Abstract
In the present study, processing parameters for the extraction of phenolic rich sea buckthorn seed (SBTE) extract were optimised using response surface method and subjected for in vitro efficacy viz. total phenolic, ABTS, DPPH and SASA activity. The optimised model depicted MeOH as a solvent at 60% concentration level with a reaction time of 20 min and extracting temperature of 55 °C for the highest yield and total phenolic content. The efficacy of different concentration of obtained SBT was evaluated in raw ground pork as a model meat system on the basis of various physico-chemical, microbiological, sensory quality characteristics. Addition of 0.3% SBTE significantly reduced the lipid peroxidation (PV, TBARS and FFA) and improved instrumental colour (L*, a*, b*) attributes of raw ground pork during refrigerated storage of 9 days. Results concluded that SBTE at 0.3% level can successfully improve the oxidative stability, microbial, sensory quality attributes in the meat model system.
Keywords: Phyto-extracts, Antioxidant, Pork, Sea buckthorn, RSM
Introduction
Sea buckthorn (Hippophae rhamnoides L.) is a deciduous and thorny bush, belongs to Eurasian nitrogen-fixing actinomycetes family and found naturally at high altitude of above 7000 ft. Sea buckthorn are among the most nutritious and vitamin-rich berries found in the plant kingdom (Li and Schroeder 1996). Recently, it has attracted considerable attention from researchers around the world mainly due its nutritional and medicinal value for use in foods, cosmetics, and medicinal materials. It has been reported to contain various bioactive ingredients in the juice, fruit, pulp etc. (Wagh et al. 2015; Beveridge et al. 1999).
The retrieval of any marked compound from plant/food products can be done in 5 different phases that follow the analytical chemistry. These include: (a) pre-treatment, (b) isolation of high-molecular from low-molecular compounds, (c) extractions, (d) purification/isolation and (e) encapsulation (Galanakis 2014). Out of which extraction plays most important role in the recovery and purification of active ingredients. Various techniques have been employed to extract bioactive ingredients from sea buckthorn juice, roots, fruits, seeds include Soxhlet extraction (Negi et al. 2005), supercritical CO2 extraction etc. However, the efficiency of extraction protocols varied with many factors such as the type of extraction solvent, temperature, time, number of steps, matrices of material and particle size and hence, optimization of these factors for efficient recovery of potential bioactive ingredients is of paramount concern.
Lipid oxidation is regarded as one of the main reason for functional, sensory and nutritional quality deterioration in meat and meat products. Natural antioxidants are considered as safe option to retard the rate of lipid oxidation in meat and meat products. In the recent years, various plant based antioxidant has been explored for extending the storage stability of meat products (Wagh et al. 2015; Kumar et al. 2015; Ye et al. 2015).
The present study was focused on the optimization of extraction conditions (solvent concentration, temperature and extraction time) to extract bioactive compounds from sea buckthorn seeds using a RSM with employing Central Composite Rotatable Design (CCD), in order to develop the best extraction conditions to obtain phenolic rich antioxidants from sea buckthorn seeds, evaluated on the basis of total phenolic content (TP), 2,2-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) radical scavenging activity (ABTS), 2,2-Diphenyl-1-picrylhydrazyl radical scavenging ability (DPPH) and superoxide radical scavenging activity (SASA). The efficacy of the developed sea buckthorn seed extract (SBTE) was further evaluated in model meat system using raw ground pork in relation to physico-chemical qualities, oxidative stability and instrumental colour attributes microbiological and sensory characteristics during refrigerated storage (4 ± 1 °C) under aerobic packaging conditions.
Materials and methods
Plant material and extraction process
Sea buckthorn berries were collected from the North-Western Himalayan region (Himachal Pradesh, India) (Latitude: Longitude N 34° 0′ 0″: E 78° 0′ 0″34) and seeds were obtained after deseeding the berries by passing through stainless steel sieve and dried in a cabinet dryer. Dried seeds were powdered into a particle size of 60–80 mesh in Willey Grinder (Arthur H. Thomas Type), New Delhi, India and stored in dark prior to analysis.
The extracting methanol concentration, time and temperature were predetermined using RSM software (Design Expert 9.0, Stat ease Inc., Minneapolis, USA) as per the experimental design. Ten grams of the sea buckthorn dried powder was weighed and mixed with 100 mL of designed extracting solvent conc. in a conical flask. The mixture was shaken at constant rate of 300 rpm using a shaker (Narang Scientific works Pvt. Ltd., New Delhi, India) for overnight. The obtained extracts were filtered through a Whatman filter paper No. 1 in order to obtain a particle free extract and evaporated using a rotary evaporator (Yamato Scientific America, Inc., Santa Clara, USA) under vacuum at pre-designed temperature and a rotation speed of 100 rpm for a pre-designed period. The resultant clear solution was collected in an amber coloured reagent bottle. The extract was stored at −20 °C for further analysis. Before the incorporation of SBTE into raw ground pork, methanol was completely removed from SBTE in a rotary evaporator until methanol condensation on the cooling coil of the evaporator was no longer observed.
Raw ground pork
Castrated Large White Yorkshire pigs (8–10 months’ age, weighing 80–100 kg) were selected from Instrumental Livestock Farm, Guru Angad Dev Veterinary and Animal Sciences University (GADVASU), Ludhiana, India and humanly slaughtered as per standard procedure in accordance with animal welfare and ethics guidelines. The dressed carcasses were brought to laboratory and hot deboned manually. After removal of external fat, fascia and connective tissue meat was packaged in low density polyethylene film (LDPE) bags and stored at −18 ± 1 °C till further use.
Before use, frozen deboned meat was placed at refrigerated temperature for partial thawing, then cut into small cubes and minced by using 6 mm grinder plate in a meat mincer (ESKIMO grinder, MEW 714-H82, MADO GmbH, Dornhan, Germany) to obtain raw ground pork. The obtained ground meat was divided into four different batches and was minced with pork back fat (10%), ice (5%) and salt (2%). The first batch designated as control (without SBTE) samples while the other three batches contained 0.1%; (T1), 0.2%; (T2) and 0.3% (T3) SBT extracts, respectively.
Analytical methods
Total phenolic
The polyphenol content of SBTE was quantified by Folin–Ciocalteau’s reagent assay and expressed as gallic acid equivalents (mgGAE/g) (Yuan et al. 2005). Briefly, 100 µl of extract was mixed with 2 ml of 2.0% Na2CO3 buffer and incubated at room temperature for 2 min. The total volume was made to 2.4 ml by adding distilled water. After addition of 100 µl of 1 N Folin–Ciocalteau’s reagents the reactions tube was further incubated for 30 min at room temperature, and the absorbance was read at 720 nm. The amount of total phenolic content was determined by a standard calibration curve (y = 0.001x−0.009 and r2 = 0.992; where y = absorbance, x = gallic acid concentration, and r2 = correlation coefficient) constructed using standard gallic acid solutions from 250 to 5000 µg/g concentrations.
