Abstract
Electropulsation is one of the nonviral methods successfully used to deliver genes into living cells in vitro and in vivo. This approach shows promise in the field of gene and cellular therapies. The present review focuses on the processes supporting gene electrotransfer in vitro. In the first part, we will report the events occurring before, during, and after pulse application in the specific field of plasmid DNA electrotransfer at the cell level. A critical discussion of the present theoretical considerations about membrane electropermeabilization and the transient structures involved in the plasmid uptake follows in a second part.
Keywords: Electropermeabilization, Electroporation, Gene electrotransfer, Biophysical mechanisms, Membranes
Introduction
Electropermeabilization results from a controlled application of electric pulses to cells, leading to their transient and reversible membrane permeabilization (Neumann et al. 1989; Teissié et al. 2005; Escoffre et al. 2007). This process brings new properties to the cell plasma membrane, which, besides being permeabilized, becomes fusogenic and allows exogenous proteins to be inserted in it. Electropermeabilization is used to introduce a large variety of molecules into many different cells in vitro (Orlowski and Mir 1993; Eynard et al. 1997). Clinical applications of the method are now under development as results of the EU Cliniporator and ESOPE programs. A local anti-tumoral drug delivery to patients (a method called electrochemotherapy) is in clinical trials in tens of hospitals through Europe (Mir et al. 1995; Cemazar and Sersa, 2007; Sersa et al. 2008; Mir et al. 1998, 2006). The most frequent application of electric field-induced membrane permeabilization is the transfer and expression of genes into mammalian cells. However, this application not only involves the introduction of DNA into cells, but also depends on subsequent cellular processes (Weaver and Chizmadzhev 1996). The transfer of naked DNA plasmid and the expression of the gene of interest are enhanced by electropulsation into different tissues, including the skeletal muscle (Aihara and Miyazaki 1998; Mir et al. 1999), liver (Heller et al. 1996; Liu and Huang 2002), skin (Titomirov et al. 1991; Vandermeulen et al. 2007), and tumors (Rols et al. 1998a). The transfection efficiency of this physical method in vivo is still low compared to the viral vectors. However, due to the ease with which it is performed and its speed, reproducibility, and safety, gene electrotransfer holds a great potential for clinical application.
One of main limits of the widespread use of electropermeabilization is that very little is known about the biophysical mechanisms supporting the reorganization of the cell membrane (pore, electropore, defects?). The molecular target of the field effect remains unclear (Teissié et al. 2005). The other main limit in gene electrotransfer comes from the transfer inside the nucleus. In nondividing cells, the nuclear envelope is an especially problematic hurdle to gene transfer, which should take place through the nuclear pore complex (Van der Aa et al. 2006, 2007; Pouton et al. 2007). No direct biophysical method to alter the nuclear envelope or pore has been reported (yet). A successful approach is in modifying plasmid (pDNA) vectors to enhance nuclear import through the NPC (Miller and Dean 2009).
The present review focuses on the processes supporting gene electrotransfer in vitro. The events occurring before, during, and after pulse application leading to gene electrotransfer will be described. Theoretical considerations about membrane structures involved in the plasmid uptake will be described in a (very) critical manner. In a companion paper, in vivo gene electrotransfer and its clinical applications will be addressed.
Biophysical mechanisms
In vitro biophysical considerations
Gene electrotransfer to mammalian cells is obtained by mixing cells and plasmids in a biocompatible buffer (control of pH, osmolarity, conductivity), then by applying a well-controlled electric field pulse train (shape of pulses, choice of field strength, pulse duration, number of pulses, delay between pulses) and finally bringing the mixture into a culture medium. Expression of the coded activity can be detected after a couple of hours but reaches a high level after 24 h (Golzio et al. 2004). Transfer of a large polyelectrolyte across the membrane requires a transient loss of cohesion of the proteolipidic assembly. This dramatic event should nevertheless preserve the cell viability to observe the expression of the activity coded by the plasmid.
Experimental facts: events before, during, and after electropulsation
The plasma membrane is not permeable to hydrophilic molecules such as nucleic acids. Plasmid molecules, due to their negative charge, cannot interact with the cell plasma membrane that bears the same charge. DNA molecules therefore cannot get access to the cytoplasm. No transfected cells are detected in the absence of plasmid or when plasmid is added after the electropulsation. The prepulse incubation time is not very important but to avoid a negative effect of exogenous nucleases should be kept short and processed at a low temperature (Rols et al. 1994). Indeed, gene expression is obtained after applying electric pulses to plasmid/cell mixture after a short incubation (Neumann et al. 1982; Klenchin et al. 1991; Wolf et al. 1994). These results are the same whatever the cell types (bacteria, yeast, and mammalian cells) (Golzio et al. 1998; Eynard et al. 1992; Ganeva et al. 1995). Preadsorption of DNA on the mammalian cell surface is not requested and was indeed reported to prevent expression (Neumann et al. 1982).
