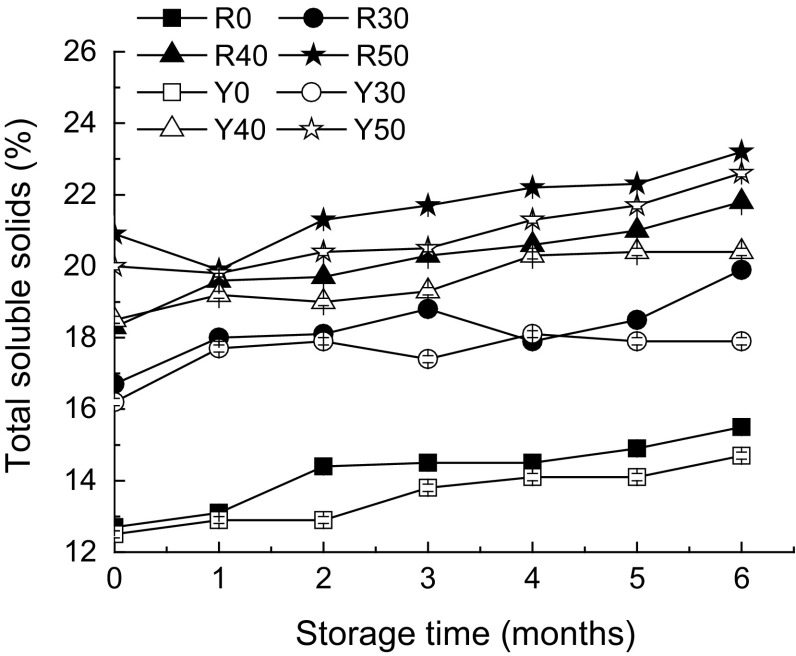
Although TSS is not totally sugar, it is often used as an estimate of sugar content (Martínez et al. 2013). Figure 3 shows the TSS changes of mangoes in different osmotic concentrations during storage at −55 °C for 6 months. In general, the TSS levels of osmotic-dehydrated samples increased with increasing osmotic concentrations at the same storage period, which could be attributed to the removal of more water and intake of more sugars, which are provided by the higher osmotic pressure during osmotic pretreatment (Suresh Kumar and Sagar 2014). Softer and riper fruit had higher initial TSS levels. Free sugars, such as glucose, fructose, and sucrose, increase during ripening, and the sucrose content can increase three- to four-fold (Rincon and Kerr 2010). Therefore, the TSS level on the sun-exposed side (riper) of most mangoes was significantly (P < 0.05) higher than that on the shaded side (except in the first and fourth months of storage). During frozen storage, there was a gradual increase of the TSS level. The changes in soluble solids could be due to the effect of frozen storage on the content of total sugars (Grzeszczuk et al. 2007). This increase may be caused by the generation of soluble solids from reserve starch, as well as additional gain from the surrounding media (Rincon and Kerr 2010). Additionally, the intake of solids increases after storage, as the permeability to sugar transport increases in the disrupted fruit tissue (Rincon and Kerr 2010).
Fig. 3.
Changes of total soluble solids in frozen mangoes with different osmotic pretreatments during long-term storage (−55 °C) in the glassy state
In general, increasing trends in TSS levels were observed both at −18 °C (Zhao et al. 2014b) and −55 °C during storage. There was no significant difference in the TSS levels of frozen mangoes subjected to glassy state storage (at −55 °C) and rubbery state storage (at −18 °C). Similarly, Rizzolo et al. (2003) and Syamaladevi et al. (2011) also reported that the storage temperature exhibited no significant influence on the degradation of anthocyanins in blueberry juices and raspberries, respectively, during long-term frozen storage, although the storage temperatures were greater than and less than the T g ′.
Conclusion
Osmotic pretreatment was demonstrated to be useful for preventing quality loss in frozen mangoes compared with untreated ones during long-term storage. Dehydration in a solution of 40% concentration was recommended as the most favorable pretreatment condition for the frozen storage of mangoes. The quality attributes of mangoes on the shaded side were better than those on the sun-exposed side during frozen storage.
