Abstract
The present paper has been designed to evaluate phytochemical profile, in vitro free radical scavenging activity, cytotoxicity of methanolic extract and in vivo antioxidant activity of polyphenolic fraction of Acalypha indica leaves. Methanolic extract of A. indica leaves (MEAIL) contained rich amount of phenols, flavonoids and saponins. The GC–MS analysis of extract revealed 13 compounds, whereas HR-LC/Q-TOF/MS showed 87, and all were coincided with functional groups identified by FTIR. The extract showed good scavenging activity on DPPH, H2O2, hydroxyl radicals and metal ions. The Polyphenolic fraction induced the antioxidant enzymes in Diabetic rats. The extract also potentially showed cytotoxic (LC50: 140.02 µg/mL) activity against brine shrimp. Based on these analytical results, in vitro and in vivo experiments, it was concluded that the MEAIL has encompassed rich amount of polyphenols (antioxidants) and cytotoxic compounds for their respective activities. Polyphenolic fraction has the induction capacity to elevate cellular antioxidant enzymes in diabetic animals.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2590-z) contains supplementary material, which is available to authorized users.
Keywords: Acalypha, FTIR, GC–MS, HR-LC/Q-TOF/MS, Antioxidants
Introduction
In aerobic life, a cell has been struggling against atoms or groups of atoms with an odd (unpaired) number of electrons by means of free radicals related to oxygen and nitrogen species. Free radicals such as hydroxyl radical, superoxide radical and peroxyl radical are generated by biotic and abiotic factors. In foods, these free radicals cause lipid peroxidation (Harsha and Anilakumar 2014). Though, most of the foods contain very low levels (<1%) of lipids, are quite susceptible for oxidation process leads to food spoilage. Lipid peroxidation in food results depletion of flavour, colour, nutrient values and deposition of toxic compounds, are detrimental to the consumer’s health (Erwin et al 2004). Free radicals excess production and long lasting action on cellular biomolecules results lipid peroxidation, protein degradation, and oxidation of DNA eventually elevate oxidative stress and cell death in the human body (Wan et al. 2011). Cells respond to oxidative stress ultimately encourage the development of many chronic diseases such as cancer, atherosclerosis, diabetes, ischemic-reperfusion, aging and degenerative diseases in humans (Cai et al. 2004).
In general, to maintain homeostasis, the human body developed an endogenous antioxidant defense system in the form of enzymatic and non-enzymatic machinery to overcome the deleterious effects of free radicals. The unexpected increase of these free radicals in oxidative stress and diseases, the body intended to require either acceleration of endogenous defense system or outside supplementation of antioxidants (Richa et al. 2014). Similarly, though, the food sources have rich amount of antioxidants, however, during food processing and preservation, we should supplement the antioxidants to reduce free radical effect on foods for long lasting maintenance of quality. Outside supplementation of synthetic antioxidants (BHA, BHT, TBHQ, EQ and propyl gallate) effectively attenuate the action of free radicals but their prescription has been restricted for food storage and human relief from stress due to fears over their toxic and carcinogenic effects (Kozarski et al. 2015). Instead of focusing on synthetic antioxidants, food and pharmaceutical industries are paying attention towards natural antioxidant compounds as supplements which are identified and isolated from medicinal plants for the sake of human health (Gulçin et al. 2010).
Acalypha indica Linn. (Family: Euphorbiaceae), popularly called as Kuppichettu by the local people of Andhra Pradesh State, a weed plant extensively available throughout the plains of India. Traditionally A. indica has been used to treat many ailments such as pneumonia, asthma, rheumatism, also used as anti-venom, anti-inflammatory and anti-oxidative agent (Kanchana et al. 2014). It showed bactericidal activity against wound invading gram positive and gram negative bacterial pathogens. Wound healing activity, analgesic effects, strong anti-helminthic property and its synthesized nanoparticles have anticancer activity in breast cancer cell lines also reported (Seebaluck et al. 2015).
Natural antioxidant compounds have been identified, isolated and characterized from plants through various chromatographic techniques. Gas chromatography and Liquid chromatography coupled with Mass spectroscopy are very sensitive, fastest and reliable methods for identification of antioxidants in plant complex mixtures (extracts) (Severine et al 2010). Plant extracts have mixture of low polar to high polar antioxidant phytochemicals. Both low and high polar individual compound identification in plant extracts can be done by employing Gas chromatography and Liquid chromatography techniques respectively.
Based on the plant source importance in food and pharmaceutical industries, we hypothesized that the A. indica plant leaves may have rich amount of phytochemical compounds, which can reduce the free radicals, induce antioxidant enzymes and may have cytotoxic property. To prove this hypothesis, present study has been designed to explore phytochemical compounds through qualitative, quantitative analysis and profiling by analytical techniques in methanolic extract of A. indica leaves (MEAIL); and also, to study the in vitro free radical scavenging activity, cytotoxicity of the extract and in vivo antioxidant enzyme induction capacity of the separated polyphenol fraction from leaves in diabetic rats.
Materials and methods
Preparation of plant extract
Acalypha indica plant leaves were collected in the campus of Sri Venkateswara University, Tirupati and its surrounding area. The leaves were identified by the taxonomist of the university and the specimen was deposited in SV University Botany herbarium (voucher no: SVUBOT-926). The leaves were washed cleanly with tap water and powdered after shade dried. The Powder was filled in amber coloured bottle and stored in dark condition until it was used for analysis.
Leaf powder (200 g) was soaked in 1000 mL of methanol solvent (1:5) and incubated for 24 h under dark conditions with occasional stirring. Solvent extract obtained by muslin cloth filtration (700 mL), was sub filtered with non-absorbent cotton and then final filtration was done with Whatman No.1 filter paper. The obtained filtrate was concentrated in rotary evaporator method; rotary evaporation of filtrate was done at 50–55°C of internal temperature in the round bottom flask of rotary evaporator instrument (Raaman 2006).
Qualitative phytochemical screening
The standard protocols described by Abha et al. (2015) for the identification of phytochemicals qualitatively in MEAIL, which includes phenolic compounds, flavonoids, alkaloids, tannins, terpenoids, cardiac glycosides, saponins, anthraquinones, anthocyanins, coumarins and reducing sugars were used.
Quantification of total phenols, total flavonoids and total saponins
The presence of total phenolic content in MEAIL was quantified by Folin–Ciocalteu method (Gutierrez and Navarro 2010). The Gallic acid was taken as standard and its equivalent phenol content in one gram dry weight of the extract was determined at 765 nm in UV–Vis spectrophotometer.
The UV-VIS spectroscopic quantification of flavonoid content in MEAIL was done through aluminium chloride method (Jia et al. 1999). Epicatechin equivalent flavonoid content in extract (1 g) was determined through a standard curve of Epicatechin.
The total saponin content quantification in 20 g of Acalypha leaf powder was determined using protocol described by Edeoga et al. (2005).
Separation of polyphenols
Leaf powder (30 g) was defatted with dichloromethane. After drying, the defatted powder was extracted with methanol/water (70:30 v/v). The organic solvent evaporated with a rotary evaporator, and then, the rest of water extract pH was adjusted to 4. In this extract, 5 g of PVPP (Polyvinylpolypyrrolidine) was added to get separate polyphenols present in the extract by adsorption mechanism. The PVPP adsorbed polyphenols was obtained in filter paper after filtration. The PVPP residue was now extracted with acetone/water (70:30 v/v). Acetone was evaporated, remaining solution was further concentrated and separated polyphenols was tested using the Folin–Ciocalteu method (Paulo et al. 2010).
FTIR analysis for detection of functional groups
The functional groups of chemical constituents present in the MEAIL were analyzed in Alpha Fourier Transform-Infrared Spectrometer (FTIR) (BRUKER). Plant extract of 10 mg/mL dissolved in HPLC grade methanol and complete analysis was done by default program.
