Abstract
Effect of olive leaf extract rich in oleuropein on the quality of virgin olive oil was investigated. After extracting the dried and ground olive leaves with the assistance of homogenizer, the dried extract was partially dissolved into the oil to increase the oxidative stability of the oil. A face central composite design through response surface methodology was used to investigate the effects of enrichment conditions (extract content, time and mixing speed) on the responses, total phenolic content and oleuropein concentration of the enriched olive oil. Furthermore, antioxidant activity of the oil was determined by 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt method. Additionally, oxidative stability of the enriched oil was assessed by the Rancimat method. Total carotenoid content, peroxide value, α-tocopherol and chlorophyll were also measured, respectively. Addition of 0.15% natural antioxidant increased the stability of the oil (≈46%). The antioxidant capacity of the enriched oil was almost 2.5 times higher than that of the untreated oil. Furthermore, olive leaf extract improved the quality of the virgin olive oil with respect to tocopherol, carotenoid and chlorophyll contents and peroxide value, respectively. The leaf sampling was also performed both in the autumn and summer to evaluate the possible seasonal effects on phenolic profile in order to be careful for selecting the proper harvesting time to apply the extract into the oil.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2607-7) contains supplementary material, which is available to authorized users.
Keywords: Olive oil, Antioxidants, Oxidative stability, Optimization, Rancimat
Introductıon
The consumption of edible oils has increased dramatically in the past century. However, lipid oxidation is the most qualitative problem in food products containing fat such as vegetable oils. This situation leads to great economic loss in food industry. Antioxidants are added to foods containing fats in order to prevent unpleasantness and formation of toxic components resulting from lipid oxidation. Therefore, new procedures based on a green process have been developing in order to improve the quality of edible oils by enrichment with natural antioxidants in recent years (Paiva-Martins et al. 2007; Farag et al. 2007; Salta et al. 2007; Japón-Luján et al. 2008; Japón-Luján and Luque de Castro 2008; Jimenez et al. 2011; Sánchez de Medina et al. 2011; Achat et al. 2012; Rafiee et al. 2012; Jaber et al. 2012; Taghvaei and Mahdi Jafari 2013; Mei et al. 2014; Delfanian et al. 2015; Castañeda Peñalvo et al. 2016; Karoui et al. 2016; Yang et al. 2016; Franco et al. 2016). Several mechanical methods such as ultrasound (Japón-Luján et al. 2008; Achat et al. 2012), homogenizer (Salta et al. 2007), magnetic stirring (Japón-Luján and Luque de Castro 2008; Sánchez de Medina et al. 2011; Jaber et al. 2012) and vertical stirring (Castañeda Peñalvo et al. 2016) have been applied to accomplish the enrichment of various edible oils with natural antioxidants.
In this study, olive tree (Olea europaea) leaf has been chosen as the research material due to its importance from the point of agriculture and economy in Turkey. Turkey has potency over the other competing countries with its geographical position, climate and olive cultivation customs since ancient times. Therefore, olive cultivation sector is of great significance to the agriculture and industry. Olive leaves consisting of 10% of total weight of harvested olives remain as an agricultural waste, if they are not assessed. It is of great importance to evaluate available and economically unvaluable resources. So, reduction of waste disposal will be productive with respect to both economy and ecology. On the other hand, this biomass contains considerable phenolic compounds having a strong radical scavenging activity (El and Karakaya 2009; Ritchason 1999; Pereira et al. 2007; Altıok et al. 2008; Japón-Luján and Luque de Castro 2008; Mavrakis 2009). Those natural antioxidants have been of great value after understanding of the toxic effects of synthetic antioxidants (Olmedo et al. 2015).
Rancimat is an accelerated method for evaluating the oxidative stability of fats and oils, leading to prediction of shelf-life (Farhoosh 2007). It is based on the measurement of the induction time, which decreases the aging and degradation of oil. The induction time depends on oil type, degree of unsaturation and food additives such as antioxidants. Therefore, several parameters like phenolic content, antioxidant activity, peroxide value and β-carotene content are applied for evaluating the quality of the edible oils as well as induction time.
