Abstract
In this study, yogurt was supplemented with 1.5 and 3.0 g L−1 of grape extract, inoculated culture containing Streptococcus thermophilus, Lactobacillus bulgaricus, Lactobacillus acidophilus and Bifidobacterium bb12 bifidum, fermented and stored at 4 °C. Acid production, microbial growth, gel strength, syneresis, rheological and sensory properties were studied. An increase in grape extract concentration extended fermentation time. Bacterial strains were found in at least 109 CFU100 g−1 of yogurt showing the possibility of probiotic yogurt production with grape extract. Gel strength decreased with increasing concentration of grape extract while syneresis increased. The addition of grape extract changed the dilatant behavior to a pseudoplastic behavior, decreased yield stress, whereas k values increased. Sensory attributes (color, flavor, taste, texture and appearance) didn’t differ significantly.
Keywords: Probiotic yogurt, Grape extract, Sensory evaluation
Introduction
The consumption of fermented dairy products, especially yogurts containing probiotic bacteria, has increased markedly over the years (Zare et al. 2011). Probiotics bacteria are defined as live microorganisms that when administered in sufficient amounts, provide health benefits on the host.
Yogurt is a milk protein network aggregated through isoelectric precipitation by lactic acid bacteria. Streptococcus thermophilus and Lactobacillus bulgaricus are used to produce lactic acid from lactose during fermentation (Sun-Waterhouse et al. 2013). Probiotic yogurt- contacting probiotic strains such as Lactobacillus acidophilus and Bifidobacterium bb12 bifidum- is claimed to offer many health benefits such as improve lactose utilization and inhibit cancer (de Vrese et al. 2001; Rafter 2003).
Phenolic compounds (PCs) are also known for their health benefits, especially for the prevention of chronic diseases as cancer (Hervert-Hernández et al. 2009). PCs are produced by plants as their secondary metabolites to protect themselves from oxidative stress (Larrauri et al. 1998; Ignea et al. 2013).
Supplementation of probiotic yogurt with phenolic compounds can be a great alternative for optimizing benefits of yogurt and phenolic compounds intakes. Adding those compounds has been proposed for many products such as dairy beverage (Boroski et al. 2012), milk powder (Gad and El-Salam 2010), probiotic petit Suisse cheese (Pereira et al. 2016) and cheese (Felix da Silva et al. 2015). However, yogurt physical and sensory properties are important aspects for consumer acceptability and can be altered by the addition of some ingredients. Acid production, microbial growth during fermentation and storage, yogurt texture and flavor can be modified by the addition of phenolic compounds-rich ingredients due to interactions with proteins.
The interaction between phenolic compounds and proteins depends on their molecular properties, molar ratio and pH (Gad and El-Salam 2010). Grape extract is a great sources of polyphenols. It is sold commercially and has Generally Recognized as Safe (GRAS) status authorized by the United States Food and Drug Administration (FDA) in 2003. They have shown antioxidant activities in various food formulations (Hu et al. 2004; Shaker 2006; Brannan and Mah 2007).
The objective was to focus on the effect of adding different concentrations of commercial grape extract to probiotic yogurt. Acid production during fermentation, survival of microorganisms, gel strength, syneresis, rheological and sensory attributes were determined.
Materials and methods
Material
Grape extract from Vitis vinifera was kindly provided by Ethical Naturals® (Sunnyvale, CA). According to Ethical Naturals® specifications, the extract was prepared using a mixture of ethanol and distilled water (50:1 volume ratio) and lyophilized. Whole pasteurized milk (Lactobom®) containing 3.51% fat, 3.3% protein was purchased in the local market. Mixed yogurt culture (Chr. Hansen A/S, Denmark) containing S. thermophilus, L. bulgaricus, L. acidophilus and Bifidobacterium bb12 bifidum was used. All other reagents were of analytical grades.
Yogurt production
Whole pasteurized milk was used to prepare yogurt formulations. Three formulations were prepared: control (0 g L−1), 1.5 and 3.0 g L−1 of grape extract. For the starter culture activation, whole pasteurized milk was inoculated with 1% of freeze-dried culture and incubated at 43 °C until pH 5.3 was reached. Whole pasteurized milk was heated to 90 °C for 3 min and then cooled to 43 °C, inoculated at 3% (v/v) with starter culture and placed in 50 mL plastic pots and falcon tubes until pH 4.6 was reached. The yogurts samples were cooled to 4 °C aiming to stop fermentation. The acidification trend during fermentation was measured. Yogurt analyses were performed in triplicate after 24 h of storage at 4 °C. Viable cell counting analyses were done after 3, 7 and 12 days of cold storage.
Yogurt physicochemical characterization
Titratable acidity (g acid lactic 100 g−1) was measured based on the volume of sodium hydroxide (0.1 M) used to neutralize acids present in yogurt. The following equation was used to calculate titratable acidity:
V: sodium hydroxide 0.1 M volume; f: molarity of sodium hydroxide solution; 0.9 = conversion factor for lactic acid; m: mass (g) of yogurt sample.
Ashes, fat, pH and moisture were also measured (AOAC 1990). The protein analyses were performed based on the determination of nitrogen by the micro-Kjeldahl method (AOAC 1990). The nitrogen-to-protein conversion value used was 6.38.