GC-MS and HR-LC/Q-TOF/MS analysis for the identification of antioxidant compounds
Nonpolar and semi polar phytochemical antioxidants present in the MEAIL were identified by Gas Chromatography instrument JEOL GCMATE II GC–MS (Agilent Technologies 6890N Network GC system). The instrument equipped with secondary electron multiplier, fused silica 50 m × 0.25 mm I.D in the column and splitless injector. Different temperature conditions in column and time intervals were used to analyze volatilization of compounds of the extract. Total run time was 22 min. Chromatography peaks obtained from related compounds are coupled with Mass Spectroscopy. The mass and structure of the volatile phytochemicals of the extract were identified using library search in National Institute of Standard and Technology (NIST).
Subsequent to the GC–MS, The antioxidant phytochemical profile was analysed in HR-LC/Q-TOF/MS (model: Agilent 1290 Infinity UHPLC System). Programmed 3 µL injection volume of the sample was injected by auto sampler into a C18 column (ZORBAX 2.1 × 50 mm 1.8 Micron). The auto sampler capacitized with 100 µL/mL auxiliary draw and ejects speed of the sample. Method flexible pursued two binary pumps (G4220B) comprises in one housing, are delivered desired mobile phase gradient ratios (solvent A: 0.1% Formic Acid (FA) in water and B: 90% Acetonitrile + 10% H2O + 0.1% FA). Total run time was 30 min, in which the solvent A-95% + B-5% was started at 2 min, at 20 min solvent A gradient ratio turned into 5%, finally from 26 to 30 min, the solvent A 95% was maintained. Binary pump pressure maintained at 1200 bar constantly and flow rate was 0.300 mL/min. Agilent G6550A Q-TOF Mass Spectrophotometer with Dual AJS ESI ion source connected with LC used to find a phytochemicals profile in the extract. The conditions set in the Q-TOF as follows; MS minimum range (m/z) 50, MS maximum range (m/z) 1000, MS and MS/MS scan rate 1, gas temperature 250 °C, gas flow 13 L/min, nebulizer 35 psig, sheath gas temperature 300 and sheath gas flow 11. Mass hunter workstation software was taken to get advantage in identification of accurate MS and MS/MS for LC profile of the extract.
The phytochemical structural profile identified in GC–MS and HR-LC/Q-TOF/MS was structurally drawn and its antioxidant descriptors such as hydrogen bond donor count (HBDC) and hydrogen bond acceptor count (HBAC) were predicted using Marvin Sketch 16.3.21 version, a Chemo informatics tool.
DPPH radical scavenging, hydrogen peroxide scavenging and metal reduction power
To assess free radical scavenging activity and metal reduction power of the MEAIL, we carried out assays against different radicals and metal ions at their respective wave length absorbance to declare potentiality. Increase in concentrations (10–50 µg/mL) of the extract against 1,1,-diphenyl-2-picryl-hydrazil (DPPH) radical (wavelength absorbance at 517 nm) was tested according to the method of Gyamfi et al. (1999). Hydrogen peroxide (10 mM in phosphate buffer) scavenging capacity of the extract with increased concentrations of 20–100 µg/mL was determined at the 230 nm wavelength absorbance and also, Fe3+ reduction capacity for the extract concentrations of 20–100 µg/mL was carried out at 700 nm using the method of Mohammad and Ali (2010). Molybdenum reduction (Total antioxidant) property for 20–100 µg/mL of the extract concentrations was done at 695 nm method described by Prieto et al. (1999).
Lipid peroxidation and 2-Deoxy ribose sugar damage mitigation
Lipid peroxidation induced by FeSO4 in egg yolk homogenate and its inhibition by MEAIL was determined using thiobarbituric acid-reactive species (TBARS) method described by Upadhyay et al. (2014). The lipid peroxidation control (without plant extract) chromogen, test samples included with increased concentrations of the extract (20–100 µg/mL) resulted chromogen were measured at 532 nm. 2-Deoxy ribose sugar protection property of the MEAIL from hydroxyl radicals was analysed using Deoxy ribose method described by Nagai et al. (2005). The source of hydroxyl radical was Fenton’s reagent, the amount of 2-Deoxy ribose sugar was 10 mM (150 µL) and the extract concentrations ranging from 20–100 µg/mL were used against hydroxyl radicals. The optical density values of reaction final product were read at 520 nm.
Induction of diabetes
The animals were fasted overnight and diabetes was induced by a single intra peritoneal injection of a freshly prepared solution of Streptozotocin (STZ) [50 mg/kg body weight (b.w)] in 0.1 M cold citrate buffer (pH 4.5). The animals were considered as diabetic when their blood glucose values were above 250 mg/dL on the third day after STZ injection.
Grouping of animals
The rats were divided into five groups of six rats each and the treatment was given every day via orally for 1 month.
Group I, Normal Control (NC): Rats received 0.9% saline and feed with normal diet.
Group II, Diabetic Control (DC): Streptozotocin (STZ 50 mg/kg b.w.) was given intra peritoneally for the induction of diabetes to this group.
Group III, Polyphenolic fraction treatment (Pt): Rats received Polyphenolic fraction (100 mg/kg b.w.) orally for a period of one month.
Group IV, Diabetics + Polyphenolic fraction treatment (D + Pt): Diabetic rats received Polyphenolic fraction, as described in group III, for a month treatment.
Group V, Diabetics + Glibenclamide treatment (D + Glbt): Diabetic rats received Glibenclamide (20 mg/kg b.w.) for one month treatment.
Antioxidant enzymes estimation
The antioxidant enzymes estimation was carried out in liver tissue. Superoxide Dismutase (SOD) activity was assayed in the mitochondrial fraction by the method of Misra and Fridovich (1972) at 480 nm for 4 min in UV–Vis spectrophotometer (Hitachi U-2000). Activity was expressed as the amount of enzyme that inhibits the oxidation of epinephrine by 50%, which is equal to 1 U per milligram of protein. Catalase (CAT) activity was determined at room temperature by using the modified method of Aebi (1984) and absorbance of the samples were measured at 240 nm for 1 min in UV–Vis spectrophotometer. Activity of glutathione peroxidase (GPx) was determined by the method of Flohe and Gunzler (1984) in the presence of NADPH and absorbance was measured at 340 nm using hydrogen peroxide. Glutathione reductase (GR) enzyme activity was determined according to the method of Carlberg and Mannervik (1985).
Cytotoxicity assay
The cytotoxic potential of MEAIL was tested on phototrophic brine shrimp larvae using the adopted protocol described by Meyer et al. (1982). Brine shrimp eggs were hatched in 1 L artificially prepared sea water as optimal growth media (38 g of normal sea salt was dissolved in 1 L of sterile double distilled water, the pH of the water was adjusted to 8.5 by 1 N NaOH). To get development of worthy brine shrimp larvae called nauplii, aeration was supplied with the aid of air pump and light source provided by a lamp. After 48 h of the hatching time in continuation supply of air and light regime the eggs were grown up to nauplii. Developed nauplii were collected using pipette and sprinkled in petri dish contained growth media. Ten nauplii were counted in one mL tip of slashed mouth using micropipette and transferred to 4 mL sea water contained experimental test tubes. Different concentrations (200, 400, 600, 800 and 1000 µg/mL) of MEAIL were added to experimental test tubes and made up to 5 mL with growth media, control tubes were prepared as such (5 mL) but without the extract. The experimental tubes were aerated with air pump and kept under a light source for the period of 24 h at room temperature to quantify the lethal concentration of the extract. The lethality (LC50) of the extract was calculated by using linear correlation.
Results and discussion
Qualitative analysis of phytochemicals
Preliminary phytochemical screening is a very useful initial step for identification of phyto-constituents and quantitative estimation of rich chemical compounds in plants, also the isolation of pharmacologically active natural principles. Phenolics, flavonoids, alkaloids, tannins, terpenoids, cardiac glycosides, saponins, anthraquinones, anthocyanins, coumarins and reducing sugars were found in MEAIL. Overall, the phenolics, flavonoids and saponins were observed rich in chemical reaction test.
