Abstract
In this study a novel biodegradable edible film based on Alyssum homolocarpum seed gum (AHSG) was fabricated and characterized. Glycerol at three levels (25, 35, and 45% based on dried AHSG) as plasticizer were added. The microstructure and barrier, electromagnetic, mechanical, and thermal properties of the film were characterized. Results showed that permeability to both oxygen and water vapor, increased as the plasticizer content increased from 25 to 45%. The mechanical properties of AHSG films were comparable to those of polysaccharide films. Results showed that the glycerol content significantly decreased the glass-transition temperature of the film. The color measurement indicated that increasing the plasticizer content augmented the b* and L* values. Results of the field emission scanning electron microscopy revealed a uniform and smooth surface morphology and an absence of phase separation among the film compositions. The findings demonstrated that AHSG has the potential to fabricate edible films with enhanced quality characteristics.
Keywords: Edible films, Gums, Biodegradable packaging, Differential scanning calorimetry, Carbohydrates
Introduction
Synthetic packaging has been increasingly utilized in recent decades. Currently, a popular trend in food technology is the use of synthetic materials as substitute for naturally biodegradable materials (Alipoormazandarani et al. 2015; López et al. 2017; Shaili et al. 2015). Biodegradable films and coatings receive growing interest recently because of concerns on environmental threats and costs (Ghasemlou et al. 2013; Spotti et al. 2016). As barriers that control the transfer of moisture, flavors, lipids, and oxygen, edible coatings and films can significantly extend the shelf life of food products (Hazaveh et al. 2015; Neethirajan and Jayas 2010).
Edible coatings and films can be manufactured from different materials, including proteins, polysaccharides, and lipids (Fakharian et al. 2015; Jafarzadeh et al. 2016). However, the exploration of other new resources to produce biodegradable and edible films has received renewed interest in recent years. Some of the new polysaccharides used recently to fabricate edible films include sago starch (Mohammadi Nafchi et al. 2011), cress seed gum (Jouki et al. 2013a), mung bean (Maznabi and Mohammadi Nafchi 2013), soluble soybean polysaccharide (Tajik et al. 2013), and Quince seed mucilage (Jouki et al. 2014). Compared with synthetic films, polysaccharide-based films are relatively hydrophilic and stiff. Polyols have been widely used as plasticizers to overcome the brittleness and flexibility of films (Mohammadi Nafchi et al. 2011; Talja et al. 2007).
Indigenous to Egypt, Iraq, Iran, and Pakistan, alyssum is a genus with approximately 100–170 species of flowering plants in the Brassicaceae family. Alyssum homalocarpum is an annual herbaceous plant with white, stellate hairs that reach 10–20 cm in length, white flowers, and oblong-linear or oblanceolate leaves. The locules of this plant each have two broad, round, pale pink, and extremely narrow margined seeds 1.5–2.5 mm in length (Koocheki and Kadkhodaee 2011). Known as Qudum-e Shirazi in Iran and used traditionally as medicine, Alyssum homolocarpum seeds contain high amounts of mucilaginous substances (Koocheki et al. 2010), which disperse when soaked in water. Hydrated gum, which displays pseudoplastic behavior, is a potential thickening agent in food applications (Koocheki et al. 2009a). Based on its high extraction yield (287.3 g/kg), Alyssum homolocarpum seed gum (AHSG) is an alternative material for some commercial gums (Koocheki et al. 2009b). AHSG have a small molecular weight (3.66 × 105 g/mol) with intrinsic viscosity (in deionized water at 25 °C) equal to 18.34 dl/g (Hesarinejad et al. 2015).
AHSG has 85.33% of total carbohydrate content [including mannose (3.04%), galactose (82.97%), xylose (2.72%), rhamnose (5.04), glucose (5.70%), and arabinose (0.53%)], which is similar to those of locust bean gum (85.1–88.7%), xanthan gum (87.4%), and Arabic gum (85.7%). This high total sugar content, which is primarily comprised of carbohydrates, indicates that AHSG has high purity. The AHSG is a galactan-type polysaccharide unlike most of seeds gum that they are in form of galactomannan or glucomannan. In addition, AHSG consisted of around 5.63% of uronic acids, that illustrating its weak polyelectrolyte nature in the gum. A broad band at approximately 895 cm−1 dominates the FTIR spectrum of AHSG owing to the presence of β-d-manopyranose units (Hesarinejad et al. 2015). This FTIR spectrum features the usual bands and peaks that characterize polysaccharides.
Preliminary studies have shown that Alyssum homolocarpum seed gum (AHSG) can produce films with excellent appearance and can be easily cast with satisfactory mechanical properties. However, to our knowledge, the film formability and characterization of AHSG have not been researched yet. Therefore, the current research intends to develop a novel AHSG-based biodegradable film that has a practical application as edible film in food-packaging materials. Given that this study is the first to report the film formability of AHSG, the effects of glycerol as plasticizer on the physical, thermal, mechanical, barrier, and microstructural properties of the resulting films are investigated.
