Abstract
Blueberries are eaten fresh or after storing at room temperature, refrigerator or freezer but little is known about changes in food values of wild blueberries due to harvest dates and storage conditions. The aim of this study was to investigate the effect of harvest date and storage conditions of wild blueberries on berry quality and health related chemistry. We analyzed Vaccinium angustifolium, V. angustifolium var. nigrum, and V. myrtilloides native to NW Ontario, Canada harvested early and late in the season for total phenol (TP), anthocyanin contents (AC), and soluble solids to titratable acidity ratio storing at room temperature, refrigerator and freezer temperature. We also determined their antioxidant content and activity (ORAC). Late harvest and low temperature storage significantly increased TP and AC for most genotypes. In V. myrtilloides TP increased by 50, 44 and 45% respectively at late harvest, 14 days refrigerator and 90 days freezer storage. It also had significantly higher ORAC (22 and 33%) than the other two genotypes. Wild blueberry pickers and consumers can optimize health benefits and quality attributes of blueberries by customizing harvest protocols and choice of cultivar and storage in household fridge and freezer. Blueberry storage, at household fridge and freezer temperature, does not reduce its health benefits.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2586-8) contains supplementary material, which is available to authorized users.
Keywords: Vaccinium angustifolium, V. angustifolium var. nigrum, V. myrtilloides, ORAC, Total phenols, Total anthocyanins
Introduction
Blueberries are consistently ranked at the top of many health foods for their good taste and high antioxidant capacity (also called oxygen radical absorbance capacity, ORAC) (Prior et al. 1998; Kalt et al. 1999a, b). Dietary consumption of blueberry is beneficial for human health. Several antioxidant compounds of blueberry, both enzymatic and non-enzymatic can combat enhanced production of reactive oxygen species (ROS), which can damage proteins, lipids, and DNA. Enhanced ROS production in cells may cause schizophrenia (Mahadik and Mukherjee 1996), Alzheimer’s disease (Butterfield et al. 2002), and cancer (Waris and Ahsan 2006). Supplemented with polyphenol rich blueberry extract, mice exhibited significant cognitive enhancement and higher brain antioxidant properties (Papandreou et al. 2009). Recently Louis et al. (2014) reported that blueberry polyphenols prevent cardiomyocyte death by preventing calpain activation and oxidative stress in human cells.
Phenolics and anthocyanins are common secondary compounds in blueberries, which are strongly correlated with antioxidant activity (Kalt et al. 1999a; Connor et al. 2002a; Srivastava et al. 2007). The degree of antioxidant activity in foods depends on their antioxidant content and their structure (Zheng and Wang 2003). Both genetics and environmental conditions can alter these characteristics. Kalt and McDonald (1996) reported up to a 30% difference in the anthocyanin content of select varieties of V. angustifolium between two years of study, suggesting that environment plays a role in berry anthocyanin levels. Wang et al. (2008) found that organically grown high bush blueberries (V. corymbosum L.) contain significantly higher anthocyanins and phenolics, and higher antioxidant capacity than those grown conventionally. Environmental factors such as herbivory, light intensity, temperature, soil moisture, and soil fertility all can potentially alter the production of phenolic compounds (Jones and Hartley 2002). Some genotypes of blueberry vary in their capacity to synthesize phenolic compounds under different growing conditions (Howard et al. 2003). These authors found significantly higher variation in antioxidant activity and phenolic content between genotypes than between harvest years, suggesting that the genotype has a greater influence on these characteristics than the environment.
Three wild blueberry genotypes of lowbush blueberry [V. angustifolium Ait. and V. angustifolium var. nigrum (Wood) Dole] and velvetleaf blueberry, V. martilloides Michx are reported from NW Ontario (Moola and Mallik 1998; Mallik 2013). But, little is known about the berry chemistry of V. angustifolium var. nigrum and V. martilloides.
Antioxidant activity in blueberry can be affected by fruit maturity at harvest, berry size and post-harvest storage conditions (Prior et al. 1998; Kalt and McDonald 1996). The effect of fruit maturity at harvest varies by species and cultivar. For example, V. corymbosum showed higher levels of phenolics and antioxidant activity than V. angustifolium when harvested green or under ripe and the levels generally decline as the fruit matures, or remain relatively stable as ripening progress from purple to blue (Castrejon et al. 2008). In contrast, Prior et al. (1998) found a marked increase in phenolics and antioxidant activity in rabbit eye blueberries (V. ashei Reade) during ripening. In the case of V. angustifolium, anthocyanin content was found to be highest in ripe, lowest in under ripe, and intermediate in overripe berries (Kalt and McDonald 1996). These findings suggest that maturity at harvest plays a significant role in antioxidant contents and activity.
Berry size can affect total phenolics, total anthocyanins, and total antioxidant activity because phenolic compounds and anthocyanins are concentrated in the epidermal tissues of blueberries. Therefore, smaller berries with a larger surface area to volume ratio tend to have higher levels of these compounds than larger berries (Prior et al. 1998).
Storage conditions also influence total phenolics, total anthocyanins, and antioxidant activity in fruits. High bush blueberries stored between 1 and 20 °C showed a slight increase in antioxidant attributes (Kalt et al. 1999a; Connor et al. 2002a). In low bush blueberry (V. angustifolium) one study found no significant change (Kalt et al. 1999a), while another study (Kalt and McDonald 1996) found up to 18% increase in anthocyanin after two weeks of storage at 1 °C. Freezer storage (−18 to −35 °C) maintains antioxidant attributes at, or near, constant levels in highbush blueberry (Lohachoompol et al. 2004). Kevers et al. (2007) reported that fruits visually spoil, when stored at room temperature, before any significant lose of antioxidant activity. However, no blueberry genotypes were included in that study.