Yogurt microbiological test
Bacterial counts were carried out in triplicate after 3, 7 and 12 days. One mL of sample was diluted with 9 mL of 1 g L−1 sterile peptonized water. Eight serial dilutions were done and each microorganism was counted in the three most appropriate dilutions. M17 agar culture media was used for quantifying viable cells of S. thermophilus. The pour plate technique was used. After inoculation, the plates were incubated at 37 °C for 48 h. For L. bulgaricus was used acidified MRS agar glucose as culture media. After inoculation, the plates were incubated inverted in anaerobic jars containing Anaerobac generator (Probac) at 37 °C for 72 h.
Bifidobacterium viable cells count was done with De Man, Rogosa Sharp MRS-IM culture media added glucose, dicloxacillin solution of lithium chloride and cysteine hydrochloride. The plates were incubated in anaerobic jars containing Anaerobac generator (Probac) at 37 °C for 72 h. For Lactobacillus acidophilus was used De Man, Rogosa Sharp MRS-IM with maltose. Pour plate technique was used and incubated inverted in anaerobic jars containing Anaerobac generator (Probac®) at 37 °C for 72 h (Kailasapathy et al. 2008; Ejtahed et al. 2011; Shori and Baba 2015a).
Gel strength and syneresis
Gel strength (N) of yogurt formulations were obtained using a CT3 Texture Analyzer (Brookfield Engineering Laboratories, Inc., Middleboro, USA) using a cylindrical acrylic probe (d = 35 mm) in a penetration test with the following settings: test normal; trigger 15 grams; deformation 5.0 mm; and speed 1.0 mm s−1. Gel strength was measured based on the ratio of deaf peak and work. To evaluated syneresis of yogurt, the percentage of whey released after centrifugation was measured. Yogurt was prepared directly 50 mL falcon tubes and centrifuged at 500×g for 10 min at 4 °C. The syneresis was expressed in percentage of whey released after centrifugation (Robitaille et al. 2009).
Rheological properties
The flow curves measurements were performed using a controlled stress rheometer Mars II (Haake®, Thermo Fisher Scientific Inc., Newington, Germany) fitted with a 35 mm diameter cone-plate with a gap of 0.052 mm. The analysis was performed in triplicate for each formulation at 10 and 20 °C. The shear rate varied from 0 to 22 s−1 in up-down-up steps. The flow curve data were fitted to the Herschel-Bulkley model as suggested by many authors (Eq. 1) by a nonlinear regression analysis using Origin Pro 2016 (Northampton, Massachusetts, USA) (Silva et al. 2010; Regina et al. 2014; Pereira et al. 2016).
| 1 |
where σ is the shear stress (Pa), σ0 is the yield stress (Pa), k is the consistency index (Pa.sn), γ is the shear rate (s−1), and n is the flow behavior index (dimensionless).
Sensory testing
Color, flavor, texture and overall appearance of yogurt supplemented with grape extract were evaluated by 120 untrained panelists using a 9-point hedonic scale. Panelists were asked to score samples from extremely dislike (1 point) to extremely like (9 points). The yogurt samples were served in white plastic pots in individual booths under light exposure. The temperature was kept at 10 °C. The variance analysis of the results was carried out from the randomized block design, using the Tukey test considering a significance level of P ≤ 0.05. Sensory evaluation was carried out 3 days after production.
Statistical analysis
Yogurts preparation and analyses were carried out in triplicate and variance analysis was conducted using Statistical Analysis System (SAS) version 9.1 (SAS Institute Cary, NC, USA). Differences were considered significant when P < 0.05. Standard errors were obtained from the statistical model and indicated in tables or in graphics as error bars.
Results and discussion
Characterization of grape extract
In a previous study (Felix da Silva et al. 2015) we have characterized different grape extracts. The solubility of 85.6 ± 3.62, moisture of 2.6 ± 0.35, total phenolic compounds (mg GAE g−1) of 962 ± 8.27, total flavonoids (mg QE g−1) of 111 ± 4.22 and DPPH (IC450, µg mL−1) of 78 ± 0.41 was observed.
Yogurt physicochemical characterization
As shown in Table 1, pH, titratable acidity (g lactic acid 100 g−1 yogurt), protein, ash, fat and moisture content were not significantly different when compared with the samples with 0, 1.5 and 3.0 g L−1 of grape extract (P < 0.05). These results were similar to those found in a study on yogurt added flavoring caramel (Ramírez-Sucre and Vélez-Ruiz 2013).
Table 1.