Gene electrotransfer is only detected for electric field values leading to membrane permeabilization (Ec > Ep). Transfection threshold values are the same as the ones for cell permeabilization when millisecond pulses are applied (Rols and Teissié 1998). Field strength is observed to have a critical role. Plasmid molecules, negatively charged, migrate when submitted to an electric field (Wolf et al. 1994; Neumann et al. 1992). In the “low” electric field regime (i.e., Ec < Ep), plasmid simply electrophoretically flows along the cell membrane towards the anode. It can be trapped when divalent ions are present (Frantescu et al. 2005; Xie and Tsong 1993).
However, beyond a critical permeabilizing field value (Ec > Ep), two main processes occur: (1) plasma membrane is permeabilized (at the microsecond scale); (2) plasmid undergoes the electrophoretic migration (at the millisecond scale) and interacts with permeabilized membrane (Golzio et al. 2002). Metastable plasmids/membrane complexes are formed and grow as local aggregates (at the millisecond scale). But once the field is turned off, the growth of the plasmid aggregates is stopped. The plasmid/membrane interaction is not homogeneously distributed on the permeabilized areas but is detected in association with membrane competent-like sites whose sizes range from 0.1 to 0.5 μm. This interaction takes place only when the plasma membrane is under permeabilization (a field over Ec must be present) (Golzio et al. 2002). It does not occur if the plasmid molecules are added after electropermeabilizing cells (Neumann et al. 1982; Tsong 1991; Klenchin et al. 1991). Recent works on electric field vectoriality show that the plasmid/membrane interaction is not a simple accumulation of plasmid at the membrane surface level, but a plasmid “irreversible” insertion occurs into the permeabilized membrane. No free plasmid diffusion into the cytoplasm is detected as was proposed in older works (Klenchin et al. 1991).
Under permeabilizing field conditions, the pulse duration plays a critical role in the formation of the plasmid/membrane complexes. These complexes are easily detected when the pulse duration is at least 1 ms, but highly sensitive technologies show that they are formed with shorter pulses (unpublished data). This suggests that the density of defects is critical in the plasmid/membrane interaction. Furthermore, this interpretation is supported by the observation that the plasmid content in the complex is under the control of the field strength (E), the number of successive pulses (N), and the pulse duration (T) (Golzio et al. 2002). The time for reaction with the membrane of the plasmid dragged against permeabilized membrane under the electrophoretic migration is increased by long pulse durations. This again is involved in the positive role of the pulse duration in gene electrotransfer. This contribution of the pulse duration to the plasmid/membrane interaction has already been illustrated by a complex dependence of the gene expression (Wolf et al. 1994). The associated gene expression Expr is shown to obey the following equation:
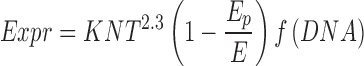 |
as long as the cell viability is not affected to a large extent by the pulse duration (Golzio et al. 2002). All parameters are as described above, with K being a constant and N, the number of electric pulses. Its dependence on the plasmid concentration is rather complex, as high levels of plasmids appear to be toxic (Rols et al. 1992). The practical conclusion is that in vitro an effective transfer is obtained by using long pulses in order to drive the plasmid towards the permeabilized membrane but with low field strength to preserve the cell viability (Rols and Teissié 1998; Kubiniec et al. 1990). Nevertheless, the gene transfection was obtained with short long pulses in the pioneering experiments (Neumann et al 1982), in a very recent report (Kanduser et al. 2009), and with stem cells (Ferreira et al. 2008).
Plasmid/membrane complex remains accessible to DNase I, up to 60 s after the pulsation in the case of CHO cells. The plasmid aggregates, which are anchored in the membrane after the electric field application, remain sensitive to the degrading action of the nucleases that were added externally post-pulse, which are known not to cross the membrane (Eynard et al. 1997). Nevertheless, the opposite observation, reported as a protective effect against DNAse, was detected when using a different protocol (Klenchin et al. 1991). More than 2 s appears to be needed to get a stable plasmid/membrane complex after a 5 ms pulse (Phez et al. 2005). Several minutes after the electropulsation, plasmids are still present on/in the cell surface. The biophysical structure of the membrane plasmid complex has to be explained. Plasmids leave the complex and diffuse in the cytoplasm. Then, plasmids are present at the nucleus surface, but only a small fraction cross the nuclear envelope to be expressed a few hours after the electropulsation (Golzio et al. 2002). These intracellular steps remain rather poorly understood as already mentioned. A positive effect of working at 37°C in all these post-pulse steps was observed (Rols et al. 1994). Hypo-osmolar buffers gave an increase in expression (Golzio et al. 1998). Starving cells prevented expression, suggesting an active contribution of the cell metabolism in transfer and/or expression (Rols et al. 1998b) (Table 1).
