Abstract
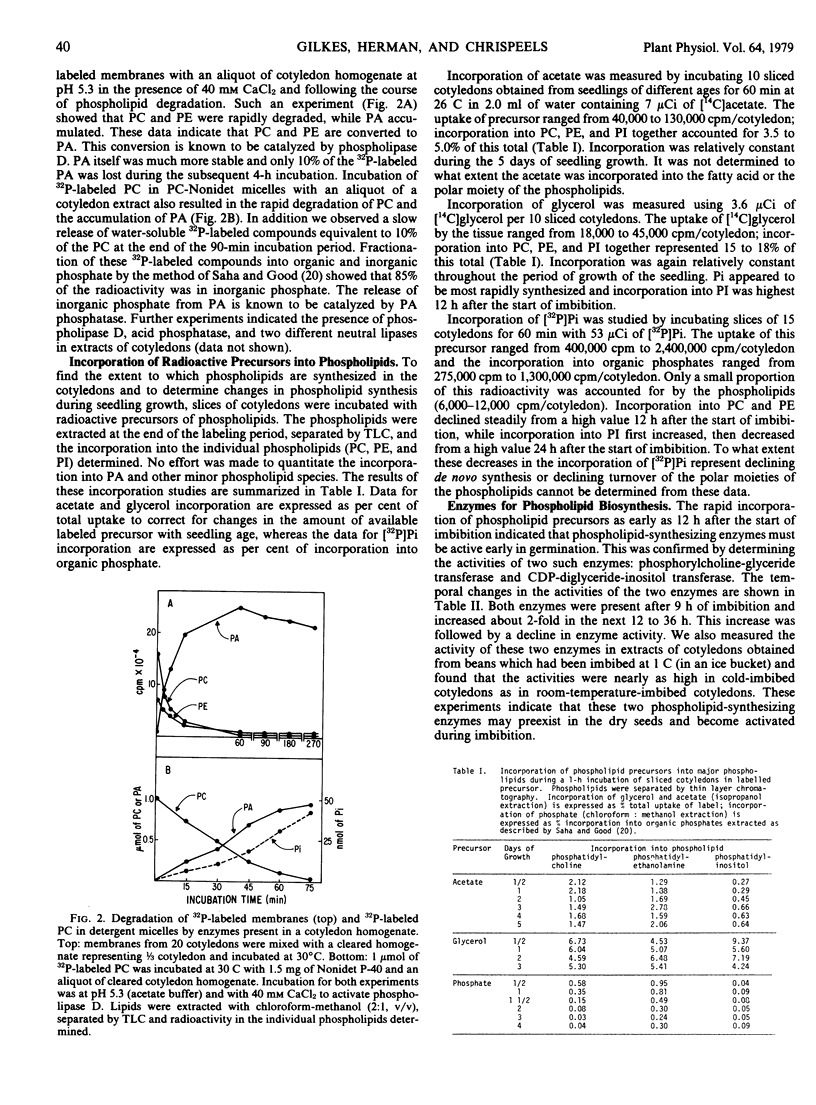
Seedling growth of mung bean is accompanied by the rapid catabolism of the three major phospholipids in the cotyledons (phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol). The decline starts 24 hours after the beginning of imbibition and by the 4th day of growth more than 50% of the phospholipids have been catabolized. Extracts of cotyledons of 24-hour-imbibed beans contain enzymes capable of degrading membrane-associated phospholipids in vitro. This degradation involves phospholipase D and phosphatase activity.
Studies with radioactive acetate, glycerol, and orthophosphate indicate that the three major phospholipids are also synthesized in the cotyledons. Incorporation of glycerol and acetate into phospholipids of cotyledons is relatively constant throughout seedling growth, while the incorporation of [32P]orthophosphate steadily declines from a high value 24 hours after the start of imbibition. The newly synthesized phospholipids become associated with membranous organelles, especially the endoplasmic reticulum, and have an in situ half-life of 2 to 2.5 days.
Determination of the activities of two enzymes involved in phospholipid biosynthesis (phosphorylcholine-glyceride transferase and CDP-diglyceride-inositol transferase) shows that the enzymes have their highest activities 12 hours after the start of imbibition. High activities for both enzymes were found in cotyledons of beans incubated at 1 C, indicating that the enzymes may preexist in the dry seeds.
The experiments demonstrate that cotyledons start synthesizing new phospholipids immediately after imbibition, but that the rate of phospholipid catabolism far exceeds the rate of synthesis long before the cotyledons start to senesce.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Baumgartner B., Tokuyasu K. T., Chrispeels M. J. Localization of vicilin peptidohydrolase in the cotyledons of mung bean seedlings by immunofluorescence microscopy. J Cell Biol. 1978 Oct;79(1):10–19. doi: 10.1083/jcb.79.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Development of phospholipid synthesizing enzymes in castor bean endosperm. FEBS Lett. 1975 Jan 1;49(3):369–371. doi: 10.1016/0014-5793(75)80787-3. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Baumgartner B., Harris N. Regulation of reserve protein metabolism in the cotyledons of mung bean seedlings. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3168–3172. doi: 10.1073/pnas.73.9.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Firn R. D., Kende H. Some effects of applied gibberellic Acid on the synthesis and degradation of lipids in isolated barley aleurone layers. Plant Physiol. 1974 Dec;54(6):911–915. doi: 10.1104/pp.54.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern R., Chrispeels M. J. Influence of the axis on the enzymes of protein and amide metabolism in the cotyledons of mung bean seedlings. Plant Physiol. 1978 Nov;62(5):815–819. doi: 10.1104/pp.62.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler D. E., Varner J. E. Hormonal control of orthophosphate incorporation into phospholipids of barley aleurone layers. Plant Physiol. 1973 Sep;52(3):208–214. doi: 10.1104/pp.52.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher B. A., Brown C. P., McManus T. T., Mudd J. B. Studies on Phospholipid-synthesizing Enzyme Activities during the Growth of Etiolated Cucumber Cotyledons. Plant Physiol. 1975 Jan;55(1):130–136. doi: 10.1104/pp.55.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- Moore T. S., Lord J. M., Kagawa T., Beevers H. Enzymes of phospholipid metabolism in the endoplasmic reticulum of castor bean endosperm. Plant Physiol. 1973 Jul;52(1):50–53. doi: 10.1104/pp.52.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S. Phospholipid turnover in soybean tissue cultures. Plant Physiol. 1977 Nov;60(5):754–758. doi: 10.1104/pp.60.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Good N. E. Products of the photophosphorylation reaction. J Biol Chem. 1970 Oct 10;245(19):5017–5021. [PubMed] [Google Scholar]
- Sumida S., Mudd J. B. The structure and biosynthesis of phosphatidyl inositol in cauliflower inflorescence. Plant Physiol. 1970 Jun;45(6):712–718. doi: 10.1104/pp.45.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Phosphatidic Acid synthesis in castor bean endosperm. Plant Physiol. 1977 Mar;59(3):459–463. doi: 10.1104/pp.59.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]