To the best of our knowledge, there have been no studies done on the optimization of enrichment conditions of olive oil with olive leaf extracts through homogenizer. Furthermore, the extracts added to the oil were obtained from olive leaves from the same cultivar harvested both in the autumn and in the summer to determine the possible seasonal effects on phenolic profile. This study is a comprehensive research with focus on oil quality parameters such as total phenolic and carotoneid contents, oleuropein (major individual phenolic compound of olive leaf), α-tocopherol, antioxidant activity, peroxide value and induction time.
Experimental work
Materials
Samples of the olive leaf and virgin olive oil were provided by Özgün Olive, Olive Oil Co.’s relevant departments. The leaves were harvested from Ayvalik in Agean of Turkey both in the autumn and in the summer, respectively.
Ethanol, methanol, hexane were provided from Merck and were of >99.5%, >99.8% and >99% mass purity, respectively. Redistilled water was used in all experiments. Folin-Ciocalteu reagent, sodium carbonate, gallic acid, 2,2′-azino-bis- (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), verbascoside and luteolin7-O-glucoside were purchased from Sigma–Aldrich. Oleuropein standard was obtained from Extrasynthese (Genay, France). 18 mΩ deionised water from Human Power I water purification system was used to prepare mixtures analyses.
Extraction of olive leaves
500 mg of dried and ground leaf samples were extracted three times with 30 mL of ethanol solution (%10, v/v) by blending in a homogeniser (IKA T25, ULTRA-TURRAX) at 4000 rpm and 45 s (three times at 10 s intervals). The mixture was centrifuged (Nüve, CN 180) at 5000×g for 25 min. After the solvent was evaporated, the dried extracts were kept in dark and stored at −20 °C.
Preparation of enriched olive oil
The dried extract was partially dissolved into the olive oil by means of solid- liquid extraction method. Olive leaf extracts were added to the oil at concentrations of 1000-1500 ppm, and mixed vigorously at several conditions (4000–10,000 rpm and 30–90 s) by means of homogenizer.
Extraction of polyphenols from enriched olive oil
2.5 mL hexane were added into 1 g of olive oil, and extracted with methanol twice at 7000 rpm and 60 s. The extracts were combined, washed with hexane and then filtered through a 0.45 μm syringe filter and stored at −20 °C until analysis for the biochemical measurements.
Determination of total phenolic content
The concentration of total phenols in the extracts were measured by UV- spectrophotometry (PG Instruments, T60/Leicestershire, England), based on calorimetric oxidation/reduction reaction. The total phenolic content was determined according to the Folin-Ciocalteu method at a wavelength of 765 nm by following the procedure of Malik and Bradford (Malik and Bradford 2006).
Determination of antioxidant capacity by ABTS assay
Free radical scavenging activity of the extracts by ABTS assay was done according to the modified method of Re et al. (Re et al. 1999). After addition of 150 µL of sample solution to 2850 µL of diluted ABTS solution, absorbance was measured at 10th minute at 734 nm against a blank sample without ABTS. The ability to scavenge the ABTS radical was calculated by using the equation below:
| 1 |
where A control is the absorbance of the control (ABTS solution without sample) and A sample is the absorbance of the test sample.
HPLC analysis
Analyses of oleuropein were conducted on an Agilent 1260 chromatographic system (Agilent, Waldbronn, USA) equipped with quaternary pump, a degasser, manual injector and a diode-array-detector (DAD). Agilent Eclipse Plus C18 RRHD 18 column (3.0 mm × 5.0 mm id, 1.8 μm particle size) was used to separate the extracts. The column temperature was maintained at 40 °C with a gradient elution of (A) 0.1% formic acid in H2O and (B) 0.1% formic acid in acetonitrile. A gradient program was written according to the following profile: 0–14 min 0% B, 14–14.2 min 40% B, 14.2–17.2 min 100% B, 17.4–20 min 0% B. Injection volume was 20 μL and the detection wavelength was set at 276 nm.
Agilent LiChrospher DIOL coloumn (4 × 250 mm, 5 µm) was used for α-tocopherol analysis by following IUPAC Standard Method (IUPAC, 1992).
Rancimat method
The stability of olive oil before and after enrichment was evaluated by Rancimat method at 130 °C in a Rancimat 892 apparatus (Metrohm). The oxidation process was monitored upon 3 g oil sample at air velocity 20 L/h. The stability of the oils was expressed as the induction time (IT).