Materials and methods
Materials
To ensure the Alyssum homolocarpum seeds featured the same physicochemical characterization as the extracted gum, the seeds were purchased from a local medicinal plant market in Torghabeh (Mashhad, Iran). All foreign matters, including broken, chaffed, and immature seeds, were removed from the seeds. All other chemicals were of analytical grade.
AHSG extraction and edible film preparation
Alyssum homolocarpum seed gum was extracted based on the optimized method described previously by Koocheki et al. (2010) that modified by Hesarinejad et al. (2015). Briefly, deionized water (MΩ-cm) was used to extract Alyssum homolocarpum seed from whole seeds until a water-to-seed ratio of 30:1 and a pH of 8 were reached. The pH, which was monitored constantly, was adjusted by adding 0.1 mol/L NaOH and/or HCl. The water bath temperature was set to 48 °C. An electric mixing paddle was used to stir the seed—water slurry during the extraction process, which lasted 1.5 h. Next, the seeds were disposed of, and the final dispersion was dried overnight in a laboratory oven at 45 °C. The dried gum was milled and sieved using a sifter with mesh 18.
The extraction yield of crude gum was estimated as the percentage weight of dried powder gum over the total seeds weight. The extraction yield was 20 g AHSG/100 g seeds.
A dispersion with 1.2% (w/v) AHSG concentration and glycerol (25, 35, and 45% (w/w dried AHSG)) was prepared, while stirring (400 rpm) for 30 min at 50 ± 5 °C. This plasticizer range was generated from preliminary experiments and literature data according to the optimum mechanical properties of the AHSG-based films (Jouki et al. 2013a; Marvizadeh et al. 2017; Talja et al. 2007). The film-forming dispersions were degassed in an ultrasonic bath for 3 min to remove air bubbles and make a clear dispersion.
A total of 150 g AHSG dispersion was placed on Perspex plates fitted with rims around the edge to produce a film-forming area 16 cm2 × 16 cm2 in size. The generated films were then dried for 24 h at 30% relative humidity (RH) and 40 °C. The dried films were peeled off the casting surface and then stored at 55 ± 5% RH and 25 ± 2 °C until further use. All the films were fabricated in triplicate.
Physical properties of AHSG films
Film thickness and density
A micrometer (Mitutoyo Co., Tokyo, Japan) was utilized to measure film thickness. Each film was measured a total of eight times, and the average values of the thickness measurements were recorded based on the barrier and mechanical properties.
Moreover, 3 cm × 3 cm samples were conditioned in a desiccator with Mg(NO3)2 (53% RH) for a week, and then weighed to determine the AHSG film density. The film densities were then calculated by dividing the mass by the occupied volume of the films (Tajik et al. 2013).
Moisture content
Thermogravimetric analysis (Pyris1 TGA, Perkin-Elmer, Massachusetts, USA) was utilized to determine the films moisture content, following the method of Mohammadi Nafchi and Karim (2013). Next, ramp temperature was applied from room temperature to 130 °C at 3 °C/min and then maintained for 1 h at 130 °C. A mass loss that was equal to the moisture content was obtained; the residual mass of the AHSG films was constant afterwards. Three replicate specimens were used for moisture content analysis.
Water solubility and moisture uptake
The solubility of the AHSG films in water was determined following Mohammadi Nafchi et al. (2012). Sample pieces measuring 2 cm × 3 cm were sliced from each film and then stored for 2 days in desiccators with P2O5 (0% RH). Afterwards, these samples were weighed, placed in beakers containing 80 mL deionized water (18 MΩ), and then shaken regularly for 2 h at room temperature. The rest of the film pieces were filtered using filter paper (Whatman No. 4) after soaking and then dried in an oven at 60 °C until a constant weight was reached. Measurement of the samples was conducted in triplicate. The formula for the percentage of total soluble matter (% solubility) is as follows:
| 1 |
Instead of the water immersion technique, the moisture uptake was evaluated according the method of Tajik et al. (2013) because of the high sensitivity of AHSG to water. The film samples were cut to 2.0 cm × 2.0 cm, conditioned using P2O5 (RH = 0%) for at least 48 h, and then weighed to obtain the dried film (W i). Afterwards, the films were conditioned along with a K2SO4-saturated solution in a desiccator for 48 h at 25 °C to ensure RH = 97%. Upon reaching the target RH, the film samples were weighed (W f). Three replicate specimens were used for moisture uptake characterization. The moisture uptake values of the samples were estimated through the following equation:
| 2 |
Contact angle measurement
To evaluation of the hydrophobicity of the AHSG films, contact-angle of water droplet was measured by a static contact angle meter (CAM-PLUS, Tantec, Germany). The data shown are the mean of 10 measurements at different sites. Immediately after the addition of 1 μL deionized water using the Sessile Drop, Half-Angle™, the contact angles on each surface of films were measured by tangent line method (Mohammadi Nafchi et al. 2012).