Our objective was to determine the effect of harvest date and storage conditions on quality and health related chemical contents of wild blueberry genotypes of NW Ontario. We hypothesized that: (1) blueberries of the same genotype, harvested later in summer, will have higher total phenols, anthocyanines and antioxidants than those harvested earlier because berry ripening stage and environmental conditions play a role in their synthesis, (2) low storage temperature will protect blueberry antioxidant and other chemical properties because metabolic processes are slow at low temperature, preventing breakdown of antioxidants, sugars and other compounds, and (3) atioxidant activity and other chemical characteristics of blueberry will vary among genotypes because genotypic differences can affect berry chemical profiles.
Materials and methods
Blueberry collection
Three wild blueberry genotypes (V. angustifolium, V. angustifolium var. nigrum, and V. myrtilloides) were collected by hand picking from 10 random locations in a 10 year-old clearcut forest near Nipigon, Ontario (49°1′39N, 87°52′21W; 463 m asl). Blueberry species and genotypes were identified using dichotomous keys. The berry samples for each genotype were pooled together for chemical analysis. Early summer harvesting was done on August 8, 2012 when the berries were firm and >90% turned blue. Late summer harvesting was done on August 27, 2012 when the berries were over ripe and soft. We placed the harvested blueberries in a cooler, brought back to laboratory and removed any under-ripe (green to pink) or damaged berries.
Storage treatments
In the laboratory berries of each genotype were divided into three portions, the first portion was kept at room temperature (21 °C) on a laboratory bench on a sheet of paper covered with another sheet of paper to protect from light, the second and third portions were stored in refrigerator (2 °C) and freezer (−15 °C) respectively keeping in thick plastic bags. Berries of the second harvest were also treated similarly. Storage times at room temperature were 0 (fresh), 4, 8 and 12 days; refrigerator 0 (fresh), 2 and 3 weeks; and freezer temperature 0 (fresh), 3 weeks and 3 months.
Fresh weight and size of berries
Fresh weight of 25 randomly picked berries per genotype was determined on a digital balance. Berry size was determined by measuring each of the 25 berries, (1) from stalk base to the tip and (2) perpendicular to this measurement using digital callipers. The two measurements were averaged to get mean diameter of the berries. Berry size was calculated from spherical volume (1.333 × π(pi) × (d/2)3) based on the average diameter.
Sample preparation for chemical analyses
Thirty grams sample of each berry genotype was put in a Magic Bullet blender and pulverized for 10 s. The sample was then made fine paste using a mortar and pestle. A sub-sample was taken for chemical extraction and the remainder was squeezed through two layers of cheesecloth, the juice was used for quality attributes as follows.
Total phenolic content
We followed Singleton and Rossi (1965) to determine total phenol. Briefly, blueberry extracts diluted with phosphate buffer at 1:2 ratio (100 µl), were mixed with 6 ml water and 500 µl Folin Ciocalteu reagent (Sigma Aldrich). After 2 min, 1.5 ml sodium carbonate (20% w/v) was added. The mixture was capped, shaken, and stored at room temperature in the dark, for 2 h. We measured the absorbance at 765 nm using a Genesys 10 UV Spectrophotometer. Gallic acid (3,4,5-trihydroxybenzoic acid) was used as standard. The results were expressed in mg gallic acid equivalents (GAE)/100 g fresh weight.
Total anthocyanin content
We determined total monomeric anthocyanin content using the pH differential method described by AOAC International (2005). Briefly, the content of monomeric anthocyanins was calculated using the extinction coefficient for cyanidin 3-glycoside (29,600 à) and expressed as Cy-3-Glu equivalents, mg/100 g fresh weight. Separate blueberry extract samples were diluted with pH 1.0 buffer (potassium chloride, 0.025 M) and pH 4.5 buffer (0.4 M sodium acetate), at a 1:12 ratio for the reading to fit within the linear range of the spectrophotometer. The absorbance of the diluted solutions was measured at 520 and 700 nm using a Genesys 10 UV spectrophotometer within 5 min of preparation. All test portions were read against a blank cell filled with distilled water.
Total soluble solids and titratable acidity
We used a handheld refractometer to measure total soluble solids of blueberry juice prepared as mentioned above at room temperature, 21 °C. We used approximately 1 g blueberry juice diluted to 25 ml with distilled water and titrated with 0.1 M NaOH to a pH 8.2 using 0.3 ml 1% phenolphthalein indicator. The amount of titrant required to reach this pH was used to calculate the titratable acid of the blueberry juice expressed in % citric acid. This was done in triplicate for each sample. Results for titratable acidity were expressed as g/l.
Antioxidant activity and antioxidant content
Extraction of antioxidants was done fresh every time in triplicate for each genotype. We used 1 g blueberry homogenate per extraction. Each 1 g sample was diluted with 10 ml 70% acetone, 28% distilled water, 2% formic acid (v/v/v) extraction solution. The mixtures were left at room temperature for 1 h, stirred every 5 min. After 1 h, the mixtures were filtered using Whatman Grade 1 (11 μm) qualitative filter paper. The solids were discarded. The extracts were used to determine total phenol and anthocyanin contents and antioxidant activity.