ABTS radical scavenging activity
The free radical scavenging activity of sample extracts was determined by the ABTS radical cation decolourisation assay (Re et al. 1999). ABTS was dissolved in water to a 7 mM concentration. The ABTS radical cation (ABTS) was produced by reacting ABTS stock solution with 2.45 mM potassium persulphate (final concentration) and the mixture was allowed to stand in the dark at room temperature for 16 h before use. Because ABTS and potassium persulphate react stoichiometrically at a ratio of 1:0.5 (mol/mol), this results in complete oxidation of ABTS. Oxidation of ABTS commenced immediately, but the absorbance was not maximum and stable until 6 h had elapsed. The radical was stable in this form more than 2 days, when stored in the dark at room temperature. Prior to use, the stock solution was diluted with ethanol to an absorbance of 0.70 at t0 (t = 0 min) and equilibrated at 30 °C exactly 6 min after the initial mixing. About 4.9 ml of ABTS working standard solution was mixed with 100 μl of plant extract and the absorbance was measured after 20 min (t20) at 734 nm. The ABTS activity was calculated by using the formula: ABTS activity (%) = [(At0–At20)/At0] × 100. Gallic acid (200–500 μM/ml) was used as a standard.
DPPH radical scavenging activity
The DPPH radical scavenging activity of extracts was estimated by the method of Kato et al. (1988). DPPH can make stable free radicals in aqueous or ethanol solution. However, fresh DPPH solution was prepared in ethanol before every measurement. About 3.9 ml of DPPH (250lM) solution was taken in a test tube, diluted with 1 ml of 0.1 M Tris–HCl buffer (pH 7.2) and then mixed well with 100ll of curry/mint leaf extracts. The absorbance in time t = 0 min (t0) was measured at 517 nm. The sample tubes were then incubated at room temperature (27 ± 1 °C) under dark for measuring the absorbance in time t = 20 min (t20). Ethanol was used as blank sample. The free radical scavenging activity was calculated as a decrease in absorbance from the equation: Scavenging activity (%) = 100-(At20/At0)100. Gallic acid (50–250 μM/ml) was used as a standard antioxidant.
Superoxide anion scavenging activity (SASA)
Superoxide anionic scavenging activity was determined based on the reduction of nitro blue tetrazolium (NBT) in the presence of NADH and phenozoniummethosulphate (PMS) under aerobic condition (Kumar and Chattopadhyay 2007). The reduction mixture contained 150 µl NBT (100 µM), 450 µl NADH (100 µM) with 400 µl sample extract. Total volume was made up to 1 ml with distilled water and then added 1.9 ml of Tris–HCl buffer (0.02 M, pH 8.0). The reaction was started with the addition of 100 µl of PMS (100 µM) in tubes and were kept under aerobic condition, and finally the absorbance change was recorded at 560 nm after 20 min. Percent inhibition was calculated against a blank without the extract. The superoxide anion scavenging activity was calculated using the formula: SASA = Absorbance of control- Absorbance of test/Absorbance of control X 100
Analytical methods for raw ground pork
pH and water activity (aw)
The pH (n = 6) was estimated using digital pH meter (Lab-India Analytical Instruments Pvt. Ltd, Thane, India). Ten gram of sample was homogenized in homogenizer (T-25D S22 digital ultra-TURRAX, Staufen, Germany) with 50 ml distilled water. The pH of the suspension was recorded by dipping combined glass electrode into it. Water activity was determined by using hand held portable digital water activity meter (ROTRONIC Hygro-Palm AW1, Bassersdorf, Switzerland). Samples were filled up to (≈80%) in moisture free sample cup and water activity was recorded as per specifications.
Peroxide value (PV) and free fatty acid (FFA)
The peroxide value and free fatty acid were measured as per procedure described by Koniecko (1979).
Thiobarbituric acid reactive substances (TBARS)
The evaluation of lipid stability was performed by measuring TBARS during storage following the method of Witte et al. (1970) with suitable modifications. Briefly, 10 g of sample were triturated with 25 ml of precooled 20% trichloroacetic acid (TCA) for 2 min. The content was then quantitatively transferred into a beaker by rinsing with 25 ml of chilled distilled water. They were well mixed and filtered through Whatman filter paper No. 1. Three millilitres of TCA extract (filtrate) were mixed with 3 ml of TBA reagent (5 mM) in test tubes and placed in a dark room for 16 h. A blank sample was made by mixing 3 ml of 10% TCA and 3 ml of 5 mM TBA reagent. The absorbance was measured at a fixed wavelength of 532 using a UV–VIS spectrophotometer (SHIMADZU, UV spectrophotometer UV-1800, Kyoto, Japan). The TBA value was calculated as mg malonaldehyde per kg of sample by multiplying the absorbance value with a factor of 5.2.
Instrumental colour profile
Instrumental colour profile was measured using Lovibond’s Tintometer (Lovibond RT-300, Reflectance Tintometer, Amesbury, UK) set at 2° of cool white light (D65) and known as ‘L*’, a*, and b* values. ‘L*’ value denotes (brightness 100) or lightness (0), a* (+redness/−greenness) and b* (+yellowness/−blueness). The mean (n = 24) and standard error were estimated accordingly.
Sensory evaluation
The sensory panel consisted of seven experienced and trained members selected among the pool of faculty members and postgraduate students. The test samples were presented to the panellists after assigning the suitable codes and evaluated on 1, 3, 5, 7 and 9 days of storage for appearance and colour, odour and overall acceptability using 5-point descriptive scale ranging from 1 as extremely undesirable to 5 as extremely desirable (Keeton 1983). The samples were evaluated in three sitting for this experiment and 21 observations were recorded.
Microbiological qualities
Standard Plate Counts (SPC), Psychrophilic count, Yeast and Mould Counts and Coliforms Count in the samples were enumerated following the methods as described by American Public Health Association (APHA 2001) and data were expressed as log10 colony forming units (CFU) per gram sample.
Experimental design
Central composite design (CCD) employed using RSM to identify optimum levels of three variables: methanol concentration (%), extraction temperature (°C) and extraction time (min.) regarding four responses: Total phenolic contents (TP), 2-2-azinobis-3ethylbenthiazole-6-sulphonic acid radical cations (ABTS), 1,1-diphenyl-2-picryl-hydrazil radical (DPPH) and superoxide anion scavenging activity (SASA) radical scavenging activity in the sea buckthorn extracts. Ranges of methanol concentration (X1), temperature (X2) and time (X3) and the central points were selected based on preliminary experimental results. The run (20 runs) of the experiments were conducted in a random order and the data was analysed by multiple regressions using least-square method as per method given by Myers and Montgomery (2002). The response function (Y) was partitioned into linear, quadratic, and interactive components and the experimental data were fitted to the second-order regression equation as follows:
where, Y = Independent responses; βo, βi, βii and βij = Regression coefficient of the process variables for the intercept, linear, quadratic, and cross product terms, respectively. Xi and Xj are coded independent variables. The fitness of the polynomial model equation to the responses was evaluated by the correlation coefficient i.e. R2 as well as by the lack of fit using the F-test with 5% level of significance.