Quantitative analysis of phytochemicals
Phenolics as secondary metabolites in plants, are possessing one or more hydroxyl groups on the aromatic ring. Among phenolics, half of the numbers have been occupied by flavonoids. The Plant produced saponins are surface active glycosides, with characteristics of stable and soap like foam formation structures. Phenols, flavonoids and saponins are having large spectrum of biological importance such as antioxidant, anti-inflammatory, anti-cancer, anti-ulcer, effect on neuronal diseases and cardio vascular diseases, etc. (Gulcin et al. 2004; Piacente et al. 2005; Vijayalaxmi et al. 2015). Based on the biological importance of phenols, flavonoids and saponins of the plants, which are also observed rich in phytochemical screening parameter in this study, we attempted to estimate those phytochemicals quantitatively in MEAIL. The total phenolic content has 111.321 mg/g and this quantification been equivalent to the Gallic acid, a standard phenolic compound. The total flavonoid content has been observed as 29.896 mg/g and it was equivalent to the Epicatechin, a flavonoid compound, while saponins have been quantified as 322 mg in 20 g of leaf dry powder.
Functional group identification by FTIR
FTIR spectrum for MEAIL was analysed between wave number 500 to 4000 cm−1. Extract absorbed IR light at different wave number cm−1. The absorbance at specific wave number is indicative of its chemical bond stretching of testing sample. The broad peaks in spectra indicative for huge amount of respective chemical functional groups, whereas the shorter peaks are for limited amount of functional groups. The online resource 1 represents the IR absorbance of extract indicative of respective functional groups, which are as follows: 3318 cm−1(O–H stretch, H–bonded = alcohols, phenols or N–H bond stretch = 1°, 2° amines and amides); 2930 cm−1(C–H bond stretch = alkanes); 2108 cm−1 (–C≡C– bond stretch = alkynes); 1636 cm−1 (N–H bend = 1° amines); 1363 cm−1(C–H rock = alkanes); 1244 cm−1 (C–H wag –CH 2 X = alkyl halides or C–N stretch = aliphatic amines); 1043 cm−1 (C–N stretch = aliphatic amines); 921 cm−1 (O–H bend = carboxylic acids); 828 cm−1 (C–H “oop” = aromatics or C–Cl stretch = alkyl halides); 768 cm−1 (C–Cl stretch = alkyl halides) and 646 cm−1 (–C≡C–H: C–H bend = alkynes or C–Br stretch = alkyl halides).
GC–MS and HR-LC/Q-TOF/MS analysis for phytochemical profile identification
GC–MS and HR-LC/Q-TOF/MS are analytical tools, which can identify the chemical components in food, plant extracts and other test samples. GC–MS facilitate the analysis of volatile and semi volatile compounds. In our analysis 13 volatile compounds have been identified in the extract. Among those, seven compounds were volatile fatty acids, the rest were non-fatty acids (Table 1), whereas, GC–MS chromatogram and its structural profile of the extract have been provided in online resource 2 and 3 respectively. HBDC and HBAC descriptors prediction for phytochemical profile revealed that Imidazoles, 4-fluoro-5-hydroxyazomethyl-; n-hexadecanoic acid and 9-octadecenoic acid [Z], 2-hydroxy-1-(hydroxymethyl) ethyl ester found to have both HBDC and HBAC. HR-LC/Q-TOF/MS examination of the MEAIL resulted total 87 phytochemicals (Table 2). Of these 87 phytochemicals, mebeverine metabolite, His-Ala-Ala, 3-Deoxyguanosine, N-Tris[hydroxymethyl]methyl2-aminoethanesulfonic acid, Val-Trp-Thr, Fenoprofenglucuronide, 1-[[2-(2,3-dihydro-2-oxo-1Hindol-4yl)ethyl]propylcarbamate] glucuronide, Tyr-Phe-Tyr, Catechin, Leukotriene F4, Lys-His-Cys, 5,6-DiHETrE-EA and 5-Methyltetrahydropteroyltri L-glutamate have contained more HBDC and HBAC than others. Interestingly, many of them are polyphenols. It has been reported that, the compounds with more HBDC and HBAC have been facilitated to rich scavenging capability on free radicals (Riccardo and Luca 2012). In the present study we also observed that the MEAIL has rich amount of Mebeverine metabolite (1.523%), Catechin (1.523%), Leukotriene F4 (1.523%), Ramipril glucuronide (4.524%), Enkephaline, (D-Ala) 2-Leu (4.520%) and Trandolapril glucuronide (2.304%). This might be a reason for the functional properties of MEAIL, whereas, HR-LC/Q-TOF/MS chromatogram and chemical compound structures of the extract have provided in online source 4 and 5 respectively. We also observed that the GC–MS and HR-LC/Q-TOF/MS resulted phytochemical profile functional groups of MEAIL corresponded with the FTIR results.
Table 1.
GC–MS analysis of the MEAIL
| S. No | RT | Formula | Mass | Phyto chemical name | (%) | Chemical descriptors | |
|---|---|---|---|---|---|---|---|
| HBDC | HBAC | ||||||
| 1 | 8.32 | C4H5FN4O | 144.109 | Imidazole, 4-fluoro-5-hydroxyazomethyl- | 3.466 | 2 | 5 |
| 2 | 13.27 | C13H20O3 | 224.300 | 2-propenoic acid, 3-[5-acetyl-2,2- dimethylcyclopentyl], methyl ester,[1a(E),5a]- | 1.742 | 0 | 4 |
| 3 | 14.52 | C14H22O | 206.329 | 1-oxaspiro [2,5] octane, 5,5-dimethyl-4-(3-methyl-1,3-butadienyl)- | 1.143 | 0 | 2 |
| 4 | 16.2 | C14H16N2O | 228.295 | 2-formyl-5,7dimethyl-1,2,3,4-tetrahydropyrimido(3,4-a) indole. | 3.109 | 0 | 2 |
| 5 | 17.03 | C17H34O2 | 270.457 | Pentadecanoic acid, 14-methyl-, methyl ester | 2.787 | 0 | 2 |
| 6 | 17.72 | C16H32O2 | 256.430 | n-hexadecanoic acid | 14.676 | 1 | 4 |
| 7 | 18.48 | C19H34O | 278.480 | 13-hexyloxacyclotridec10-en-2-one | 3.341 | 0 | 2 |
| 8 | 18.8 | C19H36O2 | 296.495 | 10-octadecenoic acid, methyl ester | 1.712 | 0 | 2 |
| 9 | 19.02 | C19H38O2 | 298.511 | Heptadecanoic acid, 16-methyl-methyl ester | 0.768 | 0 | 2 |
| 10 | 19.62 | C18H36O2 | 284.484 | Hexadecanoic acid, 14-methyl, methyl ester | 1.667 | 0 | 2 |
| 11 | 20.6 | C20H38O3 | 326.521 | Nonadecanoic acid, 18-oxo, methyl ester | 1.682 | 0 | 4 |
| 12 | 22.87 | C23H39NO2 | 361.570 | 14-methylhexadecanoic acid, picolinyl ester | 1.810 | 0 | 5 |
| 13 | 25.5 | C21H40O4 | 356.547 | 9-octadecenoic acid [Z], 2-hydroxy-1-(hydroxymethyl) ethyl ester | 0.857 | 2 | 6 |
The HBDC, HBAC are calculated for phytochemical profile computationally using Marvin sketch, a chemoinformatics tool
RT retention time, % percentage in the extract, HBDC hydrogen bond donor count, HBAC hydrogen bond acceptor count
Table 2.