Oxygen radical absorbance capacity (ORAC) assay
This was done in a Perkin Elmer Luminescence Spectrometer LS50B (UK), fitted with a 4-position automatic cell changer connected to a 37 °C water bath according to Gillespie et al. (2007) modified for the Perkin Elmer LS50B. Fluorescein sodium salt solution was used as the fluorescent probe, 2,2′-azobis-2-methyl-propanimidamide dihydrochloride (AAPH) as the peroxyl radical generator, and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) as the standard. All solutions were prepared using 75 mM phosphate buffer, pH 7.0. The blueberry extract samples were diluted as required (V. angustifolium and V. angustifolium var. nigrum 1:300, V. myrtilloides 1:500) with phosphate buffer. Four hundred microliters of diluted sample, blank (phosphate buffer), or Trolox calibration solution (6.25–50 µmol) was mixed with 2.4 ml 0.08 μM Fluorescein solution in a 10 mm cuvette and incubated at 37 °C for 10 min. Then, 400 µl of 150 mM AAPH solution was added to the cuvette and fluorescence was recorded immediately, and every two min for up to 1 h. Each blueberry genotype, at each interval was tested in triplicate. The final ORAC values were calculated from the net area under the fluorescence decay curves and expressed as Trolox equivalents (TE) per gram fresh weight. FLWinLab var. 4.00.03 data acquisition software was designed for the Perkin Elmer LS50B.
Statistical analyses
The R statistical package (R Development Core Team 2009) was used for all statistical analyses. Analysis of variance (ANOVA) was used to evaluate genotypic response to harvest dates, and storage conditions with respect to berry size, total phenolic content, total anthocyanin content, total soluble solids to titratable acidity ratio and ORAC. Raw data were used for all parameters except total phenols data, which were Ln transformed to meet the normality requirement for ANOVA. Where differences were significant, we used Tukey’s HSD test to identify homogeneous subsets. ANOVA was also used to evaluate storage treatment effects on berry chemical characteristics. Pearson correlation coefficient among the variables was also determined. In all cases, p < 0.05 was considered significant.
Results
Effect of harvest date on berry quality and chemistry
The fresh weight and size of blueberry did not differ significantly among the genotypes in early harvest (p = 0.828, p = 0.777, respectively) (Table 1). However, at late harvest, fresh weight of V. myrtilloides was 33% lower (p < 0.0005), and their size was 32% lower (p = 0.001) than early harvest (Table 1). At the late harvest there was also a significant difference in berry fresh weight and size among genotypes (p < 0.0005), in the order of V. myrtilloides < V. angustifolium < V. angustifolium var. nigrum for both traits.
Table 1.
Fresh weight and size of three wild blueberry genotypes of NW Ontario harvested early (August 8) and late (August 28) in the season
| Berry genotype | Fresh weight (g) | Berry size (mm3) | ||
|---|---|---|---|---|
| Harvest date | Harvest date | |||
| Early | Late | Early | Late | |
| V. angustifolium | 0.425 ± 0.029b | 0.391 ± 0.033b | 161.20 ± 15.99b | 153.56 ± 15.11b |
| V. angustifolium var. nigrum | 0.436 ± 0.028b | 0.436 ± 0.030b | 155.54 ± 14.56b | 176.31 ± 15.98b |
| V. myrtilloides | 0.405 ± 0.023b | 0.270 ± 0.024a | 139.86 ± 9.36ab | 94.43 ± 10.46a |
Values represent mean ± standard error of mean for 25 randomly sampled berries. Letters in superscripts indicate significant difference (p < 0.05) among genotypes and harvest dates
Between harvest dates, for all genotypes fresh weight of berries was positively correlated with size and the total soluble solid to titratable acidity ratio. Fresh weight was negatively correlated with total anthocyanin and total phenolic contents. Berry size showed the same relationships as fresh weight (Appendix I of Electronic supplementary material).
Berry chemistry
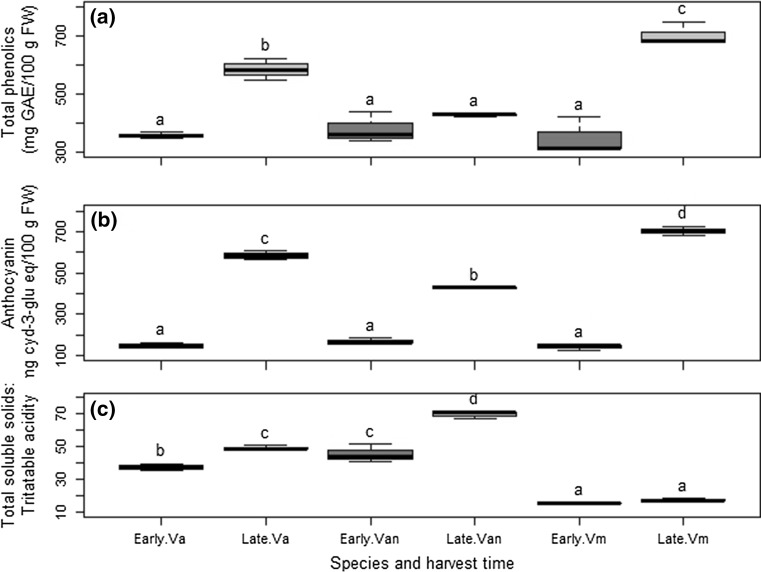
Total phenolic content of the blueberries increased between harvest dates for all genotypes (Fig. 1a). There was 50 and 39% increase in total phenols of V. myrtilloides and V. angustifolium respectively from early to late harvest (p = 0.001, p = 0.002, respectively). The increase in total phenolics of V. angustifolium var. nigrum berries was not significant between the two harvest dates. The total phenolic content of the berries varied between the three genotypes at the second harvest in the order V. myrtilloides > V. angustifolium > V. angustifolium var. nigrum (p < 0.05).
