Abstract
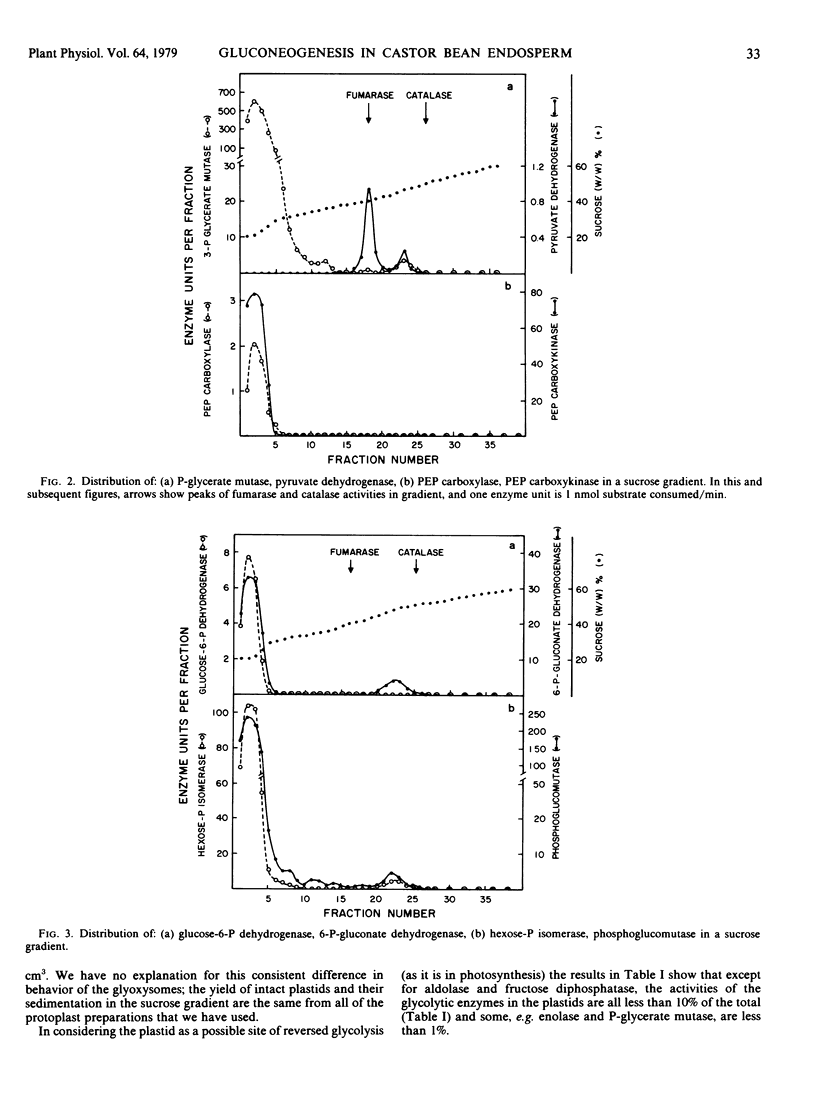
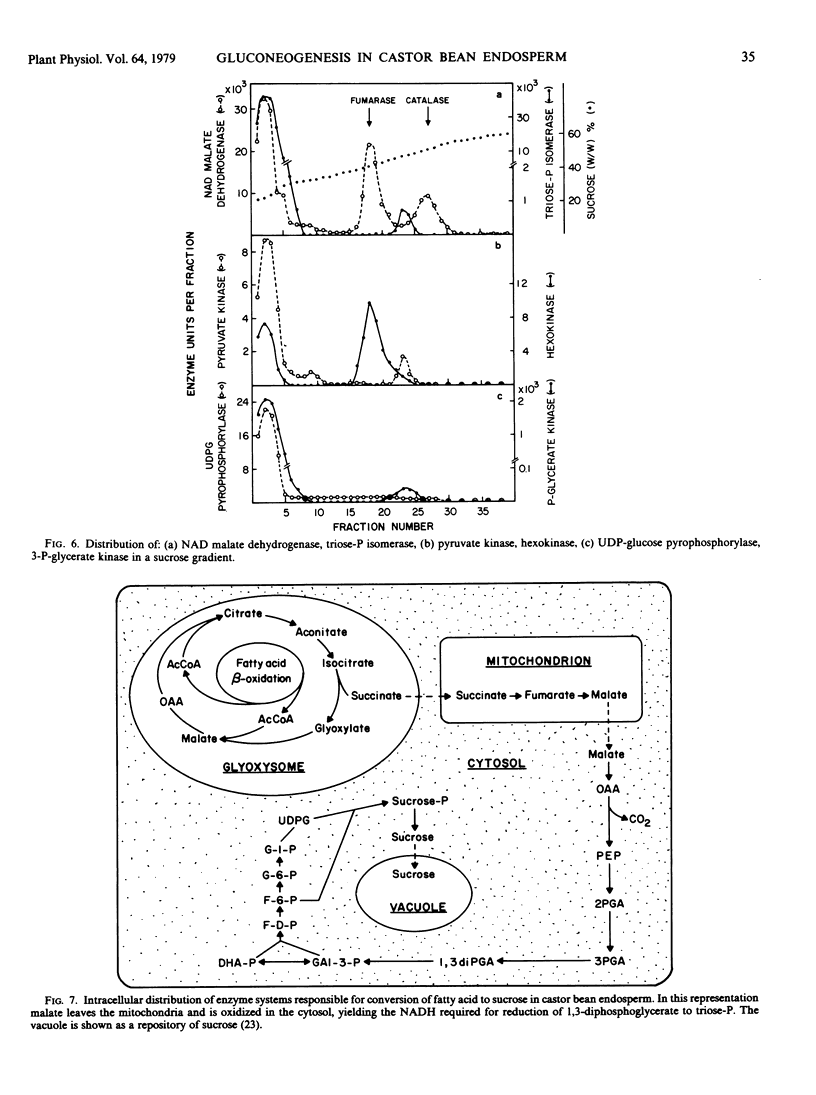
The intracellular distribution of enzymes capable of catalyzing the reactions from oxaloacetate to sucrose in germinating castor bean endosperm has been studied by sucrose density gradient centrifugation. One set of glycolytic enzyme activities was detected in the plastids and another in the cytosol. The percentages of their activities in the plastids were less than 10% of total activities except for aldolase and fructose diphosphatase. The activities of several of the enzymes present in the plastids seem to be too low to account for the in vivo rate of gluconeogenesis whereas those in the cytosol are quite adequate. Furthermore, phosphoenolypyruvate carboxykinase, sucrose phosphate synthetase, and sucrose synthetase, which catalyze the first and final steps in the conversion of oxaloacetate to sucrose, were found only in the cytosol. It is deduced that in germinating castor bean endosperm the complete conversion of oxaloacetate to sucrose and CO2 occurs in the cytosol. The plastids contain some enzymes of the pentose phosphate pathway, pyruvate dehydrogenase and fatty acid synthetase in addition to the set of glycolytic enzymes. This suggests that the role of the plastid in the endosperm of germinating castor bean is the production of fatty acids from sugar phosphates, as it is known to be in the endosperm during seed development.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ap Rees T., Thomas S. M., Fuller W. A., Chapman B. Location of gluconeogenesis from phosphoenolpyruvate in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1975 Mar 14;385(1):145–156. doi: 10.1016/0304-4165(75)90082-3. [DOI] [PubMed] [Google Scholar]
- Asahi T., Nishimura M. Regulatory function of malate dehydrogenase isoenzymes in the cotyledons of mung bean. J Biochem. 1973 Feb;73(2):217–225. [PubMed] [Google Scholar]
- Benedict C. R., Beevers H. Formation of sucrose from malate in germinating castor beans. I. Conversion of malate to phosphoenol-pyruvate. Plant Physiol. 1961 Sep;36(5):540–544. doi: 10.1104/pp.36.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- De Luca V., Dennis D. T. Isoenzyme of pyruvate kinase in proplastids from developing castor bean endosperm. Plant Physiol. 1978 Jun;61(6):1037–1039. doi: 10.1104/pp.61.