Statistical analysis
The experimental results of the response surface design were analysed using Design Expert® 9.0 (Stat-Ease Inc., Minneapolis, MN, USA) programs. The significance level was based on a confidence level of 95.0%. Data obtained during ground pork storage analysis was analysed by two-way analysis of variance (ANOVA) using SPSS V. 22 (SPSS Institute Inc., Cary, NC, USA) software package and differences among mean values were obtained by Duncan’s multiple range test. The whole set of the experiment was repeated three times and each parameter was evaluated in duplicate and results were expressed as mean ± standard error (n = 6). Significance was defined at a level of p < 0.05 as per the standard methods.
Results and discussion
Optimization of extraction protocols by RSM
Fitting the models
Each response was evaluated as a function of linear, quadratic and interaction of the independent variables including total phenolic contents (Y1), ABTS inhibition (Y2), DPPH scavenging ability (Y3) and SASA activity (Y4) in sea buckthorn seeds extracts. The quality of the generated model was evaluated by ANOVA, R2, and the lack of fit of the model. ANOVA results in Table 1 suggested that the model had very high F-values (9.34–17.11) and very low p values (<0.0008) for all four responses. The coefficients of multiple determinations (R2) of 0.9081, 0.9390, 0.8937 and 0.9329 were obtained for the response of TPC, ABTS, DPPH and SASA activity, respectively. It indicated that the second-order polynomial models were adequately represented by the respective experimental data.
Table 1.
ANOVA and regression coefficients of the second-order polynomial model for the response variables (actual values)
| Source | df | TP (mg GAE/100 g) | ABTS (%) | DPPH (%) | SASA (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | Sum of squares | p value | Coefficient | Sum of squares | p value | Coefficient | Sum of squares | p value | Coefficient | Sum of squares | p value | ||
| Model | 9 | 128.23 | 310.07 | 0.0004 | 85.32 | 940.69 | <0.0001 | 67.56 | 161.17 | 0.0008 | 77.35 | 4.72 | <0.0001 |
| Linear | |||||||||||||
| X1 | 1 | 2.39 | 77.82 | 0.0006 | 5.51 | 415.03 | <0.0001 | 1.18 | 19.10 | 0.0102 | 0.30 | 1.22 | 0.0001 |
| X2 | 1 | 0.42 | 2.39 | 0.4031 | 0.78 | 8.28 | 0.2714 | 0.50 | 3.48 | 0.2077 | 0.052 | 0.037 | 0.3187 |
| X3 | 1 | 1.68 | 38.65 | 0.0056 | 1.56 | 33.26 | 0.0418 | 0.79 | 8.56 | 0.0607 | 0.21 | 0.60 | 0.0018 |
| Quadratic | |||||||||||||
| X1 X2 | 1 | 0.060 | 0.029 | 0.9256 | −0.25 | 0.49 | 0.7839 | 0.82 | 5.41 | 0.1239 | 7.500 | 4.500 | 0.9106 |
| X1 X3 | 1 | −1.96 | 30.73 | 0.0107 | −1.73 | 23.98 | 0.0757 | −2.32 | 42.97 | 0.0008 | −0.24 | 0.48 | 0.0037 |
| X2 X3 | 1 | −0.78 | 4.87 | 0.2414 | −1.80 | 25.88 | 0.0666 | 0.18 | 0.26 | 0.7208 | −0.097 | 0.076 | 0.1654 |
| Interaction | |||||||||||||
| X21 | 1 | −3.27 | 153.64 | <0.0001 | −5.25 | 397.61 | <0.0001 | −2.31 | 77.06 | <0.0001 | −0.40 | 2.27 | <0.0001 |
| X22 | 1 | −0.027 | 0.010 | 0.9556 | 0.59 | 5.07 | 0.3836 | 1.566 | 3.533 | 0.9967 | 8.030 | 9.292 | 0.8719 |
| X23 | 1 | −0.51 | 3.71 | 0.3024 | 0.69 | 6.84 | 0.3150 | −0.70 | 7.07 | 0.0839 | −0.052 | 0.039 | 0.3085 |
| Residual | 10 | 31.39 | 61.10 | 19.18 | 0.34 | ||||||||
| Lack of fit | 5 | 10.44 | 0.7686 | 25.50 | 0.6383 | 10.47 | 0.4220 | 0.16 | 0.5445 | ||||
| Pure error | 5 | 20.95 | 35.60 | 8.70 | 0.18 | ||||||||
| Total | 19 | 341.46 | 1001.79 | 180.35 | 5.06 | ||||||||
| R2 | 0.9081 | 0.9390 | 0.8937 | 0.9329 | |||||||||
| Adj. R2 | 0.8253 | 0.8841 | 0.7979 | 0.8725 | |||||||||
| C.V. % | 1.28 | 2.99 | 2.11 | 0.24 | |||||||||
TP Total phenolic content (GAE/100g); ABTS (%) 2-2-azinobis-3ethylbenthiazoline-6-sulphonic acid radical activity; DPPH (%) 1, 1-diphenyl-2-picrylhydrazyl radical scavenging activity; SASA (%) superoxide anion scavenging activitys
Total phenolic contents (TPC)
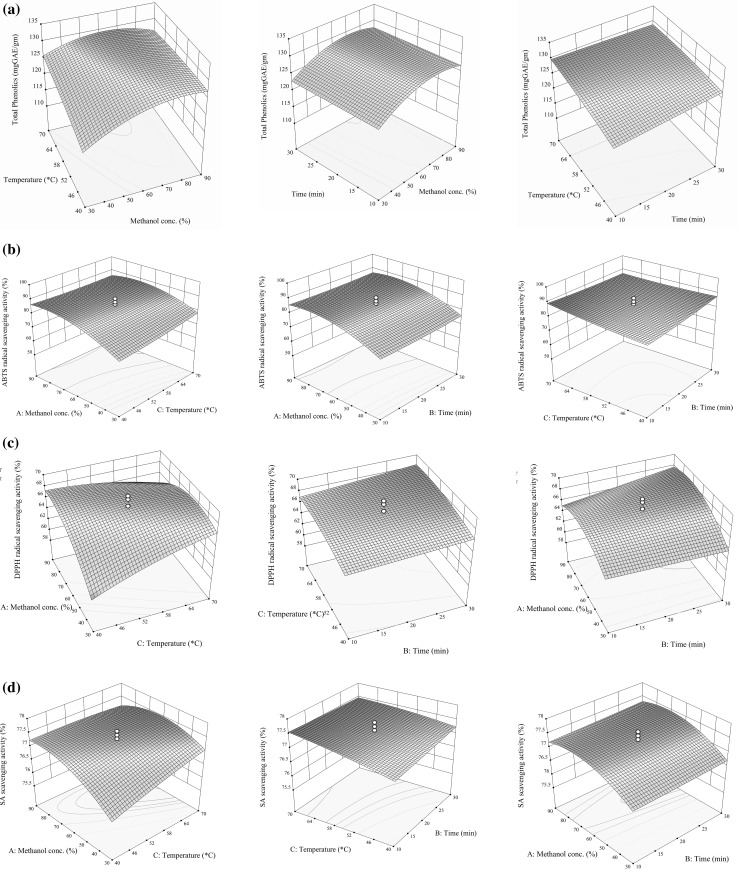
Table 1 and Fig. 1a illustrated the effect of methanol conc., time and temperature of extraction process on the TPC of SBTE. The second-order regression model equations predicting effect of extracting variables is as follows;
Fig. 1.