Table 1.
Steps in gene electrotransfer. The central column describes the events that occur before, during, and after the electropulsation. The right column reports their time scale
| Steps | Events | Time scale |
|---|---|---|
| Before electropulsation | Plasma membrane is not permeable. Plasmid cannot get access to the cytoplasm. | |
| During electropulsation | Electropermeabilization takes place. | Microseconds |
| Plasmid molecules are electrophoretically driven into contact with the cell surface. | Milliseconds | |
| Metastable complexes are formed between plasmid molecules and the localized electropermeabilized part of cell membrane. | Milliseconds | |
| After electropulsation | Stable complexes result. | Seconds |
| Plasmids leave the complexes and diffuse in the cytoplasm. | Minutes | |
| A small fraction of the plasmid molecules cross the nuclear envelope to be expressed. | Hours | |
| Gene expression occurs. | Days |
Theories of DNA plasmid electroentry
Although the first pioneering report on gene electrotransfer in cells was published more than 25 years ago by E. Neumann, the molecular basis behind the process of gene electrotransfer is highly debated. Different scenarios have been proposed (Fig. 1):
-
(i)
Krassowska’s model supports the simplest mechanism, in which plasmid enters the 5 nm thick membrane through stable macropores (i.e., 20 to 200 nm Ø) (Smith et al. 2004; Krassowska and Filev 2007). The electrically induced defects result from the field-associated membrane potential changes. Their model relies on tension-coupled pores that do not bring membrane rupture [a dramatic feature of the classical Chizmadzhev model (Weaver and Chizmadzhev 1996)]. It predicts a post-pulse growth of macropores on the seconds time scale fairly consistent with experimental evidence (Neu and Krassowska 2003). This model predicts pores large enough to permit the plasmid uptake, even in its circular or supercoiled conformation (Blackburn and Gait 1996). These pores remain open for the entire duration of electropulsation providing adequate time for the plasmid to enter the cell (Sukharev et al. 1994).
Fig. 1.
Differents models of gene electrotransfer. Model i: a The electric pulse induces a macrodefect (2r > 20 nm) and an electrophoretically mediated DNA accumulation. b After the pulse, a free diffusion of DNA takes place across the long-lived macropore. Model ii: a DNA is preadsorbed with the plasma membrane by an interaction with cationic lipids, sphingosines. b DNA is transiently inserted in plasma membrane. c DNA is electrophoretically pulled through the permeabilized zones. d DNA leaves the membrane and enters into the cell. Model iii: a The electric pulses induce the membrane permeabilization. b DNA is concentrated near the membrane surface and pushed through the putative electropores by electrophoretic forces. c The mechanical interaction between the pores and the DNA driven by electrophoretic forces induces an adjustment of pore sizes. c DNA enters into the cell. Model iv: a DNA remains at the interfacial region when no pore is present. b Under high electric field, the DNA diffuses towards the interior of the bilayer after a pore is created beneath it. c Diffusion of the strand toward the interior of the membrane leads to a complex DNA/lipid in which the lipid head groups encapsulate the DNA. d After the electric field pulse, the DNA is translocated. e DNA enters into the cell
This model remains an attractive model in spite of the existence of many experimental contradictions. Indeed, until now, no study made it possible to visualize these membrane pores. This validation appears impossible (Weaver and Chizmadzhev 1996). Moreover, the resealing time of pores appears to be shorter in this model than in experiments (e.g., seconds rather than minutes) (Bier et al. 1999; Golzio et al. 1998; Satkauskas et al. 2002; Tekle et al. 1991). In addition, in the case of CHO cells, plasmid accessibility to DNAase I in the minute following the end of electropulsation shows that the plasmid transfer inside the cell occurs after the electropulsation (Eynard et al. 1997). Of course, no selectivity of the macropore is predicted meaning that DNAases have free access. To date, theoretical models could predict stable pores of only a few nanometers in radius; larger pores are unstable (Freeman et al. 1994; Joshi and Schoenbach 2000) These models are confirmed by some experiments in which high-voltage, short pulses are used that must have created a large number of pores with radii of about 1 nm (Glaser et al. 1988; Kakorin and Neumann 2002; Schwister and Deuticke 1985). To reconcile these results with the experimental evidence of plasmid translocation after electropulsation, some researchers postulated that plasmid entry into cells relies on the plasmid/membrane interactions, which may be facilitated by a coalescence of many small, 1 nm pores (Neumann et al. 1996; Rols and Teissié 1998; Rols et al 1998b; Sukharev et al 1992, 1994). The slow transport of DNA across the electropermeabilized membrane reflects a highly interactive electrotransfer, where many small pores coalesced into large pores that were transiently occluded by DNA (Neumann et al. 1999).