A storage temperature of −55 °C (in the glassy state) was used for long-term storage of frozen mangoes, providing better retention of quality than −18 °C (in the rubbery state) with prolonged storage time. Although the mangoes were in the glassy state during frozen storage, the quality attributes continued to change. However, the changes of quality for osmotic-dehydrated samples were slower than those of the untreated ones. The findings of the current work indicate that both osmotic dehydration pretreatment technology and glassy state storage could enhance the quality attributes of frozen mangoes during long-term storage.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 31501546) and the Agricultural Science and Technology Innovation Program (ASTIP) from the Chinese Central Government.
References
- Akkose A, Aktas N. Determination of glass transition temperature of beef and effects of various cryoprotective agents on some chemical changes. Meat Sci. 2008;80:875–878. doi: 10.1016/j.meatsci.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Aleksandar J, Julianna G, Ljubinko L, Zoltan Z. Osmotic dehydration of sugar beet in combined aqueous solutions of sucrose and sodium chloride. J Food Eng. 2007;78:47–51. doi: 10.1016/j.jfoodeng.2005.09.003. [DOI] [Google Scholar]
- AOAC (2000). Official methods of analysis of the association of official analytical chemists. Official method 967.21. Washington, DC: AOAC International
- Chottanom P, Srisa-ard M. Osmotic dehydration as a factor in freezing of tomato. Am J Food Technol. 2011;6:483–491. doi: 10.3923/ajft.2011.483.491. [DOI] [Google Scholar]
- Dermesonlouoglou EK, Giannakourou MC, Taoukis P. Stability of dehydrofrozen tomatoes pretreated with alternative osmotic solutes. J Food Eng. 2007;78:272–280. doi: 10.1016/j.jfoodeng.2005.09.026. [DOI] [Google Scholar]
- Dermesonlouoglou EK, Pourgouri S, Taoukis PS. Kinetic study of the effect of the osmotic dehydration pre-treatment to the shelf life of frozen cucumber. Innov Food Sci Emerg Technol. 2008;9:542–549. doi: 10.1016/j.ifset.2008.01.002. [DOI] [Google Scholar]
- FAO (2014) FaoStat database. http://faostat3.fao.org
- Forni E, Torregiani D, Crivelli G, Mastrelle A, Bertolo G, Santelli F. Influence of osmosis time on the quality of dehydrofrozen kiwi fruit. Acta Hortic. 1990;282:425–434. doi: 10.17660/ActaHortic.1990.282.54. [DOI] [Google Scholar]
- Grzeszczuk M, Jadczak D, Podsiadlo C. The effect of blanching, freezing and freeze-storage on changes of some chemical compounds content in New Zealand spinach. Veg Crops Res Bull. 2007;66:95–103. [Google Scholar]
- Guizani N, Al-Saidi GS, Rahman MS, Bornaz S, Al-Alawi AA. State diagram of dates: glass transition, freezing curve and maximal-freeze concentration condition. J Food Eng. 2010;99:92–97. doi: 10.1016/j.jfoodeng.2010.02.003. [DOI] [Google Scholar]
- Hagiwara T, Mao J, Suzuki T, Takai R. Ice recrystallization in sucrose solutions stored in a temperature range of −21 °C to −50 °C. Food Sci Technol Res. 2005;11:407–411. doi: 10.3136/fstr.11.407. [DOI] [Google Scholar]
- Jha SN, Jaiswal P, Narsaiah K, Kaur PP, Singh AK, Kumar R. Textural properties of mango cultivars during ripening. J Food Sci Technol. 2013;50(6):1047–1057. doi: 10.1007/s13197-011-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Sun DW. Novel methods for rapid freezing and thawing of foods—a review. J Food Eng. 2002;54:175–182. doi: 10.1016/S0260-8774(01)00209-6. [DOI] [Google Scholar]