Composition of yogurt control (0.0 g L−1) and supplemented with grape extract (1.5 and 3.0 g L−1)
| Concentration of grape extract added | |||
|---|---|---|---|
| 0. g L−1 | 1.5 g L−1 | 3.0 g L−1 | |
| pH | 4.63 ± 0.047a,* | 4.65 ± 0.047a | 4.65 ± 0.047a |
| Acidity (°Dornic) | 92.70 ± 0.998a | 92.05 ± 0.998a | 92.10 ± 0.998a |
| Protein (%) | 4.35 ± 0.048a | 4.27 ± 0.048a | 4.30 ± 0.048a |
| Ashes (%) | 0.98 ± 0.093a | 0.89 ± 0.093a | 0.83 ± 0.093a |
| Fat (%) | 3.10 ± 0.208a | 3.80 ± 0.208a | 3.40 ± 0.208a |
| Moisture (%) | 81.56 ± 0.312a | 80.60 ± 0.312a | 81.76 ± 0.312a |
S.E. standard error
* Means within a row with different superscripts are significantly different (P < 0.05)
Acidification trend during yogurt fermentation and microbiological tests
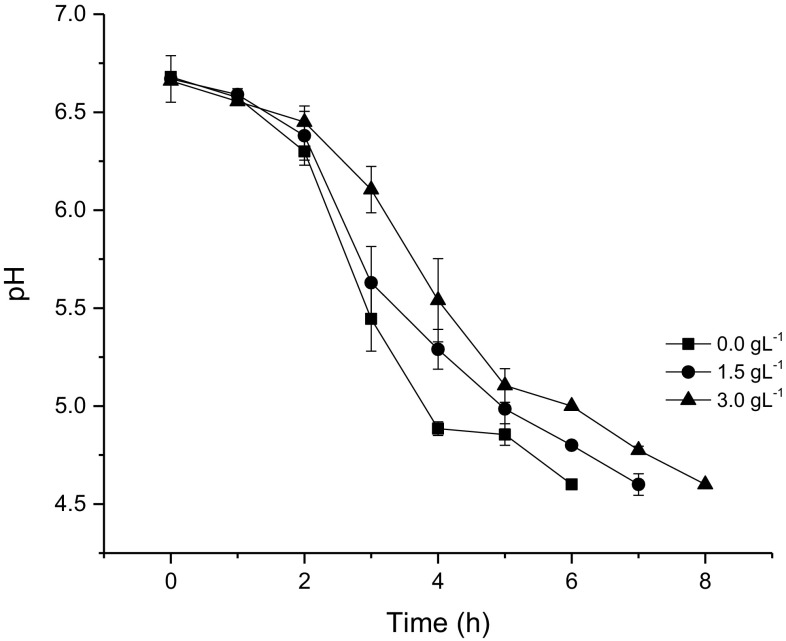
The grape extract was mixed (1.5 and 3.0 g L−1) with milk and inoculated with the microorganism culture. Also, control (without grape extract) sample was produced. The pH monitored during fermentation and acidification trend is presented in Fig. 1. An increase in grape extract concentration extended fermentation time. The initial pH of the milk was 6.68, the addition of 1.5 and 3.0 g L−1 of grape extract did not reduce the initial pH of the milk significantly (P ≤ 0.05).
Fig. 1.
Change in pH as a function of time during acidification of probiotic yogurt control (0 g L−1) and supplemented with grape extract (1.5 and 3.0 g/L). Error bars represent the standard error obtained from the statistical model
The formulation supplemented with 3.0 g L−1 of grape extract showed a decrease in acidification rate, as compared to the control. This effect was statistically significant after 3 h of fermentation (0.0 g L−1 pH = 5.4; 1.5 g L−1pH = 5.6, 3.0 g L−1 pH = 6.1). The addition of 1.5 g L−1 also resulted in lower acidification rate which became significant after 4 h of incubation (0 g L−1 pH = 4.9; 1.5 g L−1 pH = 5.3, 3.0 g L−1 pH = 5.54). The time taken for supplemented yogurt to reach pH 4.6 was 7 and 8 h for 1.5 and 3.0 g L−1 respectively. The effects of grape attract addition on probiotic yogurt have not been studied earlier thus, the comparison of our results with literature data was not possible.
The effect of antioxidant compounds on fermentation time during yogurt production has been studied (Jaziri et al. 2009; Amirdivani and Baba 2011). The authors have shown that the different interactions between phenolic compounds from different sources may occured. Yogurt supplemented with peppermint extract (M. piperita) showed a longer fermentation time compared to yogurt supplemented with dill extract (A. graveolens) (Amirdivani and Baba 2011). No differences were found in the fermentation time of yogurt supplemented with 2 and 4% of black and green tea (Jaziri et al. 2009). Those results suggested that different antioxidant source may affect the fermentation time in a different way. In this study, the addition of grape extract extended the fermentation time.
Overall, the survival of functional bacteria in fermented dairy products depends on numerous environmental and technological factors (Lourens-hattingh and Viljoen 2001). Streptococcus thermophilus and L. bulgaricus have associated growth in yogurt (Sun-Waterhouse et al. 2013), but the presence of different strains as L. acidophilus and Bifidobacterium can interfere with this associate microbial growth. The microorganism can continue to grow during storage. The number of viable microorganisms is an important factor in the final product in terms of acidification and nutritional health benefits attributed to yogurt. It is recommended that yogurt or fermented milk should contain at least 108 CFU/g (EFSA 2010). The stimulatory or inhibitory effect of grape extracts on microorganisms growth can be affected by its composition and phenolic profile (Rodríguez et al. 2009).
The effect of the grape extract on the survival of yogurt microorganisms, viable cells count of L. acidophilus, Bifidobacterium, S. thermophilus and L. bulgaricus were determined after 3, 7 and 12 days. The results for L. acidophilus, Bifidobacterium bb12 bifidu, S. thermophilus and L. bulgaricus viable cells are shown in Table 2.
Table 2.