Peroxide value
The peroxide value in mg oxygen equivalent per kg of oil (meq-O2/kg-oil) was determined by titration of 0.1 N KI saturated solutions of the oil with 0.1 N Na2S2O3 and starch as indicator (Sánchez de Medina et al. 2011).
Determination of carotenoid
Total carotenoid content (TCC) of the oil was determined by following the slightly modified method of Chuang and Brunner at 450 nm (Chuang and Brunner 2006). The carotenoids content was calculated using the calibration curve, obtained using known concentration of β-carotene dissolved in hexane.
Determination of chlorophylls
Chlorophyll content was determined at 670 nm in cyclohexane by using exactly the same method of Jaber et al. The chlorophyll concentration is expressed as mg of pheophytin a per kg of oil (Jaber et al. 2012).
Experimental design
A face central composite design (FCCD) was performed with three variables to explore the effect of variables on the response (Online Resource 1). Total phenolic content (Y 1) and oleuropein (Y 2) were the responses, respectively. Mixing speed (X 1), time (X 2) and solid mass (X 3) were independent variables, selected based on the preliminary experiments including three levels. In order to apply the FCCD, Design-Expert 9.0.6 software (trial version) was used. Twenty experiments were conducted with six replications at the center values to evaluate the pure error sum of squares.
Experimental data were fitted to the quadratic model. The quadratic model proposed was shown as follows in Eq. (2):
| 2 |
where Y is the response, β 0 is the constant coefficient often described as intercept, X i (i = 1–3) is the non-coded variable, β i is the linear, and β ii is the quadratic, and β ij (i and j = 3) is the second order interaction coefficients.
Statistical analysis
Three replicate experiments were carried out for each of the samples followed by a minimum of three spectrophotometric measurements from each sample. The analysis of variance (ANOVA) test was applied to identify the interaction between the variables and the response using Design-Expert program.
Results and dıscussıons
Phenolic profile of olive leaf extracts
Bioactive material composition of plant extracts can be affected by so many factors such as leaf age, degree of ripeness, cultivar, geographical origin, sampling conditions, phonological stage during sampling, proportion of brunches on the tree, moisture content, degree of contamination with soil, cultivation methods and industrial processes employed for extraction (Şahin et al. 2012; Bilgin and Şahin 2013). In this study, olive leaves were extracted by means of homogenizer assisted extraction (HAE). Optimization studies of enrichment conditions for olive oil were evaluated according to the maximum total phenolic content (TPC) and oleuropein concentration of the oil, since oleuropein is the major compound contributing to the total phenols in the leaf (Online Resource 5).
Leaf samples were picked up both in the autumn and in the summer to determine the possible seasonal effects on phenolic profile. Table 1 indicates the levels of basic phenolic compounds of olive leaf extract obtained by HAE depending on the harvesting period. The highest level of oleuropein was found in autumn leaf samples, and the levels were significantly lower in samples harvested in the summer. This is mostly attributable to the fact that the concentration of phenolic compounds have a tendency to decrease in hot summer days (Şahin et al. 2012). This information should be valuable for anyone trying to apply the extract as food additive at commercial scale.
Table 1.
Concentrations of phenolic compounds in olive leaves from 2 different seasons
| Harvesting time | Oleuropein (ppm) | Verbascoside (ppm) | Luteolin 7-O-glucoside (ppm) |
|---|---|---|---|
| Autumn | 271.66 ± 10.11 | 31.85 ± 1.10 | 17.30 ± 0.80 |
| Summer | 133.54 ± 5.30 | 18.62 ± 1.01 | 13.13 ± 0.66 |
Data are expressed as the mean (n = 3) ± SD
Optimization of enrichment conditions for olive oil
The enrichment process was carried out by means of solid–liquid extraction, in which solid olive leaf extract was partially dissolved into the olive oil. In order to optimize the process conditions, possible interactions among the operating parameters such as mixing time, speed of the homogenizer and the quantity of the extract should be considered depending on the preliminary experiments. 20 experimental runs were chosen randomly with Design Expert 9.0.6 as seen in Online Resource 2.