-
(ii)
Other data report that gene electrotransfer through lipid bilayer could be mediated by transient complexes between plasmid and the lipids in the pore edges of elongated, electropercolated hydrophilic pore zones (Hristova et al. 1997). This is present with specific lipids. Moreover, the plasmid association with a lipid bilayer greatly facilitates the transport of small ions. This suggests a locally conductive plasmid/lipid interaction zone where parts of the plasmid may be inserted in the bilayer, leaving other parts of the plasmid probably protruding out from the outer surface of the bilayer. Plasmid is not only transiently inserted in, but also actually electrophoretically pulled through the permeabilized zones onto the other membrane side ultimately leaving the bilayer structure basically intact (Spassova et al. 1994). With such a model, in the case of mammalian cells, the resting potential difference should be the driving force for plasmid translocation after the pulse-induced insertion. This has not been checked yet.
-
(iii)
Previous works suggested that electric pulses induce the membrane permeabilization, then plasmid molecules are concentrated near the membrane surface and pushed through the putative electropores by electrophoretic forces (Winterbourne et al. 1988; Klenchin et al. 1991; Sukharev et al. 1992; Tekle et al. 1994). The mechanical interaction between the pores and the plasmid driven by electrophoretic forces induces an adjustment of pore sizes and/or lifetimes to allow plasmid entry in the cell. The plasmid may interact with the electropermeabilized membrane in three possible ways: (1) The plasmid coil is aligned in an electric field, and at the appropriate pulse polarity it moves toward the permeabilized membrane. The plasmid may interact with a single membrane defect; it becomes enhanced upon plasmid interaction by the action of electrophoretic forces. (2) The passage of the (linearized) plasmid coil can be initiated by penetration of one end of the thread, which then leads the whole molecule through one pore. (3) The plasmid molecule can be involved in two pores (or more) and moving with electrophoretic forces, it cuts the membrane between these pores as a sharp thread can do (Sukharev et al. 1992). Transfer is dependent on electrophoretic forces and is complete at the end of the pulse. Interestingly, hypothesis (1) suggests a deformation of the electric field distribution close to the membrane. The large membrane defect is associated with enhanced membrane conductivity. The electric field lines will focus on the defect carrying more plasmids to it. This is in agreement with the formation of the experimentally detected plasmid aggregates (Golzio et al. 2002).
If the electrophoretic forces are the only driving forces of the plasmid transfer into the cell, similar transfection efficiencies should be obtained for equal ET values (i.e., E, field strength and T, pulse duration). However, in the case of HeLa cells, the number of transfected clones as a function of ET values is different according to whether short or long electric pulses are used (Hui 1995). Then, when the ENT value is constant, transfection rate depends preferentially on T (Rols and Teissié 1998). Therefore, the electrophoretic migration cannot be the only driving force of the plasmid transfer into the cells but clearly supports the formation of aggregates.
-
(iv)
A molecular dynamic approach gives a mechanism by which plasmids do not translocate across the membrane during the electropulsation (Tarek 2005). The DNA/lipid system simulation was undertaken starting from a well-equilibrated 12 bp DNA duplex placed near a model POPC bilayer. The perturbation of the system under a 1.0 V nm−1 transverse electric field is followed for 2 ns. Under this high electric field, the DNA duplex diffuses towards the interior of the bilayer only after the creation of a pore beneath it, and within the same timescale, it remains at the interfacial region when no pore is present. Diffusion of the strand toward the interior of the membrane leads to a DNA/lipid complex in which the lipid head groups encapsulate the strand. The partial charges carried by the zwitterionic phosphatidylcholine groups of the lipids are known to be efficient for neutralizing the charges carried by the DNA (Bandyopadhyay et al. 1999). Such interactions between the plasmid and the lipids contribute to the effective screening of DNA charges and therefore to the stabilization of the complex.