Lactobacillus acidophilus, Bifidobacterium, S. thermophilus (A) and L. bulgaricus viable cells counting after 3, 7 and 12 days of cold storage in yogurt control (0.0 g L−1) and supplemented with grape extract (1.5 and 3.0 g L−1)
| g L−1 | Day 3 | Day 7 | Day 12 | Day 3 | Day 7 | Day 12 |
|---|---|---|---|---|---|---|
| Lactobacillus acidophilus | Bifidobacterium | |||||
| 0.0 | 220 ± 8.2a | 48.33 ± 6.7a | 10.67 ± 3.3a | 162.3 ± 5.4c | 9.97 ± 2.7c | 6.30 ± 2.3b |
| 1.5 | 31.34 ± 3.6b | 12.47 ± 2.8c | 3.2 ± 2.0b | 323.3 ± 7.9a | 37.67 ± 5.1b | 15.90 ± 3.8a |
| 3.0 | 33.67 ± 4.1b | 19.67 ± 4.6b | 4.2 ± 0.1b | 205 ± 9.2b | 220 ± 5.8a | 12.6 ± 3.4a |
| Streptococcus thermophilus | Lactobacillus bulgaricyus | |||||
| 0.0 | 36.27 ± 7.2a | 0.77 ± 0.12b | 0.73 ± 0.16b | 69.67 ± 6.7a | 65.34 ± 5.8a | 6.20 ± 3.4b |
| 1.5 | 8.9 ± 2.4b | 3.23 ± 1.2a | 0.74 ± 0.22b | 49.37 ± 4.8b | 52.67 ± 6.3b | 6.5 ± 0.40b |
| 3.0 | 8.8 ± 3.3b | 3.11 ± 1.4a | 2.43 ± 1.4a | 27.64 ± 3.7c | 23.7 ± 7.7c | 18.40 ± 2.6a |
Results are given in 108 CFU/g
* Means within a row with different superscripts are significantly different for same microorganism (P < 0.05)
In the present study, the added phenolic compounds changed yogurt starter culture activity but enabled their survival and growth up to 12 days (Table 2). Different phenolic compounds and their metabolites produced by the starter cultures can affect differently the bacterial growth. It is possible that phenolic compounds are transformed into more active derivatives (e.g. aglycones) under certain conditions, which might enhance starter culture activity (Sun-Waterhouse et al. 2013). However, the counting of Lactobacillus acidophilus in supplemented yogurts showed lower counts after 3 days of cold storage compared to control samples. L. acidophilus viable cells counts showed difference (P < 0.05) comparing control (0.0 g L−1) with supplemented (1.5 and 3.0 g L−1) formulations. Control (0.0 g L−1) showed the highest viable cell counts at days of analyses. The supplementation of yogurt with grape extract decreased the number of L. acidophilus but did not show the difference between 1.5 and 3.0 g L−1 formulations (Table 2). It suggests that grape extract may have an antimicrobial effect.
In this study, the supplementation of probiotic yogurt with grape extract affected the time for yogurt production but did not interfere in the number of viable bacterial cells required for health benefits (Grajek et al. 2005). Phenolic compounds have different levels of antimicrobial effects (Rauha et al. 2000; Välimaa et al. 2007). Varied effects of added bioactive ingredients on different microorganisms were reported, which can be associated with the differences in chemical structure and antioxidant activity (Sun-Waterhouse et al. 2013). Bacteria type may also determine the antimicrobial effect (Alberto et al. 2001; Lee et al. 2006).
Bifidobacterium showed a higher viable cells counts in the presence of 1.5 g L−1 grape extract at day 3 followed by a decrease at day 7 and 12 of storage while 3.0 g L−1 had a decrease in counts between day 7 and 12 (Table 2). In this study, grape extract increased the fermentation time, i.e., the time to reach pH 4.6 acting as a buffering (higher pH), what allows the growth of Bifidobacterium (Shori and Baba 2015b). Therefore, at least 108 CFU/g yogurt were present ensuring the yogurt properties.
Studies have shown the effect of grape extract, grape residues extract and standard compounds as quercetin, gallic acid, catechin, tannin acid on the growth of L. acidophilus in agar diffusion tests. The authors did not find an inhibitory effect on L. acidophilus CECT 903 growth, showing that this microorganism was not affected by the presence of a concentration of 500 μg/mL of grape pomace (Hervert-Hernández et al. 2009). Grape extract and grape pomace contain a wide array of phenolic compounds.
A study with L. acidophilus ATCC 4356 showed resistance behavior in the presence of tannic acid at a concentration up to 1000 µg/mL (Chung et al. 1998). Other authors suggested that a higher concentration would be required to inhibit the growth of this lactic acid bacteria strain. This finding suggests that L. acidophilus CECT 903 strain, as well as other lactic acid bacteria, possesses the ability to degrade tannic acid and obtain energy from it (Rodríguez et al. 2009).
In this study (Table 2), the counts of S. thermophilus viable cells remained constant for supplemented formulations while the control formulation had a significant decrease after 7 and 12 days (P ≤ 0.05). A study showed that yogurt supplementation with pycnogenol extract did not affect the growth of Streptococcus and Lactobacillus, while isoflavones and phytosterols did (Ramchandran and Shah 2008). Blackcurrant extract and cyanidin added before yogurt fermentation affected differently the colony number starter cultures S. thermophilus and L. bulgaricus (Sun-Waterhouse et al. 2013). In a yogurt matrix, various interactions could occur such as bindings between phenolic compounds and milk proteins or polysaccharides (Papadopoulou and Frazier 2004).
Grape extracts are known for their high content of total phenolic compounds mainly oligomeric proanthocyanidins (74–78%) and less than approximately 6% of free flavanol monomers (Perumalla and Hettiarachchy 2011). Studies have shown the influence of adding phenolic compounds on the chemical, physical and microbiological properties of drinking yogurt showing that those compounds can be successfully incorporated into drinking yogurts with no decrease in survival and growth of Streptococcus and Lactobacillus (Sun-Waterhouse et al. 2013).