ANOVA results for the quadratic equations of Design Expert 9.0.6 for the TPC and oleuropein responses are given in Online Resources 3 and 4, respectively. The models derived for TPC and oleuropein were found significant (p < 0.05) to display the relationship between the response and independent variables. The ANOVA result also showed that the experimental data had correlation coefficients (R 2) of 0.8193 and 0.8909 with the calculated models, accounting for the 81.93 and 89.09% of the results, respectively. The quadratic models for TPC (Y 1) and oleuropein (Y 2) were derived as given in the equations below:
| 3 |
| 4 |
The independent and dependent variables were tested for lack of fit on the quadratic response surface models. The first model derived for TPC had a non-significant lack of fit value (p > 0.05), showing that the model accurately fits the data (Peretto et al. 2014). However, the lack of fit value of the other model calculated for oleuropein were found significant (p < 0.05). The same results were also observed by Saldaňa-Robles et al. (2014), Danish et al. (2014), Kittisuban et al. (2014) and Elsen and Ramesh (2015). Kittisuban et al. asserted that a model with a significant lack of fit could still be used when large amounts of data were included in the analysis. On the other hand, Adequate Precision measures the signal to noise ratio. Both of the predicted models had ratios greater than 4, which is desirable in order to indicate adequate model discrimination. Therefore, the models can be utilized for navigation the design space (Elsen and Ramesh 2015).
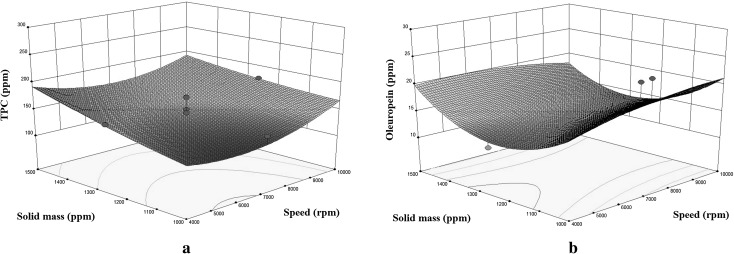
In order to explore the potential relationships between the variables, three dimensional (3D) response surface plots (Figs. 1, 2, 3) are constructed according to the quadratic Eqs. (3) and (4). Expectedly, yield of TPC increased by extract quantity as seen in Fig. 1a. This is in agreement with the results of Franco et al., who found out that increasing potato peel extract concentration would have improved the control of oil oxidation (Franco et al. 2016). Mei et al. (2014) also suggested higher concentrations of the rambutan extract into sunflower oil in order to improve the stability of the oil. On the other hand, oleuropein yield had a tendency to decrease until a point, then it started to increase (Fig. 1b). This is in agreement with the results of Ballard et al. (2010)and Şahin (2015). Ballard et al. observed a 35.8% reduction in TPC when extracting phenolic antioxidants from peanut skins in the mass of the skins from 1.5 to 3.5 g. Şahin showed almost 22 and 13% decrease in the TPC and TFC yields of mandarin leaf exracts obtained by microwave–assisted extraction. This is mostly attributable to the surface area. Increasing the sample mass decreases the surface area, which is unfavourable for the penetration of the sample into the oil.
Fig. 1.
Response surface plots for the TPC (a) and oleuropein (b) of enriched olive oil as a function of solid mass to mixing speed (time = 60 s)
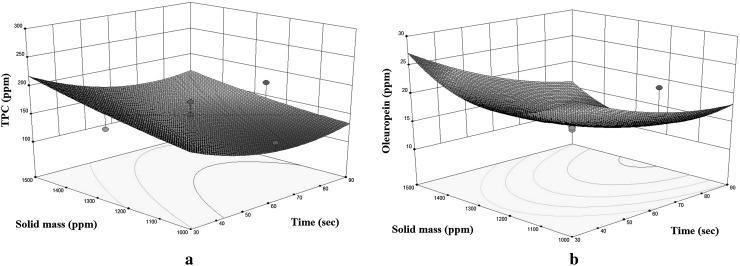
Fig. 2.