The process described herein provides support to the gene delivery model by Golzio and collaborators (Golzio et al. 2002), in which it is proposed that only localized parts of the cell membrane brought to the permeabilized state are competent for transfer and that the proper transfer of plasmid—that does not require that the electric pulse be maintained—is preceded by an “anchoring step,” connecting the plasmid to the permeabilized membrane, which takes place during the pulse. One should not forget that electropulsation-mediated gene delivery concerns much larger supercoiled plasmids than the 12 bp construct considered here. Transfer of such plasmids is certainly a complex process and all aspects may not be addressed by simulations (Tarek 2005). Nevertheless this model shows that the plasmid is stabilized in the membrane core after electropulsation in agreement with the overall experimentally observed process of DNA translocation. DNA migration from the outer side of the cell membrane to the cytoplasm is beyond this simulation study, and no calculation was carried out to follow the resealing process.
Conclusions
The molecular mechanisms involved in the phenomenon of gene electrotransfer remain poorly understood. The study of the mechanisms of gene electrotransfer shows that permeabilization is necessary but that the mechanism of the transfer of the DNA molecules is different from that of the small molecules (Table 1) (Teissié et al. 2005). The current models described in this review are sometimes contradictory and do not always fit the experimental facts (Fig. 1). Indeed, experimental observations showed that the electrotransfer is a slow mechanism. This is in contradiction with the prediction of models i and iii. Models iii and iv are in agreement with a localized plasmid/membrane interaction (so-called competent membrane sites). These sites appear to be created by the electropermeabilization process by altering the field distribution (Freeman et al. 1994). This occurs through the perturbation of the transmembrane voltage by a conducting defect. A spatially inhomogeneous electric field is expected within the outer buffer near electrodefects (Pastushenko and Chizmadzhev 1982; Powell and Weaver 1986; Barnett and Weaver 1991 ; Weaver 1993). This brings a nonzero component of the electric field parallel to the membrane surface, i.e., the equipotentials near the membrane have significant, nonlinear gradients. For this reason, the current flowing through a membrane electrodefect not only results in a potential drop within the membrane, but also in the buffer near the defects (Weaver and Chizmadzhev 1996). Model ii suggests a putative method for transmembrane transfer and needs further investigations. One major problem with these theoretical models is that they consider only the lipid assembly description of a membrane while the presence of membrane proteins may affect the electropermeabilization of lipid bilayers by changing their mechanical properties (Troiano et al. 1999).
The knowledge and the control of the biophysical mechanisms of gene electrotransfer are necessary to evaluate their consequences in biological and physiological terms and their effectiveness for the development of clinical and biotechnological protocols.
Acknowledgments
This work was supported by the CNRS, the AFM (Association Française pour les Myopathies), the ANR Cemirbio, and the région Midi-Pyrénées.
Footnotes
J.-M. Escoffre and C. Mauroy have contributed equally to this work.
Contributor Information
Marie-Pierre Rols, Email: rols@ipbs.fr.
Justin Teissié, Email: Justin.Teissie@ipbs.fr.
References
- Aihara H, Miyazaki JI. Gene transfer into muscle by electroporation in vivo. Nature Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Tarek M, Klein ML. Molecular dynamics study of lipid-DNA complexes. J Phys Chem B. 1999;103:10075–10080. doi: 10.1021/jp9927496. [DOI] [Google Scholar]
- Barnett A, Weaver JC. Electroporation: a unified, quantitative theory of reversible electrical breakdown and rupture. Bioelectrochem Bioenerg. 1991;25:163182. doi: 10.1016/0302-4598(91)87001-W. [DOI] [Google Scholar]
- Bier M, Hammer SM, Canaday DJ, Lee RC. Kinetics of resealing for transient electropores in isolated mammalian skeletal muscle cells. Bioelectromagnetics. 1999;20:194–201. doi: 10.1002/(SICI)1521-186X(1999)20:3<194::AID-BEM6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Blackburn GM, Gait MJ. Nucleic acids in chemistry and biology. Oxford: Oxford University Press; 1996. [Google Scholar]
- Cemazar M, Sersa G. Electrotransfer of therapeutics molecules into tissues. Curr Opin Mol Ther. 2007;9:554–562. [PubMed] [Google Scholar]
