Abstract
Ultrafiltration and diafiltration of skim milk altered delicate salt equilibrium and composition of 5× UF retentate (5× UFR), and thus adversely affected the reconstitutional and functional properties of milk protein concentrate (MPC) powders. It might be due to interaction and aggregation of proteins during spray drying. Therefore, this study was envisaged to investigate the effect of disodium phosphate (DSP) addition, diafiltration and homogenization of retentates on physico-chemical, functional and rheological properties of MPC60 powders. Solubility of fresh control powder was significantly lower than MPC60-H powder; at par with that of MPC60-DSP and MPC60-Na–K, but remained minimum after 60 days of storage at 25 ± 1 °C. The pH (6.6) adjustment of 5× UFR with DSP, significantly enhanced the dispersability, wettability, specific surface area (SSA), heat coagulation time (HCT), emulsification capacity and stability; buffer index of MPC60-DSP powder over control. Diafiltration of 5× UFR with NaCl and KCl, significantly (P < 0.05) decreased calcium content, but enhanced pH and mineral content of MPC60-Na–K powder. This treatment led to significant improvement in dispersability, SSA, emulsification capacity and stability, HCT and oil binding properties. Flowability, wettability, dispersability, HCT, foaming capacity, emulsification capacity and stability were also improved significantly in MPC60-H powder made from homogenized 5× UFR. Rheological behavior of reconstituted powder samples exhibited pseudoplastic behavior, best explained by Hershel Bulkley model. These MPC60 powders with improved functional properties can be used for the improvement of quality attributes of various food formulations.
Keywords: Milk protein concentrate 60, Homogenization, Disodium phosphate, Reconstitutional, Functional and rheological properties
Introduction
Milk protein concentrates (MPCs) are protein rich powders; produced by employing the ultrafiltration (UF), diafiltration (DF) of milk followed by evaporation (optional) and spray drying. These powders differ significantly from skimmed milk powder (SMP) and whole milk powder (WMP) in their proximate composition, physico-chemical and functional properties. Ratio of casein to whey protein in MPCs are identical to native milk. The protein content of MPCs may ranges from 40 to ≤89% on dry matter (DM) basis and most common MPC types includes MPC42, MPC60, MPC70, MPC80 and MPC85, however definite standards for proper identification of MPCs still does not exists worldwide. Wide variation in proximate composition particularly in protein contents and functional properties of MPC powders still exists. Sikand et al. (2011) has attempted to categorize MPC powders into three main type’s i.e. (a) low-protein powder (≤40% protein content), (b) medium protein powder (60–70% protein content) and, (c) high-protein powder (≥80% protein content).
As high quality milk protein ingredient, demand of MPCs is growing rapidly worldwide. Launching of about 900 different (high protein and low lactose, specialty foods, sports drinks, energy and nutrition bars and other) products in market using MPCs as a key ingredient, indicate their popularity among stakeholders (Agarwal et al. 2015). It has been estimated that production of MPCs will grow more than 40,000 MT by 2020 and the same may expand its market volume by displacing casein in specific applications (Lagrange et al. 2015).
Solubility of protein-rich powders, is a pre-requisite for their effective utilization as functional properties ingredient. Key processes used for the production of MPCs i.e. UF, DF and spray drying have been reported to alter the delicate salt equilibrium between colloidal and soluble phases of the protein stabilization system. Any deviation may not only induce a detrimental impact on milk proteins environment (Singh 2007), but also adversely affects the functional properties like solubility. MPC powders with higher protein content have poor solubility, thus, restricting their wider usage in several potential food applications (De Castro-Morel and Harper 2002). The observed variation in the solubility of commercial MPC samples was identified as the biggest obstacle in realization of the global market potential (De Castro-Morel and Harper 2002; Huppertz and Gazi 2015). Functional properties of MPCs are at par with that of calcium caseinate (except gelation), but poor than that of sodium caseinate, whey protein concentrate (WPC) and (WPI) whey protein isolates (Singh 2011). Nitrogen solubility index (NSI); dispersability and heat stability of 32 commercial MPC powders were widely varied in the range of 27–87%; 38–100% and 0–42 min, respectively (Huppertz and Gazi 2015). Baldwin (2010) reported that poor solubility of MPC powders continued to be a problem by negatively affecting other functional properties.
During drying of SMP and WMP, lactose prevents protein–protein interaction as it acts as a mechanical spacer; forms hydrogen bonds with protein chains and also provides channel for absorption of water molecules within casein micelles during reconstitution process, resulting in better solubility (Baldwin 2010). Passage of lactose into permeate during UF treatment, minimizes retentates protection during drying; resulted in poor solubility of MPC powders. Insolubility reaction can take place either on the particle surface or between casein micelles, but detailed scientific data for the complete elucidation of insolubility development mechanism is still not clear (Baldwin 2010). Therefore, improving the solubility of MPC powders remains a key challenge to improve its functionality and uses. Various technological approaches based on chemical, physical, and enzymatic methods have been attempted to improve of the solubility of different MPC powders by several researchers, however, effect of stabilizing salts and mechanical shearing has not been studied so far. Further, most of the research has been focused on high-protein powder (MPC80–MPC89) rather than medium (60–70%) protein powders. Few attempts for manufacturing MPC could not succeeded due to lack of standardized process. However, dairy processors willing to diversify towards high value added products such as MPC owing to its export potential as SMP production has no more a viable option due to its fluctuating demand and the stiff competition in international market.
Therefore, current investigation was aimed to investigate the effect of UF concentration along with Na2HPO4 addition, diafiltration (with 1:1 ratio 75:75 mM of NaCl and KCl) and homogenization on physico-chemical, functional and rheological properties of milk protein concentrate 60 (MPC60) powders.
Materials and methods
Ultrafiltration and diafiltration
Pasteurized cow skim milk (PCSM) (73 ± 1 °C/15 s) was procured from Experiential Dairy of ICAR-National Dairy Research Institute, Karnal (India) and ultrafiltered up to 5× concentration in a pilot UF plant (Tech-Sep., France, tubular module, 50 kDa ZrO2 membrane having 1.68 m2 surface area) at 50 ± 1 °C and 1 kg/cm2 constant transmembrane pressure (TMP) by maintaining inlet and outlet pressures at 4.2 and 3 kg/cm2, respectively as also reported by Meena et al. (2015). Concentration factor (CF) i.e. ratio of original feed volume to final feed volume was calculated for milk on weight basis as reported by Meena et al. (2016). All salts used in this study were of analytical grade and procured from Sigma-Aldrich, USA.