Response surface plots for optimization of extraction protocols of SBTE a total phenolic (mg GAE/gm) b ABTS activity (%) c DPPH activity (%) and d SASA activity (%) in function of methanol concentration (%), extraction temperature (°C) and extraction time (min)
ANOVA of the quadratic regression models for TPC shows that the model was significant with F-value and p value of 10.98 and 0.0004, respectively with satisfactory regression coefficient (R2) of 0.9081. Extracting methanol concentration had both significant linear and interaction effects (p < 0.01) excluding extraction time. Extraction temperature as well as the interaction effect of methanol conc. (X21) had the most significant effect on TP (Table 1), followed by the quadratic terms of methanol conc. and temperature (X1 X3).
From Fig. 1a, it was observed that TPC increases linearly with the increase of extraction temperature from 40 to 70 °C. Similarly, the increase in the methanolic conc. from 30 to 90% promoted recovery of higher TPC. Furthermore, the TPC also increased with the increase of extraction temperature up to about 65 °C, however subsequent increase in temperature didn’t cause a significant change in total phenolic content. As reported by Cacace and Mazza (2003), higher solubility and diffusion coefficient of polyphenols were observed with increased temperature, leading to more extraction rate.
DPPH scavenging ability
The model was significant (p < 0.05) with F-values and p values of 9.34 and 0.0008, respectively. In terms of antioxidant capacity of SBTE, only the linear and interaction terms of methanol concentration (X1) had significant effect (p < 0.05) on DPPH scavenging ability. However, the quadratic term of extraction temperature and methanol conc. (X1 X3) had significant effect at p < 0.0008 level (Table 1). The results obtained are in agreement with Wong et al. (2015) and Pompeu et al. (2009), in which the authors reported that the antioxidant activity is greatly influenced by the concentration of solvent used. The R2 was 0.8937 for DPPH scavenging ability and the lack of fit was highly non-significant (0.4220), verifying that a good fitness of the model. The effect of three independent variables on the DPPH scavenging ability was reported by the coefficient of the second-order polynomial regression equations as follows;
Figure 1c showed the combined effect of methanol concentration, extraction time and extraction temperature on the DPPH scavenging ability, and it revealed that at low levels of the methanol concentration and extraction temperature and time the DPPH scavenging ability was minimal.
Al-Farsi and Lee (2008) documented that increasing extraction temperature will lead to enhance the solubility of solute and increase the extraction coefficient, but temperature above 50 °C will affect the stability of the phenolic compounds and the alteration of plant’s membrane integrity may affect the antioxidant capacity. Figure 1c clearly indicated that at fix methanol conc. level, increase in extraction temperature and time resulted in increase in DPPH activity of the SBTE.
SASA scavenging ability
The RSA (Table 1 and Fig. 1d also demonstrated a high regression value (R2 = 0.9329) for the SASA scavenging ability in the SBTE. The ANOVA of the quadratic regression models for SASA showed that the model was significant (p < 0.05) with F-values and p values of 15.45 and <0.0001, respectively and equation shows the relationship as follows;
Perusal of Table 1 showed that extracting methanol conc. had significant (p < 0.0001) linear and interaction effect on SASA activity, followed by quadratic effect of methanol conc. and extracting temperature (p < 0.05). Linear term effect of extracting temperature also had significant (p < 0.005) effect on overall SASA activity.
The effect of extraction parameters on the SASA activity of SBTE and their interactions were shown in Fig. 1d. Increasing extraction temperature and methanol conc. showed linear increase in SASA activity as per Fig. 1d, which is also true for extraction time with constant temperature.
Optimization of extraction parameters
Numerical optimization procedures were carried out using the Design-Expert 9.0 software for predicting the optimum level of independent variables for obtaining extracts with maximum values of TPC, ABTS, DPPH and SASA. The optimal conditions obtained using the model was as follows: methanol concentration, 60%; extraction temperature, 55 °C; and extraction time, 20 min. Under optimal conditions, the model predicted a maximum response. The experimental values of 128.23 mg GAE/g (Y1), 87.13% (Y2), 66.11% (Y3), and 77.24% (Y4), were found close to the predicted values derived from the respective regression models with the CV ranging from 0.24% to 2.99% as shown in Table 1. With the use of these extracting conditions phenolic rich sea buckthorn extract can be produced, ultimately leading to highest yield of proven natural antioxidants for future studies
Effect of incorporation of different concentration of phenolic rich SBTE into model meat system during storage
The effect of different levels of SBTE i.e. (T1: 0.1%, T2: 0.2% and T3: 0.3%) obtained from optimization experiment on various physico-chemical, microbiological and storage quality are presented in the Tables 2 and 3.
Table 2.
Effect of the different levels of SBTE on the physico-chemical and colour attributes of raw ground pork during refrigerated aerobic storage (mean ± S.E.)