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P. Membrane lipid metabolism in germinating castor bean endosperm. Plant Physiol. 1976 Apr;57(4):510–515. doi: 10.1104/pp.57.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Bowman P. D., Beevers H. Immunological and biochemical studies on isozymes of malate dehydrogenase and citrate synthetase in castor bean glyoxysomes. Plant Physiol. 1974 Sep;54(3):364–367. doi: 10.1104/pp.54.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobr M. J., Beevers H. Gluconeogenesis in the castor bean endosperm: I. Changes in glycolytic intermediates. Plant Physiol. 1971 Jan;47(1):48–52. doi: 10.1104/pp.47.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood R. C., ap Rees T. Phosphoenolpyruvate carboxykinase and gluconeogenesis in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1978 May 11;524(1):207–218. doi: 10.1016/0005-2744(78)90119-5. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Hydrolases in vacuoles from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Isolation of intact plastids from protoplasts from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):40–43. doi: 10.1104/pp.62.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Graham D., Akazawa T. Isolation of intact chloroplasts and other cell organelles from spinach leaf protoplasts. Plant Physiol. 1976 Sep;58(3):309–314. doi: 10.1104/pp.58.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Takabe T., Sugiyama T., Akazawa T. Structure and function of chloroplast proteins. XIX. Dissociation of spinach leaf ribulose-1,5-diphosphate carboxylase by p-mercuribenzoate. J Biochem. 1973 Nov;74(5):945–954. [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Reid E. E., Thompson P., Lyttle C. R., Dennis D. T. Pyruvate dehydrogenase complex from higher plant mitochondria and proplastids. Plant Physiol. 1977 May;59(5):842–848. doi: 10.1104/pp.59.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys. 1973 Jan;154(1):438–448. doi: 10.1016/0003-9861(73)90077-5. [DOI] [PubMed] [Google Scholar]
- Simcox P. D., Dennis D. T. Isoenzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinis communis L. Plant Physiol. 1978 Jun;61(6):871–877. doi: 10.1104/pp.61.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Fatty Acid synthesis in endosperm of young castor bean seedlings. Plant Physiol. 1978 Aug;62(2):173–178. doi: 10.1104/pp.62.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]