Fig. 1.
Effect of early and late harvest on a total phenol content, b anthocyanin content, and c total soluble solids to titratable acidity ratio of three wild blueberry genotypes of NW Ontario. Different letters above boxplots indicate significant difference (p < 0.05) among genotype and harvest dates. Va Vaccinium angustifolium, Vn V. angustifolium var. nigrum and Vm V. myrtilloides. Error bars ± 1 SD
Total anthocyanin content generally differed between harvest dates and berry genotypes (p = 0.006, p = 0.001, respectively) (Fig. 1b). In early harvest none of the blueberry genotypes differed significantly in total anthocyanin content but in the late harvest, total anthocyanin content of V. myrtilloides was significantly higher (35 and 46%, respectively) than V. angustifolium and V. angustifolium var. nigrum (p < 0.0005). Berries of V. angustifolium var. nigrum did not show any significant change in total anthocyanin content between the harvest dates. Total anthocyanin content and total phenolic content were positively correlated. Total anthocyanin content and total soluble solid to titratable acidity ratio were negatively correlated (Appendix I of Electronic supplementary material).
Vaccinium myrtilloides had markedly lower ratio of total soluble solids to titratable acidity than V. angustifolium var. nigrum and V. angustifolium (Fig. 1c). The ratio increased significantly between harvest dates for V. angustifolium var. nigrum and V. angustifolium (Fig. 1c). The order of increase in the ratio of total soluble solids to titratable acidity in the three genotypes were V. angustifolium var. nigrum > V. angustifolium > V. myrtilloides respectively 35, 24 and 12%.
Effect storage temperature and duration on blueberry
Room temperature storage
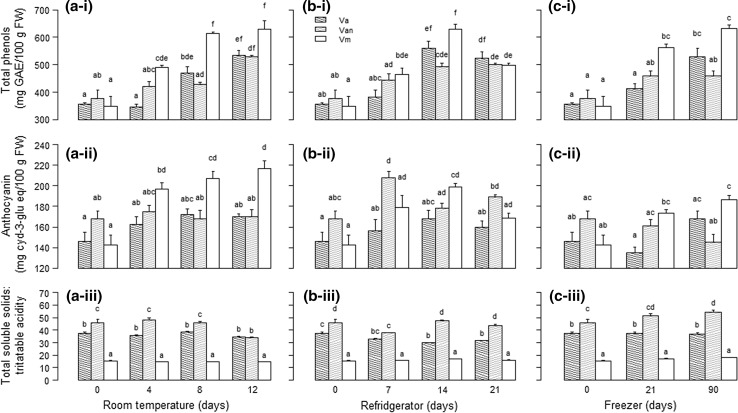
Total phenolic content of V. myrtilloides increased significantly during room temperature storage up to 8 days (Fig. 2a-i). In V. angustifolium and V. angustifolium var. nigrum the increases were slower than V. myrtilloides. Total phenolic content of V. angustifolium did not increase significantly until after 8 and 12 days of storage (p = 0.001). Significant increase in total phenolic content of V. angustifolium var. nigrum happened after 12 days (p = 0.009).
Fig. 2.
Effect of storage a at room temperature, b in refrigerator and c in freezer on i total phenol content, ii total anthocyanin content and iii total soluble solids to titratable acidity ratio of three wild blueberry genotypes of NW Ontario. Different letters above a histogram indicate significant difference (p < 0.05) among genotype and storage time. Va Vaccinium angustifolium, Vn V. angustifolium var. nigrum and Vm V. myrtilloides. Error bars ± 1 SD
Total anthocyanin content of V. myrtilloides differed significantly (p = 0.003) between storage durations. Its anthocyanin content was increased after 4 days of storage and continued to increase after 8 and 12 days of storage. On the other hand, V. angustifolium var. nigrum and V. angustifolium berries did not show significant change (p = 0.921, p = 0.189, respectively) in total anthocyanin content in room temperature storage (Fig. 2a-ii).
Vaccinium myrtilloides had significantly lower ratio of total soluble solids to titratable acidity than V. angustifolium var. nigrum and V. angustifolium (Fig. 2a-iii). The ratio of total soluble solids to titratable acidity did not change significantly in V. angustifolium berries during room temperature storage. This ratio decreased slightly for V. myrtilloides (p = 0.006) after 4 days of storage and remained relatively stable throughout the rest of the storage period. In V. angustifolium var. nigrum the ratio remained relatively stable for the first 8 days of room temperature storage then decreased significantly (p = 0.048) from day 8 to day 12 (Fig. 2a-iii).
Total anthocyanin and total phenolic content were positively correlated, and both were negatively correlated with the ratio of total soluble solids to titratable acidity (Appendix II of Electronic supplementary material).