Phytochemical profile of the MEAIL
| S. No | RT | Formula | m/z | Mass | Phytochemical name | % | Chemical descriptors | |
|---|---|---|---|---|---|---|---|---|
| HBDC | HBAC | |||||||
| 1 | 0.439 | C7 H9 N O2 S | 176.013 | 171.034 | O-Toluenesulfamide | 0.386 | 1 | 2 |
| 2 | 0.493 | C5 H14 N O | 104.108 | 104.108 | Choline | 0.397 | 1 | 2 |
| 3 | 0.5 | C15 H18 O10 | 381.081 | 358.091 | Mebeverine metabolite (Veratric acid glucuronide) | 1.523 | 5 | 9 |
| 4 | 0.521 | C7 H9 N O3 | 138.056 | 155.059 | Ethosuximide M5 | 2.145 | 1 | 3 |
| 5 | 0.535 | C8 H15 N O3 | 156.103 | 173.107 | Methyl N-(amethylbutyryl)glycine | 0.648 | 1 | 2 |
| 6 | 0.603 | C17 H17 N3 O | 262.130 | 279.133 | Desmethylondansetron | 0.110 | 1 | 2 |
| 7 | 0.606 | C12 H19 N5 O4 | 280.141 | 297.144 | His Ala Ala | 0.115 | 5 | 6 |
| 8 | 0.617 | C10 H19 N O | 174.125 | 169.146 | Lupinine | 0.126 | 1 | 2 |
| 9 | 0.62 | C17 H17 N3 O2 | 318.120 | 295.131 | 6-Hydroxydesmethylondansetron | 0.132 | 2 | 3 |
| 10 | 0.622 | C10 H13 N5 O4 | 268.106 | 267.096 | 3-Deoxyguanosine | 0.137 | 4 | 8 |
| 11 | 0.642 | C18 H19 N3 O | 276.146 | 293.149 | Ondansetron | 0.143 | 0 | 2 |
| 12 | 0.656 | C14 H24 O2 | 229.156 | 224.178 | 3,4-Tetradecadienoic acid | 0.154 | 1 | 2 |
| 13 | 0.668 | C11 H14 O3 | 199.073 | 194.094 | Orthothymotinic Acid | 0.165 | 2 | 3 |
| 14 | 0.675 | C4 H9 N O2 | 86.061 | 103.064 | Dimethylglycine | 0.171 | 1 | 3 |
| 15 | 0.685 | C14 H20 O | 209.130 | 204.152 | 13-Tetradecen-2,4-diyn-1-ol | 0.176 | 1 | 1 |
| 16 | 0.75 | C6 H11 N O4 | 144.066 | 161.069 | L-2-Aminoadipic acid | 0.182 | 3 | 5 |
| 17 | 0.759 | C7 H11 N O4 | 156.067 | 173.070 | 2,6-Piperidinedicarboxylic acid | 0.187 | 3 | 5 |
| 18 | 0.861 | C6 H15 N O6 S | 230.068 | 229.061 | N-Tris[hydroxymethyl]methyl2-aminoethanesulfonic acid | 0.193 | 5 | 7 |
| 19 | 0.902 | C19 H17 N3 | 310.130 | 287.141 | Pararosaniline | 0.198 | 3 | 3 |
| 20 | 1.633 | C6 H11 N O3 | 128.072 | 145.075 | Propionylglycine methyl ester | 1.556 | 1 | 2 |
| 21 | 3.257 | C17 H14 O4 | 283.094 | 282.087 | Dehydrovariabilin | 0.160 | 0 | 3 |
| 22 | 3.425 | C17 H20 N2 O2 | 289.128 | 284.149 | Tropicamide | 0.165 | 1 | 3 |
| 23 | 3.516 | C19 H20 O7 | 343.115 | 360.119 | Elephantopin | 0.171 | 1 | 5 |
| 24 | 4.665 | C10 H8 O4 | 193.051 | 192.044 | 4-Methyldaphnetin | 0.342 | 2 | 3 |
| 25 | 4.839 | C20 H17 F O2 S | 341.099 | 340.092 | Sulindac sulfide | 0.342 | 1 | 2 |
| 26 | 4.95 | C13 H18 O2 | 189.129 | 206.132 | Ibuprofen | 0.353 | 1 | 2 |
| 27 | 4.955 | C20 H28 N4 O5 | 409.184 | 404.206 | Val TrpThr | 0.364 | 6 | 6 |
| 28 | 5.002 | C21 H22 O9 | 401.120 | 418.124 | Fenoprofen glucuronide | 0.342 | 5 | 8 |
| 29 | 5.677 | C11 H16 O3 | 197.119 | 196.112 | Benzenemethanol, 2-(2aminopropoxy)-3-methyl | 1.241 | 2 | 3 |
| 30 | 5.68 | C9 H9 N O2 | 146.062 | 163.065 | 4-(3-Pyridyl)-3-butenoic acid | 0.273 | 1 | 3 |
| 31 | 5.878 | C11 H12 N4 O2 S | 287.057 | 264.068 | Sulfamerazine | 0.330 | 2 | 5 |
| 32 | 6.053 | C12 H20 O4 | 233.1172 | 228.138 | Traumatic acid | 0.342 | 2 | 4 |
| 33 | 6.054 | C12 H18 O3 | 193.1246 | 210.127 | (3S,7R)-epi-jasmonic acid | 0.331 | 1 | 3 |
| 34 | 6.414 | C20 H26 N2 O9 | 421.1625 | 438.165 | 1-[[2-(2,3-dihydro-2-oxo-1Hindol-4yl)ethyl]propylcarbamate] glucuronide | 0.331 | 5 | 8 |
| 35 | 6.6 | C15 H18 O7 | 293.1043 | 310.107 | Picrotin | 0.375 | 2 | 6 |
| 36 | 6.68 | C10 H8 O4 | 193.0518 | 192.044 | 2,10-Dihydroxy-4,6,8decatriynoic acid | 0.297 | 3 | 4 |
| 37 | 7.079 | C18 H21 N O5 | 314.1404 | 331.143 | Ambelline | 0.307 | 1 | 6 |
| 38 | 7.095 | C27 H29 N3 O6 | 496.180 | 491.201 | Tyr Phe Tyr | 0.165 | 6 | 7 |
| 39 | 7.315 | C13 H24 N O6 | 272.1456 | 290.156 | 3-Methylglutarylcarnitine | 0.342 | 1 | 6 |
| 40 | 8.425 | C18 H28 O3 | 275.2028 | 292.206 | 13-Keto-9Z,11E,15Zoctadecatrienoic acid | 0.224 | 1 | 3 |
| 41 | 8.425 | C20 H32 O6 | 351.216 | 368.219 | PGG2 | 0.297 | 2 | 6 |
| 42 | 8.843 | C14 H12 O4 | 245.0833 | 244.076 | Thyroacetic acid | 0.273 | 2 | 3 |
| 43 | 8.843 | C15 H14 O6 | 291.0884 | 290.081 | Catechin | 1.523 | 5 | 6 |
| 44 | 9.037 | C28 H44 N2 O8 S | 573.2534 | 568.274 | Leukotriene F4 | 1.523 | 6 | 9 |
| 45 | 9.988 | C18 H30 O5 | 291.1976 | 308.200 | 2R-hydroperoxy-9Z,12Z,15Zoctadecatrienoic acid | 0.735 | 3 | 5 |
| 46 | 10.587 | C15 H26 N6 O4 S | 387.1821 | 386.174 | Lys His Cys | 0.342 | 7 | 7 |
| 47 | 11.262 | C15 H16 O4 | 261.1145 | 260.107 | Peucenin | 0.309 | 2 | 4 |
| 48 | 11.901 | C39 H54 O11 | 699.3566 | 698.349 | Heudelottin C | 0.297 | 2 | 7 |
| 49 | 11.902 | C19 H26 N2 O2 S | 715.3302 | 346.169 | Pergolide sulfone | 0.847 | 1 | 3 |
| 50 | 11.903 | C18 H30 O2 | 261.2235 | 278.226 | 9Z,12Z,15E-Octadecatrienoic acid | 0.847 | 1 | 2 |
| 51 | 12.802 | C18 H30 O3 | 277.2186 | 294.221 | 13-Oxo-ODE | 0.384 | 1 | 3 |
| 52 | 13.068 | C27 H53 N O8 P | 532.3491 | 550.360 | GPCho(16:0/3:1(2E)) | 0.674 | 1 | 7 |
| 53 | 13.07 | C30 H44 O9 | 553.2782 | 548.299 | Peruvoside | 0.674 | 3 | 8 |
| 54 | 13.074 | C19 H38 O4 | 353.270 | 330.281 | 1-Hexadecanoyl-sn-glycerol | 0.674 | 2 | 4 |
| 55 | 13.879 | C20 H34 O4 | 321.243 | 338.247 | 8,9-Dihydroxy-5,11,14eicosatrienoic acid | 0.297 | 3 | 4 |