Response surface plots for the TPC (a) and oleuropein (b) of enriched olive oil as a function of solid mass to mixing time (mixing speed = 7000 rpm)
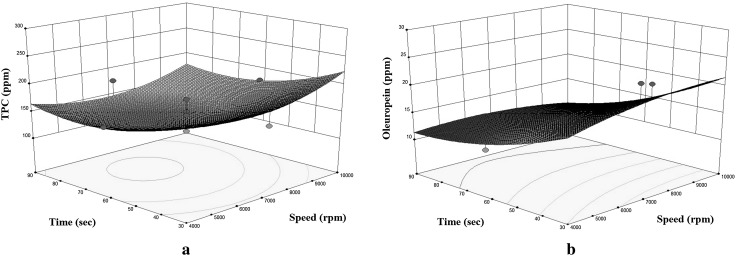
Fig. 3.
Response surface plots for the TPC (a) and oleuropein (b) of enriched olive oil as a function of mixing time to speed (solid mass = 1250 ppm)
Time had a negative effect on the enrichment of olive oil for both variables (Fig. 2a, b). This result is most probably due to the degradation of the components caused by overexposure to the heating of the long mixing time (Chan et al. 2009). This might be explained by the fact that the major phenolic compound, oleuropein and the other polyphenols of the olive leaf are not thermally stable. Expectedly, mixing speed indicated an increase in the oil enriched by TPC and oleuropein while extraction time gave rise a drop under a constant solid mass.
As seen in Fig. 3a, b, enrichments by TPC and oleuropein were both increased with mixing speed of homogenizer. Diffusion of the target compounds into the oil is expected to be increased with stirring (Ho and Hsieh 2001). Moreover, agitation also contributes to the favourable extraction with its additional energy and promoting the homogeneity (Georgiev et al. 2009; Bilgin and Şahin 2013).
Validation of the optimum conditions
The results of the present study suggested that 10,000 rpm of mixing speed, 30 s of time and 1500 ppm of olive leaf extract should be employed as optimal operating conditions in order to enrich the greatest TPC (247.168 ppm) and oleuropein (26.471 ppm) in olive oil. On the other hand, Table 2 presents the experimental results of TPC (246 ppm) and oleuropein (25.98 ppm) in addition to α-tocopherol, antioxidant activity, carotene and chlorophyll contents, peroxide value and induction time at the optimum conditions of olive oil enrichment. The quadratic models have proven to be adequate to predict the optimal conditions.
Table 2.
Changes of olive oil stability parameters before and after their enrichment with the olive leaf extract at the optimum conditions
| TPC (ppm) | Oleuropein (ppm) | α-Tocopherol (ppm) | AA (%) | TCC (mg-β carotene/kg-oil) | Chlorophyll (mg-pheophytin/kg-oil) | PV (meq-O2/kg-oil) | IT (h) | |
|---|---|---|---|---|---|---|---|---|
| Pure olive oil | 132 ± 3.19 | 6.46 ± 0.19 | 114.38 ± 0.54 | 13.45 ± 1.15 | 2.78 ± 0.07 | n.d | 9.09 ± 0.40 | 3.41 ± 0.17 |
| Enriched olive oil | 246 ± 4.29 | 25.98 ± 1.30 | 127.83 ± 0.75 | 34.09 ± 1.05 | 7.89 ± 0.52 | 28.17 ± 0.22 | 7.07 ± 0.54 | 4.98 ± 0.20 |
Data are expressed as the mean (n = 3) ± SD
n.d not detected
Efficiency of the proposed method at the optimum conditions
Table 2 summarizes the results of enrichment process applied at regarding quality parameters such as TPC, oleuropein, α-tocopherol, antioxidant activity (AA), carotene (TCC) and chlorophyll contents, peroxide value (PV) and IT.
Total phenolic content increased almost 2 times over the pure olive oil whereas oleuropein concentration increased approximately 3.5 times as seen in Table 2. Achat et al. (2012) put 150,000 ppm olive leaf into the olive oil and got 50.7 and 111 mg oleuropein per gram of olive oil by optimized ultrasound assisted extraction and conventional methods, respectively. Salta et al. (2007) added olive leaf extract containing 1680 ppm oleuropein into the sunflower oil and achieved 74.9 ppm oleuropein supplementation in the oil. However, our extract had 272 ppm oleuropein depending on the method, cultivar, harvesting time and etc. Considering the restrictions of the Joint FAO/WHO Expert Committee on food additives, only 1500 ppm extract was applied in the samples. Therefore, our oleuropein enrichment in the olive oil is reasonable comparing to those of Achat et al. and Salta et al. On the other hand, Japón-Luján et al. used 100,000 ppm olive leaf to increase the quality of the sunflower oil, and obtained 10.21 mg oleuropein per liter of oil (Japón-Luján et al. 2008).