Barrier properties
Water vapor permeability
Water vapor permeability (WVP) tests were performed on the films by following the modified method (Yu et al. 2009) of the American Society for Testing and Materials (ASTM) standard E96-05. A total of 20 g silica gel (desiccant) was placed in each test cup to produce 0% RH below the film. The measurements for water vapor transmission rates (WVTR) of each film were obtained at 53 ± 2% RH and 25 ± 2 °C. The WVP of the AHSG films were calculated by multiplying the film thickness by the steady WVTR, and dividing by the films area and water vapor pressure difference across the film. Three samples were tested per treatment.
Oxygen permeability
The ASTM standard method D3985-05 with a Mocon Oxtran 2/21 (Minneapolis, USA) equipped with WinPermTM permeability software and a patented colorimetric sensor (Coulox®) was applied to perform oxygen permeability measurements. This method was adopted by Mohammadi Nafchi et al. (2013a) for biopolymeric films. The oxygen permeability tests were conducted under the following conditions: atmospheric pressure, 50% RH, 21% oxygen as test gas, and 25 °C temperature. A “convergent by hour” approach was used to conduct the measurements and reach steady-state of gas transmission. Oxygen permeability coefficients were calculated according to the steady state rate of oxygen by considering the film thicknesses (cm3-μm/(m2 day atm)). Three replicate specimens were used for oxygen permeability measurements.
Mechanical properties
The ASTM D882-10 method was adopted to determine the mechanical properties following the method described by Sadegh-Hassani and Mohammadi Nafchi (2014). The film strips were cut into a length of 15 cm and a width of 2.5 cm and then conditioned at 53% RH and 25 °C for at least 48 h. The mechanical properties of the films were measured via a texture analyzer (TA.XT2, Stable Micro System, Surrey, UK). The crosshead speed was 3 cm/min, and the initial grip separation was 10 cm. Stress–strain data obtained by the software were utilized to estimate elongation at break (EB), tensile strength, and Young’s modulus. Eight replicate specimens were used for mechanical characterization.
Modulated differential scanning calorimetry
A differential scanning calorimeter (DSC-Q100, TA Instruments, Newcastle, DE, USA) was used for differential scanning calorimetric analysis. The samples, which were prepared in triplicate at 10–20 mg each, were analyzed under modulation mode. After equilibration at 0 °C, the samples were heated up to 180 °C at 2 °C/min, with 60 s modulation, 0.318 °C amplitude, and an empty aluminum pan was used as reference. On the basis of reverse heat flow thermograms, the midpoints between the onset and end temperatures of step changes in the heat flow during heating were recorded as the glass transition temperatures, which were considered as 2nd order transitions (Mohammadi Nafchi et al. 2013b).
Transmittance colorimetry
AHGS films were investigated using a colorimeter in triplicate (Minolta CM-3500D; Minolta Co. Ltd., Osaka, Japan). Calibration of the colorimeter was conducted using air as a fully transmittance and CM-A100 as a zero transmittance calibration plate. The CIE color L*, a*, b* values were measured.
Film morphology
The conditioned AHSG film samples were vacuum-coated with gold for field emission scanning electron microscopy (FESEM). The surface microstructure of the films was investigated using a Leo Supra 50 VP FESEM (Carl-Ziess. SMT, Oberkochen, Germany) equipped with an Oxford INCA 400 energy dispersive system (Ghazihoseini et al. 2015).
Statistical analysis
The quantitative data were presented as mean ± SD of the replicated determinations and were analyzed through the ANOVA procedure by GraphPad Prism 6 software (GraphPad Inc., La Jolla, USA). The means were compared using Duncan’s least significant test at a significance of 5%. The differences were considered significant at p value <0.05.
Results and discussion
AHSG film appearance and formulation
The films formulated without plasticizer or were lower than 25% (w/w) were brittle, and were difficult to handle and remove from the casting plate. The plasticizer concentration range for the film preparation was determined through preliminary experiments. An increase in plasticizer concentration increased the transparency, flexibility, and homogeneity of the films, as well as resulted in smooth surfaces without pores or cracks. An over 50% (w/w) increase in glycerol concentration caused the AHSG films to very soft and sticky.
Physical properties of AHSG films
The effects of glycerol concentration on the physical properties of the AHSG films are reported in Table 1. Significant differences were observed in the film thickness among the different plasticizer concentrations. Reports of other researchers also showed that increasing the plasticizer concentration thickened the starch films (Mohammadi Nafchi et al. 2011). The density of the AHSG films decreased upon adding the plasticizer, and a slight variation was evident. The moisture content of the films significantly increased (p < 0.05) from 14 to 19%. This moisture increase was likely caused by the water holding capacity of glycerol (Cheng et al. 2006). The increase in moisture content was consistent with the increase in film thickness.