- Liang DW, Lin FY, Yang GM, Yue XJ, Zhang QK, Zhang ZQ, Chen HB. Advantages of immersion freezing for quality preservation of litchi fruit during frozen storage. LWT-Food Sci Technol. 2015;60:948–956. doi: 10.1016/j.lwt.2014.10.034. [DOI] [Google Scholar]
- Liu FX, Fu SF, Bi XF, Chen F, Liao XJ, Hu XS, Wu JH. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chem. 2013;138:396–405. doi: 10.1016/j.foodchem.2012.09.111. [DOI] [PubMed] [Google Scholar]
- Marani CM, Agnelli ME, Mascheroni RH. Osmo-frozen fruits: mass transfer and quality evaluation. J Food Eng. 2007;79:1122–1130. doi: 10.1016/j.jfoodeng.2006.03.022. [DOI] [Google Scholar]
- Martínez S, Pérez N, Carballo J, Franco I. Effect of blanching methods and frozen storage on some quality parameters of turnip greens (“grelos”) LWT-Food Sci Technol. 2013;51:383–392. doi: 10.1016/j.lwt.2012.09.020. [DOI] [Google Scholar]
- Nambi VE, Thangavel K, Jesudas DM. Scientific classification of ripening period and development of colour grade chart for Indian mangoes (Mangifera indica L.) using multivariate cluster analysis. Sci Hortic. 2015;193:90–98. doi: 10.1016/j.scienta.2015.05.031. [DOI] [Google Scholar]
- Pott I, Marx M, Neidhart S, Muhlbauer W, Carle R. Quantitative determination of β-carotene stereoisomers in fresh, dried, and solar-dried mangoes (Mangifera indica L.) J Agric Food Chem. 2003;51:4527–4531. doi: 10.1021/jf034084h. [DOI] [PubMed] [Google Scholar]
- Rahman MS. Applications of macro-micro region concept in the state diagram and critical temperature concepts in determining the food stability. Food Chem. 2012;132:1679–1685. doi: 10.1016/j.foodchem.2011.09.092. [DOI] [Google Scholar]
- Rahman MS, Sablani SS. Structural characteristics of freeze-dried abalone porosimetry and puncture test. Food Bioprod Process. 2003;81:309–315. doi: 10.1205/096030803322756394. [DOI] [Google Scholar]
- Ramallo LA, Mascheroni RH. Dehydrofreezing of pineapple. J Food Eng. 2010;99:269–275. doi: 10.1016/j.jfoodeng.2010.02.026. [DOI] [Google Scholar]
- Rincon A, Kerr WL. Influence of osmotic dehydration, ripeness and frozen storage on physicochemical properties of mango. J Food Process Preserv. 2010;34:887–903. doi: 10.1111/j.1745-4549.2009.00404.x. [DOI] [Google Scholar]
- Rizzolo A, Nani RC, Viscardi D, Bertolo G, Torreggiani D. Modification of glass transition temperature through carbohydrates addition and anthocyanin and soluble phenol stability of frozen blueberry juices. J Food Eng. 2003;56:229–231. doi: 10.1016/S0260-8774(02)00257-1. [DOI] [Google Scholar]
- Sablani SS, Syamaladevi RM, Swanson BG. A review of methods, data and applications of state diagrams of food systems. Food Eng Rev. 2010;2:168–203. doi: 10.1007/s12393-010-9020-6. [DOI] [Google Scholar]
- Sriwimon W, Boonsupthip W. Utilization of partially ripe mangoes for freezing preservation by impregnation of mango juice and sugars. LWT-Food Sci Technol. 2011;44:375–383. doi: 10.1016/j.lwt.2010.08.012. [DOI] [Google Scholar]