| Treatments/days | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 |
|---|---|---|---|---|---|
| pH | |||||
| C | 5.79 ± 0.04Aa | 5.83 ± 0.04Ab | 5.88 ± 0.09Bb | 5.90 ± 0.04Cc | 5.98 ± 0.05Cd |
| T-1 | 5.73 ± 0.05Aa | 5.82 ± 0.08Aab | 5.83 ± 0.05Ab | 5.85 ± 0.08Bb | 5.90 ± 0.04Bc |
| T-2 | 5.75 ± 0.01Aa | 5.82 ± 0.08Ab | 5.83 ± 0.01Abc | 5.84 ± 0.08ABc | 5.85 ± 0.05Ad |
| T-3 | 5.74 ± 0.02Aa | 5.81 ± 0.01Ab | 5.82 ± 0.02Ab | 5.83 ± 0.01Ac | 5.84 ± 0.01Ad |
| Water activity (a W ) | |||||
| C | 0.910 ± 0.002Ae | 0.908 ± 0.004Ad | 0.901 ± 0.007Ac | 0.891 ± 0.008Ab | 0.879 ± 0.003Aa |
| T-1 | 0.922 ± 0.012Be | 0.914 ± 0.008Bd | 0.909 ± 0.005Ac | 0.904 ± 0.007Bb | 0.897 ± 0.006Aa |
| T-2 | 0.926 ± 0.016Bd | 0.918 ± 0.008Bc | 0.916 ± 0.008ABbc | 0.915 ± 0.004Cb | 0.904 ± 0.004Ba |
| T-3 | 0.929 ± 0.008Be | 0.925 ± 0.002Cd | 0.920 ± 0.004Bc | 0.917 ± 0.001Cb | 0.913 ± 0.002Ba |
| L* (Lightness) | |||||
| Control | 56.89 ± 0.31Ad | 56.48 ± 0.56Ad | 54.81 ± 1.78Ac | 53.54 ± 1.10Ab | 51.93 ± 0.19Aa |
| T-1 | 56.41 ± 0.51Ad | 56.22 ± 1.12Ad | 55.59 ± 0.54Bc | 54.23 ± 1.15Bb | 53.44 ± 0.15Ca |
| T-2 | 56.26 ± 0.48Ad | 56.14 ± 0.14Ad | 55.46 ± 1.02Bc | 54.36 ± 0.25Bb | 53.68 ± 0.05Ba |
| T-3 | 56.12 ± 0.82Ac | 56.01 ± 0.45Ac | 55.24 ± 0.10Bb | 54.69 ± 0.98Bab | 54.07 ± 0.12Ca |
| a* (Redness) | |||||
| Control | 13.87 ± 0.84Ad | 13.58 ± 0.11Ad | 12.63 ± 0.10Ac | 11.48 ± 0.24Ab | 10.26 ± 0.15Aa |
| T-1 | 13.90 ± 0.54Ad | 13.78 ± 0.61Acd | 13.12 ± 0.15Bc | 12.53 ± 0.64Bb | 11.15 ± 0.48Ba |
| T-2 | 13.92 ± 0.11Ac | 13.82 ± 0.46Abc | 13.20 ± 0.54Bb | 12.82 ± 0.25Ca | 12.17 ± 0.54Ca |
| T-3 | 13.95 ± 1.74Ac | 13.84 ± 0.16Abc | 13.24 ± 0.21Bb | 12.97 ± 0.10Ca | 12.25 ± 0.41Ca |
| b* (Yellowness) | |||||
| Control | 15.17 ± 0.44Ae | 15.01 ± 0.09Ad | 14.84 ± 0.01Ac | 14.09 ± 0.64Ab | 13.86 ± 1.46Aa |
| T-1 | 15.39 ± 0.51Ad | 15.23 ± 0.10Bcd | 15.06 ± 0.10Bc | 14.58 ± 0.46Bb | 14.19 ± 0.16Ba |
| T-2 | 15.54 ± 0.57BCd | 15.36 ± 0.40BCcd | 15.11 ± 0.51Cc | 14.63 ± 0.54BCb | 14.38 ± 0.96BCa |
| T-3 | 15.85 ± 0.16Cd | 15.44 ± 0.55Ccd | 15.26 ± 0.85Dc | 15.04 ± 0.55Cb | 14.85 ± 0.12Ca |
n = 6; Control: (without any phyto-extract); T-1 = 0.1% SBTE, T-2 = 0.2% SBTE; T-3 = 0.3% SBTE
* Mean ± S.E. with different superscripts row wise (small alphabets) and column wise (capital alphabets) differ significantly (p < 0.05)
Table 3.
Effect of different levels of SBTE on the sensory and microbiological quality parameters of raw ground pork during refrigerated aerobic storage (mean ± S.E.)
| Treatments/days | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 |
|---|---|---|---|---|---|
| Colour | |||||
| Control | 4.17 ± 0.01Ae | 4.01 ± 0.05Ad | 3.97 ± 0.01Ac | 3.63 ± 0.01Ab | 3.04 ± 0.01Aa |
| T-1 | 4.19 ± 0.02Be | 4.16 ± 0.05Bd | 4.05 ± 0.03Bc | 3.94 ± 0.08Bb | 3.53 ± 0.08Ba |
| T-2 | 4.33 ± 0.01BCe | 4.25 ± 0.01Cd | 4.11 ± 0.01BCc | 4.01 ± 0.01Cb | 3.84 ± 0.06Ca |
| T-3 | 4.34 ± 0.08Ce | 4.30 ± 0.03Bd | 4.14 ± 0.08Cc | 4.09 ± 0.05Db | 3.98 ± 0.04 Da |
| Odour | |||||
| Control | 4.05 ± 0.02Ae | 3.81 ± 0.04Ad | 3.75 ± 0.05Ac | 2.47 ± 0.02Ab | 2.06 ± 0.01Aa |
| T-1 | 4.11 ± 0.01Be | 4.07 ± 0.05Bd | 3.84 ± 0.04Bc | 3.25 ± 0.01Bb | 2.98 ± 0.05Ba |
| T-2 | 4.39 ± 0.01Cd | 4.30 ± 0.03Ccd | 4.26 ± 0.09Cc | 4.08 ± 0.04Cb | 3.08 ± 0.09Ca |
| T-3 | 4.73 ± 0.03Dd | 4.68 ± 0.04Dcd | 4.59 ± 0.04Dc | 4.21 ± 0.05Db | 3.91 ± 0.02 Da |
| Overall acceptability | |||||
| Control | 4.15 ± 0.03Ae | 4.05 ± 0.01Ad | 3.77 ± 0.04Ac | 3.09 ± 0.04Ab | 2.97 ± 0.04Aa |
| T-1 | 4.22 ± 0.01Be | 4.17 ± 0.08Bd | 4.00 ± 0.08Bc | 3.46 ± 0.07Bb | 3.30 ± 0.09Ba |
| T-2 | 4.39 ± 0.05Cd | 4.28 ± 0.09Ccd | 4.09 ± 0.07Bc | 3.63 ± 0.06Cb | 3.45 ± 0.07Ba |
| T-3 | 4.47 ± 0.05Dd | 4.33 ± 0.01Ccd | 4.27 ± 0.04Cc | 3.97 ± 0.04Db | 3.89 ± 0.04Ca |
| SPC (log 10 cfu/g) | |||||
| Control | 3.49 ± 0.12Ca | 5.38 ± 0.09Cb | 6.78 ± 0.07Cb | 7.13 ± 0.20Cc | 7.76 ± 0.12Cd |
| T-1 | 2.97 ± 0.22Ba | 4.44 ± 0.21Bb | 5.12 ± 0.15Cc | 6.48 ± 0.13Bcd | 6.73 ± 0.09Bd |
| T-2 | 2.51 ± 0.10Ba | 3.86 ± 0.11Aa | 4.39 ± 0.08Bb | 5.77 ± 0.17Ab | 6.07 ± 0.17Bc |
| T-3 | 2.26 ± 0.14Aa | 3.44 ± 0.18Aab | 4.86 ± 0.19Ab | 5.34 ± 0.07Ac | 5.67 ± 0.01Ac |
| Coliforms count (log 10 cfu/g) | |||||
| Control | 1.92 ± 0.04Ca | 1.98 ± 0.03Ba | 2.13 ± 0.01Bb | 2.19 ± 0.02Cbc | 2.95 ± 0.02Cd |
| T-1 | 1.83 ± 0.01Ba | 1.91 ± 0.04Bab | 1.98 ± 0.02Ab | 2.08 ± 0.05Bc | 2.26 ± 0.09Bd |
| T-2 | 1.82 ± 0.02Ba | 1.89 ± 0.01Aa | 1.97 ± 0.06Ab | 2.01 ± 0.01Bbc | 2.22 ± 0.05Bc |
| T-3 | 1.73 ± 0.05Aa | 1.81 ± 0.02Aab | 1.93 ± 0.01Ab | 1.98 ± 0.02Ac | 2.07 ± 0.02Ac |
| Psychrophilic count (log 10 cfu/g) | |||||
| Control | 2.37 ± 0.15Ba | 3.83 ± 0.10Ba | 4.17 ± 0.22Cb | 6.13 ± 0.25Cc | 6.59 ± 0.24Cd |