Table 1.
Moisture content, density, moisture uptake, and water solubility of AHSG films
| Glycerol (%) | Thickness (mm) | Moisture content (%) | Moisture uptake (g/g dried film) | Water solubility (%) | Density (g/cm3) | Contact angle (°) |
|---|---|---|---|---|---|---|
| 0 | 0.092 ± 0.013d | 14.24 ± 0.03d | 6.90 ± 0.46d | 38.16 ± 1.02d | 1.29 ± 0.02a | 75.6 ± 2.8a |
| 25 | 0.112 ± 0.014c | 16.04 ± 0.08c | 7.35 ± 0.27c | 45.04 ± 2.24c | 1.25 ± 0.01b | 64.3 ± 2.1b |
| 35 | 0.124 ± 0.011b | 17.34 ± 0.03b | 7.98 ± 0.15b | 49.42 ± 1.42b | 1.23 ± 0.03b | 57.5 ± 1.7c |
| 45 | 0.134 ± 0.014a | 19.09 ± 0.06a | 8.43 ± 0.31a | 53.21 ± 1.27a | 1.18 ± 0.01c | 48.8 ± 3.6d |
Values are mean (n = 5) ± SD. Different letters in each column represent significant difference at 5% level of probability among AHSG films
The water solubility of the AHSG films is also shown in Table 1. The results indicated that increasing the glycerol content from 25 to 45% augmented the film solubility. The obtained results were directly related to the hydrophilic nature of glycerol in the AHSG films. If the packaged film would be consumed simultaneously with food, then a high degree of solubility would be acceptable. Similar results were also observed by Tajik et al. (2013) on soluble soybean polysaccharide, in which increasing the glycerol content in films was suggested to increase the water solubility of the films. Adding glycerol also significantly affected the moisture uptake. The moisture uptake increased significantly as the glycerol concentration rose (Table 1).
These results were consistent with those of Jouki et al. (2013a), who reported that higher concentrations of glycerol in cress seed gum-based films exhibited a higher moisture uptake. Conversely, the density and static contact angle of AHSG films decreased as the glycerol concentration increased. This finding was related to the increase in moisture content. Increasing the moisture and glycerol contents decreased the contact angle (Table 1) and density. Given the hydrophilic nature of glycerol, reducing the hydrophobicity would consequently decrease the contact angle of AHSG films (Jouki et al. 2013b).
Barrier properties of AHSG films
The water vapor permeability (WVP) and oxygen permeability (OP) values of the AHSG films are presented in Table 2. A significant increase in both WVP and OP was observed after adding glycerol.
Table 2.
Water vapor permeability, oxygen permeability, and transmittance colorimetric parameter of AHSG films
| Glycerol (%) | WVP × 1011 (g/m/s/Pa) | O.P (cm3 μm/m2 day atm) | L* | a* | b* |
|---|---|---|---|---|---|
| 0 | – | – | 65.56 ± 0.24d | −0.75 ± 0.02a | 4.11 ± 0.48a |
| 25 | 4.93 ± 0.14c | 67.36 ± 4.14c | 67.13 ± 0.28c | −0.76 ± 0.03b | 5.05 ± 0.32a |
| 35 | 5.36 ± 0.05b | 84.12 ± 3.16b | 72.23 ± 0.16b | −0.78 ± 0.07c | 4.72 ± 0.29a |
| 45 | 6.41 ± 0.18a | 102.27 ± 1.58a | 74.14 ± 0.21a | −0.87 ± 0.083d | 4.64 ± 0.32a |
Values are mean (n = 5) ± SD. Different letters in each column represent significant difference at 5% level of probability among AHSG films
The predominantly hydrophophilic behavior of biopolymers, such as polysaccharides, resulted in poor water barrier characteristics. The WVP of films is a characteristic that depends on the diffusion rate and solubility of water in the film. Thus, the WVP of plasticized films would increase by adding plasticizer to the film matrix. Increasing the glycerol concentration decreased the intermolecular forces among the biopolymer chains, and increased the segmental motions and free volume, allowing water molecules to diffuse more easily and providing a higher WVP (Jouki et al. 2013b; Sothornvit and Krochta 2001).
Given the significance of oxygen in the oxidation of food, the oxygen permeability of food packaging materials is greatly important in food preservation. The oxygen and aroma permeability of polysaccharide-based films is very low given large amounts of hydrogen bonds. The biopolymer chain structure and plasticizer distribution within the biopolymer matrix are important in the permeability of films. Increasing the glycerol levels from 25 to 45% augmented the OP values drastically. This finding could be attributed to an increase in chain mobility of the biopolymer and the creation of void spaces in the film matrix (Jouki et al. 2013b). The OP of AHGS films at 50% RH (67.36–102.27 cm3 μm/m2 day atm) was higher than that of films made from polyvinylidene chloride (5.1 cm3 μm/m2 day atm), and polyethylene terephthalate films (13 cm3 μm/m2 day atm), polyethylene (PE) films (18 cm3 μm/m2 day atm), although the values were lower than those of high-density PE (HDPE) films (427 cm3 μm/m2 day atm) and low-density PE films (LDPE) (1870 cm3 μm/m2 day atm) (Miller and Krochta 1997).