Refrigerator storage
The total phenolic content of both V. angustifolium and V. myrtilloides berries increased (p = 0.008 and 0.027, respectively) during refrigerator storage (Fig. 2b-i). For V. angustifolium, total phenolic content at 14 and 21 days of storage was significantly increased from day one and the respective increases were 36 and 32% compared to the fresh samples. The same was also true for V. myrtilloides, with respective increases of 44 and 30%. Total phenolic content of V. angustifolium var. nigrum however, did not differ significantly between refrigerator temperature storage dates, until after 21 days (Fig. 2b-i).
The total anthocyanin content of V. angustifolium and V. myrtilloides did not change significantly (p = 0.477, and p = 0.092, respectively) during refrigerator storage (Fig. 2b-ii). However, in V. angustifolium var. nigrum, there was a significant (19%) increase (p = 0.043) in total anthocyanin content between day 1 and day 7 when it reached a peak in total anthocyanin content following that the values did not differ significantly from day 1.
As for the total soluble solids to titratable acidity ratio during refrigerator storage, V. myrtilloides had much lower value than the other two genotypes (Fig. 2b-ii). The ratio for V. angustifolium (p = 0.015) and V. myrtilloides (p = 0.012) changed significantly between storage dates but for V. angustifolium var. nigrum the change was not significant (p = 0.071). For V. angustifolium, this ratio was significantly lower on day 14 and 21, than day 1 (16 and 20% respectively). For V. myrtilloides, there was hardly any change in the ratio during the entire storage period (Fig. 2b-iii).
Freezer storage
After 90 days freezer temperature storage, all the blueberry genotypes had increase in total phenolic content with highest increase in V. myrtilloides followed by V. angustifolium (Fig. 2c-i). The increases were 45, 33 and 18% for V. myrtilloides, V. angustifolium and V. angustifolium var. nigrum respectively. However, for V. angustifolium var. nigrum the increases were not significant and the same was true for V. angustifolium up to 21 day storage (Fig. 2c-i).
In freezer temperature, total anthocyanin content of V. myrtilloides was increased 24% between 1 and 90 days of storage, V. angustifolium did not exhibit significant change (p = 0.058) but for V. angustifolium var. nigrum it was decreased by 14% (p = 0.037) (Fig. 2c-ii). In freezer temperature, the ratio of total soluble solids to titratable acidity was the lowest in V. myrtilloides. The ratio did not change significantly for V. angustifolium var. nigrum or V. angustifolium (p = 0.143 and p = 0.608, respectively) but for V. myrtilloides, the ratio was significantly higher between day 1 and day 21 (14%), and between day 1 and day 90 (14%) (Fig. 2c-iii).
ORAC of NW Ontario wild blueberries
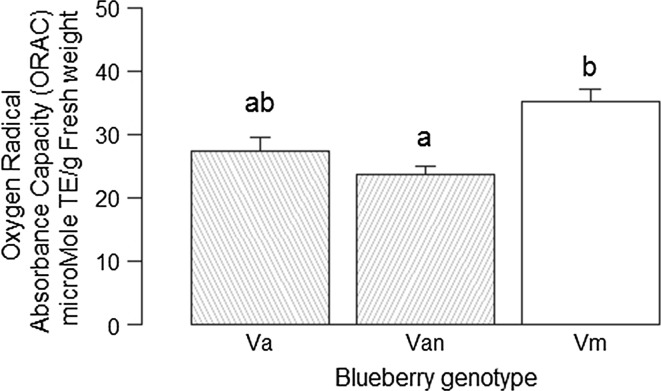
Berries of V. myrtilloides showed the highest antioxidant activity (35.13 µmol TE/g FW ± 1.91 SEM): 33% higher than V. angustifolium var. nigrum (23.57 µmol TE/g FW ± 1.39 SEM) and 22% higher than V. angustifolium (27.44 µmol TE/g FW ± 2.07 SEM) (Fig. 3). The ORAC value of V. angustifolium was similar to both V. angustifolium var. nigrum (p = 0.353) and V. myrtilloides (p = 0.054) but that of V. angustifolium var. nigrum and V. myrtilloides was significantly different (p = 0.010). Antioxidant activity was strongly positively correlated with total anthocyanin content and total phenolic content and it was strongly negatively correlated with the ratio of total soluble solids to titratable acidity (Appendix III).
Fig. 3.
Oxygen radical absorbance capacity (ORAC) of three NW Ontario blueberry genotypes determined from frozen berries and expressed as micromole trolox equivalents/g fresh weight. Different letters above histogram indicate significant difference (p < 0.05) among genotypes. Va Vaccinium angustifolium, Vn V. angustifolium var. nigrum and Vm V. myrtilloides. Error bars ± 1 SD
Discussion
Early and late harvest effects on berry quality and chemistry
For both V. angustifolium and V. myrtilloides, total phenol and anthocyanin contents increased significantly from early to late harvest dates. Vaccinium myrtilloides exhibited the most significant increase. These results support our first hypothesis that blueberries of the same genotype, harvested later in the season, would have increased total phenol, anthocyanin and antioxidant levels than those harvested earlier in the season. Our results correspond with others who noted significant increases in total anthocyanin and total phenolic compounds in successive harvests of mature blueberries (Prior et al. 1998; Castrejon et al. 2008). The large decrease in fresh weight and surface area between harvest dates for V. myrtilloides may relate to more substantial increases in antioxidant content. The decreases in berry weight and size reflect a decrease in water content of late harvest fruits and, thus, an increase in the concentration of anthocyanins and phenols within individual berries (Prior et al. 1998). However, this change in berry fresh weight and surface area does not completely explain the differences in total anthocyanin and total phenolic content. For example V. angustifolium showed increased antioxidants but did not show a significant change in fresh weight or surface area. Connor et al. (2002b) also noted that changes in berry size do not fully account for relatively higher antioxidant activity in small blueberry species such as V. myrtilloides. The continued increase in antioxidant levels may be a result of continued exposure to environmental stressors, such as light intensity and low soil moisture, which enhance the production of antioxidants within plant tissues. The more significant increases in V. myrtilloides may be a result of genotypic differences in the ability to synthesize phenolic compounds (Howard et al. 2003). Other potential sources of variation in antioxidant levels can be berry shape and skin thickness, which was not investigated in the present study.