Production of MPC60 powders
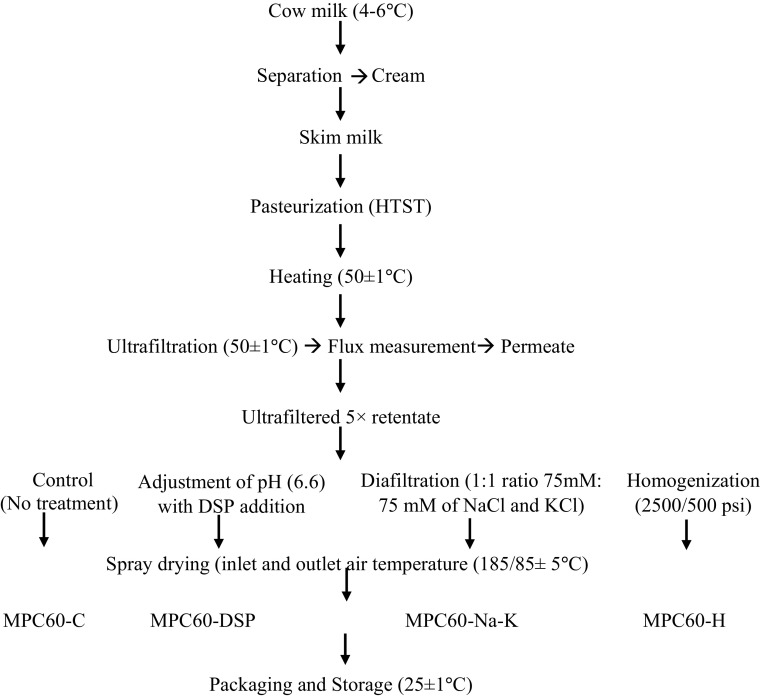
Retentate recovered after 5× UF concentration of feed was collected in pre-sterilized stainless steel cans. A part of 5× UF retentate obtained without applying treatment was considered as control and termed as 5× UFR, while a part of 5× UFR was homogenized at 2000 psi and 500 psi pressure in double stage homogenizer (APV Crepaco, Inc. Chicago, ILL. U.S.A., capacity-50 kg/h) and termed as 5× HUFR. The pH of a part of 5× UFR was adjusted to 6.6 using 10% DSP solution and termed as 5× DSP retentate. Total 150 mM solution containing 75 mM solution of NaCl and KCl (1:1 ratio) was added in a part of 5× UFR during DF. DF retentate thus obtained was named as 5× Na–K.
The 5× UFR, 5× HUFR, 5× DSP and 5× Na–K retentates were forewarmed to 40 ± 1 °C and spray dried in a pilot scale spray drier (Jektron Pvt. Ltd. Pune, feed rate 110 kg/h; atomizer diameter-0.17 mm) at 185 ± 5 °C inlet air and 85 ± 5 °C outlet air temperatures and referred as MPC60-C (control), MPC60-H, MPC60-DSP and MPC60-Na–K powders, respectively. All experiments were conducted in triplicate. Powders were packed in metalized polyester-LDPE laminates and stored at 4 ± 1 °C in a refrigerator until analyzed. To determine solubility after 60 days, powders were also stored at 25 ± 1 °C.
Compositional analysis
Total solids (TS) and ash contents of all powders were determined by gravimetric method of BIS (2001b). Crude protein content of powders was determined using Macro Kjeldahl Method (IDF 1993) using 6.38 as conversion factor. Fat contents of MPC powders were estimated by the BIS method (BIS 1986: 11721). Lactose contents of the powders were determined by difference i.e. by subtracting protein, fat and ash from TS contents of respective powders as earlier reported for retentates by Meena et al. (2016). Calcium content of all powders were analyzed in a Shimadzu AA-7000, atomic absorption spectrophotometer (AAS) using the method of AOAC (2005) adopting the procedure earlier reported by Kaushik et al. (2014).
pH measurement and its adjustment by DSP addition
pH of retentates and all reconstituted (10 g powder, volume made-up to 100 mL) MPC60 powder solutions were measured using Eutech pH meter (make-Thermo Scientific, model—cyberscan 1100) at 20 ± 1 °C. The pH of 5× DSP retentate was rechecked and corrected after 1 h to nullify the effect of buffering action of milk proteins.
Determination of physical and reconstitutional properties of MPC60 powders
Loose and packed bulk densities (g/mL), wettability (min), dispersability (%) and flowability expressed as angle of repose (θ°) of MPC60 powders were determined as per the methods described by Sjollema (1963), Muers and House (1962), American Dry Milk Institute (ADMI 1965) and Sjollema (1963), respectively. Different parameters related to powder particle size such as specific surface area (SSA, m2/kg), median particle size distribution (d10, d50 and d90, μm) and volume mean diameter (D4,3 and D3,2) were determined using Laser-Light-Scattering technique in a Malvern Mastersizer 3000 (Malvern Instruments Ltd., Malvern, Worcestershire, UK) adopting the procedure reported by Crowley et al. (2014). Color values (L*, a*, b*) of MPC60 powders were determined using a Tristimulus spectrophotometer Hunter Lab model Colour Flex® (Hunter Associates Laboratory Inc., VA, U.S.A.), while water activity (aw) values were determined using Aqua lab (U.S.A.).
Determination of functional properties of MPC60 powders
Solubility of MPC60 powders were determined adopting the method reported by Haque et al. (2012) with slight modification. Total 100 mL, 5% (w/v) solution of MPC60 powders was continuously stirred (400 rpm) at 25 ± 1 °C for 30 min (bulk solution); 40 mL bulk solution was transferred to two 50 mL falcon tubes and centrifuged at 1000×g for 10 min at 20 °C. TS contents of bulk and supernatant solutions were determined as per the BIS method (2001b) and then, solubility of MPC60 powders were calculated using the following equation:
Foam capacity of MPC60 powders were determined as per the method reported by Shilpashree et al. (2015). Foam capacity (overrun) was calculated as follows:
where, A-Volume of liquid before whipping (mL); B—total volume (foam plus liquid) obtained immediately after whipping (mL), while foam stability was determined as the volume of foam that remained after 30 min (30 ± 2 °C) and expressed as a percentage of the initial foam volume. Emulsion capacity of MPC60 powders were determined as per the method reported by Shilpashree et al. (2015) with some modifications. Blender (250 W orpat motor, RPM-18000) was used instead of probe sonicator for the preparation of emulsion. Emulsion capacity was calculated using the following equation:
Emulsion stability (ES) was determined by heating the emulsion at 80 °C for 30 min before centrifuging at 1100×g for 5 min and calculated as follows:
Water and oil binding capacities of MPC60 powders were determined as per the procedure reported by Shilpashree et al. (2015) and expressed as g of water or g of oil per g of protein. Buffer index (dB/dH) of MPC60 powders were determined by method suggested by Van Slyke (1922) and calculated using the following equation:
Reconstitution of MPC60 powders were carried out following the method of Crowley et al. (2014) and their HCT was measured at 140 °C as mentioned by Khatker et al. (2014). Rennet coagulation time (RCT) of reconstituted (3.5% protein, stirred at 20 °C/30 min) MPC60 powders were determined by the addition of 0.035 international milk clotting units (IMCU) per gram rennet (Mucor Miehei, Sigma-Aldrich) as per the protocol of Martin et al. (2010). Ten mL reconstituted powder solutions were transferred in test tubes and placed in a water bath maintained at 30 ± 1 °C, without external addition of calcium chloride. The time taken to form visible coagulation was recorded and expressed as RCT in min.