| 56 | 14.663 | C22 H39 N O4 | 364.286 | 381.289 | 5,6-DiHETrE-EA | 0.165 | 4 | 4 |
| 57 | 14.886 | C16 H24 O5 | 301.1428 | 296.164 | Lactone of PGF-MUM | 0.320 | 1 | 4 |
| 58 | 14.896 | C8 H6 O4 | 149.025 | 166.028 | Terephthalic acid | 0.154 | 2 | 4 |
| 59 | 15.064 | C16 H35 N O2 | 278.2496 | 273.270 | C16 Sphinganine | 0.268 | 3 | 3 |
| 60 | 15.295 | C25 H36 N8 O12 | 623.2499 | 640.253 | 5-MethyltetrahydropteroyltriL-glutamate | 0.172 | 7 | 16 |
| 61 | 15.398 | C22 H36 O3 | 349.2752 | 348.267 | Larixol Acetate | 0.264 | 2 | 3 |
| 62 | 15.64 | C19 H35 N O2 | 292.2653 | 309.268 | Dicyclomine | 0.220 | 0 | 2 |
| 63 | 15.691 | C23 H38 O3 | 367.2634 | 362.284 | 24-Nor-5beta-chol-22-ene3alpha,7alpha,12alpha-triol | 0.176 | ||
| 64 | 16.125 | C17 H26 N4 O | 16.125 | 302.210 | Emedastine | 0.126 | 0 | 4 |
| 65 | 16.428 | C35 H42 N2 O9 | 639.281 | 634.302 | Rescinnamine | 0.203 | 1 | 8 |
| 66 | 16.802 | C18 H32 O3 | 279.233 | 296.236 | 12-Hydroxy-10-octadecynoic acid | 1.026 | 2 | 3 |
| 67 | 17.079 | C18 H28 O2 | 259.2074 | 276.210 | 6,11-Octadecadiynoic acid | 1.655 | 1 | 2 |
| 68 | 17.514 | C33 H36 N4 O6 | 607.2557 | 584.266 | Bilirubin | 0.132 | 6 | 6 |
| 69 | 17.535 | C26 H46 O5 | 461.3243 | 438.335 | 27-Nor-5b-cholestane3a,7a,12a,24,25-pentol | 0.139 | 5 | 5 |
| 70 | 17.580 | C18 H37 N O2 | 282.2806 | 299.283 | N-(2hydroxyethyl)palmitamide | 0.121 | 2 | 2 |
| 71 | 17.799 | C22 H43 N5 O13 | 608.2627 | 585.273 | Amikacin | 0.821 | 13 | 15 |
| 72 | 17.799 | C35 H36 N4 O6 | 609.2701 | 608.263 | Harderoporphyrin | 0.993 | 5 | 9 |
| 73 | 18.259 | C29 H40 N2 O11 | 615.2574 | 592.268 | Ramipril glucuronide | 4.524 | 5 | 10 |
| 74 | 18.267 | C29 H39 N5 O7 | 592.264 | 569.280 | Enkephaline, (D-Ala)2-Leu | 4.520 | 5 | 8 |
| 75 | 18.851 | C30 H40 O7 | 535.2697 | 512.280 | 3-Deoxo-3betaacetoxydeoxydihydroge Dunin | 0.507 | 0 | 4 |
| 76 | 19.449 | C19 H34 O3 | 293.2482 | 310.251 | Methoprene (S) | 0.450 | 0 | 2 |
| 77 | 19.555 | C32 H42 O9 | 553.2801 | 570.283 | Deoxykhivorin | 0.123 | 0 | 4 |
| 78 | 19.825 | C30 H46 O4 | 493.3287 | 470.339 | (22R)-1alpha,22,25trihydroxy-26,27-dimethyl23,24-tetradehydro-24ahomo-20-epivitamin D3 / (22R)-1a | 0.060 | 4 | 4 |
| 79 | 20.018 | C30 H42 N2 O11 | 607.2903 | 606.283 | Trandolapril glucuronide | 2.304 | 5 | 10 |
| 80 | 20.177 | C40 H56 O3 | 567.4182 | 584.421 | Flavoxanthin | 2.295 | 2 | 3 |
| 81 | 20.181 | C40 H56 O2 | 551.4237 | 568.427 | Tunaxanthin J/ Chiriquixanthin B | 2.065 | ||
| 82 | 20.475 | C39 H64 O5 | 613.482 | 612.474 | 1-(9Z-hexadecenoyl)-2(5Z,8Z,11Z,14Z,17Zeicosapentaenoyl)-snglycerol | 0.437 | 1 | 3 |
| 83 | 20.654 | C24 H40 O5 | 413.266 | 408.287 | 3beta,6alpha,7alphaTrihydroxy-5beta-cholan-24oic Acid | 0.551 | 4 | 5 |
| 84 | 20.943 | C41 H74 O8 P | 708.5096 | 725.513 | 1-Octadecanoyl-2(5Z,8Z,11Z,14Zeicosatetraenoyl)-sn-glycero3-phosphate | 0.476 | 2 | 5 |
| 85 | 21.661 | C45 H74 O10 | 797.5159 | 774.526 | 1,2 Di-(9Z,12Z,15Zoctadecatrienoyl)-3-O-Beta-Dgalactosyl-sn-glycerol | 0.337 | 4 | 8 |
| 86 | 21.896 | C45 H78 N O8 P | 792.5601 | 791.552 | GPEtn(18:0/22:6(4Z,7Z,10Z,1 3Z,16Z,19Z)) | 0.264 | 2 | 5 |
| 87 | 26.117 | C2 H6 Al N O4 | 139.9883 | 135.007 | Dihydroxyaluminumaminoacetate | 0.158 | 3 | 4 |
The analysis was performed using HR-LC/Q-TOF/MS and prediction of HBDC, HBAC were done using Marvin sketch,a chemoinformatics tool
RT retention time, % percentage of compound present in the extract, HBDC hydrogen bond donor count, HBAC hydrogen bond acceptor count, m/z mass to charge ratio
DPPH radical, hydrogen peroxide scavenging and metal reduction power
The poisonous effects of free radicals on bio-molecules have been attenuated by antioxidants by means of many synthetic and natural sources. Plant compounds as natural antioxidants have an advantage in relaxation of many deleterious effects of free radicals in food and in humankind. Assessment of free radical scavenging activity of natural antioxidant potency in in vitro is not able to conclude only by means of a single method. Because of different radicals have differed in their sensitivity for mitigation by antioxidants, means, different methods have their unique character (Gulcin et al. 2005). Plant antioxidant agents are tested for its radical scavenging activity using free radical DPPH, which is most widely used, simple and acceptable method (Lalita et al. 2014). DPPH is a stable diamagnetic free radical, dissolved in organic solvents such as methanol and ethanol. Dissolved DPPH radical in solvents visible as intense violet colour (oxidised state) and they would be stable by accepting hydrogen or electron followed by proton from donor gives a colourless product (reduced state) (Saini et al. 2014). The increase intensity of colourless product decreases the absorbance, whereas, the decrease in colourless intensity increases the absorbance at wavelength 517 nm. The MEAIL produced stable DPPH product when increased in concentration. The IC50 for DPPH radical scavenging concentration of the extract showed at 28.330 µg/mL. The standard antioxidant ascorbic acid has showed rich DPPH radical scavenging activity at lesser concentrations than the extract (Table 3).
Table 3.