Effects of glycerol on the color parameters of AHSG films
The transparent color values L*, a*, and b* of the films with and without plasticizer are presented in Table 2. Incorporating glycerol to the AHSG films mostly affected the lightness index (L*). Increasing the glycerol concentration increased L* significantly, but a* and b* had no significant difference. Apparently, a* statistically showed a significant difference, but the change was minimal for the human eyes to detect. These results agreed with the visual observation of AHSG films. Other researchers have also reported similar results on incorporating glycerol to other types of biopolymers (Jouki et al. 2013a, b; Nouri and Mohammadi Nafchi 2014; Tajik et al. 2013).
Mechanical properties of AHSG films
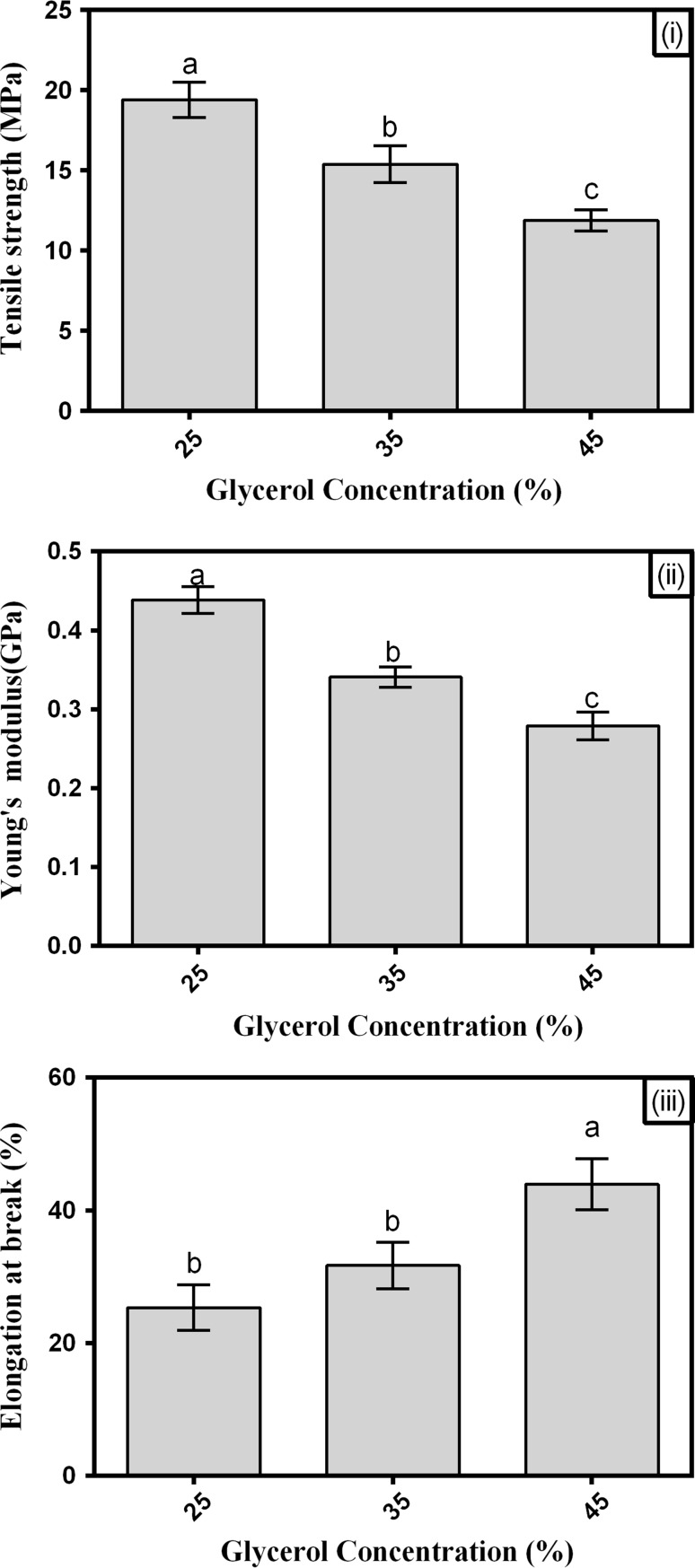
Tensile strength (TS), EB values, and Young’s modulus (YM), which are the respective indicators of strength, flexibility, and stiffness, were considered to characterize the mechanical properties of the films. The effect of the plasticizer level on the mechanical properties of AHSG-based films is shown in Fig. 1. The TS and YM of the plasticized AHSG films significantly decreased when the plasticizer content increased from 25% (19.39 MPa and 0.438 GPa) to 45% (11.87 MPa, 0.279 GPa). Meanwhile, by adding glycerol, the EB values of the films significantly increased from 25 to 43%. Therefore, the increase in glycerol concentration of the AHSG films weakened the films but enhanced their flexibility. The typical effects of plasticizers on mechanical properties, such as decreasing the tensile strength and Young’s modulus, and increasing the EB, have been extensively discussed in literature (Cheng et al. 2006; Ghasemlou et al. 2013). As stated in the physical properties, glycerol interferes with polymer chains and decreases intermolecular forces. Moreover, plasticizers such as glycerol can absorb more moisture, and water can also function as a plasticizing agent. The tensile strength values of the AHSG films were comparable to both LDPE (9–17 MPa) and HDPE (16–41 MPa), as well as to cress seed gum (7–13 MPa), soybean polysaccharide films (6–18 MPa), and starch films (5–12 MPa) (Ghasemlou et al. 2013; Jouki et al. 2013a; Mohammadi Nafchi et al. 2014). The EB values of the AHSG films were higher than those of polystyrene (1–10%) and cellophane (15–25%) but were comparable to those of soluble soybean polysaccharide (SSPS) and cress seed gum (Tajik et al. 2013).