Measurement of viscosity and rheological properties
Ten percent (w/v) solution of MPC60 powders were prepared using MilliQ water and continuously stirred using a mechanical stirrer (400 rpm) at 25 ± 1 °C for 30 min. Flow behavior of the samples were performed at 20 °C using Rheometer (MCR 52, Anton Paar, Germany) attached with cone plate CP75-1° (SS) probe at variable shear rate ranging from 1 to 1000 s−1. The rheological data obtained in the range of 1–1000 s−1 were fitted to following rheological models:
where, σ is the shear stress (Pa), σo is the yield stress, is the shear rate (s−1), K is the consistency index (Pa sn) and n is the flow behaviour index.
Statistical analysis
Results obtained during the present investigation (mean value, n = 3) were subjected to one-way analysis of variance (ANOVA) using SAS Enterprise guide (5.1, 2012) developed by SAS Institute Inc., North Carolina, USA (SAS 2008) and represented using Tukey’s Studentized Range (HSD) test as per Meena et al. (2016).
Results and discussion
Proximate composition of MPC60 powders
Chemical composition, calcium contents and pH values of MPC60-C, MPC60-DSP, MPC60-Na–K and MPC60-H powders have been reported in Table 1. The contents of TS, protein, ash, lactose, fat, calcium and pH values of MPC60 powders were in the range of 95.21–97.45, 58.43–62.60, 7.12–8.12, 24.35–28.40, 1.63–2.07, 2.40–2.66 and 6.72–6.85%, respectively and observed variations were in board agreement with the earlier published values by Crowley et al. (2014) and Huppertz and Gazi (2015). Significant (P < 0.05) difference in TS and protein contents of all powders were observed that might be due to the variation approaches employed during their production. Compared to other MPC60 powders, MPC60-Na–K sample had higher TS, protein, ash and fat, but lower lactose contents, which indicate that substantial lactose is moves towards permeate during DF. Adjustment of pH (6.6) of 5× UFR with DSP might be responsible for higher ash and pH value, but lower protein, lactose, fat and calcium contents of the MPC60-Na–K powder. The observed difference in pH values of MPC60 powders could be ascribed to the addition of DSP and DF of the retentate with NaCl and KCl. Calcium contents of MPC60 powders were significantly (P < 0.05) different with each other except MPC60-C and MPC60-H, but in broader agreement with the reported values by (Floris et al. 2007) and it plays a pivotal role in determining functional properties of these powders. Further, the lower Ca content in the MPC60-Na–K powder is due to the loss of calcium in permeate during the diafiltration of UF retentate with NaCl-KCl that is related to changes in the casein micelles structure and it facilitate calcium passage into permeate (Sikand et al. 2013). Calcium content of MPC powders containing 56.9–60% protein were in the range of 14.44–18.89 mg/g of powder (Floris et al. 2007).
Table 1.
Composition of MPC60 powders
| Powders | Total solids | Protein | Ash | Lactose | Fat | Calcium | pH |
|---|---|---|---|---|---|---|---|
| % | – | ||||||
| MPC60-C | 97.45 ± 0.02a | 60.13 ± 0.02b | 7.12c ± 0.01 | 28.40 ± 0.02a | 1.80 ± 0.06b | 2.66 ± 0.00a | 6.72c ± 0.00 |
| MPC60-DSP | 95.21d ± 0.02 | 58.43d ± 0.04 | 8.04 ± 0.03b | 27.02 ± 0.04b | 1.72 ± 0.01b | 2.40c ± 0.01 | 6.85 ± 0.00a |
| MPC60-Na–K | 97.14 ± 0.01b | 62.60 ± 0.06a | 8.12 ± 0.01a | 24.35c ± 0.07 | 2.07 ± 0.03a | 2.58 ± 0.01b | 6.73 ± 0.00b |
| MPC60-H | 96.84c ± 0.01 | 59.86c ± 0.01 | 7.13c ± 0.01 | 28.22 ± 0.02a | 1.63 ± 0.03b | 2.65 ± 0.01a | 6.72c ± 0.00 |
Mean values ± S.E. (n = 3), values in a particular column followed by different letters differ significantly (P < 0.05) with each other
Physical and reconstitutional properties of MPC60 powders
Final quality of MPC powders is markedly affected by the existing inter-relationship among their physico-chemical, functional and rheological properties. Physical and functional properties of MPC powders plays a key role during reconstitution and products formulation. Physical properties of MPC60-C, MPC60-DSP, MPC60-Na–K and MPC60-H powders were determined and presented in Table 2. Bulk density (loose and packed), dispersability, flowability (angle of repose, θ), wettability, color values (L*, a*, b*), aw, SSA, median particle size (i.e. particle size distribution in terms of d10, d50, and d90) and average particle size (either D3,2 or D4,3) of MPC60 powders were in the range of 0.34–0.43, 0.46–0.60 g/mL; 75.94–94.51%;36.74°–43.11°; 0.72–2.15 min; 87.93–88.76, −2.06 to −1.51, 12.65–13.86; 0.27–0.34,73.57–88.45 m2/kg; 34.17–42.35, 89.83–107.40, 225.41–234.56 and 67.83–81.56, 115.00–125.12 μm, respectively.
Table 2.