Radical scavenging activity of MEAIL, comparision with standard ascorbic acid
| A) DPPH scavenging activity | B) Hydrogen peroxide scavenging activity | ||||
|---|---|---|---|---|---|
| Concentration (µg) | % Scavenge leaves | % Scavenge standard | Concentration (µg) | % Scavenge leaves | % Scavenge standard |
| 10 | 33.076 ± 1.386 | 51.282 ± 0.800 | 20 | 22.136 ± 0.887 | 22.530 ± 1.410 |
| 20 | 47.051 ± 0.967 | 62.564 ± 0.967 | 40 | 34.741 ± 2.151 | 45.066 ± 1.405 |
| 30 | 52.051 ± 0.587 | 71.660 ± 0.971 | 60 | 41.310 ± 2.153 | 56.333 ± 0.810 |
| 40 | 60.769 ± 1.017 | 77.943 ± 1.822 | 80 | 49.765 ± 2.153 | 64.316 ± 1.622 |
| 50 | 62.948 ± 0.587 | 79.483 ± 1.598 | 100 | 53.990 ± 3.544 | 68.540 ± 0.810 |
| IC50 | 28.330 | 4.106 | IC50 | 84.415 | 57.560 |
All values denoted in triplicates Mean ± SD
Hydrogen peroxide is endogenously produced in cell, regulates various important physiological processes (Autreaux and Toledano 2007). Nevertheless, it’s over abundance in a cell causes cellular damage because of its interaction with cellular metal ions generates highly reactive hydroxyl radicals. The hydroxyl radical’s adverse effects on biomolecules will be discussed in the next section. Hydrogen peroxide changed into water in the presence of electron donor by cellular enzymes and external antioxidants (Ruch et al. 1984). The MEAIL converted the hydrogen peroxide into water on concentration dependent manner. The increased concentrations of the extract have shown an increase in hydrogen peroxide scavenging activity and its IC50 value was 84.415 µg/mL. Standard antioxidant ascorbic acid IC50 showed at 57.560 µg/mL (Table 3).
The antioxidant property of active compounds in MEAIL was confirmed by DPPH radical and Hydrogen peroxide assays. These antioxidants in the extract as reductants were tested for conversion of Fe trivalent ion state (Fe3+ state) to Fe divalent ion (Fe2+ state). The increase in extract concentration and reference active moiety ascorbic acid generated an increase in green colour Fe2+ product was directly proportional to the absorbance and reducing state of metal. All five concentrations of MEAIL have their sole metal reduction property, whereas the ascorbic acid showed better power than the extract (Table 4). Apart from the ferrous reduction test, we also performed another chelation assay by the reference of molybdenum metal. Different concentrations of MEAIL at acidic pH reduced Mo (VI) into green phosphate/Mo (V) complex. The importance of this test is likely to evaluate the total antioxidant activity because it predicts both fat soluble antioxidant and water soluble antioxidant properties (Abubakar et al. 2013). The Mo metal reduction power of MEAIL has concentration dependent and observed good reduction power in each concentration but somewhat lower when compared with the standard ascorbic acid (Table 4).
Table 4.
Metals reduction power of the MEAIL and standard ascorbic acid
| A) Ferrous reduction test | B) Molybdenum reduction test | ||||
|---|---|---|---|---|---|
| Concentration (µg) | OD values leaves | OD values Standard | Concentration (µg) | OD values leaves | OD values standard |
| 20 | 0.103 ± 0.001 | 0.267 ± 0.002 | 20 | 0.081 ± 0.003 | 0.222 ± 0.001 |
| 40 | 0.161 ± 0.001 | 0.372 ± 0.004 | 40 | 0.100 ± 0.002 | 0.312 ± 0.002 |
| 60 | 0.230 ± 0.002 | 0.395 ± 0.002 | 60 | 0.150 ± 0.003 | 0.306 ± 0.001 |
| 80 | 0.274 ± 0.004 | 0.401 ± 0.001 | 80 | 0.211 ± 0.003 | 0.342 ± 0.002 |
| 100 | 0.326 ± 0.004 | 0.402 ± 0.001 | 100 | 0.291 ± 0.002 | 0.353 ± 0.002 |
The optical density values are represented with Mean ± SD measurements
Lipid peroxidation and 2-Deoxy ribose sugar damage mitigation
The disturbance of bio-molecules such as lipids and sugars by hydroxyl free radicals are estimated and assessed their protection by MEAIL using TBA method. Fenton’s reagent (results of H2O2 and metals = Hydroxyl radicals) react with 2-Deoxy ribose sugar generates malondialdehyde (MDA) like products of 2-Deoxy ribose damage and this can be quantified using the thiobarbituric acid (TBA) at 532 nm. The 2-Deoxy ribose sugar is one of the backbone moieties of DNA strand. The mechanism of hydroxyl radical induced DNA strand breaks initiated by hydrogen atoms abstraction in 2-Deoxy ribose sugars. Hydrogen atom abstraction in sugar leads to loss of nucleosides in DNA strand results strand break with phosphate ends (Balasubramanian et al. 1998; Pogozelski and Tullius 1998). The protective role of MEAIL and ascorbic acid on Fenton’s radicals (hydroxyl) induced sugar damage was studied at different concentrations. Increase in concentration leads to decrease in sugar damage due to its radical mitigation property. The IC50 concentration value for the extract was noticed at 35.933 µg/mL and for ascorbic acid at 5.278 µg/mL (Table 5).
Table 5.
TheMEAIL and standard ascorbic acid bio molecular protection property against hydroxyl radicals
| A) Ribose protection from hydroxyl radicals | B) Lipid peroxidation prevention | ||||
|---|---|---|---|---|---|
| Concentration (µg) | % Scavenge leaves | % Scavenge standard | Concentration (µg) | % Scavenge leaves | % Scavenge standard |
| 20 | 39.012 ± 0.427 | 53.811 ± 0.858 | 20 | 20.623 ± 1.811 | 31.065 ± 2.389 |
| 40 | 46.419 ± 0.855 | 61.888 ± 1.198 | 40 | 31.654 ± 2.158 | 41.043 ± 3.423 |
| 60 | 53.456 ± 0.770 | 68.486 ± 0.858 | 60 | 41.486 ± 2.723 | 48.979 ± 2.721 |
| 80 | 58.518 ± 0.641 | 82.363 ± 1.042 | 80 | 48.441 ± 2.526 | 56.462 ± 2.452 |
| 100 | 62.222 ± 0.979 | 84.414 ± 1.612 | 100 | 54.916 ± 1.497 | 62.131 ± 3.490 |
| IC50 | 35.933 | 5.278 | IC50 | 84.775 | 65.321 |
All Values are triplicates and calculated with Mean ± SD
Lipids are a class of molecules put on show diverse in structure, biological functions of cellular and sub cellular membranes. The mechanism of lipid peroxidation is well known and is very harmful to the structure of the cell because it causes membrane damage results failure in function of normal membrane fluidity, permeability, ion transportation and inhibition of metabolic events in food and humans (Kosugi et al. 1987). In this study, we observed the effect of MEAIL on hydroxyl radical (Fenton’s reagent) induced formation of MDA pink chromogen with TBA in egg homogenate and it has noted as concentration dependent. The extract anti-lipid peroxidation IC50 value concentration was noticed at 84.775 µg/mL, whereas the ascorbic acid observed at 65.321 µg/mL (Table 5).
Overall comparison of radical scavenging (DPPH, H2O2, hydroxyl) and metal chelation (Fe and Mo) properties of MEAIL with standard ascorbic acid showed that, the plant extract has shown somewhat lower activity than standard, though it has a huge number of antioxidant compounds (found in GC–MS and HR-LC/Q-TOF/MS). This difference could be due to the presence of individual antioxidant compounds in the extract which are lower at given concentrations, whereas ascorbic acid is a standard and completely individual compound hence, it ultimately showed rich antioxidant activity at tested concentrations than MEAIL.