- Suresh Kumar P, Sagar VR. Drying kinetics and physico-chemical characteristics of Osmo-dehydrated Mango, Guava and Aonla under different drying conditions. J Food Sci Technol. 2014;51(8):1540–1546. doi: 10.1007/s13197-012-0658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syamaladevi RM, Sablani SS, Tang J, Powers J, Swanson BG. State diagram and water adsorption isotherm of raspberry (Rubus idaeus) J Food Eng. 2009;91:460–467. doi: 10.1016/j.jfoodeng.2008.09.025. [DOI] [Google Scholar]
- Syamaladevi RM, Sablani SS, Tang J, Powers J, Swanson BG. Stability of anthocyanins in frozen and freeze-dried raspberries during long-term storage: in relation to glass transition. J Food Sci. 2011;76:414–421. doi: 10.1111/j.1750-3841.2011.02249.x. [DOI] [PubMed] [Google Scholar]
- Syamaladevi RM, Manahiloh KN, Muhunthan B, Sablani SS. Understanding the influence of state/phase transitions on ice recrystallization in Atlantic Salmon (Salmo salar) during frozen storage. Food Biophys. 2012;7:57–71. doi: 10.1007/s11483-011-9243-y. [DOI] [Google Scholar]
- Tedjo W, Taiwo K, Eshtiaghi M, Knorr D. Comparison of pretreatment methods on water and solid diffusion kinetics of osmotically dehydrated mangos. J Food Eng. 2002;53:133–142. doi: 10.1016/S0260-8774(01)00149-2. [DOI] [Google Scholar]
- Tovar B, Garcie H, Mata M. Physiology of pre-cut mango II. Evolution of organic acids. Food Res Int. 2001;34:705–714. doi: 10.1016/S0963-9969(01)00092-8. [DOI] [Google Scholar]
- Wen X, Hu R, Zhao JH, Peng Y, Ni YY. Evaluation of the effects of different thawing methods on texture, colour and ascorbic acid retention of frozen hami melon (Cucumis melo var. saccharinus) Int J Food Sci Technol. 2015;50:1116–1122. doi: 10.1111/ijfs.12755. [DOI] [Google Scholar]
- Xiao HW, Bai JW, Sun DW, Gao ZJ. The application of superheated steam impingement blanching (SSIB) in agricultural products processing-a review. J Food Eng. 2014;132:39–47. doi: 10.1016/j.jfoodeng.2014.01.032. [DOI] [Google Scholar]
- Xiao HW, Law CL, Sun DW, Gao ZJ. Color change kinetics of American ginseng (Panax quinquefolium) slices during air impingement drying. Dry Technol. 2014;32:418–427. doi: 10.1080/07373937.2013.834928. [DOI] [Google Scholar]
- Yadav AK, Singh SV. Osmotic dehydration of fruits and vegetables: a review. J Food Sci Technol. 2014;51(9):1654–1673. doi: 10.1007/s13197-012-0659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang H, Wang L, Gao H, Guo XN, Yao HY. Improvement of texture properties and flavor of frozen dough by carrot (Daucus carota) antifreeze protein supplementation. J Agr Food Chem. 2007;55:9620–9626. doi: 10.1021/jf0717034. [DOI] [PubMed] [Google Scholar]
- Zhao JH, Hu R, Xiao HW, Yang Y, Liu F, Gan ZL, Ni YY. Osmotic dehydration pretreatment for improving the quality attributes of frozen mango: effects of different osmotic solutes and concentrations on the samples. Int J Food Sci Technol. 2014;49:960–968. doi: 10.1111/ijfs.12388. [DOI] [Google Scholar]
- Zhao JH, Wen X, Peng Y, Meng XX, Kang JQ, Ni YY. Effects of osmo-dehydration on quality attributes of mangoes during frozen storage. Mod Food Sci Technol. 2014;30:225–968. [Google Scholar]
- Zhao JH, Liu F, Wen X, Xiao HW, Ni YY. State diagram for freeze-dried mango: freezing curve, glass transition line and maximal-freeze-concentration condition. J Food Eng. 2015;157:49–56. doi: 10.1016/j.jfoodeng.2015.02.016. [DOI] [Google Scholar]