Fig. 1.
Effects of glycerol concentration on tensile strength (i), Young’s modulus (ii), and elongation at breaks of AHSG films
Glass transition temperature
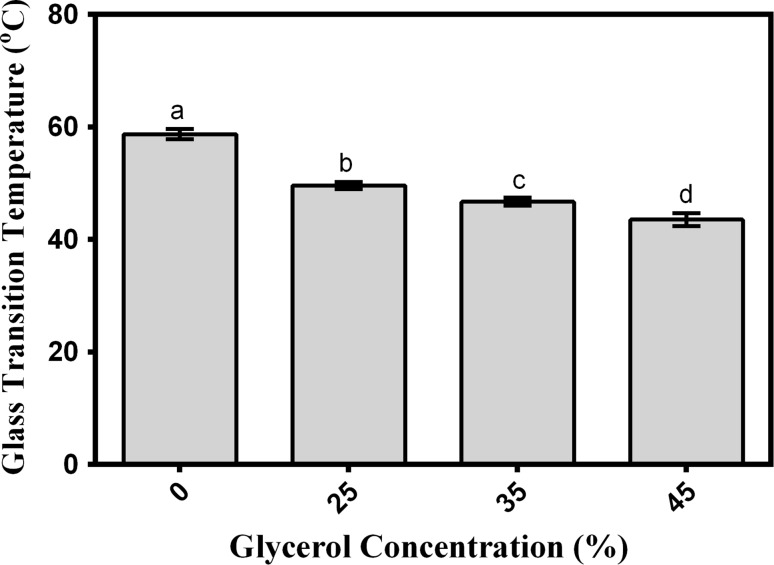
One of the important thermal properties of materials is in the packaging industry is glass transition temperature (Tg). Modulated differential scanning calorimetry is a common method used to determine the Tg of biopolymeric films because of the complexity and aging of biopolymer structures. The glass transitions of the AHSG films are shown in Fig. 2. The results indicated that adding glycerol significantly decreased the Tg of the films from 58 to 43 °C. A negative relationship was found between thermal properties and moisture content of the biopolymer films (Mohammadi Nafchi et al. 2011). Similar to the findings in physical properties, the addition of glycerol increased the films’ moisture content and then decreased Tg.
Fig. 2.
Effects of glycerol concentration on glass transition temperature of AHSG films
AHSG films morphology
The microstracture and surface morphology of the films were mostly characterized via scanning electron microscopy (SEM). The AHSG films surface without plasticizer appeared smooth, but that of the plasticized AHSG films exhibited certain differences in terms of surface microstructure (results not shown). This result was likely caused by the high moisture content absorbed by glycerol, which is hydrophilic in nature. Increasing the concentration of glycerol from 25 to 45% resulted in a rougher film surface compared with that of control films. The pores on the film surface could be the binding site for water during moisture uptake. This qualitative result may explain the increasing in gas permeability and moisture content of AHSG films by plasticizers incorporation. Similar results were reported by Tajik et al. (2013) on soluble soybean polysaccharide (SSPS) films morphology.
Conclusion
As a novel biopolymer basis for edible films, the film formability of Alyssum homolocarpum seed gum (AHSG) was found in this study. The qualitative and quantitative characterizations of AHSG films indicated that the functional properties of this novel film were comparable to the common biopolymers such as starch or gelatin. Glass transition temperature of the AHSG films was around 43–58 °C and permeability to oxygen was 67.36–102.27 (cm3 μm/m2 day atm). Mechanical properties and water vapor permeability of AHSG films are comparable to those common polysaccharides in film preparation. The results of this study suggested that AHSG is a promising raw material for the preparation of edible coatings and films. As a common problem in biopolymeric films; further studies should be conducted to improve the hydrophilicity of AHSG such as incorporation of nanoparticles or mixing with other biopolymers.