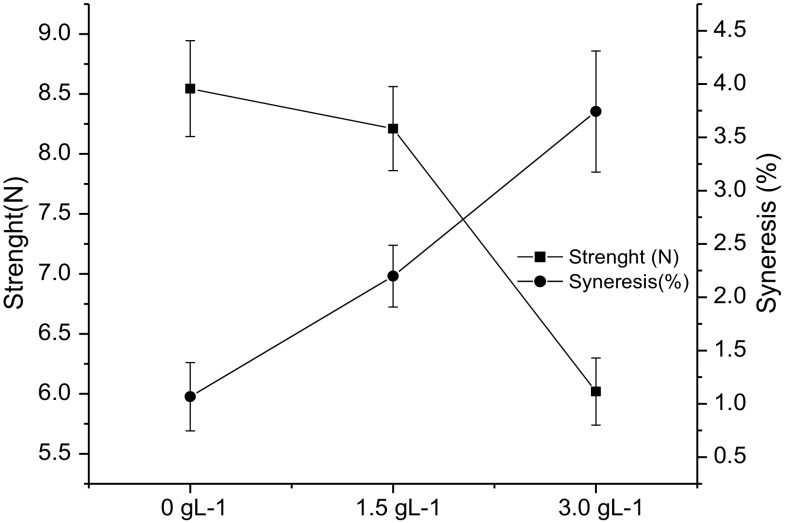
Gel strength and syneresis
The yogurt gel strength and yogurt syneresis are important parameters to measure yogurt quality. The values of gel strength after force compression and syneresis percentage are shown in Fig. 2. The addition of grape extract influenced both the texture and the syneresis (P ≤ 0.05). Phenolic compounds are a group of plants secondary metabolites responsible for its defense mechanisms, which has strong ability to associate with primary metabolites such as proteins and carbohydrates (McManus et al. 1985; Papadopoulou and Frazier 2004; Han et al. 2011a, b).
Fig. 2.
Gel strength (N) and syneresis (%) of yogurt control (0.0 g L−1) and supplemented with grape extract (1.5 and 3.0 g/L). Error bars represent the standard error obtained from the statistical model (P ≤ 0.05)
The structure of milk protein network is a result of non-covalent interactions, mainly, hydrophobic interactions between the side chains of the amino acids (Han et al. 2011a). The addition of phenolic compounds in milk may induce interactions with the hydrophobic surface of milk protein, which would reduce hydrophobic interactions between the amino acid side chains. A reduction in the number of available hydrophobic functional groups could result in a decreased of water binding (Han et al. 2011b).
Syneresis is controlled by the balance between attraction and repulsion forces within the casein network and the rearrangement capacity of the network bonds (Matumoto-Pintro et al. 2011; Giroux et al. 2014). In this study, syneresis was significantly affected by the grape extract concentration (P ≤ 0.05). This effect may be explained by the structural difference in the gels induced by phenolic compounds. Polyphenols may increase rearrangements, which would results in larger pore size in the gel matrix which is associated with higher syneresis. Han et al. (2011a, b) reported results indicating that milk gel syneresis increased in the presence of commercial grape extract. Interactions between phenolic compounds and yogurt proteins allow water not connect strongly to the network proteins (Han et al. 2011a, b).
Gel strength decreased with increasing concentration of the grape extract, which means that a lower force was required for deformation (Fig. 2). Studies have shown that polyphenols interact with milk protein and can induce crosslinking and network bonds destabilization (O’Connell and Fox 1999; Han et al. 2011b). Reduced gel strength in the presence of phenolic compounds indicates a weak gel structure. This could be explained by reduced repulsion between the proteins of the gel matrix. In a study using different commercial grape extracts in cheese making, this results indicated that milk gel syneresis increased in the presence of commercial grape due to hydrophobic bonds (Han et al. 2011b). Studies with probiotic petit Suisse cheese supplemented with phenolic compounds rich extract (jabuticaba- Plinia trunciflora extract) showed a decreased in the gel strength, suggesting that the addition of antioxidant interfere in the formation of protein network (Pereira et al. 2016).
Rheological properties
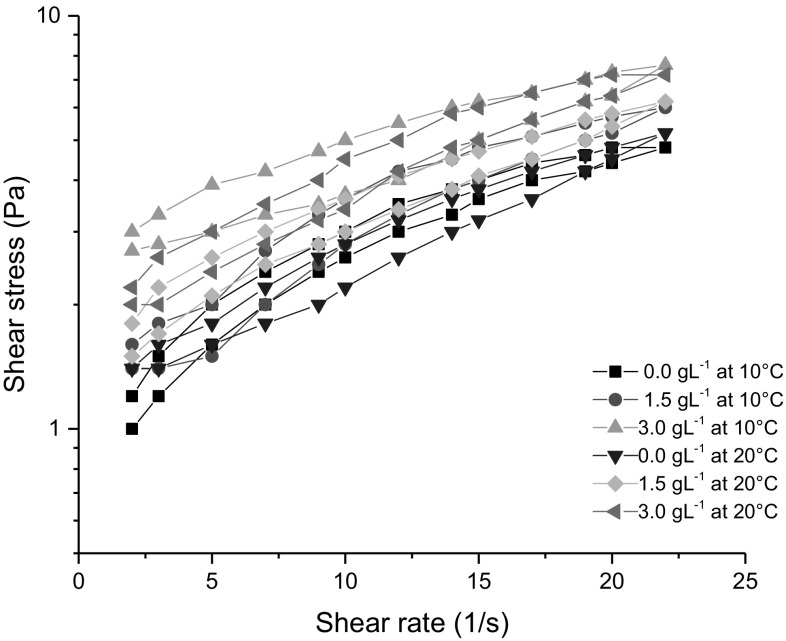
The flow curves for the samples at 10 and 20 °C were presented in Fig. 3 showing a thixotropic non-Newtonian behavior. The samples also showed the presence of yield stress related to the existence of an interactive or crosslinked structure (Fangary et al. 1999). The yield stress can be used to characterize the firmness of the yogurts (Smith 1978). The control sample (0.0 g L−1) presented the highest value of yield stress while the yogurt supplemented with 3.0 g L−1 presented the lowest value at 10 and 20 °C confirming a weaker gel (Fig. 2). Studies with petit suisse and cheese with antioxidant compounds have shown similar results regarding the wreaking of gel structure (Han et al. 2011a; Pereira et al. 2016). The samples showed a non-Newtonian behavior and the shear rate curves were modeled mathematically using the Herschel-Bulkley equation (Fangary et al. 1999) obtaining the parameters presented in Table 3. The data were well fitted showing a R2 higher than 0.96. The control sample (0.0 g L−1) at 10 °C showed dilatant behavior (n > 1). The addition of grape extract has changed this behavior to pseudoplastic. This change in behavior may be related to the change in the network interaction between milk protein and phenolic compounds. The addition of the antioxidant extract may induce the decrease of yield stress, whereas k values increased with the supplementation. Similar results were found in petit suisse supplemented with ascorbic acid, glucose oxidase, cysteine and jabuticaba extract (Pereira et al. 2016).