| T-1 | 2.03 ± 0.11Ba | 2.77 ± 0.24Bab | 3.86 ± 0.07Bb | 4.21 ± 0.11Bc | 5.68 ± 0.44Bc |
| T-2 | 1.24 ± 0.14Aa | 1.97 ± 0.48Aa | 2.81 ± 0.20Bb | 3.18 ± 0.31Abc | 4.26 ± 0.19Ac |
| T-3 | 1.07 ± 0.26Aa | 1.83 ± 0.24Aab | 2.21 ± 0.17Ab | 3.07 ± 0.28Ac | 4.12 ± 0.11Ac |
| Yeast and molds count (log 10 cfu/g) | |||||
| Control | ND | ND | 1.36 ± 0.02Ae | 2.76 ± 0.02Ae | 2.93 ± 0.01Be |
| T-1 | ND | ND | ND | ND | 1.04 ± 0.01A |
| T-2 | ND | ND | ND | ND | ND |
| T-3 | ND | ND | ND | ND | ND |
n = 6; Control: (without any phyto-extract); T-1 = 0.1% SBTE, T-2 = 0.2% SBTE; T-3 = 0.3% SBTE
* Mean ± S.E. with different superscripts row wise (small alphabets) and column wise (capital alphabets) differ significantly (p < 0.05)
ND Not detected. Sensory: 5-point descriptive scale; 1 as extremely undesirable to 5 as extremely desirable
Physico-chemical quality parameters
The critical analyses of Table 2 revealed that the pH of raw ground pork varied significantly (p < 0.05) amongst treatments and days of storage. Initially the pH was comparable in all treated samples thereafter followed an increasing trend during storage, irrespective of the added amount of phyto-extract. The pH was significantly (p < 0.05) lower for T-3 and T-2 than T-1 on the 9th day of storage and significantly (p < 0.05) higher in control than all the treatments throughout the storage period. Demeyer et al. (1986) reported that increase in the pH during storage might be due to the low carbohydrate content, formation of N non-protein compounds and basic ammonium ions coupled with buffering action of protein. Wardlaw et al. (1973) further explained that this could be due to the accumulation of basic compounds such as ammonia, derived from microbial action.
It has been widely recognized that the concept of water activity (aw) is of great importance in meat preservation, because measured values generally correlated well with the potential for growth and metabolic activity of microorganisms (Chirife and Buera 1995). Water activity varied significantly (p < 0.05) with the progress of storage period (Table 2). The rate of decrease in water activity during storage was lower in T-3 followed by T-2 and T-1. The aW was reported highest in T-3 and lowest in control on last (9th) day of storage. This might be due to antimicrobial properties of the added SBTE into the raw ground pork. An antimicrobial property of sea buckthorn seeds has been widely documented by various authors (Wagh et al. 2015; Kumar et al. 2015).
Instrumental colour profile parameters
Instrumental colour profile (Table 2) varied within SBTE-pork samples and storage days. L* value followed a declining trend with the increase in the level of incorporation of SBTE, however, the decrease was (p > 0.05) among treatments. The rate of decrease in treatments was lower than control, which might be due to the ability of the SBTE to maintain the colour of the product by retarding the oxidation reaction (Kumar et al. 2015).
Lightness (L* value) reported higher in SBT-pork samples than control and exhibited declining trend during storage, which is attributed to the fact that salt greatly enhanced the process of meat discoloration due to the pro-oxidative activity as well as its ability to release iron from heme pigments (Devatkal et al. 2014).
Redness (a* value) is an indicator of freshness of the meat and criteria for quality evaluation by the consumers. Redness (a*) value showed the pattern T-3 > T-2 > T-1 amongst treatments and followed a decreasing trend in all the treatments throughout the storage. This might be due to gradual oxidation of myoglobin and accumulation of metmyoglobin with storage time (Mancini and Hunt 2005). However, the a* value was higher in SBTE treated samples than control throughout the storage period and rate of decrease in a* value was significantly (p < 0.05) lower in treated products than control. Several authors have studied the colour of meat and meat products and reported that meat oxidation causes a decrease in a∗ value (Lavieri and Williams 2014; Kumar et al. 2015).
The b* values also followed a decreasing trend for all the treated samples as well as control throughout the storage period irrespective of the level of incorporation of SBTE (Table 2). Realini et al. (2015) also reported a decrease in b* values of beef patties containing Acerola fruit extract as a natural antioxidant.
Oxidative stability parameters
The oxidative stability of raw ground pork added with SBTE during storage was evaluated on the basis of TBARS, peroxide value and free fatty acid content. Results of oxidative stability attributes are presented in (Figs. 2, 3).
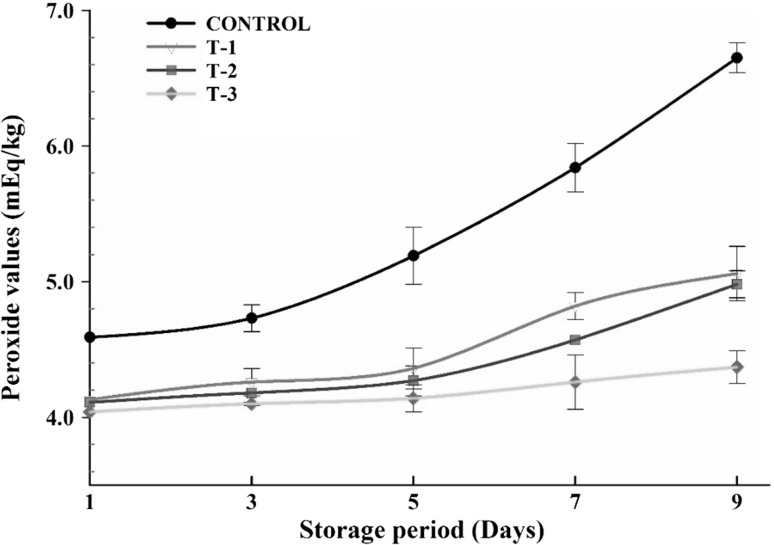
Fig. 2.