From early to late harvest dates V. angustifolium var. nigrum showed a decrease in total anthocyanin content. It is possible that this genotype reached a peak in anthocyanin production and shifted toward synthesis of phenolic content other than anthocyanin later in the season. This is supported by the increase in total phenolic content of V. angustifolium var. nigrum between harvest dates. Because anthocyanins are a type of phenolic compound, the increase in total phenolic content would be a result of an increase in non-anthocyanin phenolic compounds and would have to make up for the decrease in anthocyanins. Perhaps pigment development reaches a maximum more quickly in this genotype and anthocyanin levels begin to decline as they are oxidized by ROS.
Late harvest of blueberries showed an increase in the ratio of total soluble solids to titratable acidity in all genotypes. Several authors have reported higher percent of soluble solids in overripe than in under ripe blueberries (Kalt and McDonald 1996; Castrejon et al. 2008). Increases in sugar concentration may be a result of increased conversion of starch to sugars as part of the natural ripening process of climacteric fruit (Beckles 2012). Sugar accumulation in fruits can also be influenced by genetic background and environmental factors such as solar radiation, temperature, and soil mineral content (Giovannoni 2001; Beckles 2012). In addition, decreases in titratable acidity have been reported during fruit ripening (Castrejon et al. 2008). Organic acids may provide carbon for the synthesis of phenolic compounds, so a decrease in titratable acidity could be the result of increased phenolic compound synthesis (Kalt et al. 1999a). Increases in total soluble solids and decreases in titratable acidity combine to increase the ratio of total soluble solids to titratable acidity. The large difference in this ratio between V. myrtilloides and V. angustifolium is likely a result of genetic differences in sugar accumulation (Giovannoni 2001). A higher ratio of total soluble solids-to-titratable acidity received higher consumer acceptability in terms of flavor (Vasquez-Araujo et al. 2010). Considering the increase in flavor acceptability and general increase in antioxidant levels, from a marketing perspective it may be beneficial for producers to alter their harvest practices to optimize these parameters.
Effect storage conditions on berry quality and chemistry
The results of storage treatments on blueberry chemical contents provide partial support to our second hypothesis that low storage temperature protects blueberry antioxidant and other chemical properties. During refrigerator storage, total anthocyanin content of V. myrtilloides increased significantly, while no significant changes in anthocyanin content occurred in either of the other genotypes. After 90 days freezer temperature storage, all the three blueberry genotypes had significant increase in total phenolic content. The increases were 45, 33 and 18% for V. myrtilloides, V. angustifolium and V. angustifolium var. nigrum respectively (Fig. 2). Due to genetic similarity it is expected that berries of V. angustifolium and V. angustifolium var. nigrum would respond similarly to storage conditions and it is not surprising that V. myrtilloides responds differently. After harvest, respiratory metabolism continues in blueberries at room temperature and antioxidant synthesis continues in V. myrtilloides correspondingly.
Increases in total phenolic content were also observed in all three genotypes during room temperature storage. The increases are not likely a result of water loss during storage as Connor et al. (2002a) also saw increases in total phenolic content of blueberries with corrections for changes in fresh weight. The reason for increases in total phenolic content during room temperature storage of the berries is unclear but might be related to water loss from the berries and metabolic breakdown due to respiration.
The increases in total phenolic content and lack of increase in total anthocyanin content exhibited by both V. angustifolium and V. angustifolium var. nigrum suggests that phenolic compounds play an important role in these genotypes after berry maturation. These results, combined with the decrease in total anthocyanin content of V. angustifolium var. nigrum between harvest dates, also suggest that a peak is reached in anthocyanin production in V. angustifolium genotypes, after which there is a possible shift to production of non-anthocyanin phenolic compounds.
During room temperature storage, V. angustifolium var. nigrum and V. myrtilloides both showed decrease in the ratio of total soluble solids to titratable acidity as a result of significant increase in titratable acidity. This may be a result of the breakdown of cell walls as the fruit softens, making acids more accessible compared to fresh berries (Kalt et al. 2003).
During freezer storage, there were no significant increases in total anthocyanin content. While total phenolic content did increase for all three genotypes, the increases were much smaller than those observed during room temperature storage. This is likely a result of slow respiratory metabolism at lower temperature storage (Kalt et al. 1999a). As respiratory metabolism slows down, there is a concomitant decrease in available energy for antioxidant synthesis and a decreased need for the synthesis of antioxidants because the production of ROS is also slowed (Mittler et al. 2004).