- Escoffre JM, Hubert M, Dean DS, Rols MP, Favard C. Membrane perturbation by an external electric field: a mechanism to permit molecular uptake. Eur Biophys J. 2007;36:973–983. doi: 10.1007/s00249-007-0194-7. [DOI] [PubMed] [Google Scholar]
- Eynard N, Sixou S, Duran N, Teissié J. Fast kinetics studies of Escherichia coli electrotransformation. Eur J Biochem. 1992;209:431–436. doi: 10.1111/j.1432-1033.1992.tb17306.x. [DOI] [PubMed] [Google Scholar]
- Eynard N, Rols MP, Ganeva V, Galutzov B, Sabri N, Teissié J. Electrotransformation pathways of prokaryotic and eukaryotic cells: recents developments. Bioelectrochem Bioenerg. 1997;44:103–110. doi: 10.1016/S0302-4598(97)00038-X. [DOI] [Google Scholar]
- Ferreira E, Potier E, Logeart-Avramoglou D, Salomskaite-Davalgiene S, Mir LM, Petite H. Optimization of a gene electrotransfer method for mesenchymal stem cell transfection. Gene Ther. 2008;15:537–544. doi: 10.1038/gt.2008.9. [DOI] [PubMed] [Google Scholar]
- Frantescu A, Kakorin S, Toensing K, Neumann E. Adsorption of DNA and electric fields decrease the rigidity of lipid vesicle membranes. Phys Chem Chem Phys. 2005;7:4126–4131. doi: 10.1039/b510882a. [DOI] [PubMed] [Google Scholar]
- Freeman SA, Wang MA, Weaver JC. Theory of electroporation of planar bilayer membranes: predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys J. 1994;67:42–56. doi: 10.1016/S0006-3495(94)80453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeva V, Galutzov B, Teissié J. Fast kinetics studies of plasmid DNA transfer in intact yeast cells mediated by electropulsation. Biochem Biophys Res Commun. 1995;214:825–832. doi: 10.1006/bbrc.1995.2361. [DOI] [PubMed] [Google Scholar]
- Glaser RW, Leikin SL, Chernomordik LV, Pastushenko VF, Sokirko AI. Reversible electrical breakdown of lipid bilayer: formation and evolution of pores. Biochim Biophys Acta. 1988;940:275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Golzio M, Mora MP, Raynaud C, Delteil C, Teissié J, Rols MP. Control by osmotic pressure of voltage-induced permeabilization and gene transfer in mammalian cells. Biophys J. 1998;74:3015–3022. doi: 10.1016/S0006-3495(98)78009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio M, Teissié J, Rols MP. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc Natl Acad Sci USA. 2002;99:1292–1297. doi: 10.1073/pnas.022646499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio M, Rols MP, Teissié J. In vitro and in vivo electric-field-mediated permeabilization, gene transfer, and expression. Methods. 2004;33:126–135. doi: 10.1016/j.ymeth.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Heller R, Jaroszeski M, Atkin A, Moradpour D, Gilbert R, Wands J, Nicolau C. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 1996;389:225–228. doi: 10.1016/0014-5793(96)00590-X. [DOI] [PubMed] [Google Scholar]
- Hristova NI, Tsoneva I, Neumann E. Sphingosine-mediated electroporative DNA transfer through lipid bilayers. FEBS Lett. 1997;415:81–86. doi: 10.1016/S0014-5793(97)01097-1. [DOI] [PubMed] [Google Scholar]
- Hui SW. Effects of pulse length and strength on electroporation efficiency. Methods Mol Biol. 1995;55:29–40. doi: 10.1385/0-89603-328-7:29. [DOI] [PubMed] [Google Scholar]
- Joshi RP, Schoenbach KH. Electroporation dynamics in biological cells subjected to ultrafast electrical pulses: a numerical simulation study. Phys Rev E. 2000;62:1025–1033. doi: 10.1103/PhysRevE.62.1025. [DOI] [PubMed] [Google Scholar]
- Kakorin S, Neumann E. Ionic conductivity of electroporated lipid bilayer membranes. Bioelectrochem. 2002;56:163–166. doi: 10.1016/S1567-5394(02)00040-3. [DOI] [PubMed] [Google Scholar]
- Kanduser M, Miklavcic D, Pavlin M. Mechanisms involved in gene electrotransfer using high- and low-voltage pulse—an in vitro study. Bioelectrochem. 2009;74:265–271. doi: 10.1016/j.bioelechem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Klenchin VA, Sukharev SI, Serov SM, Chernomordik LV, Chizmadzev YuA. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys J. 1991;60:804–811. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassowska W, Filev PD. Modeling electroporation in a single cell. Biophys J. 2007;92:404–417. doi: 10.1529/biophysj.106.094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiniec RT, Liang H, Hui SW (1990) Effects of pulse length andpulse strength on transfection by electroporation. BioTechniques8:16 –20 [PubMed]
- Liu F, Huang L. Electric gene transfer to the liver following systemic administration of plasmid DNA. Gene Ther. 2002;9:1116–1119. doi: 10.1038/sj.gt.3301733. [DOI] [PubMed] [Google Scholar]