Fig. 3.
Flow curves of the probiotic yogurt control (0 g/L) and supplemented with grape extract (1.5 and 3.0 g L−1)
Table 3.
Rheological parameters estimated by Herschel–Bulkley model and hysteresis area for flow curves of the probiotic yogurt supplemented with grape extract
| Samples | σ0 (Pa) | k (Pa.sn) | n | Hysteresis area (Pa s−1) |
|---|---|---|---|---|
| 0.0 g L−1 at 10 °C | 2.73 ± 0.51a | 0.06 ± 0.01f | 1.36 ± 0.20b | 19.5 ± 1.2a |
| 1.5 g L−1 at 10 °C | 0.96 ± 0.23e | 0.189 ± 0.01b | 1.06 ± 0.21d | 12.25 ± 1.1c |
| 3.0 g L−1 at 10 °C | 0.38 ± 0.04f | 0.404 ± 0.02a | 0.78 ± 0.1e | 7.7 ± 0.78e |
| 0.0 g L−1 at 20 °C | 1.64 ± 0.25b | 0.178 ± 0.01c | 1.12 ± 0.3c | 15.25 ± 1.1b |
| 1.5 g L−1 at 20 °C | 1.45 ± 0.16c | 0.13 ± 0.01d | 1.15 ± 0.1c | 10.75 ± 1.2d |
| 3.0 g L−1 at 20 °C | 1.28 ± 0.14d | 0.04 ± 0.01e | 1.47 ± 0.1a | 7.7 ± 0.88e |
Means within a column with different superscripts are significantly different (P < 0.05)
A thixotropic phenomenon can be explained by the change in viscosity when a shear force is applied, first, the gel network tends to resist to the shear force and then, the breakdown occurs after a critical shear rate application (Bourne 2002). This behavior can be estimated using the difference between the areas under the curves of shear rate versus shear stress, once the energy required for disrupting the gel structure is proportional to the area of the hysteresis (Bourne 2002). The hysteresis area data is shown in Table 3. Thixotropy of control sample (0.0 g L−1) was higher than the other samples. Hysteresis area value decreased as the increase of grape extract addition indicating a weaker structure of supplemented yogurt. These results agree with the previous data from the texture analysis.
Sensory properties
Yogurt control and supplemented with grape extracts were ranked in terms of color, flavor, taste, texture and overall appearance and results are presented in Table 4. The lowest numbers represent less desirable and the highest represent the most desirable formulations [i.e., extremely dislike (1 point) until extremely like (9 points) with a score of 5 points representing neither like or dislike]. None of the sensory attributes differ significantly (P ≤ 0.05). Based on sensory properties, it was possible to supplement yogurt with 1.5 and 3.0 g L−1 of grape extract without significant sensory changes.
Table 4.
Sensory properties of yogurt control (0.0 g L−1) and supplemented with grape extract (1.5 and 3.0 g L−1) according to 9 points hedonic scale (1-extremely dislike to 9-extremely like)
| Concentration of grape extract added | |||
|---|---|---|---|
| 0.0 g L−1 | 1.5 g L−1 | 3.0 g L−1 | |
| Color | 6.78 ± 1.34a | 6.23 ± 1.34a | 5.56 ± 1.34a |
| Flavor | 6.79 ± 1.23a | 6.29 ± 123a | 6.06 ± 1.23a |
| Taste | 7.20 ± 1.01a | 6.48 ± 1.01a | 6.13 ± 1.01a |
| Texture | 7.27 ± 1.16a | 6.88 ± 1.16a | 6.19 ± 1.164a |
| Overall appearance | 7.30 ± 0.85a | 6.63 ± 0.85a | 6.14 ± 0.85a |
* Means within a row with different superscripts are significantly different (P < 0.05)
Conclusion
The grape extract can be used as an ingredient in probiotic yogurt containing S. thermophilus, L. bulgaricus, L. acidophilus and Bifidobacterium bb12 bifidum. The grape extract increased fermentation time. The addition of grape extract did alter significantly the yogurt gel strength and syneresis compared with control formulation. Those differences resulted in a supplemented yogurt with softer texture. Syneresis increased in yogurts formulated with grape extract and the dilatant behavior has changed to a pseudoplastic behavior, decrease of yield stress, whereas k values increased with the supplementation. However, from a sensory stand point no differences were found between the samples. The production of supplemented probiotic yogurt- up to 3.0 g L−1 grape extract- is feasible in industrial and consumer point of view, once did not show significant sensory or microbiological activities changes.
