Abstract
Sugar transport has been directly observed in isolated higher plant vacuoles for the first time. The latter were released from protoplasts isolated from the mesophyll of Pisum sativum L.
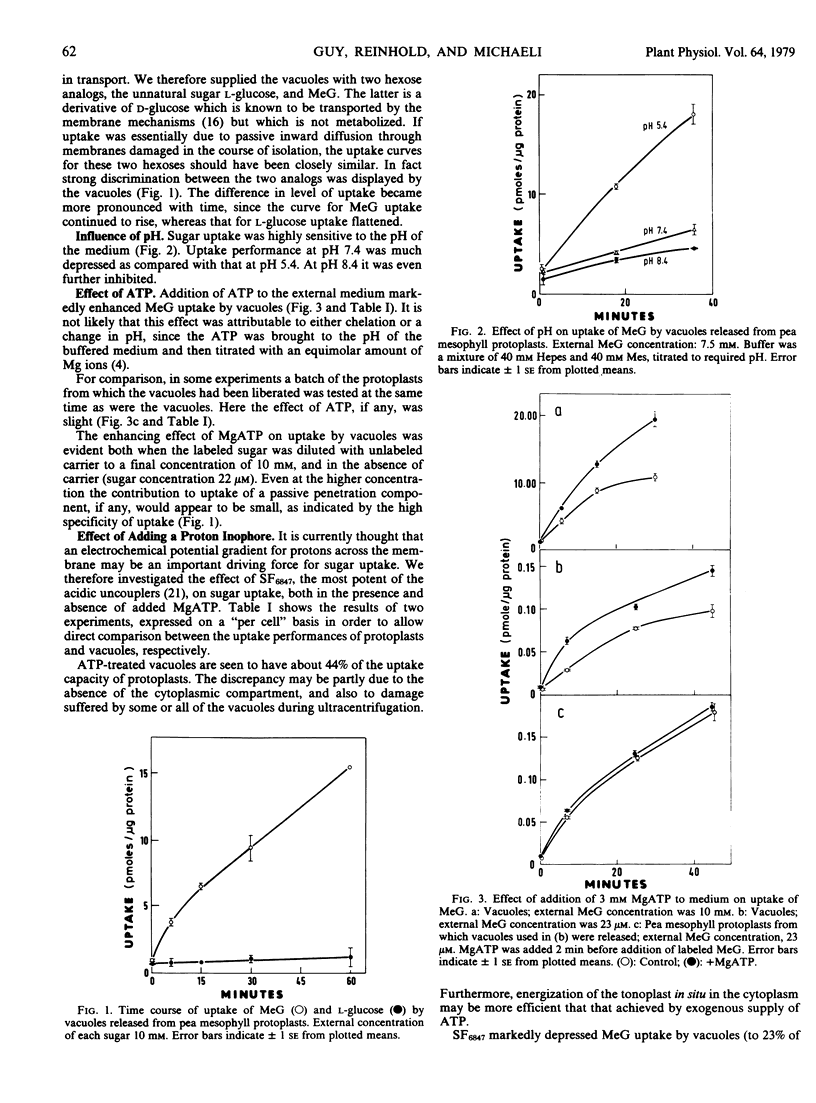
Uptake of l-glucose by the vacuoles was very slight in comparison with that of the d-glucose analog 3-O-methyl glucose (MeG), indicating, first, that a highly selective sugar uptake mechanism is seated in the tonoplast; and, second, that the mechanism was functioning in the isolated vacuoles.
MeG uptake was markedly sensitive to the pH of the medium, falling as the external pH rose. Addition of MgATP to buffered medium strongly promoted MeG uptake by vacuoles, but not by the protoplasts from which they were released. Treatment with the proton ionophore SF6847 drastically reduced uptake by the vacuoles, but had a lesser effect on uptake by the protoplasts. The inhibitory effect of SF6847 on uptake by the vacuoles was countered to a substantial degree by the addition of MgATP.
The influence of pH, the stimulatory effect of ATP, and the ATP-reversible inhibition by SF6847 all strengthen the conclusion that the observed sugar uptake reflected membrane function and was not due to a diffusional inward leak through damaged membranes.
The results are discussed in the light of currently held concepts regarding the driving force for sugar transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar L., Reinhold L. Loss of membrane transport ability in leaf cells and release of protein as a result of osmotic shock. Plant Physiol. 1973 Apr;51(4):620–625. doi: 10.1104/pp.51.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Boos W., Schwartz M., Szmelcman S. Energy-coupling of the transport system of Escherichia coli dependent on maltose-binding protein. Eur J Biochem. 1977 May 2;75(1):187–193. doi: 10.1111/j.1432-1033.1977.tb11516.x. [DOI] [PubMed] [Google Scholar]
- Guy M., Reinhold L. Membrane transport of sugars and amino acids in isolated protoplasts. Plant Physiol. 1978 Apr;61(4):593–596. doi: 10.1104/pp.61.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol. 1974 Nov;64(5):568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemiosmotic processes. Annu Rev Biochem. 1977;46:996–1005. doi: 10.1146/annurev.bi.46.070177.005024. [DOI] [PubMed] [Google Scholar]
- Reinhold L., Eshhar Z. Transport of 3-o-Methylglucose Into and Out of Storage Cells of Daucus carota. Plant Physiol. 1968 Jul;43(7):1023–1030. doi: 10.1104/pp.43.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. B., Epstein W. Energy coupling to net K+ transport in Escherichia coli K-12. J Biol Chem. 1977 Feb 25;252(4):1394–1401. [PubMed] [Google Scholar]
- Terada H. Some biochemical and physiochemical properties of the potent uncoupler SF 6847 (3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile). Biochim Biophys Acta. 1975 Jun 17;387(3):519–532. doi: 10.1016/0005-2728(75)90090-0. [DOI] [PubMed] [Google Scholar]