- Miller AM, Dean DA. Tissue-specific and transcription factor-mediated nuclear entry of DNA. Adv Drug Deliv Rev. 2009;61:603–613. doi: 10.1016/j.addr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Mir LM, Roth C, Orlowski S, Quintin Colonna F, Fradelizi D, et al. Systemic antitumor effects of electrochemotherapy combined with histocompatible cells secreting interleukin-2. J Immunother Emphasis Tumor Immunol. 1995;17:30–38. doi: 10.1097/00002371-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Mir LM, Glass LF, Sersa G, Teissié J, Domenge C, Miklavcic D, et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br J Cancer. 1998;77:2336–2342. doi: 10.1038/bjc.1998.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir LM, Gehl J, Sersa G, Collins CG, Garbay JR, Billard V, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur J Cancer. 2006;42(suppl 4):14–25. [Google Scholar]
- Neu JC, Krassowska W. Modeling postshock evolution of large electropores. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;67:021915. doi: 10.1103/PhysRevE.67.021915. [DOI] [PubMed] [Google Scholar]
- Neumann E, Shaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E, Sowers AE, Jordan CA. Electroporation and electrofusion in cell biology. New York: Plenum press; 1989. [Google Scholar]
- Neumann E, Werner E, Sprafke A, Kruger K. Electroporation phenomena. Electrooptics of plasmid DNA and of lipid bilayer vesicles. In: Jennings BR, Stoylov SP, editors. Colloid and molecular electro-optics. Bristol: IOP; 1992. pp. 197–206. [Google Scholar]
- Neumann E, Kakorin S, Tsoneva I, Nikolova B, Tomov T. Calcium-mediated DNA adsorption to yeast cells and kinetics of cell transformation by electroporation. Biophys J. 1996;71:868–877. doi: 10.1016/S0006-3495(96)79288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E, Kakorin S, Toensing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem Bioenerg. 1999;48:3–16. doi: 10.1016/S0302-4598(99)00008-2. [DOI] [PubMed] [Google Scholar]
- Orlowski S, Mir LM. Cell electropermeabilization: a new tool for biochemical and pharmacological studies. Biochim Biophys Acta. 1993;1154:51–63. doi: 10.1016/0304-4157(93)90016-h. [DOI] [PubMed] [Google Scholar]
- Pastushenko VF, Chizmadzhev YAQ. Stabilization of conducting pores in BLM by electric current. Gen Physiol Biophys. 1982;1:43–52. [Google Scholar]
- Phez E, Faurie C, Golzio M, Teissié J, Rols MP. New insights in the visualization of membrane permeabilization and DNA/membrane interaction of cells submitted to electric pulses. Biochim Biophys Acta. 2005;1724:248–254. doi: 10.1016/j.bbagen.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pouton CW, Wagstaff KM, Roth DM, Moseley GW, Jans DA. Targeted delivery to the nucleus. Adv Drug Deliv Rev. 2007;59:698–717. doi: 10.1016/j.addr.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Powell KT, Weaver JC. Transient aqueous pores in bilayer membranes: a statistical theory. Bioelectrochem Bioenerg. 1986;15:211–227. doi: 10.1016/0302-4598(86)80029-0. [DOI] [Google Scholar]
- Rols MP, Teissié J. Electropermeabilization of mammalian cells to macromolecules: control by pulse duration. Biophys J. 1998;75:1415–1423. doi: 10.1016/S0006-3495(98)74060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rols MP, Coulet D, Teissié J. Highly efficient transfection of mammalian cells by electric field pulses. Application to large volumes of cell culture by using a flow system. Eur J Biochem. 1992;206:115–121. doi: 10.1111/j.1432-1033.1992.tb16908.x. [DOI] [PubMed] [Google Scholar]
- Rols MP, Delteil C, Serin G, Teissié J. Temperature effects on electrotransfection of mammalian cells. Nucleic Acid Res. 1994;22:540. doi: 10.1093/nar/22.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rols MP, Delteil C, Golzio M, Dumond P, Cros S, Teissié J. In vivo electrically mediated protein and gene transfer in murine melanoma. Nat Biotechnol. 1998;16:168–171. doi: 10.1038/nbt0298-168. [DOI] [PubMed] [Google Scholar]
- Rols MP, Delteil C, Teissié J. Control by ATP and ADP of voltage-induced mammalian-cell-membrane permeabilization, gene transfer and resulting expression. Eur J Biochem. 1998;254:382–388. doi: 10.1046/j.1432-1327.1998.2540382.x. [DOI] [PubMed] [Google Scholar]
- Satkauskas S, Bureau MF, Puc M, Mahfoudi A, Scherman D, Miklavcic D, Mir LM. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol Ther. 2002;5:133–140. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- Schwister K, Deuticke B. Formation and properties of aqueous leaks induced in human erythrocytes by electrical breakdown. Biochim Biophys Acta. 1985;816:332–348. doi: 10.1016/0005-2736(85)90501-2. [DOI] [PubMed] [Google Scholar]