During freezer storage, production of phenolic compounds was further slowed, by the decreased rate of respiratory metabolism. The increases witnessed after 12 days at room temperature were just reached by V. myrtilloides at 3 months storage in the freezer and were not reached by the other genotypes. There were no significant increases in anthocyanin content during freezer storage, but V. angustifolium var. nigrum did experience a significant decrease in total anthocyanin content by the third month of storage. The stability of anthocyanins is strongly influenced by their structure (Srivastava et al. 2007). Perhaps, the structures of V. angustifolium var. nigrum anthocyanins are such that they are less stable than the anthocyanins of the other genotypes under the influence of freeze and thaw processes. Vaccinium angustifolium var. nigrum also had the highest ratio of total soluble solids to titratable acidity, which has been linked with decreased shelf-life in blueberries (Ballinger and Kushman 1970). Increased fruit decay, resulting from a high ratio of total soluble solids to titratable acidity, is another possible explanation for the decrease in total anthocyanin content.
Lastly, there was an increase in the total soluble solids to titratable acidity ratio in V. myrtilloides and V. angustifolium var. nigrum during freezer storage. This is opposed to the decreases witnessed during room and refrigerator temperature storage. It is possible that freezing and thawing may have released sugars by rupturing cell walls, making them more available than in the fresh samples. These results support our second hypothesis that low storage temperature protects blueberry chemical properties but the degree of protection varies depending on the genotype.
Antioxidant activity
The antioxidant activity (ORAC) results supports our third hypothesis that ORAC and other chemical characteristics of blueberry vary among genotypes. The ORAC assay was conducted with early harvest berries that were kept frozen for three months. Although there was no significant difference in total anthocyanin content or total phenolic content at harvest, antioxidant activity of V. myrtilloides was significantly higher than the other two genotypes. The total phenolic content of frozen berries of V. myrtilloides was significantly higher than the fresh berries (Fig. 2). The total phenolic content of V. myrtilloides was 10% higher than V. angustifolium and 22% higher than V. angustifolium var. nigrum after three months of freezer storage. The strong positive relationship between ORAC and total phenolic content indicates that this increase may be due to some of the difference in ORAC between V. myrtilloides and other genotypes (Fig. 3). However, the increase in total phenolic content alone, does not fully account for the V. myrtilloides ORAC being 22 and 33% higher than V. angustifolium and V. angustifolium var. nigrum, respectively. The difference is not likely due to difference in maturity stage (berries were harvested at the same time) because the phenological fruiting stage occurs at approximately the same time in V. myrtilloides and V. angustifolium (Moola and Mallik 1998). Also, because the total anthocyanin and total phenolic content were not significantly different between genotypes at the early harvest date (Fig. 1), suggesting berries of each genotype were at approximately the same maturity stage at harvest.
Alternatively, the difference in ORAC between V. myrtilloides and the other genotypes analyzed may be a result of differences in the anthocyanin or phenolic profile of the individual genotypes, rather than the total contents. There are more than 24 different anthocyanins found within berries and each species has a different set of dominant anthocyanin compounds, with differing antioxidant abilities (Kahkonen et al. 2003). The anthocyanin profile of V. myrtilloides and V. angustifolium has been found to be quite similar (Kalt and McDonald 1996b), so it is more likely that the different ORAC values are a result of different compositions of non-anthocyanin phenolic compounds or other chemical components between species. Louis et al. (2014) showed by in vitro experiment with human heart cells that pretreatment with blueberry (V. angustifolium) polyphenols was able to reverse the oxidative stress induced by a cardiac inhibitor, norepinephrine through lowering of superoxide dismutase and catalase activities. Similar enzymatic protective mechanism might operate in other blueberry species. However, it is necessary to analyze the enzymatic profiles of wild blueberries of NW Ontario specially those of V. myrtilloides to get a full picture of enzymatic defense system of these blueberries against cellular oxidative stress.
Conclusion
We found significant difference in berry chemical composition among genotypes of NW Ontario blueberries and between early and late harvest dates. Consumers and growers can optimize health benefits and quality attributes by customizing blueberry harvest protocols and choice of genotype. The storage treatment results indicate that one can expect an increase in antioxidant related health benefits by storing blueberries in household fridge and freezer. There were some decreases in the total soluble solids to titratable acidity ratio that may affect blueberry test and flavor, but these decreases varied by genotype and storage condition and did not occur across all treatments. Antioxidant activity (ORAC) of V. myrtilloides was significantly higher than that of V. angustifolium and V. angustifolium var. nigrum. Marketing initiatives can be extended to indicate this and also noting that blueberry storage, at any temperature, does not reduce its health benefits.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by a Grant from the Agricultural Research Institute of Ontario (Grant # 9227) awarded to AUM. We thank Toby Braithwaite, Veronica Berini and Saiful Khan for field assistance and data analysis.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2586-8) contains supplementary material, which is available to authorized users.