Physical and functional properties of MPC60 powders
| Powders | Physical properties | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bulk density (g/mL) | Dispersability (%) | Flowability, (θ°) | Wet. (min) | Color values | aw | SSA (m2/kg) | Particle size distribution (μm) | ||||||
| LBD | PBD | L* | a* | b* | d10 | d50 | d90 | ||||||
| MPC60-C | 0.34b ± 0.01 | 0.46b ± 0.02 | 75.94d ± 0.34 | 41.53c ± 0.02 | 1.17c ± 0.01 | 87.93d ± 0.01 | −1.51 ± 0.03a | 13.50c ± 0.12 | 0.26b ± 0.02 | 77.65c ± 0.00 | 38.82b ± 0.00 | 107.40 ± 0.00a | 225.49c ± 0.00 |
| MPC60-DSP | 0.40 ± 0.00ab | 0.60 ± 0.00a | 94.51 ± 0.04a | 43.11 ± 0.00a | 1.33b ± 0.01 | 88.42b ± 0.03 | −2.06d ± 0.00 | 13.63b ± 0.04 | 0.27b ± 0.00 | 83.89b ± 0.00 | 36.99c ± 0.00 | 95.97c ± 0.00 | 225.93b ± 0.00 |
| MPC60-Na–K | 0.34b ± 0.02 | 0.54 ± 0.02ab | 88.77b ± 0.10 | 42.58b ± 0.07 | 2.15 ± 0.01a | 88.76 ± 0.01a | −1.72b ± 0.02 | 12.65c ± 0.01 | 0.27b ± 0.01 | 88.45 ± 0.00a | 34.17d ± 0.00 | 89.83d ± 0.00 | 225.41d ± 0.00 |
| MPC60-H | 0.43 ± 0.02a | 0.54 ± 0.03ab | 84.52c ± 0.15 | 36.74d ± 0.03 | 0.72d ± 0.01 | 88.14c ± 0.06 | −1.93c ± 0.01 | 13.86 ± 0.01a | 0.34 ± 0.02a | 73.57d ± 0.00 | 42.35 ± 0.00a | 106.49b ± 0.00 | 234.56 ± 0.00a |
| Powders | Functional properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average. particle size (μm) | HCT (min) | Viscosity (mPa s) | WBC (g/g protein) | OBC (g/g protein) | EC (%) | ES (%) | FC (%) | FS (%) | RCT (min) | ||
| D3,2 | D4,3 | ||||||||||
| MPC60-C | 77.27b ± 0.00 | 123.84b ± 0.00 | 32.00d ± 0.00 | 33.50 ± 0.00a | 5.22 ± 0.02a | 3.38b ± 0.03 | 35.67b ± 0.01 | 33.50b ± 0.03 | 82.74 ± 0.03a | 55.86b ± 0.01 | 210.00 ± 0.00a |
| MPC60-DSP | 71.52c ± 0.00 | 116.49c ± 0.00 | 55.00 ± 0.00a | 23.30c ± 0.00 | 5.11 ± 0.00a | 3.37b ± 0.00 | 43.50 ± 0.76a | 41.25 ± 0.72a | 24.62d ± 0.22 | 43.75c ± 0.72 | 210.00 ± 0.00a |
| MPC60-Na–K | 67.83d ± 0.00 | 115.00d ± 0.00 | 36.00c ± 0.00 | 19.30d ± 0.00 | 4.61b ± 0.03 | 3.73 ± 0.02a | 35.70b ± 0.01 | 33.62b ± 0.09 | 75.65c ± 0.03 | 35.87d ± 0.02 | 210.00 ± 0.00a |
| MPC60-H | 81.56 ± 0.00a | 125.12 ± 0.00a | 37.00b ± 0.00 | 25.50b ± 0.00 | 3.85c ± 0.04 | 3.27d ± 0.06 | 37.62b ± 1.22 | 33.84b ± 0.07 | 80.96b ± 0.02 | 68.15 ± 0.00a | 210.00 ± 0.00a |
Mean values ± S.E. (n = 3), values in a particular column followed by different superscript letters differ significantly (P < 0.05) with each other
LBD loose bulk density, PBD packed bulk density, SSA specific surface area, WBC water binding capacity, OBC oil binding capacity, EC emulsification capacity, ES emulsification stability, FC foaming capacity, FS foam stability, RCT rennet coagulation time
Loose bulk density of MPC60-H powder was significantly (P < 0.05) differed with that of MPC60-C, MPC60-Na–K, but close to that of MPC60-DSP powder. However, MPC60-C and MPC60-Na–K—powders have non-significant difference in their loose bulk densities. Packed bulk densities of MPC60-DSP and MPC60-H differed significantly (P < 0.05) with each other, but statistically there was no significant difference was observed with that of MPC60-H and MPC60-Na–K powders in their packed bulk densities. Lower TS contents (≤25%) of retentates (than TS of concentrated milk ~48–50%) used to produce MPC powders might be responsible for lower bulk density of MPC60 powders than SMP (0.53 g/mL). Particle density and tapped density of MPC 60 was 1.12 and 0.54 g/mL, respectively (Crowley et al. 2014). The observed difference among the loose and packed bulk densities of MPC60 powders could be attributed to the existing difference in the feed (treated with different treatments) characteristics particularly in TS and viscosity of 5× UFR as earlier reported by Meena et al. (2016) that might have altered the atomization and could resulted in altered density, occluded and interstitial air content, SSA and particle size distribution of the resultant powders. Bulk density of powders being complex property cannot be correlated with single factor only as it is affected by feed properties, atomizer type, particle size (distribution) and density; occluded and interstitial air contents (Schuck 2013). The loose and packed bulk density of MPC60 powders are similar to the reported values for other milk protein preparations such as dairy whitener (Khatkar and Gupta 2012), micellar casein isolate (MCI) and (MPI) milk protein isolate (Schuck 2013) were 0.27, 0.24, 0.29 and 0.37, 0.29, 0.35 (g/mL), respectively.
Upon contact with water, the ease with which powder particles get dispersed in water is referred as dispersability and it has significance in the reconstitution properties of powder. MPC60-DSP, MPC60-Na–K and MPC60-H powders exhibited significantly (P < 0.05) improved dispersability than control powder (Table 2) and reflected that applied technological approaches might be responsible for the marked increase. Casein rich powders such as micellar casein isolates (MCI) and MPCs have lower dispersibility indexes (Bouvier et al. 2013). Dispersibility of 32 commercial MPC powders containing protein content in the range of 55–85% were varied from 38 to 100% (Huppertz and Gazi 2015), while for MCI and milk protein isolates (MPI), it was only 5.1 and 25.6%, respectively (Schuck 2013). Lowering in calcium content has prevented the severe protein–protein interaction and aggregation by enhancing repulsive forces, especially in MPC60-DSP and MPC60-Na–K (Table 1) that resulted in their improved dispersability. However, modification of protein structure owing to high shearing and cavitation in MPC60-H powder might be responsible for its increased dispersability.