The ability to scavenge free radicals has been found almost 2.5 times higher than that of untreated oil. Olive leaf extract also enhanced the quality of the oil with respect to tocopherol, carotenoid and chlorophyll contents and peroxide value.
The time required to produce a sudden increase in conductivity, due to the formation of volatile acids, determines an induction time which can be defined as a measure of the stability of oil (Jannat et al. 2010; Yang et al. 2016). In other words, induction time is used to characterize the resistance of oil to oxidation. Online resource 6a and b show the induction times of treated and untreated oils measured at 130 °C through Rancimat method. Addition of 0.15% natural antioxidant gave rise to increase of the stability of the oil (≈46%). As stated before, increasing the proportion of extract would have improved the control of oil oxidation more. However, the limitations of the legal food and health committees on food additives have to be considered. Otherwise, the more the additive, the longer the induction time and the more stable the oil is to oxidation. Furthermore, the differences in IT between the oils could be attributed to the type and the quality of oil other than the proportions of additives and methods (De Leonardis et al. 2007).
As already mentioned above, the season of the year have remarkable effect on levels of major phenolic compounds in olive leaves. This information should be precious for anyone trying to apply the extract as food additive to improve the quality of the vegetable oils at commercial scale. Table 3 demonstrates the comparitive results of oxidative stabilities of olive oils enriched by olive leaves harvested at different seasons. The increase in the oil stability by the extract of autumn olive leaves was nearly 46%, while the value of summer remained around 6%.
Table 3.
Comparitive results of oxidative stabilities of olive oils enriched by olive leaves harvested at different seasons
| Harvesting time | IT (h) | Increase (%) |
|---|---|---|
| Autumn | 4.98 ± 0.11 | 46 |
| Summer | 3.62 ± 0.09 | 6 |
Data are expressed as the mean (n = 3) ± SD
Conclusion
The optimum conditions for HAE was found to be 1500 ppm extract at 10,000 rpm for 30 s to achieve the maximum yields of TPC and oleuropein in olive leaf. The concentration of phenolic compounds in olive leaves had a tendency to decrease in hot summer days. Therefore, care must be taken in selecting the most accurate season for harvesting time to apply the extracts into the olive oil. Olive leaf extract has proved that its phenolic content and especially major compound, oleuropein significantly contribute to the antioxidant capacity of the extract. Addition of this natural antioxidant to olive oil has enhanced the stability of the oil as well as increasing its added-value with high quality parameters, substantially.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank to The Scientific & Technological research Council of Turkey (TÜBİTAK) for the support of this Project. Project number is 114M728.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2607-7) contains supplementary material, which is available to authorized users.
References
- Achat S, Tomao V, Madani K, et al. Direct enrichment of olive oil in oleuropein by ultrasound-assisted maceration at laboratory and pilot plant scale. Ultrason Sonochem. 2012;19:777–786. doi: 10.1016/j.ultsonch.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Altıok E, Bayçın D, Bayraktar O, Ülkü S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Sep Purif Technol. 2008;62:342–348. doi: 10.1016/j.seppur.2008.01.022. [DOI] [Google Scholar]
- Ballard TS, Mallikarjunan P, Zhou K, O’Keefe S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010;120:1185–1192. doi: 10.1016/j.foodchem.2009.11.063. [DOI] [Google Scholar]
- Bilgin M, Şahin S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J Taiwan Inst Chem Eng. 2013;44:8–12. doi: 10.1016/j.jtice.2012.08.008. [DOI] [Google Scholar]