- Sersa G, Miklavcic D, Cemazar M, Rudolf Z, Pucihar G, Snoj M. Electrochemotherapy in treatment of tumours. Eur J Surg Oncol. 2008;34:232–240. doi: 10.1016/j.ejso.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Smith KC, Neu JC, Krassowska W. Model of creation and evolution of stables electropores for DNA delivery. Biophys J. 2004;86:2813–2826. doi: 10.1016/S0006-3495(04)74334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova M, Tsoneva I, Petrov AG, Petkova JI, Neumann E. Dip patch clamp currents suggest electrodiffusive transport of the polyelectrolyte DNA through lipid bilayers. Biophys Chem. 1994;52:267–274. doi: 10.1016/0301-4622(94)00097-4. [DOI] [PubMed] [Google Scholar]
- Sukharev SI, Klenchin VA, Serov SM, Chernomordik LV, Chizmadzhev YuA. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys J. 1992;63:1320–1327. doi: 10.1016/S0006-3495(92)81709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev SI, Titomirov AV, Klenchin VA. Electrically induced DNA transfer into cells. Electroporation in vivo. In: Wolff JA, editor. Gene therapeutics: methods and applications of direct gene transfer. Boston: Birkhäuser; 1994. pp. 210–232. [Google Scholar]
- Tarek M. Membrane electroporation: a molecular dynamics simulation. Biophys J. 2005;88:4015–4053. doi: 10.1529/biophysj.104.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissié J, Golzio M, Rols MP. Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of?) knowledge. Biochim Biophys Acta. 2005;1724:270–280. doi: 10.1016/j.bbagen.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Tekle E, Astumian RD, Chock PB. Electroporation by using bipolar oscillating electric field: an improved method for DNA transfection of NIH 3T3 cells. Proc Natl Acad Sci USA. 1991;88:4230–4234. doi: 10.1073/pnas.88.10.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekle E, Astumian RD, Chock PB. Selective and asymmetric molecular transport across electroporated cell membranes. Proc Natl Acad Sci USA. 1994;91:11512–11516. doi: 10.1073/pnas.91.24.11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titomirov AV, Sukharev S, Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta Rep. 1991;1088:131–134. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]
- Troiano GC, Stebe KJ, Raphael RM, Tung L. The effects of gramicidin on electroporation of lipid bilayers. Biophys J. 1999;76:3150–3157. doi: 10.1016/S0006-3495(99)77466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong TY. Electroporation of cell membranes. Biophys J. 1991;60:297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Aa MA, Mastrobattista E, Oosting RS, Hennik WE, Koning GA, Crommelin DJ. The nuclear pore complex: the gateway to successful nonviral gene delivery. Pharm Res. 2006;23:447–459. doi: 10.1007/s11095-005-9445-4. [DOI] [PubMed] [Google Scholar]
- Van der Aa MA, Mastrobattista E, Oosting RS, Hennink WE, Koning GA, Crommelin DJ. The nuclear pore complex: the gateway to successful nonviral gene delivery. Adv Drug Deliv Rev. 2007;59:698–717. doi: 10.1016/j.addr.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Vandermeulen G, Staes E, Vanderhaeghen ML, Bureau MF, Scherman D, Préat V. Optimisation of intradermal DNA electrotransfer for immunisation. J Control Res. 2007;124:81–87. doi: 10.1016/j.jconrel.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Weaver JC. Electroporation: a general phenomenon for manipulating cells and tissues. J Cell Biochem. 1993;51:426–435. doi: 10.1002/jcb.2400510407. [DOI] [PubMed] [Google Scholar]
- Weaver JC, Chizmadzhev YA. Theory of electroporation: a review. Bioelectrochem Bioenerg. 1996;41:135–160. doi: 10.1016/S0302-4598(96)05062-3. [DOI] [Google Scholar]
- Winterbourne DJ, Thomas S, Hermon-Taylor J, Hussain I, Johnstone AP. Electric-shock-mediated transfection of cells. Biochem J. 1988;251:427–434. doi: 10.1042/bj2510427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H, Rols MP, Boldt E, Neumann E, Teissié J. Control by pulse parameters of electric field-mediated gene transfer in mammalian cells. Biophys J. 1994;66:524–531. doi: 10.1016/S0006-3495(94)80805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie TD, Tsong TY. TY Study of mechanisms of electric field-induced DNA transfection. V. Effects of DNA topology on surface binding, cell uptake, expression, and integration into host chromosomes of DNA in the mammalian cell. Biophys J. 1993;65:1684–1689. doi: 10.1016/S0006-3495(93)81208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]