Contributor Information
Denise Felix da Silva, Phone: +45 50313709, Email: denise@food.ku.dk, Email: denise_felix@outlook.com.
Paula Toshimi Matumoto-Pintro, Email: ptmpintro@gmail.com.
References
- Alberto MR, Farías ME, Manca De Nadra MG. Effect of gallic acid and catechin on Lactobacillus hilgardii 5w growth and metabolism of organic compounds. J Agric Food Chem. 2001;49:4359–4363. doi: 10.1021/jf0101915. [DOI] [PubMed] [Google Scholar]
- Amirdivani S, Baba AS. Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT Food Sci Technol. 2011;44:1458–1464. doi: 10.1016/j.lwt.2011.01.019. [DOI] [Google Scholar]
- Boroski M, Giroux HJ, Sabik H, et al. Use of oregano extract and oregano essential oil as antioxidants in functional dairy beverage formulations. LWT Food Sci Technol. 2012;47:167–174. doi: 10.1016/j.lwt.2011.12.018. [DOI] [Google Scholar]
- Bourne MC. Food texture and viscosity: concept and measurement. Amsterdam: Elsevier Science & Technology Books; 2002. [Google Scholar]
- Brannan RG, Mah E. Grape seed extract inhibits lipid oxidation in muscle from different species during refrigerated and frozen storage and oxidation catalyzed by peroxynitrite and iron/ascorbate in a pyrogallol red model system. Meat Sci. 2007;77:540–546. doi: 10.1016/j.meatsci.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Chung KT, Lu Z, Chou MW. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem Toxicol. 1998;36:1053–1060. doi: 10.1016/S0278-6915(98)00086-6. [DOI] [PubMed] [Google Scholar]
- de Vrese M, Stegelmann A, Richter B, et al. Probiotics–compensation for lactase insufficiency. Am J Clin Nutr. 2001;73:421S–429S. doi: 10.1093/ajcn/73.2.421s. [DOI] [PubMed] [Google Scholar]
- Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, et al. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci. 2011;94:3288–3294. doi: 10.3168/jds.2010-4128. [DOI] [PubMed] [Google Scholar]
- Fangary YS, Barigou M, Seville JPK. Simulation of yoghurt flow and prediction of its end-of-process properties using rheological measurements. TransI Chem E. 1999;77:33–39. [Google Scholar]
- Felix da Silva D, Matumoto-Pintro PT, Bazinet L, et al. Effect of commercial grape extracts on the cheese-making properties of milk. J Dairy Sci. 2015;98:1552–1562. doi: 10.3168/jds.2014-8796. [DOI] [PubMed] [Google Scholar]
- Gad AS, El-Salam MHA. The antioxidant properties of skim milk supplemented with rosemary and green tea extracts in response to pasteurisation, homogenisation and the addition of salts. Int J Dairy Technol. 2010;63:349–355. doi: 10.1111/j.1471-0307.2010.00585.x. [DOI] [Google Scholar]
- Giroux HJ, Bouchard C, Britten M. Combined effect of renneting pH, cooking temperature, and dry salting on the contraction kinetics of rennet-induced milk gels. Int Dairy J. 2014;35:70–74. doi: 10.1016/j.idairyj.2013.10.016. [DOI] [Google Scholar]
- Grajek W, Olejnik A, Sip A. Probiotics, prebiotics and antioxidants as functional foods. Acta Biochimica Polonica. 2005;52:665–671. [PubMed] [Google Scholar]
- Han J, Britten M, St-Gelais D, et al. Polyphenolic compounds as functional ingredients in cheese. Food Chem. 2011;124:1589–1594. doi: 10.1016/j.foodchem.2010.08.021. [DOI] [Google Scholar]
- Han J, Britten M, St-Gelais D, et al. Effect of polyphenolic ingredients on physical characteristics of cheese. Food Res Int. 2011;44:494–497. doi: 10.1016/j.foodres.2010.10.026. [DOI] [Google Scholar]
- Hervert-Hernández D, Pintado C, Rotger R, Goñi I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int J Food Microbiol. 2009;136:119–122. doi: 10.1016/j.ijfoodmicro.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Hu M, McClements DJ, Decker EA. Antioxidant activity of a proanthocyanidin-rich extract from grape seed in whey protein isolate stabilized algae oil-in-water emulsions. J Agric Food Chem. 2004;52:5272–5276. doi: 10.1021/jf049486j. [DOI] [PubMed] [Google Scholar]
- Ignea C, Dorobanţu CM, Mintoff CP, et al. Modulation of the antioxidant/pro-oxidant balance, cytotoxicity and antiviral actions of grape seed extracts. Food Chem. 2013;141:3967–3976. doi: 10.1016/j.foodchem.2013.06.094. [DOI] [PubMed] [Google Scholar]
- Jaziri I, Ben Slama M, Mhadhbi H, et al. Effect of green and black teas (Camellia sinensis L.) on the characteristic microflora of yogurt during fermentation and refrigerated storage. Food Chem. 2009;112:614–620. doi: 10.1016/j.foodchem.2008.06.017. [DOI] [Google Scholar]
- Kailasapathy K, Harmstorf I, Phillips M. Survival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. lactis in stirred fruit yogurts. LWT Food Sci Technol. 2008;41:1317–1322. doi: 10.1016/j.lwt.2007.08.009. [DOI] [Google Scholar]