- Castañeda Peñalvo G, Rodríguez Robledo V, Sánchez-Carnerero Callado C, et al. Improving green enrichment of virgin olive oil by oregano. Effects on antioxidants. Food Chem. 2016;197:509–515. doi: 10.1016/j.foodchem.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Chan EWC, Lim YY, Wong SK, et al. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009;113:166–172. doi: 10.1016/j.foodchem.2008.07.090. [DOI] [Google Scholar]
- Chuang M-H, Brunner G. Concentration of minor components in crude palm oil. J Supercrit Fluids. 2006;37:151–156. doi: 10.1016/j.supflu.2005.09.004. [DOI] [Google Scholar]
- Danish M, Hashim R, Ibrahim MNM, Sulaiman O. Optimized preparation for large surface area activated carbon from date (Phoenix dactylifera L.) stone biomass. Biomass Bioenerg. 2014;61:167–178. doi: 10.1016/j.biombioe.2013.12.008. [DOI] [Google Scholar]
- De Leonardis A, Macciola V, Lembo G, et al. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007;100:998–1004. doi: 10.1016/j.foodchem.2005.10.057. [DOI] [Google Scholar]
- Delfanian M, Kenari RE, Sahari MA. Antioxidant activity of loquat (Eriobotrya japonica Lindl.) fruit peel and pulp extracts in stabilization of soybean oil during storage conditions. Int J Food Prop. 2015;18:2813–2824. doi: 10.1080/10942912.2015.1013635. [DOI] [Google Scholar]
- El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009 doi: 10.1111/j.1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- Elsen SR, Ramesh T. Optimization to develop multiple response hardness and compressive strength of zirconia reinforced alumina by using RSM and GRA. Int J Refract Met Hard Mater. 2015;52:159–164. doi: 10.1016/j.ijrmhm.2015.06.007. [DOI] [Google Scholar]
- Farag RS, Mahmoud EA, Basuny AM. Use crude olive leaf juice as a natural antioxidant for the stability of sunflower oil during heating. Int J Food Sci Technol. 2007;42:107–115. doi: 10.1111/j.1365-2621.2006.01374.x. [DOI] [Google Scholar]
- Farhoosh R. Shelf-life prediction of edible fats and oils using rancimat. Lipid Technol. 2007;19:232–234. doi: 10.1002/lite.200700073. [DOI] [Google Scholar]
- Franco D, Pateiro M, Amado IR, et al. Antioxidant ability of potato (Solanum tuberosum) peel extracts to inhibit soybean oil oxidation. Eur J Lipid Sci Technol. 2016 [Google Scholar]
- Georgiev MI, Weber J, Maciuk A. Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl Microbiol Biotechnol. 2009;83:809–823. doi: 10.1007/s00253-009-2049-x. [DOI] [PubMed] [Google Scholar]
- Ho W-H, Hsieh S-J. Solid phase microextraction associated with microwave assisted extraction of organochlorine pesticides in medicinal plants. Anal Chim Acta. 2001;428:111–120. doi: 10.1016/S0003-2670(00)01219-8. [DOI] [Google Scholar]
- Jaber H, Ayadi M, Makni J, et al. Stabilization of refined olive oil by enrichment with chlorophyll pigments extracted from Chemlali olive leaves. Eur J Lipid Sci Technol. 2012;114:1274–1283. doi: 10.1002/ejlt.201100176. [DOI] [Google Scholar]
- Jannat B, Oveisi M, Sadeghi N, et al. Shelf life prediction of infant formula by using rancidity test. Iran J Pharm Res. 2010;0:269–273. [Google Scholar]
- Japón-Luján R, Luque de Castro MD. Liquid–liquid extraction for the enrichment of edible oils with phenols from olive leaf extracts. J Agric Food Chem. 2008;56:2505–2511. doi: 10.1021/jf0728810. [DOI] [PubMed] [Google Scholar]
- Japón-Luján R, Janeiro P, de Castro MDL. Solid–liquid transfer of biophenols from olive leaves for the enrichment of edible oils by a dynamic ultrasound-assisted approach. J Agric Food Chem. 2008;56:7231–7235. doi: 10.1021/jf800748p. [DOI] [PubMed] [Google Scholar]
- Jimenez P, Masson L, Barriga A, et al. Oxidative stability of oils containing olive leaf extracts obtained by pressure, supercritical and solvent-extraction. Eur J Lipid Sci Technol. 2011;113:497–505. doi: 10.1002/ejlt.201000445. [DOI] [Google Scholar]
- Karoui IJ, Msaada K, Abderrabba M, Marzouk B. Bioactive compounds and antioxidant activities of thyme-enriched refined corn oil. J Agric Sci Technol. 2016;18:79–91. [Google Scholar]