References
- Alipoormazandarani N, Ghazihoseini S, Mohammadi Nafchi A. Preparation and characterization of novel bionanocomposite based on soluble soybean polysaccharide and halloysite nanoclay. Carbohydr Polym. 2015;134:745–751. doi: 10.1016/j.carbpol.2015.08.059. [DOI] [PubMed] [Google Scholar]
- Cheng LH, Karim AA, Seow CC. Effects of water-glycerol and water-sorbitol interactions on the physical properties of konjac glucomannan films. J Food Sci. 2006;71:E62–E67. doi: 10.1111/j.1365-2621.2006.tb08898.x. [DOI] [Google Scholar]
- Fakharian M-H, Tamimi N, Abbaspour H, Mohammadi Nafchi A, Karim AA. Effects of κ-carrageenan on rheological properties of dually modified sago starch: Towards finding gelatin alternative for hard capsules. Carbohydr Polym. 2015;132:156–163. doi: 10.1016/j.carbpol.2015.06.033. [DOI] [PubMed] [Google Scholar]
- Ghasemlou M, Aliheidari N, Fahmi R, Shojaee-Aliabadi S, Keshavarz B, Cran MJ, Khaksar R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr Polym. 2013;98:1117–1126. doi: 10.1016/j.carbpol.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Ghazihoseini S, Alipoormazandarani N, Mohammadi Nafchi A. The effects of nano-SiO2 on mechanical barrier, and moisture sorption isotherm models of novel soluble soybean polysaccharide films. Int J Food Eng. 2015 [Google Scholar]
- Hazaveh P, Mohammadi Nafchi A, Abbaspour H. The effects of sugars on moisture sorption isotherm and functional properties of cold water fish gelatin films. Int J Biol Macromol. 2015;79:370–376. doi: 10.1016/j.ijbiomac.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Hesarinejad MA, Razavi SMA, Koocheki A. Alyssum homolocarpum seed gum: dilute solution and some physicochemical properties. Int J Biol Macromol. 2015;81:418–426. doi: 10.1016/j.ijbiomac.2015.08.019. [DOI] [PubMed] [Google Scholar]
- Jafarzadeh S, Alias AK, Ariffin F, Mahmud S, Najafi A. Preparation and characterization of bionanocomposite films reinforced with nano kaolin. J Food Sci Technol. 2016;53:1111–1119. doi: 10.1007/s13197-015-2017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouki M, Khazaei N, Ghasemlou M, HadiNezhad M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr Polym. 2013;96:39–46. doi: 10.1016/j.carbpol.2013.03.077. [DOI] [PubMed] [Google Scholar]
- Jouki M, Tabatabaei Yazdi F, Mortazavi SA, Koocheki A. Physical, barrier and antioxidant properties of a novel plasticized edible film from quince seed mucilage. Int J Biol Macromol. 2013;62:500–507. doi: 10.1016/j.ijbiomac.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Jouki M, Yazdi FT, Mortazavi SA, Koocheki A. Quince seed mucilage films incorporated with oregano essential oil: physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014;36:9–19. doi: 10.1016/j.foodhyd.2013.08.030. [DOI] [Google Scholar]
- Koocheki A, Kadkhodaee R. Effect of Alyssum homolocarpum seed gum, Tween 80 and NaCl on droplets characteristics, flow properties and physical stability of ultrasonically prepared corn oil-in-water emulsions. Food Hydrocoll. 2011;25:1149–1157. doi: 10.1016/j.foodhyd.2010.10.012. [DOI] [Google Scholar]
- Koocheki A, Mortazavi SA, Shahidi F, Razavi SMA, Taherian AR. Rheological properties of mucilage extracted from Alyssum homolocarpum seed as a new source of thickening agent. J Food Eng. 2009;91:490–496. doi: 10.1016/j.jfoodeng.2008.09.028. [DOI] [Google Scholar]
- Koocheki A, Taherian AR, Razavi SMA, Bostan A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocoll. 2009;23:2369–2379. doi: 10.1016/j.foodhyd.2009.06.014. [DOI] [Google Scholar]
- Koocheki A, Mortazavi SA, Shahidi F, Razavi SMA, Kadkhodaee R, Milani JM. Optimization of mucilage extraction from Qodume Shirazi seed (Alyssum homolocarpum) using response surface methodology. J Food Process Eng. 2010;33:861–882. [Google Scholar]
- López D, Márquez A, Gutiérrez-Cutiño M, Venegas-Yazigi D, Bustos R, Matiacevich S. Edible film with antioxidant capacity based on salmon gelatin and boldine. LWT Food Sci Technol. 2017;77:160–169. doi: 10.1016/j.lwt.2016.11.039. [DOI] [Google Scholar]