Antioxidant enzymes estimation
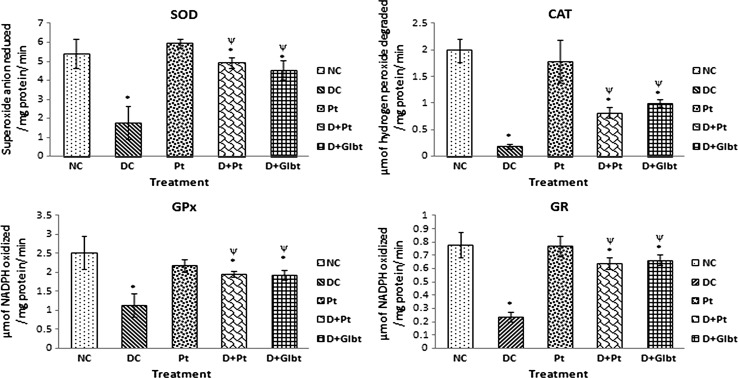
Oxidative stress is an abnormal condition due to alteration in cellular antioxidant defense system results production of excessive reactive oxygen species (ROS) which could initiate and aggravate many diseases. Streptozotocin (STZ) has been widely used for inducing type 1diabetes in a variety of animal models by affecting degeneration and necrosis of pancreatic beta cells and induction of oxidative stress (Merzouk et al. 2000). The previous studies were suggested that free radicals generated in STZ intoxication could play a crucial role in oxidative tissue damage (Brands et al. 2004; Shanmugam et al. 2011a, b). SOD, CAT, GPx and GR are enzymatic cellular antioxidants which could act as first line of defense system in cell and protects the organism from ROS induced oxidative damage (Nonaka et al. 1991). Damasceno et al. (2002) and Shanmugam et al. (2011a, b) have been reported that STZ produces oxidative stress by depletion of antioxidant systems in both blood and tissues. The results obtained from this study have in concurrence with the previous reports regarding significant depletion of antioxidant enzymes (SOD, CAT, GPx and GR) in STZ intoxicated rats. Supplementation of polyphenolic fraction of A. indica leaves has considerably reversed the STZ intoxication on antioxidant enzymes when compared with diabetic control rats (Fig. 1).Similarly, our in vitro studies and other previous studies have been reported that polyphenolic fractions from plant sources shown free radical scavenging activity, which may reflect the decrease in free radicals in STZ intoxicated liver tissue (Pillai and Mini 2016). In addition to this, in our present study, we observed the polyphenolic fraction of A. indica exert its antioxidant activity on STZ intoxication which may be due to increase in antioxidant enzyme activities (Chiang et al., 2006) and induction of synthesis of antioxidant enzymes (Chung et al. 2006).
Fig. 1.
Acceleration of antioxidant enzymes (SOD, CAT, GPx and GR) activity in polyphenolic fraction treated rat liver tissue when compared to diabetic rat liver. Data expressed as mean ± SD (n = 6). * The values are significant compared to the following: control (*p < 0.005), Diabetes (*p < 0.05) (Dunnett’s multiple comparison test)
Cytotoxicity estimation
Over the past 25 years, the compounds having cytotoxicity have been tested using brine shrimp, a model organism. Brine shrimp is a zoological organism belongs to Phylum Arthropod, is preferred in natural product cytotoxicity trials because it is an effective, simple, rapid and inexpensive method (Meyer et al. 1982). Brine shrimp lethality test for terrestrial plant extracts has been maintained good relationship in identification of anti tumoral agents (Jose et al. 2002). Cytotoxicity experiment using brine shrimp is opening feature for further experiments on mammalian models. Treatment of different concentrations of MEAIL for 24 h exhibited cytotoxicity and it’s LC50 (Lethal Concentration) value was calculated and noticed at 140.029 µg/mL (Online resource 6). The potential levels of cytotoxicity LC50 values of compounds are described by Bussmann and Co-workers (2011) that below 249 µg/mL is highly toxic, 250–499 µg/mL is moderate toxic, 500–1000 µg/mL is less toxic and above 1000 µg/mL considered as nontoxic. Based on these statistical values considerations, the MEAIL comes under highly toxic and hopefully it can be considered as a potential cytotoxic agent. The cytotoxic effect of the extract may be the presence of already known cytotoxic compounds found in HR-LC/Q-TOF/MS analysis such, as Choline, Sulindac Sulphide, Ibuprofen, Ambelline, Catechin (polyphenol), Peruvoside, Rescinnamine, Bilirubin, Amikacin and also the presence of unpredicted polyphenolic cytotoxic compounds (Table 2).
Conclusion
Having investigated on MEAIL, we conclude that the extract has quantitatively rich source of phenols, which has capacity to act as scavenger on DPPH, H2O2 and hydroxyl radical. The extract also acts as metal ion reducer and cytotoxic agent. In vivo antioxidant enzyme induction capacity by polyphenolic fraction reflects its relaxation ability in diabetic condition. Further studies are essential for the isolation of individual polyphenol compounds from the MEAIL and polyphenolic fraction for the preparation of natural antioxidant supplement and cytotoxic compounds to lend a hand for Food and Pharmaceutical Industries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The first author (SR) express sincere gratitude to the University Grants Commission New Delhi, India for their financial assistance to him through Rajiv Gandhi National Fellowship (RGNF No: F117.1/2012-13/R GNF-2012-13-SC –AND 34335), Also UGC-BSR Fellowship (F.25-1/2013-14 (BSR)/7-160/2007) and UGC-BSR- One Time Grant (No: F.19-141/2014 (BSR) dated 16-12-2014) sanctioned to the SHP and KSR respectively. The authors also thank to SAIF IIT Madras and IIT Bombay for the analysis of sample in GC–MS and HR-LC/Q-TOF/MS respectively.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2590-z) contains supplementary material, which is available to authorized users.
References
- Abha S, Swati V, Shukla RK. Phytochemical screening, proximate analysis and antioxidant activity of Dracaena reflexa Lam. leaves. Indian J Pharm Sci. 2015;77(5):640–644. doi: 10.4103/0250-474X.169035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubakar BA, Mohammed A, Ibrahim Aliyu M, Musa Aisha OM, Joyce JK, Adebayo OO. Free radical scavenging and total antioxidant capacity of root extracts of Anchomanes difformis Engl (Araceae) Acta Pol Pharm Drug Res. 2013;70(1):115–121. [PubMed] [Google Scholar]
- Aebi H. Catalase. Methods Enzymol. 1984;105:125–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Autreaux DB, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Balasubramanian B, Wendy K, Pogozelski Thomas DT. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci. 1998;95:9738–9743. doi: 10.1073/pnas.95.17.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands AMA, Kessels RPC, De HEHF, Kappelle LJ, Biessels GJ. Cerebral dysfunction in type I diabetes: effects of insulin, vascular risk factors and blood glucose levels. Eur J Pharmacol. 2004;490:159–168. doi: 10.1016/j.ejphar.2004.02.053. [DOI] [PubMed] [Google Scholar]
- Bussmann R, Malca G, Glenn A, Sharon D, Nilsen B, Parris B, Dubose D, Ruiz D, Saleda A, Maritinez M, Carillo L, Walker K, Kuhlman A, Townesmith A. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J Ethnophamacol. 2011;137(1):121–140. doi: 10.1016/j.jep.2011.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Luo Q, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/S0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Chiang A, Wu H, Yeh H, Chu C, Lin H, Lee W. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids. 2006;41:797–803. doi: 10.1007/s11745-006-5033-6. [DOI] [PubMed] [Google Scholar]
- Chung MJ, Walker PA, Hogstrand C. Dietary phenolic antioxidants, caffeic acid and trolox, protect rainbow trout gill cells from nitric oxide-induced apoptosis. Aquat Toxicol. 2006;80:321–328. doi: 10.1016/j.aquatox.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Damasceno DC, Volpato GT, Paranhos-Calderon M, Cunha-Rudge MV. Oxidative stress and diabetes in pregnant rats. Anim Reprod Sci. 2002;15(72):235–244. doi: 10.1016/S0378-4320(02)00094-5. [DOI] [PubMed] [Google Scholar]
- Edeoga HO, Okwu DE, Mbaeble BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4(7):685–688. doi: 10.5897/AJB2005.000-3127. [DOI] [Google Scholar]