It was observed that all MPC60 powders have significant (P < 0.05) difference in their flowability (θ). According to Carr (1965) classification based on θ values, only MPC60-H powder was free flowing (θ = 30–38º), while remaining powders have fair to passable flow (θ = 38–45º) characteristics. Better flowability of MPC60-H powder over other powders could be related to its higher particle size which flow easily than fine particles as evident from lower SSA and higher d10, d90, D3,2 and D4,3 values (Table 2). Similar trend was also observed in the flowability of MPC60-C, MPC60-H and MPC60-Na–K powders i.e. flowability decreased with increase in SSA, due to increase in fine particles and vice versa (Crowley et al. 2014).
Ability of powders to absorb water is referred as wettability which depends on typical attributes of a powder such as surface charge and activity, particle size, density, porosity and the presence of hydrophilic (lactose), amphiphilic (protein) and hydrophobic (fat) substances (Khatkar and Gupta 2012). Statistically significant (P < 0.05) variation was observed in the wettability of MPC60 powders. The minimum (0.72 min) wetting time observed in MPC60-H powder might be attributed lower residual fat at powder surface, while maximum (2.15 min) wetting time of MPC60-Na–K powder might be accorded to its higher fat, protein, but lesser lactose content. In general, MPC powders have comparatively higher wetting index (>120 s) than SMP and WMP (15–16 s) owing to surface composition (i.e. presence of protein load and residual load) of their particles (Bouvier et al. 2013). The MCI, MPI, NaCas and WPI also have wetting index >120 s, while wettability of granulated and non-granulated micellar casein and whey protein powders were 1, 3, 4 and 17 min, respectively (Schuck 2013).
Color of protein powders influence their acceptability. The lightness (L*), redness (a*) and yellowness (b*) values of MCI, MPI, NaCas and WPI were 69.5, −5.1, 12.0; 73.5, −5.50, 9.3; 73.2, −5.80, 10; and 73.1, −6.0, 13.1, respectively (Schuck 2013). The L*, a* and b* values of all MPC60 powders were differed significantly (P < 0.05) with each other except b* value of MPC60-C and MPC60-Na–K powders which differed non-significantly. Maximum and minimum L*, a* and b* values (Table 2) were observed in MPC60-Na–K and MPC60-C; MPC60-C and MPC60-DSP; MPC60-H and MPC60-C or MPC60-Na–K powders, respectively. Mainly due to compositional difference, high protein powders have grayish-white color yellowish-white color of skimmed milk powder. The deviation in color values of MPC60 powders compared to other protein powders are mainly attributed to the compositional difference in the retentates and variations in the manufacturing process such as type of dryer, number of drying stages and drying conditions (inlet/outlet air temperatures) employed during their manufacture.
The SSA, particle size distribution (d10, d50, and d90) and average particle size (D3,2 and D4,3) have vital role in determining the physical, reconstitutional and sensorial attributes of a particular powder. Significant (P < 0.05) variations in the SSA; d10, d50, and d90; D3,2 and D4,3 values of the MPC60 powders were observed which might be attributed to the reported variation in composition of feed and its viscosity (Meena et al. 2016) prior to its drying. The maximum and minimum values (Table 2) of SSA; d10, d50, and d90; D3,2 and D4,3 were observed in MPC60-Na–K and MPC60-H; MPC60-H and MPC60-Na–K, MPC60-C and MPC60-H, MPC60-H and MPC60-Na–K; MPC60-H and MPC60-Na–K, respectively. Crowley et al. (2014) reported that SSA and particle size (d10, d50, and d90) of MPC60 were 320 m2/kg, 18.1, 48.9, 90.6 μm respectively which is higher than the values observed in the present investigation. This difference might be due to higher TS content of the retentate (36.3%) used by Crowley et al. (2014) for drying than the <23% TS contents of retentates (Meena et al. 2016) used for manufacture of different MPC60 powders during the present investigation (Fig. 1).
Fig. 1.
Flow diagram for the production of MPC60 powders
Functional properties of MPC60 powders
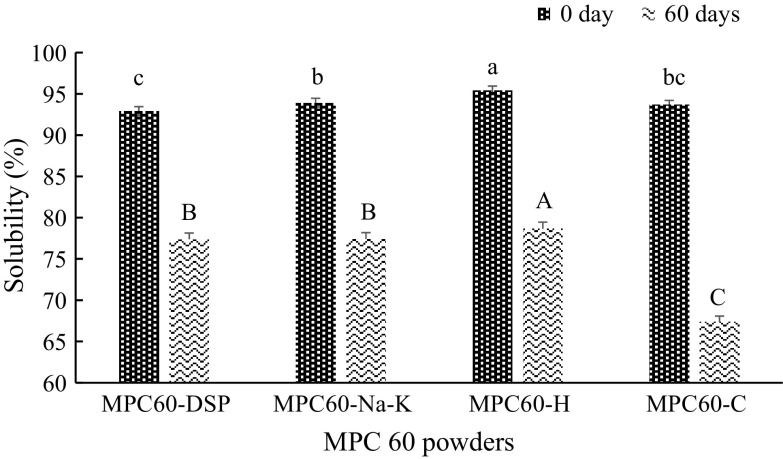
Protein rich powders delivers desirable attributes to the food formulations owing to their unique functional properties. Nitrogen solubility index (NSI) of MPC powders containing 56.9-60% protein (Floris et al. 2007) and fresh low-protein (<65% protein) powders (Huppertz and Gazi 2015) were 68–88 and 78%, respectively. Solubility of MPCs is vital for the full expression of other functional properties of these powders. Solubility of fresh MPC60-DSP, MPC60-Na–K and MPC60-H powders were significantly (P < 0.05) different with each other, but non-significant relation was observed among the solubility values of control versus MPC60-DSP and MPC60-Na–K powders (Fig. 2). Solubility of these powders varied in the range of 92.92–95.43%. Maximum solubility was observed in MPC60-H powder which might be because of the alteration in caseins structure during homogenization process owing to shearing and cavitation. Improvement in solubility of MPC80 upon homogenization (13,800 kPa) was observed by Sikand et al. (2012). However, solubility improvement was noted even during low pressure homogenization. Solubility of MPC powders has been reported to reduce drastically with the increase in duration and temperature of storage. This trend (i.e. reduction in solubility) was also observed in MPC60 powders after 60 days of storage at 25 ± 1 °C (Fig. 2), but maximum reduction (26.30%) was observed in control powder. The observed ~10% lower reduction in the solubility of MPC60-H, MPC60-DSP and MPC60-H powders after 60 days storage indicated the effectiveness of the applied technological approaches in either improvement or in better retention of the solubility of these powders than control (Fig. 2).