Effect of different levels of SBTE on peroxide value of raw ground pork during refrigerated storage. Control: Without any phyto-extracts; T-1: 0.1% SBTE; T-2: 0.2% SBTE; T-3: 0.3% SBTE. Bar represents the standard error (n = 6)
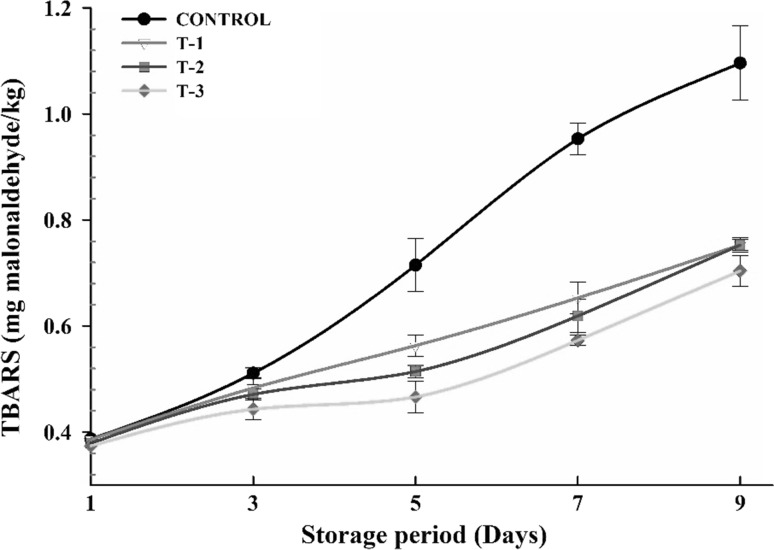
Fig. 3.
Effect of different levels of SBTE on TBARS values of raw ground pork during refrigerated storage. Control: Without any phyto-extracts; T-1: 0.1% SBTE; T-2: 0.2% SBTE; T-3: 0.3% SBTE. Bar represents the standard error (n = 6)
The peroxide value is used as a measure of primary oxidation products in meat (Gray 1978). Peroxide value (Fig. 2) of control remained higher (p < 0.05) during storage as compared to SBTE-pork samples. T-3 measured lowest PV followed by T-2 and T-1. The lower PV in SBTE-pork samples is an indication of antioxidant potential of SBTE attribute to the phenolic groups which provides a labile hydrogen atom by free radicals like alkoxyl or peroxyl radicals and get transformed into phenoxyl radical.
PV followed an increasing trend throughout storage period in both SBTE treated as well as control samples. It might be due to the formation of hydroperoxides during storage than their degradation into secondary oxidation products. These results are in consonance with Kumar et al. (2015) in pork patties added with sea buckthorn and grape seed extracts.
TBARS values followed an increasing (p < 0.05) trend throughout the storage period irrespective of added level of SBTE in the raw ground pork. TBARS value was measured lowest in T-3 and highest in T-1 among treatments and similar trend continued throughout the storage (Fig. 3). However, TBARS was significantly (p < 0.05) higher in control than SBTE treated products throughout the storage period. TBARS values were well below the permissible level of 1.0, indicator of acceptability of meat products (Witte et al. 1970). The lower TBARS value in treated products can be attributed to oxidative stability provided by sea buckthorn seed extract.
Free fatty acids (FFA) are the products of microbial or enzymatic lipolysis of fat calculated by titration of FFA present in meat. FFA followed an increasing trend during storage in both the control as well as SBTE- pork samples. FFA value measured lowest in T-3 and highest in T-1 among treatments. However, it was significantly (p < 0.05) higher in control than all treated products throughout the storage period of 9 days. The results in present study clearly indicated that 0.3% SBTE can act as an antioxidant in meat system by controlling the oxidative deterioration changes.
Sensory quality parameters
The sensory panelists awarded comparatively higher colour, odour and overall acceptability scores to all the treated samples than control on 1st day, which followed the same trend throughout the storage (Table 3). However, the rate of decrease of sensory score was significantly (p < 0.05) higher in control than treated products during storage. T-3 showed highest colour score throughout storage, which was followed by T-2 and T-1. The results can be correlated with instrumental colour profile.
Significantly higher (p < 0.05) odour score of T-3 samples implies that higher the SBTE level into the raw ground pork improves the odour of the products (Table 3). The overall acceptability of meat and meat products containing added phyto-extracts is considered to be of high importance in the development of functional meat products. The critical appraisal of sensory quality attributes revealed that the sensory panellists awarded highest overall acceptability scores to T-3 and were significantly (p < 0.05) higher than T-2 and T-1 and graded it as best among all the treatments including control.
Microbiological quality
In the present study, all three concentrations, i.e. 0.1, 0.2 and 0.3% of SBTE, were found to be significantly (p < 0.05) effective in decreasing the microbial load on raw ground pork (Table 3). The standard plate count (SPC) was significantly (p < 0.05) higher in control than all the other treatments throughout storage period of 9 days. Kumar et al. (2015) reported increase in aerobic plate count in pork patties incorporated with 0.3% SBTE and stored at 4 ± 1 °C.
Psychrophilic count (PPC) followed similar trend as that of SPC in raw ground pork during storage days. These findings are in consonance with Dhanze et al. (2013) who found that no significant difference (p < 0.05) for sensory attributes in the control and treated groups; however, scores were higher for the treated groups compared with the control group and 3% seabuckthorn leaf extract treatment resulted in significant (p < 0.05) lowering of SPC, PPC, CC and Yeast and mold count through 7 days. Coliforms count followed an increasing trend with the progress of storage period progressed and were significantly (p < 0.05) higher in control than SBTE-pork samples.
Yeast and mold count were detected on day 5th of storage in control and on 9th day in T-1 whereas, in all other treatments it was not detected even on 9th day of storage. Various workers, Lorenzo et al. (2014) and Kumar et al. (2015) in pork patties reported linear increase in standard plate count and psychrophilic count during refrigerated storage period.
Conclusion
Response surface methodology can be successfully implemented for the optimization of extraction conditions from sea buckthorn seeds to obtained phenolic rich sea buckthorn seed extracts. The optimal extraction conditions were determined as methanol concentration of 60%, extraction time of 20 min, and temperature of 55 °C. Further, the maximal responses of TP, ABTS, DPPH and SASA scavenging activity were 128.23 GAE/g, 87.13, 66.11 and 77.24%, respectively. The analytical data of raw ground pork analysis during refrigeration storage clearly indicated that incorporation of 0.3% of SBT in the raw ground pork lead to the improvement in the various physico-chemical quality, antioxidant potential, microbiological and sensory quality and stable up to 9 days of refrigerated storage.