- Erwin W, Anna G, Marzanna H, Henryk HJ, Jozef K, Maria M, Sylwia MS, Magdalena R, Urszula S, Renata ZW. Oxidation of lipids in food. Pol J Food Nutr Sci. 2004;13(54):87–100. [Google Scholar]
- Flohe L, Gunzler WA. Glutathione peroxidase. Methods Enzymol. 1984;105:115–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Gulcin I, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med. 2004;70(6):561–563. doi: 10.1055/s-2004-827158. [DOI] [PubMed] [Google Scholar]
- Gulcin I, Haci AA, Mehmet C. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull. 2005;53:281–285. doi: 10.1248/cpb.53.281. [DOI] [PubMed] [Google Scholar]
- Gulçin I, Bursal E, Sehitoglu MH, Bilsel M, Goren AC. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol. 2010;48:2227–2238. doi: 10.1016/j.fct.2010.05.053. [DOI] [PubMed] [Google Scholar]
- Gutierrez RM, Navarro YT. Antioxidant and hepatoprotective effects of the methanol extract of the leaves of Satrejamacrostema. Pharmacogn Mag. 2010;6:125–131. doi: 10.4103/0973-1296.62901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi MA, Yonamine M, Aniya Y. free radical scavenging activity of medicinal herb O Ghana: thonningia sanguine on experimentally induced liver injuries. Gen Pharmacol. 1999;32:661–667. doi: 10.1016/S0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- Harsha SN, Anilakumar KR. In vitro free radical scavenging and DNA damage protective property of Coriandrumsativum L leaves extract. J Food Sci Technol. 2014;51(8):1533–1539. doi: 10.1007/s13197-012-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Jose LCZ, Hernandez-inda Pilar P, Maria DG. Acomparision between two brine shrimp assay to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002;2:17. doi: 10.1186/1472-6750-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchana A, Jeevitha D, Isha A, Srividya S. Cytotoxicity induction by ethanolic extract of Acalypha indica loaded casein-chitosan microparticles in human prostate cancer cell line in vitro. Biomed Prev Nutr. 2014;4(3):445–450. doi: 10.1016/j.bionut.2013.03.009. [DOI] [Google Scholar]
- Kosugi H, Kato T, Kikugawa K. Formation of yellow, orange and red pigments in the reaction of alk-2-enals with 2-thiobarbituric acid. Anal Biochem. 1987;165:456–464. doi: 10.1016/0003-2697(87)90296-X. [DOI] [PubMed] [Google Scholar]
- Kozarski M, Anita K, Dragica J, Nina T, Jovana V, Predrag P, Miomir N, Miroslav M, Vrvic Leo VG. Review: antioxidants of edible mushrooms. Molecules. 2015;20:19489–19525. doi: 10.3390/molecules201019489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalita S, Sunitha T, Pabitra D, Ritu T, Anita P, Kalpana P. Antioxidant activity and phenol and flavonoid contents of eight medicinal plants from Western Nepal. J Tradit Chin Med. 2014;34(5):584–590. doi: 10.1016/S0254-6272(15)30067-4. [DOI] [PubMed] [Google Scholar]
- Merzouk H, Madani S, Chabane D, Prost J, Bouchenak M, Belleville J. Time course of changes in serum glucose, insulin, lipids and tissue lipase activities in macrosomic offspring of rats with streptozotocin-induced diabetes. Clin Sci. 2000;98:21–30. doi: 10.1042/cs0980021. [DOI] [PubMed] [Google Scholar]
- Meyer BN, Ferrigni NR, Putnam JE, Jacobson LB, Nicholas DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Mohammad AE, Ali S. Antioxidant, free radical scavenging activities of Salvia brachyantha and its protective effect against cardiac cell injury. Food Chem Toxicol. 2010;48:846–853. doi: 10.1016/j.fct.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Nagai T, Myoda T, Nagashima T. Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem. 2005;91:389–394. doi: 10.1016/j.foodchem.2004.04.016. [DOI] [Google Scholar]
- Nonaka A, Manabe T, Tobe T. Effect of new synthetic ascorbic acid derivative as a free radical scavenger on the development of acute pancreatitis in mice. Gut. 1991;32:528–532. doi: 10.1136/gut.32.5.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo JM, Joana SV, Luís MG, João GP, Luís FG, Aquiles AB. Isolation of phenolic compounds from hop extracts using polyvinylpolypyrrolidone: characterization by high-performance liquid chromatography–diode array detection–electrospray tandem mass spectrometry. J Chromatogr A. 2010;1217:3258–3268. doi: 10.1016/j.chroma.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Piacente S, Pizza C, Oleszek W. Saponins and Phenolics of Yucca schidigera Roezl: chemistry and bioactivity. Phytochem Rev. 2005;4(2):177–190. doi: 10.1007/s11101-005-1234-5. [DOI] [Google Scholar]
- Pillai SS, Mini S. Polyphenols rich Hibiscus rosasinensis Linn petals modulate diabetic stress signalling pathways in streptozotocin-induced experimental diabetic rats. J Funct Foods. 2016;20:31–42. doi: 10.1016/j.jff.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Pogozelski WK, Tullius TD. Oxidative strand scission of nucleic acids: routes initiated by hydrogen abstraction from the sugar moiety. Chem Rev. 1998;98(3):1089–1108. doi: 10.1021/cr960437i. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Raaman N. Phytochemical techniques. New Delhi: New India Publishing Agency; 2006. p. 10. [Google Scholar]
- Riccardo A, Luca V. Modulation of the antioxidant activity of phenols by non-covalent interactions. Org Biomol Chem. 2012;10:4147–4158. doi: 10.1039/c2ob25174d. [DOI] [PubMed] [Google Scholar]
- Richa U, Jitendra KC, Kavindra NT, Karuna S. Antioxidant property of aerial parts and root of Phyllanthus fraternus Webster, an important medicinal plant. Sci World J. 2014 doi: 10.1155/2014/692392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch RJ, Chug SU, Klaunig JE. Spin trapping of superoxide and hydroxyl radicals. Methods Enzymol. 1984;105:198–209. doi: 10.1016/S0076-6879(84)05026-6. [DOI] [PubMed] [Google Scholar]
- Saini R, Koushalya D, Himani S, Veena G. Antioxidant and antiproliferative activities of phenolics isolated from fruits of Himalayan yellow raspberry (Rubusellipticus) J Food Sci Technol. 2014;51(11):3369–3375. doi: 10.1007/s13197-012-0836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebaluck R, Gurib-Fakim A, Mahomoodally F. Medicinal plants from the genus Acalypha (Euphorbiaceae): a review of their ethnopharmacology and phytochemistry. J Ethnopharmacol. 2015;159:137–157. doi: 10.1016/j.jep.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Severine VD, Pascal G, Patrick F. Qualitative and quantitative Saponin contents in five sea Cucumbers from the Indian ocean. Mar Drugs. 2010;8:173–189. doi: 10.3390/md8010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam KR, Mallikarjuna K, Nishanth K, Hua Kuo C, Sathyavelu RK. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2011;124:1436–1442. doi: 10.1016/j.foodchem.2010.07.104. [DOI] [Google Scholar]
- Shanmugam KR, Mallikarjuna K, Nishanth K, Sathyavelu RK. Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49:893–897. doi: 10.1016/j.fct.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Upadhyay R, Jitendra KC, Kavindra NT, Karuna S. Antioxidant property of aerial parts and root of Phyllanthus fraternus Webster, an important medicinal Plant. Sci World J. 2014 doi: 10.1155/2014/692392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayalaxmi S, Jayalakshmi SK, Sreeramulu K. Polyphenols from different agricultural residues: extraction, identification and their antioxidant properties. J Food Sci Technol. 2015;52(5):2761–2769. doi: 10.1007/s13197-014-1295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C, Yanying Y, Shouran Z, Wei L, Shuge T, Shuwen C. Antioxidant activity and free radical-scavenging capacity of Gynura divaricata leaf extracts at different temperatures. Pharmacogn Mag. 2011;7(25):40–45. doi: 10.4103/0973-1296.75900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.