Fig. 2.
Effect of different treatments on MPC60 powders (n = 3). abcdefg, ABCDEFGMean values of solubility within a graph with different letters differ significantly (P < 0.01) for zero and 60 days of storage
Significant (P < 0.05) difference was noted in the HCT values of MPC60 powders as it is affected by pH, proteins, calcium contents and processing conditions. Heat stability of MPC powders containing 55–85% protein contents was in the range of 0–40 min (Huppertz and Gazi 2015). Heat stability of MPC60 powders were in the range of 32–55 min (Table 2). Minimum (32 min) HCT of control powder could be because of its higher calcium content which, promote casein micelles aggregation on heating. Maximum (55 min) HCT of MPC60-DSP powder could be accorded to DSP addition having ability to increase buffering capacity and to reduce Ca-ion activity (De Kort et al. 2012) by calcium chelation. Addition of phosphate ions has been reported to enhance voluminosity of casein micelles, causes non-discriminate casein solubilization and thus protects caseins towards heat instability via solubilization of the micellar minerals. Improvement in heat stability of reconstituted casein micelles dispersion was observed upon addition of sodium phosphate by Le Ray et al. (1998). Reduction in calcium content or in other terms calcium ion activity during DF is responsible for better heat stability of MPC60-Na–K powder over control. In descending order, the noted trend for HCT of these powders were MPC60-DSP > MPC60-H > MPC60-Na–K > MPC60-C, respectively.
Significant (P < 0.05) difference was observed in the viscosity of MPC60 powders which ranges from 19.30 to 33.5 mPa.s at 50 s−1 and 20 °C. Minimum and maximum viscosities were observed in MPC60-Na–K and control powders (Table 2). Lower calcium content in MPC60-Na–K powder resulted in its lower viscosity value as normally also noticed in sodium and calcium caseinates while, mediated changes in casein micelles could be considered as major factor for lower viscosity of MPC60-H.
Prevention of the release of water from a 3-dimensional structure in a food is known as water binding, water hydration capacity, water absorption and water-imbibing capacity and the same depends on physico-chemical parameters, charge and surface properties of caseins; ions and their strength, pH condition and temperature of surrounding medium. Water binding capacity of MPC60 powders differed significantly (P < 0.05) with each other except control and MPC60-DSP powders. Maximum (5.22 g/g protein) and minimum (3.85 g/g protein) water binding was observed in control and MPC60-H powders. Maximum and minimum oil binding capacity was observed in MPC60-Na–K and MPC60-H powder, which also differed significantly (P < 0.05) with other MPC60 powders having non-significant relationship among them (Table 2). Water binding and emulsification properties determine the utility of protein preparation in formulated foods.
Milk protein concentrates and calcium caseinates have poor emulsifying capability. Emulsion capacity and stability of MPC60 powders were in the range of 37.62–43.50 and 33.50–41.25%, respectively. Emulsion capacity and stability of MPC60-DSP powder was significantly (P < 0.5) higher than remaining MPC60 variants, which can be explained partially on the basis of its compositional difference and its smaller particle size as indicated by SSA, particle size distribution and average particle size (Table 2). Emulsion properties are affected by calcium and protein contents; pH and particle size of the powder i.e. lower calcium content and particle size results in finer, stable emulsions and vice versa. Improvement in emulsion capacity and stability has been reported in MPC powders with reduction in calcium (Ye 2011). Although, MPC60-Na–K powder had lowest particle size and calcium content, but presence of salts might have hindered the formulation of stable emulsions and thus resulted in its lower emulsion capacity and stability.
Ability of a protein to entrap and retain air is known as foaming ability and the same is considered as important selection criteria for their utilization in ice-cream, frozen desserts, bakery and certain confectionary products. Foam capacity and foam stability of all MPC powders were differed significantly (P < 0.05) with each other and were in the range of 24.62–82.74 and 35.87–68.15% respectively (Table 2). Maximum and minimum foam capacity and foam stability were observed in control and MPC60-DSP powder; MPC60-H and MPC60-Na–K powders, respectively. It mainly depends on heat treatment, pH, surface activity of proteins and ionic environment influence. Foaming properties are related with hydrophobicity of protein surface and same might be attributed to maximum foaming capacity of control powder (55.86%). Foam stability of MPC60 powders were significantly (P < 0.05) different with each other and varied in the range of 35.87–68.15% (Table 2). The observed values of foam capacity and stability were lower than the values reported by Huppertz and Gazi (2015). Moreover, scientific reports pertaining to foaming properties of MPC 60 powders are scanty. Non-significant variation was observed in RCT values of all MPC60 powders as the powder solutions were remain uncoagulated upon rennet addition even till >210 min. It might be attributed to the lower concentration of soluble salts particularly of calcium in MPC60 powder solutions and higher buffering capacity of milk proteins that has resisted the change in the pH.