Acknowledgements
Funding was provided by the Guru Angad Dev Veterinary and Animal Sciences University.
References
- Al-Farsi MA, Lee CY. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008;108(3):977–985. doi: 10.1016/j.foodchem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- APHA (2001) Compendium of methods for the microbiological examination of food, 4th edn. In: Speck ML (ed) American Public Health Association. Washington
- Beveridge T, Li TS, Oomah BD, Smith A. Sea buckthorn products: manufacture and composition. J Agric Food Chem. 1999;47(9):3480–3488. doi: 10.1021/jf981331m. [DOI] [PubMed] [Google Scholar]
- Cacace JE, Mazza G. Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng. 2003;59(4):379–389. doi: 10.1016/S0260-8774(02)00497-1. [DOI] [Google Scholar]
- Chirife J, Buera MP. A critical review of some non-equilibrium situations and glass transitions on water activity values of foods in the microbiological growth range. J Food Eng. 1995;25(4):531–552. doi: 10.1016/0260-8774(94)00033-6. [DOI] [Google Scholar]
- Demeyer A, Verplaetse M, Gistelinck K (1986) Fermentation of meat: an integrated process, pp 241–246. In: 32nd European meat research work, Ghent, Belgium
- Devatkal SK, Kumboj R, Paul D. Comparative antioxidant effect of BHT and water extracts of banana and sapodilla peels in raw poultry meat. J Food Sci Technol. 2014;51(2):387–391. doi: 10.1007/s13197-011-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanze H, Khurana SK, Mane BG. Effect of sea buckthorn leaf extract on microbiological quality of raw chicken during extended periods of storage. J Food Qual. 2013;36(1):59–65. doi: 10.1111/jfq.12007. [DOI] [Google Scholar]
- Galanakis CM (2014). Universal Strategy for the recovery of polyphenols: targeting industrial applications. In: 8th World congress on polyphenols applications. Book of abstracts. International Society of Antioxidants in Nutrition and Health (ISANH), the French Society of Antioxidants (SFA), and the Japanese Society of Antioxidants (JSA), 6 June 2014, Lisbon, Portugal, p 45
- Gray JI. Measurement of lipid oxidation: a review. J Am Oil Chem Soc. 1978;55(6):539–546. doi: 10.1007/BF02668066. [DOI] [Google Scholar]
- Kato K, Terao S, Shimamoto N, Hirata M. Studies on scavengers of active oxygen species. 1. Synthesis and biological activity of 2-O-alkylascorbic acids. J Med Chem. 1988;31(4):793–798. doi: 10.1021/jm00399a019. [DOI] [PubMed] [Google Scholar]
- Keeton JT. Effects of fat and NaCl/phosphate levels on the chemical and sensory properties of pork patties. J Food Sci. 1983;48(3):878–881. doi: 10.1111/j.1365-2621.1983.tb14921.x. [DOI] [Google Scholar]
- Koniecko EK. Handbook for meat chemists. Wayne: Avery Publishing Group Inc; 1979. pp. 68–69. [Google Scholar]
- Kumar A, Chattopadhyay S. DNA damage protecting activity and antioxidant potential of pudina extract. Food Chem. 2007;100(4):1377–1384. doi: 10.1016/j.foodchem.2005.12.015. [DOI] [Google Scholar]
- Kumar V, Chatli MK, Wagh RV, Mehta N, Kumar P. Effect of the combination of natural antioxidants and packaging methods on quality of pork patties during storage. J Food Sci Technol. 2015;52(10):6230–6241. doi: 10.1007/s13197-015-1734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavieri N, Williams SK. Effects of packaging systems and fat concentrations on microbiology, sensory and physical properties of ground beef stored at 4 ± 1°C for 25 days. Meat Sci. 2014;97(4):534–541. doi: 10.1016/j.meatsci.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Li TS, Schroeder WR. Sea buckthorn (Hippophae rhamnoides L.): a multipurpose plant. Hort Technol. 1996;6(4):370–380. [Google Scholar]
- Lorenzo JM, Sineiro J, Amado IR, Franco D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014;96(1):526–534. doi: 10.1016/j.meatsci.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Mancini RA, Hunt M. Current research in meat color. Meat Sci. 2005;71(1):100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Myers RH, Montgomery DC. Response surface methodology: process and product optimization using designed experiments. 2. New York: Wiley; 2002. [Google Scholar]
- Negi PS, Chauhan AS, Sadia GA, Rohinishree YS, Ramteke RS. Antioxidant and antibacterial activities of various sea buckthorns (Hippophae rhamnoides L.) seed extracts. Food Chem. 2005;92(1):119–124. doi: 10.1016/j.foodchem.2004.07.009. [DOI] [Google Scholar]
- Pompeu DR, Silva EM, Rogez H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour Technol. 2009;100:6076–6082. doi: 10.1016/j.biortech.2009.03.083. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Realini CE, Guàrdia MD, Díaz I, García-Regueiro JA, Arnau J. Effects of acerola fruit extract on sensory and shelf-life of salted beef patties from grinds differing in fatty acid composition. Meat Sci. 2015;99:18–24. doi: 10.1016/j.meatsci.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Wagh RV, Chatli MK, Ruusunen M, Puolanne E, Ertbjerg P. Effect of various phyto-extracts on physico-chemical, colour and oxidative stability of pork frankfurters. Asian Australas J Anim Sci. 2015;28(8):1178–1186. doi: 10.5713/ajas.14.0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw FB, Skelley GC, Johnson MG, Acton JC. Changes in meat components during fermentation, heat processing and drying of a summer sausage. J Food Sci. 1973;38(7):1228–1231. doi: 10.1111/j.1365-2621.1973.tb07244.x. [DOI] [Google Scholar]
- Witte VC, Krause GF, Bailey ME. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J Food Sci. 1970;35(5):582–585. doi: 10.1111/j.1365-2621.1970.tb04815.x. [DOI] [Google Scholar]
- Wong WH, Lee WX, Ramanan RN, Tee LH, Kong KW, Galanakis CM, Sun J, Prasad KN. Two level half factorial design for the extraction of phenolics, flavonoids and antioxidants recovery from palm kernel by-product. Ind Crops Prod. 2015;63:238–248. doi: 10.1016/j.indcrop.2014.09.049. [DOI] [Google Scholar]
- Ye L, Wang H, Duncan SE, Eigel WN, O’Keefe SF. Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 2015;172:416–422. doi: 10.1016/j.foodchem.2014.09.090. [DOI] [PubMed] [Google Scholar]
- Yuan YV, Bone DE, Carrington MF. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005;91(3):485–494. doi: 10.1016/j.foodchem.2004.04.039. [DOI] [PubMed] [Google Scholar]