- Larrauri A, Sanchez-Moreno C, Saura-Calixto F. Effect of temperature on the free radical scavenging capacity of extracts from red and white grape pomace peels. J Agric Food Chem. 1998;46:2694–2697. doi: 10.1021/jf980017p. [DOI] [Google Scholar]
- Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Lourens-hattingh A, Viljoen BC. Yogurt as probiotic carrier food. Int Dairy J. 2001;11:1–17. doi: 10.1016/S0958-6946(01)00036-X. [DOI] [Google Scholar]
- Matumoto-Pintro PT, Rabiey L, Robitaille G, Britten M. Use of modified whey protein in yoghurt formulations. Int Dairy J. 2011;21:21–26. doi: 10.1016/j.idairyj.2010.07.003. [DOI] [Google Scholar]
- McManus JP, Davis KG, Beart JE, et al (1985) Polyphenol interactions. Part 1. Introduction; some observations on the reversible complexation of polyphenols with proteins and polysaccharides. J Chem Soc Perkin Trans 2 28:1429. doi: 10.1039/p29850001429
- O’Connell JE, Fox PF. Proposed mechanism for the effect of polyphenols on the heat stability of milk. Int Dairy J. 1999;9:523–536. doi: 10.1016/S0958-6946(99)00124-7. [DOI] [Google Scholar]
- Papadopoulou A, Frazier RA. Characterization of protein–polyphenol interactions. Trends Food Sci Technol. 2004;15:186–190. doi: 10.1016/j.tifs.2003.09.017. [DOI] [Google Scholar]
- Pereira EPR, Cavalcanti RN, Esmerino EA, et al. Effect of incorporation of antioxidants on the chemical, rheological, and sensory properties of probiotic petit suisse cheese. J Dairy Sci. 2016;99:1762–1772. doi: 10.3168/jds.2015-9701. [DOI] [PubMed] [Google Scholar]
- Perumalla AVS, Hettiarachchy NS. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res Int. 2011;44:827–839. doi: 10.1016/j.foodres.2011.01.022. [DOI] [Google Scholar]
- Rafter J. Probiotics and colon cancer. Bailliere’s Best Pract Res Clin Gastroenterol. 2003;17:849–859. doi: 10.1016/S1521-6918(03)00056-8. [DOI] [PubMed] [Google Scholar]
- Ramchandran L, Shah NP. Effect of Versagel on the growth and metabolic activities of selected lactic acid bacteria. J Food Sci. 2008;73:21–26. doi: 10.1111/j.1750-3841.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- Ramírez-Sucre MO, Vélez-Ruiz JF. Physicochemical, rheological and stability characterization of a caramel flavored yogurt. LWT Food Sci Technol. 2013;51:233–241. doi: 10.1016/j.lwt.2012.09.014. [DOI] [Google Scholar]
- Rauha JP, Remes S, Heinonen M, et al. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 2000;56:3–12. doi: 10.1016/S0168-1605(00)00218-X. [DOI] [PubMed] [Google Scholar]
- Regina K, Guimarães F, Maria F, Lopes R. Assessing the potential of fl axseed protein as an emulsi fi er combined with whey protein isolate. FRIN. 2014;58:89–97. [Google Scholar]
- Robitaille G, Tremblay A, Moineau S, et al. Fat-free yogurt made using a galactose-positive exopolysaccharide-producing recombinant strain of Streptococcus thermophilus. J Dairy Sci. 2009;92:477–482. doi: 10.3168/jds.2008-1312. [DOI] [PubMed] [Google Scholar]
- Rodríguez H, Curiel JA, Landete JM, et al. Food phenolics and lactic acid bacteria. Int J Food Microbiol. 2009;132:79–90. doi: 10.1016/j.ijfoodmicro.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Shaker ES. Antioxidative effect of extracts from red grape seed and peel on lipid oxidation in oils of sunflower. LWT Food Sci Technol. 2006;39:883–892. doi: 10.1016/j.lwt.2005.06.004. [DOI] [Google Scholar]
- Shori AB, Baba AS. Survival of Bifidobacterium bifidum in cow- and camel-milk yogurts enriched with Cinnamomum verum and Allium sativum. J Assoc Arab Univ Basic Appl Sci. 2015;18:7–11. [Google Scholar]
- Shori AB, Baba AS. Survival of Bifidobacterium bifidum in cow- and camel-milk yogurts enriched with Cinnamomum verum and Allium sativum. J Assoc Arab Univ BASIC Appl Sci. 2015 [Google Scholar]
- Silva VM, Carla A, Sato K, et al. (2010) Original article the effect of homogenisation on the stability of pineapple pulp. 2127–2133. doi: 10.1111/j.1365-2621.2010.02386.x
- Smith EG (1978) Routine quality control technics. J Histotechnol 1:99–99. doi:10.1179/014788878794582220
- Sun-Waterhouse D, Zhou J, Wadhwa SS. Drinking yoghurts with berry polyphenols added before and after fermentation. Food Control. 2013;32:450–460. doi: 10.1016/j.foodcont.2013.01.011. [DOI] [Google Scholar]
- Välimaa AL, Honkalampi-Hämäläinen U, Pietarinen S, et al. Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms. Int J Food Microbiol. 2007;115:235–243. doi: 10.1016/j.ijfoodmicro.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Zare F, Boye JI, Orsat V, et al. Microbial, physical and sensory properties of yogurt supplemented with lentil flour. Food Res Int. 2011;44:2482–2488. doi: 10.1016/j.foodres.2011.01.002. [DOI] [Google Scholar]