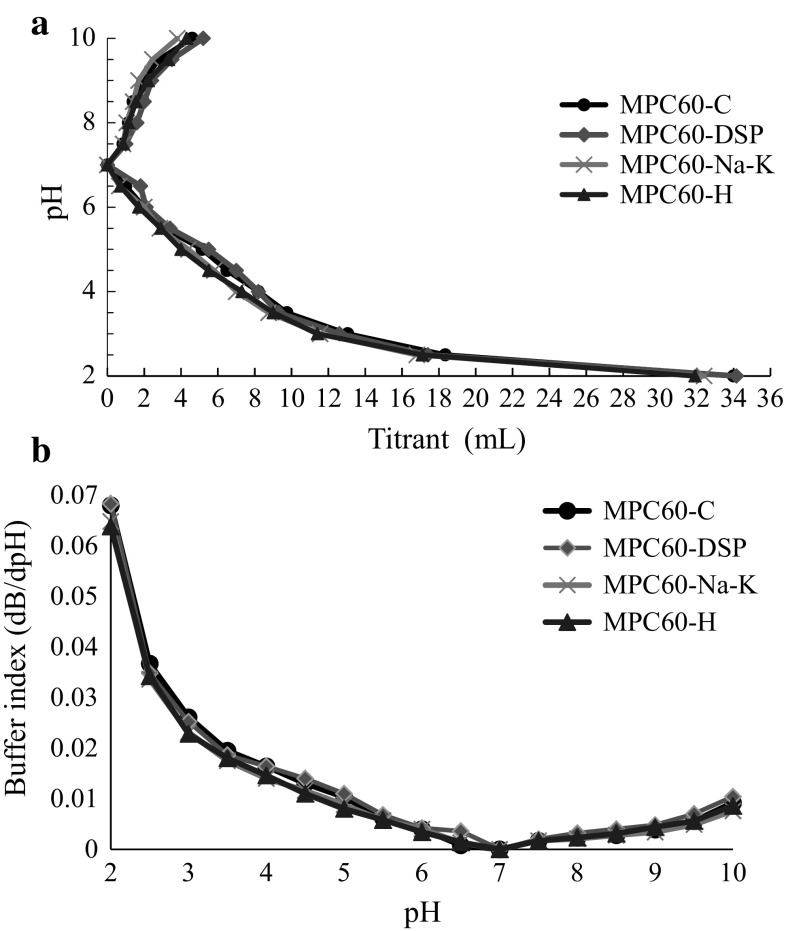
Buffering capacity is one of the important property of dairy products that resist in the change in pH during acidification and alkalization. The volume of titrants (acid or base, mL) used for the 0.5 change in the pH and concerned buffering capacity expressed as buffer index (dB/dpH) of MPC60 powders has been shown in Fig. 3a, b, respectively. Buffer index of MPC60 powders changed with the pH. Compared to MPC60-C and MPC-H powders, MPC60-DSP and MPC60-Na–K powders showed higher buffer index on majority of the pH range owing to the external addition of salts like phosphate, Na and K that have been reported to enhance the buffer index.
Fig. 3.
Titration curve (a) and buffer index (b) of alkalization-acidification with 0.1 N HCl and 0.1 N NaOH solution of MPC60 powders (0.5% protein solution)
Rheological properties of MPC60 powders
The reconstituted MPC60 samples were evaluated for their rheological behavior using various models as presented in the Table 3. The control sample exhibited the highest apparent viscosity values, however it was markedly less for remaining powders. This could be correlated with higher calcium content, lower bulk density and poor dispersability of MPC60-C, which may hold and entrap the water physically but not chemically. This could also be explained by the highest yield stress value (1.347 Pa) of MPC60-C sample. Among the various rheological models evaluated Herschel Bulkley model well explained the rheological behavior indicating yield stress values in the range of 0.787–1.347 Pa.
Table 3.
Rheological parameters modelling of reconstituted MPC60 powders
| Powders | Bingham | Herschel Bulkley | Power Law | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| σ0 | η | R2 | σ0 | k | η | R2 | k | n | R2 | |
| MPC60-C | 1.308 | 7.47 | 0.997 | 1.347 | 0.006 | 1.031 | 0.998 | 0.351 | 0.434 | 0.856 |
| MPC60-DSP | 0.875 | 5.93 | 0.997 | 0.767 | 0.012 | 0.894 | 0.999 | 0.220 | 0.465 | 0.912 |
| MPC60-Na–K | 0.745 | 4.13 | 0.997 | 0.656 | 0.009 | 0.884 | 0.999 | 0.201 | 0.430 | 0.901 |
| MPC60-H | 0.974 | 5.70 | 0.994 | 0.787 | 0.018 | 0.824 | 0.998 | 0.258 | 0.439 | 0.912 |
Where, σ is the shear stress (Pa), σo—yield stress, η-viscosity (mPa.s), and k—consistency index (Pa s), n flow behavior index and R2 is the coefficient of determination
Conclusion
Reduction in calcium contents of MPC60 powders either by exchanging calcium ions with Na and K ions during DF with NaCl-KCl salts, or chelation of calcium by DSP salt, reduced casein–casein interaction, dissociate and demineralize casein micelles via solubilization of calcium phosphate. Enhanced ionic strength and electrostatic repulsive forces, modification of the hydrophobicity of caseins, reduced casein aggregation and hydrophobic nature of these salts also resulted in marked increase in physico-functional properties of these powders. Improvement in the various properties of MPC60-H powder might have resulted from the physical modification of casein micelles during homogenization under the influence of high shearing and cavitation. Decrease in calcium content of treated powders remarkably improved their heat stability, but external calcium addition is inevitable to induce rennet coagulation in these powders. It has been established from this investigation that compared to control, MPC60 powders produced either employing homogenization or with DSP and NaCl–KCl salts, possesses improved physico-functional potential. As an ingredient, these powders could replace control powder during formulations of different foods in which functionality of milk proteins is aimed to improve end product quality.
Acknowledgements
Author are very grateful to the Director, ICAR-National Dairy Research Institute, Karnal for providing the required facilities to carrying out this work.