- Marvizadeh MM, Oladzadabbasabadi N, Mohammadi Nafchi A, Jokar M. Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int J Biol Macromol. 2017;99:1–7. doi: 10.1016/j.ijbiomac.2017.02.067. [DOI] [PubMed] [Google Scholar]
- Maznabi HS, Mohammadi Nafchi A. Investigation of the effects of rosemary extract on barrier and colorimetric properties of Mungbean starch films. JCHR. 2017;3:47–52. [Google Scholar]
- Miller KS, Krochta JM. Oxygen and aroma barrier properties of edible films: a review. Trends Food Sci Technol. 1997;8:228–237. doi: 10.1016/S0924-2244(97)01051-0. [DOI] [Google Scholar]
- Mohammadi Nafchi A, Karim AA. Mechanical, barrier, physicochemical, and heat seal properties of starch films filled with nanoparticles. J Nano Res. 2013;25:90–100. doi: 10.4028/www.scientific.net/JNanoR.25.90. [DOI] [Google Scholar]
- Mohammadi Nafchi A, Cheng LH, Karim AA. Effects of plasticizers on thermal properties and heat sealability of sago starch films. Food Hydrocoll. 2011;25:56–60. doi: 10.1016/j.foodhyd.2010.05.005. [DOI] [Google Scholar]
- Mohammadi Nafchi A, Alias AK, Mahmud S, Robal M. Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide. J Food Eng. 2012;113:511–519. doi: 10.1016/j.jfoodeng.2012.07.017. [DOI] [Google Scholar]
- Mohammadi Nafchi A, Nassiri R, Sheibani S, Ariffin F, Karim AA. Preparation and characterization of bionanocomposite films filled with nanorod-rich zinc oxide. Carbohydr Polym. 2013;96:233–239. doi: 10.1016/j.carbpol.2013.03.055. [DOI] [PubMed] [Google Scholar]
- Mohammadi Nafchi A, Tabatabaei RH, Pashania B, Rajabi HZ, Karim A. Effects of ascorbic acid and sugars on solubility, thermal, and mechanical properties of egg white protein gels. Int J Biol Macromol. 2013;62:397–404. doi: 10.1016/j.ijbiomac.2013.09.050. [DOI] [PubMed] [Google Scholar]
- Mohammadi Nafchi A, Moradpour M, Saeidi M, Alias AK. Effects of nanorod-rich ZnO on rheological, sorption isotherm, and physicochemical properties of bovine gelatin films. LWT Food Sci Technol. 2014;58:142–149. doi: 10.1016/j.lwt.2014.03.007. [DOI] [Google Scholar]
- Neethirajan S, Jayas DS. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol. 2010;4:39–47. doi: 10.1007/s11947-010-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri L, Mohammadi Nafchi A. Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int J Biol Macromol. 2014;66:254–259. doi: 10.1016/j.ijbiomac.2014.02.044. [DOI] [PubMed] [Google Scholar]
- Sadegh-Hassani F, Mohammadi Nafchi A. Preparation and characterization of bionanocomposite films based on potato starch/halloysite nanoclay. Int J Biol Macromol. 2014;67:458–462. doi: 10.1016/j.ijbiomac.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Shaili T, Abdorreza MN, Fariborz N. Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO2. Carbohydr Polym. 2015;134:726–731. doi: 10.1016/j.carbpol.2015.08.073. [DOI] [PubMed] [Google Scholar]
- Sothornvit R, Krochta JM. Plasticizer effect on mechanical properties of β-lactoglobulin films. J Food Eng. 2001;50:149–155. doi: 10.1016/S0260-8774(00)00237-5. [DOI] [Google Scholar]
- Spotti ML, Cecchini JP, Spotti MJ, Carrara CR. Brea Gum (from Cercidium praecox) as a structural support for emulsion-based edible films. LWT Food Sci Technol. 2016;68:127–134. doi: 10.1016/j.lwt.2015.12.018. [DOI] [Google Scholar]
- Tajik S, Maghsoudlou Y, Khodaiyan F, Jafari SM, Ghasemlou M, Aalami M. Soluble soybean polysaccharide: a new carbohydrate to make a biodegradable film for sustainable green packaging. Carbohydr Polym. 2013;97:817–824. doi: 10.1016/j.carbpol.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Talja RA, Helen H, Roos YH, Jouppila K. Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr Polym. 2007;67:288–295. doi: 10.1016/j.carbpol.2006.05.019. [DOI] [Google Scholar]
- Yu J, Yang J, Liu B, Ma X. Preparation and characterization of glycerol plasticized-pea starch/ZnO-carboxymethylcellulose sodium nanocomposites. Bioresour Technol. 2009;100:2832–2841. doi: 10.1016/j.biortech.2008.12.045. [DOI] [PubMed] [Google Scholar]