- Kittisuban P, Ritthiruangdej P, Suphantharika M. Optimization of hydroxypropylmethylcellulose, yeast β-glucan, and whey protein levels based on physical properties of gluten-free rice bread using response surface methodology. LWT Food Sci Technol. 2014;57:738–748. doi: 10.1016/j.lwt.2014.02.045. [DOI] [Google Scholar]
- Malik NSA, Bradford JM. Changes in oleuropein levels during differentiation and development of floral buds in “Arbequina” olives. Sci Hortic (Amsterdam) 2006;110:274–278. doi: 10.1016/j.scienta.2006.07.016. [DOI] [Google Scholar]
- Mavrakis T. Exploitation of bioactive constituents of olive leaves, grape pomace, olive mills waste water and their application in phytoprotection. England: Cranfield University; 2009. [Google Scholar]
- Mei W, Ismail A, Esa N, et al. The effectiveness of rambutan (Nephelium lappaceum L.) extract in stabilization of sunflower oil under accelerated conditions. Antioxidants. 2014;3:371–386. doi: 10.3390/antiox3020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo RH, Asensio CM, Grosso NR. Thermal stability and antioxidant activity of essential oils from aromatic plants farmed in Argentina. Ind Crops Prod. 2015;69:21–28. doi: 10.1016/j.indcrop.2015.02.005. [DOI] [Google Scholar]
- Paiva-Martins F, Correia R, Félix S, et al. Effects of enrichment of refined olive oil with phenolic compounds from olive leaves. J Agric Food Chem. 2007;55:4139–4143. doi: 10.1021/jf063093y. [DOI] [PubMed] [Google Scholar]
- Pereira AP, Ferreira IC, Marcelino F, et al. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules. 2007;12:1153–1162. doi: 10.3390/12051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto G, Du W-X, Avena-Bustillos RJ, et al. Optimization of antimicrobial and physical properties of alginate coatings containing carvacrol and methyl cinnamate for strawberry application. J Agric Food Chem. 2014 doi: 10.1021/jf4042886. [DOI] [PubMed] [Google Scholar]
- Rafiee Z, Jafari SM, Alami M, Khomeiri M. Antioxidant effect of microwave-assisted extracts of olive leaves on sunflower oil. J Agric Sci Technol. 2012;14:1497–1509. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Ritchason J. Olive leaf extract. Utah: Pleasant Grove; 1999. [Google Scholar]
- Şahin S. A novel technology for extraction of phenolic antioxidants from mandarin (Citrus deliciosa Tenore) leaves: solvent-free microwave extraction. Korean J Chem Eng. 2015;32:950–957. doi: 10.1007/s11814-014-0293-y. [DOI] [Google Scholar]
- Şahin S, Saeed N, Malik A, et al. Seasonal changes of individual phenolic compounds in leaves of twenty olive cultivars grown in Texas. J Agric Sci Technol B. 2012;2:242–247. [Google Scholar]
- Saldaña-Robles A, Guerra-Sánchez R, Maldonado-Rubio MI, Peralta-Hernández JM. Optimization of the operating parameters using RSM for the Fenton oxidation process and adsorption on vegetal carbon of MO solutions. J Ind Eng Chem. 2014;20:848–857. doi: 10.1016/j.jiec.2013.06.015. [DOI] [Google Scholar]
- Salta FN, Mylona A, Chiou A, et al. Oxidative stability of edible vegetable oils enriched in polyphenols with olive leaf extract. Food Sci Technol Int. 2007;13:413–421. doi: 10.1177/1082013208089563. [DOI] [Google Scholar]
- Sánchez de Medina V, Priego-Capote F, Jiménez-Ot C, Luque de Castro MD. Quality and stability of edible oils enriched with hydrophilic antioxidants from the olive tree: the role of enrichment extracts and lipid composition. J Agric Food Chem. 2011;59:11432–11441. doi: 10.1021/jf2020528. [DOI] [PubMed] [Google Scholar]
- Taghvaei M, Mahdi Jafari S. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J Food Sci Technol. 2013 doi: 10.1007/s13197-013-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Song X, Sui X, et al. Rosemary extract can be used as a synthetic antioxidant to improve vegetable oil oxidative stability. Ind Crops Prod. 2016;80:141–147. doi: 10.1016/j.indcrop.2015.11.044. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.