References
- Agarwal S, Beausire RL, Patel S, Patel H. Innovative uses of milk protein concentrates in product development. J Food Sci. 2015;80(S1):A23–A29. doi: 10.1111/1750-3841.12807. [DOI] [PubMed] [Google Scholar]
- American Dry Milk Institute (ADMI) (1965) Standards for grades of dry milk including methods of analysis. Bull.915, ADMI, USA
- AOAC . Official methods of analysis. The association of official analytical chemists (18th ed) Maryland: North Fredrick Avenue Gaithersburg; 2005. [Google Scholar]
- Baldwin AJ. Insolubility of milk powder products-A mini review. Dairy Sci Technol. 2010;90(2–3):169–179. doi: 10.1051/dst/2009056. [DOI] [Google Scholar]
- BIS (2001b) Condensed milk and dried milk. In: Hand book of food analysis: (Part XI) Dairy products. Bureau of Indian Standards (3rd reprint). Manak Bhavan, New Delhi, pp 120–126
- Bouvier JM, Collado M, Gardiner D, Scott M, Schuck P. Physical and rehydration properties of milk protein concentrates: comparison of spray-dried and extrusion-porosified powders. Dairy Sci Technol. 2013;93(4–5):387–399. doi: 10.1007/s13594-012-0100-7. [DOI] [Google Scholar]
- Carr RL. Evaluating flow properties of solids. Chem Engg. 1965;72(2):163–169. [Google Scholar]
- Crowley SV, Gazi I, Kelly AL, Huppertz T, O’Mahony JA. Influence of protein concentration on the physical characteristics and flow properties of milk protein concentrate powders. J Food Eng. 2014;135:31–38. doi: 10.1016/j.jfoodeng.2014.03.005. [DOI] [Google Scholar]
- De Castro-Morel M, Harper WJ. Basic functionality of commercial milk protein concentrates. Milchwissenschaft. 2002;57(7):367–370. [Google Scholar]
- De Kort E, Minor M, Snoeren T, van Hooijdonk T, Van der Linden E. Effect of calcium chelators on heat coagulation and heat-induced changes of concentrated micellar casein solutions: the role of calcium-ion activity and micellar integrity. Int Dairy J. 2012;26(2):112–119. doi: 10.1016/j.idairyj.2012.03.014. [DOI] [Google Scholar]
- Floris R, Alting A, Slangen C, van der Meulen IE, Adamse M, Klok H, Verbeek M. MPC functionality: a comparitive study of commercial samples. NIZO-Rep E. 2007;157:1–55. [Google Scholar]
- Haque E, Whittaker AK, Gidley MJ, Deeth HC, Fibrianto K, Bhandari BR. Kinetics of enthalpy relaxation of milk protein concentrate powder upon ageing and its effect on solubility. Food Chem. 2012;134(3):1368–1373. doi: 10.1016/j.foodchem.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Huppertz T, Gazi I. Milk protein concentrate functionality through optimized product-process interactions. New Food. 2015;18(1):12–17. [Google Scholar]
- IDF . Lait: De´termination de la teneur en azote; IDF Standard 20 B. Brussels: International Dairy Federation; 1993. [Google Scholar]
- Indian Standards (1986) Determination of fat content in milk powder and similar products. Bureau of Indian Standards, IS-11721, Manak Bhavan, New Delhi: BIS
- Kaushik R, Sachdeva B, Arora S, Kapila S, Wadhwa BK. Bioavailability of vitamin D2 and calcium from fortified milk. Food Chem. 2014;147:307–311. doi: 10.1016/j.foodchem.2013.09.150. [DOI] [PubMed] [Google Scholar]
- Khatkar SK, Gupta VK. Physicochemical and functional quality attributes of dairy whitener prepared from ultrafiltration process. J Food Process Pres. 2012;38:1145–1154. doi: 10.1111/jfpp.12074. [DOI] [Google Scholar]
- Khatker SK, Gupta VK, Khatker AB. Studies on preparation of medium fat liquid dairy whitener from buffalo milk employing ultrafiltration process. J Food Sci Technol. 2014;51(9):1956–1964. doi: 10.1007/s13197-014-1259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange V, Whitsett D, Burris C. Global market for dairy proteins. J Food Sci. 2015;80(S1):A16–A22. doi: 10.1111/1750-3841.12801. [DOI] [PubMed] [Google Scholar]
- Le Ray C, Maubois JL, Gaucheron F, Brulé G, Pronnier P, Garnier F. Heat stability of reconstituted casein micelle dispersions: changes induced by salt addition. Le Lait. 1998;78(4):375–390. doi: 10.1051/lait:1998437. [DOI] [Google Scholar]
- Martin GJ, Williams RP, Dunstan DE. Effect of manufacture and reconstitution of milk protein concentrate powder on the size and rennet gelation behaviour of casein micelles. Int Dairy J. 2010;20(2):128–131. doi: 10.1016/j.idairyj.2009.08.007. [DOI] [Google Scholar]
- Meena PK, Gupta VK, Meena GS, Raju PN, Parmar PT. Application of ultrafiltration technique for the quality improvement of dahi. J Food Sci Technol. 2015;52:7974–7983. doi: 10.1007/s13197-015-1951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena GS, Singh AK, Borad S, Raju PN. Effect of concentration, homogenization and stabilizing salts on heat stability and rheological properties of cow skim milk ultrafiltered retentate. J Food Sci Technol. 2016 doi: 10.1007/s13197-016-2388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muers MM, House TU (1962) A simple method for comparing wettability of instant spray dried separated milk powder. In: XVI international dairy congress, vol. 8. Copenhagen, Denmark, p 299
- Schuck P. Dairy protein powders. In: Smithers GW, Augustin MA, editors. Advances in dairy ingredients. Hoboken: Wiley and Institute of Food Technologists; 2013. pp. 1–29. [Google Scholar]
- Shilpashree BG, Arora S, Chawla P, Vakkalagadda R, Sharma A. Succinylation of sodium caseinate and its effect on physicochemical and functional properties of protein. LWT-Food Sci Technol. 2015;64(2):1270–1277. doi: 10.1016/j.lwt.2015.07.008. [DOI] [Google Scholar]
- Sikand V, Tong PS, Roy S, Rodriguez-Saona LE, Murray BA. Solubility of commercial milk protein concentrates and milk protein isolates. J Dairy Sci. 2011;94(12):6194–6202. doi: 10.3168/jds.2011-4477. [DOI] [PubMed] [Google Scholar]
- Sikand V, Tong P, Vink S, Walker J. Effect of powder source and processing conditions on the solubility of milk protein concentrates 80. Milchwissenschaft. 2012;67:300–303. [Google Scholar]
- Sikand V, Tong PS, Roy S, Rodriguez-Saona LE, Murray BA. Effect of adding salt during the diafiltration step of milk protein concentrate powder manufacture on mineral and soluble protein composition. Dairy Sci Technol. 2013;93:401–413. doi: 10.1007/s13594-013-0110-0. [DOI] [Google Scholar]
- Singh H. Interactions of milk proteins during the manufacture of milk powders. Le Lait. 2007;87(4–5):413–423. doi: 10.1051/lait:2007014. [DOI] [Google Scholar]
- Singh H. Functional properties of milk proteins. In: Fuquay JW, Fox PF, McSweeney PLH, editors. Encyclopaedia of dairy science. 2. San Diego: Academic Press; 2011. pp. 887–893. [Google Scholar]
- Sjollema A. Some investigations on the free-flowing properties and porosity of milk powders. Ned Melk-En Zuiveltijdschr. 1963;17(3):245–259. [Google Scholar]
- Van Slyke DD. On the measurement of buffer values and on the relationship of buffer value to the dissociation constant of the buffer and the concentration and reaction of the buffer solution. J Biol Chem. 1922;52(525570):20. [Google Scholar]
- Ye A. Functional properties of milk protein concentrates: emulsifying properties, adsorption and stability of emulsions. Int Dairy J. 2011;21(1):14–20. doi: 10.1016/j.idairyj.2010.07.005. [DOI] [Google Scholar]