References
- AOAC Association of Official Analytical Chemists. Official method of Analysis of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines. J Assoc Official Anal Chem. 2005;88:1269. [PubMed] [Google Scholar]
- Ballinger WE, Kushman LJ. Relationship of stage of ripeness to composition and keeping quality highbush blueberries. J Am Hort Soc. 1970;95:239–242. [Google Scholar]
- Beckles D. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharv Biol Tech. 2012;63:129–140. doi: 10.1016/j.postharvbio.2011.05.016. [DOI] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. doi: 10.1016/S0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Castrejon ADR, Eichholz I, Rohn S, Kroh LW, Huyskens-Keil S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008;109:564–572. doi: 10.1016/j.foodchem.2008.01.007. [DOI] [Google Scholar]
- Connor AM, Luby JJ, Hancock JF, Berkheimer S, Hanson EJ. Changes in fruit antioxidant activity among blueberry cultivars during cold-temperature storage. J Agric Food Chem. 2002;50:893–898. doi: 10.1021/jf011212y. [DOI] [PubMed] [Google Scholar]
- Connor AM, Luby JJ, Tong CBS. Variability in antioxidant activity in blueberry and correlations among different antioxidant activity assays J Am Hort Soc. 2002;127(2):238–244. [Google Scholar]
- Gillespie KM, Chae JM, Ainsworth EA. Rapid measurement of total antioxidant capacity in plants. Nat Protoc. 2007;2(4):867–870. doi: 10.1038/nprot.2007.100. [DOI] [PubMed] [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Ann Rev Plant Physiol Mol Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Howard LR, Clark JR, Brownmiller C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J Sci Food Agric. 2003;83:1238–1247. doi: 10.1002/jsfa.1532. [DOI] [Google Scholar]
- Jones CG, Hartley SE. A protein competition model of phenolic allocation 1999. Oikos. 2002;86:27–44. doi: 10.2307/3546567. [DOI] [Google Scholar]
- Kahkonen MP, Heinamaki J, Ollilainen V, Heinonen M. Berry anthocyanins: isolation, identification and antioxidant activities. J Sci Food Agric. 2003;83:1403–1411. doi: 10.1002/jsfa.1511. [DOI] [Google Scholar]
- Kalt W, McDonald JE. Chemical composition of lowbush blueberry cultivars. J Am Soc Hort Sci. 1996;121(1):142–146. [Google Scholar]
- Kalt W, Forney CF, Martin A, Prior RL. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J Agric Food Chem. 1999;47:4638–4644. doi: 10.1021/jf990266t. [DOI] [PubMed] [Google Scholar]
- Kalt W, McDonald JE, Ricker RD, Lu X. Anthocyanin content and profile within and among blueberry species. Can J Pl Sci. 1999;79:617–623. doi: 10.4141/P99-009. [DOI] [Google Scholar]
- Kalt W, Lawand C, Ryan DAJ, McDonald JE, Donner H, Forney CF. Oxygen radical absorbance capacity, anthocyanin and phenolic content of highbush blueberries (Vaccinium corymbosum L.) during ripening and storage 2003. J Am Soc Hort Sci. 2003;128(6):917–923. [Google Scholar]
- Kevers C, Falkowski M, Tabart J, Defraigne J, Dommes J, Pincemail J. Evolution of antioxidant capacity during storage of selected fruits and vegetables. J Agric Food Chem. 2007;55(21):8596–8603. doi: 10.1021/jf071736j. [DOI] [PubMed] [Google Scholar]
- Lohachoompol V, Srzednicki G, Craske J. The change of total anthocyanins in blueberries and their antioxidant effect after drying and freezing. J Biomed Biotech. 2004;5:248–252. doi: 10.1155/S1110724304406123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis XL, Thandapilly SJ, Kalt W, Tymchuk MV, Aloud BM, Raj P, Le H, Netticadan T. Blueberry polyphenols prevent cardiomyocyte death by preventing calpain and oxidative stress. Food Funct. 2014 doi: 10.1039/c3fo60588d. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia: a review. Schizo Res. 1996;19(1):1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Mallik AU (2013) Determining health benefits, horticultural and market potential of wild blueberry ecotypes from northwestern Ontario. OMAFRA Project No. SR0909 Progress report, March
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9(10):490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Moola FM, Mallik AU. Phenology of Vaccinium spp. in a black spruce (Picea mariana) plantation in northwestern Ontario: possible implications for the timing of forest herbicide treatments. Can J For Res. 1998;28:1579–1585. doi: 10.1139/x98-151. [DOI] [Google Scholar]
- Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, Lamari FN. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res. 2009;198(2):352–358. doi: 10.1016/j.bbr.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Prior RL, Cao G, Martin A, Sofic E, McEwen J, O’Brien C, Lischner N, Ehlenfeldt M, Kalt W, Krewer G, Mainland CM. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem. 1998;46(7):2686–2693. doi: 10.1021/jf980145d. [DOI] [Google Scholar]
- R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
- Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Ecol Viticult. 1965;16(3):144–158. [Google Scholar]
- Srivastava A, Akoh CC, Yi W, Fischer J, Krewer G. Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles. J Agric Food Chem. 2007;55:2705–2713. doi: 10.1021/jf062914w. [DOI] [PubMed] [Google Scholar]
- Vasquez-Araujo L, Chambers E, IV, Adhikari K, Carbonell-Barrachina AA. Sensory and physiochemical characterization of juices made with pomegranate and blueberries, blackberries, or raspberries. J Food Sci. 2010;75(7):S398–S404. doi: 10.1111/j.1750-3841.2010.01779.x. [DOI] [PubMed] [Google Scholar]
- Wang SY, Chen C, Sciarappa Wang CY, Camp MJ. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J Agric Food Chem. 2008;56:5788–5794. doi: 10.1021/jf703775r. [DOI] [PubMed] [Google Scholar]
- Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinogen. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Wang SY. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J Agric Food Chem. 2003;51(2):502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.